Behavioral Interactions and Mate Compatibility Influence the Reproductive Success of New England Cottontails (Sylvilagus transitionalis) in a Conservation Breeding Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Housing
2.2. NEC Observations
2.3. Interobserver Reliability
2.4. Data Analysis
3. Results
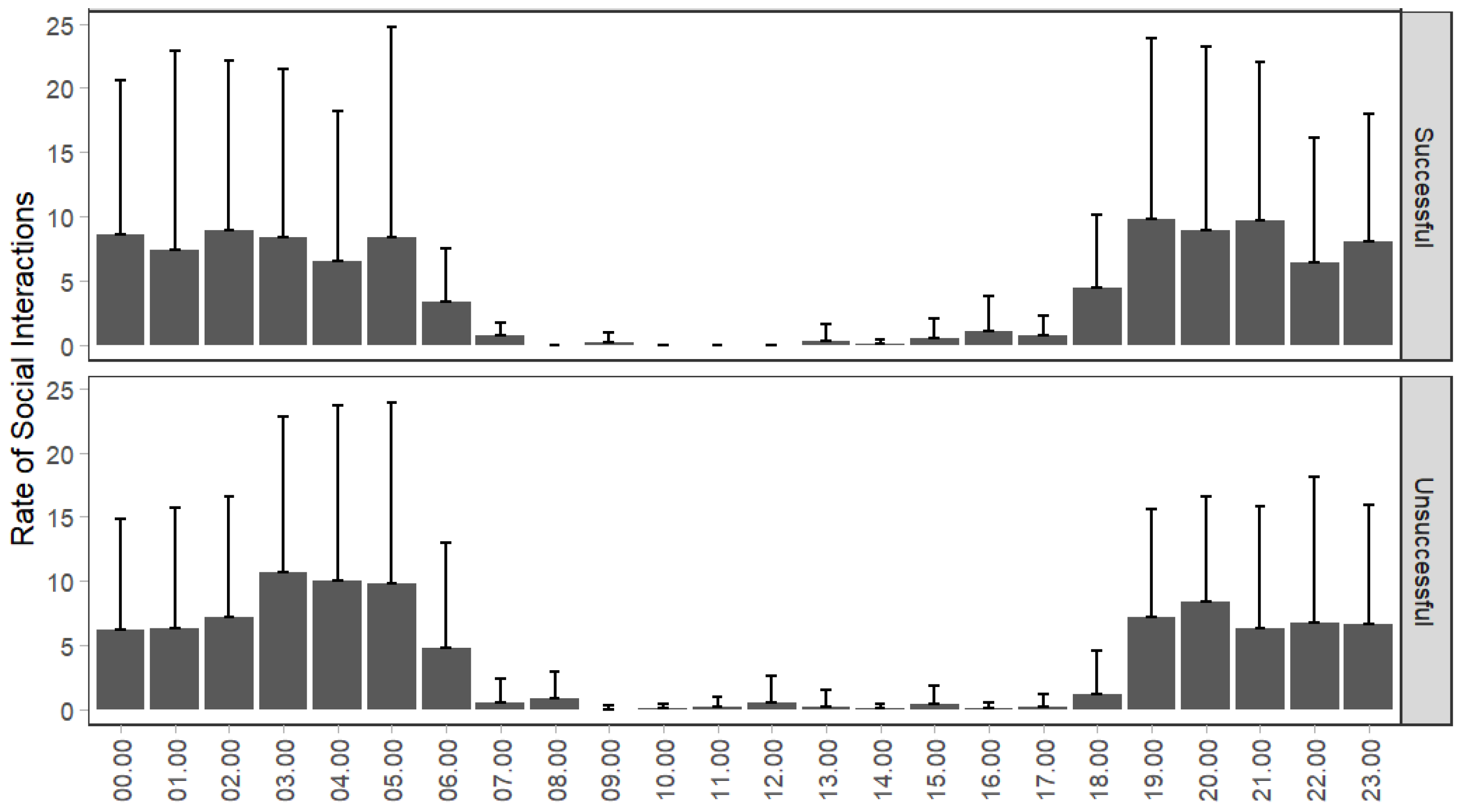
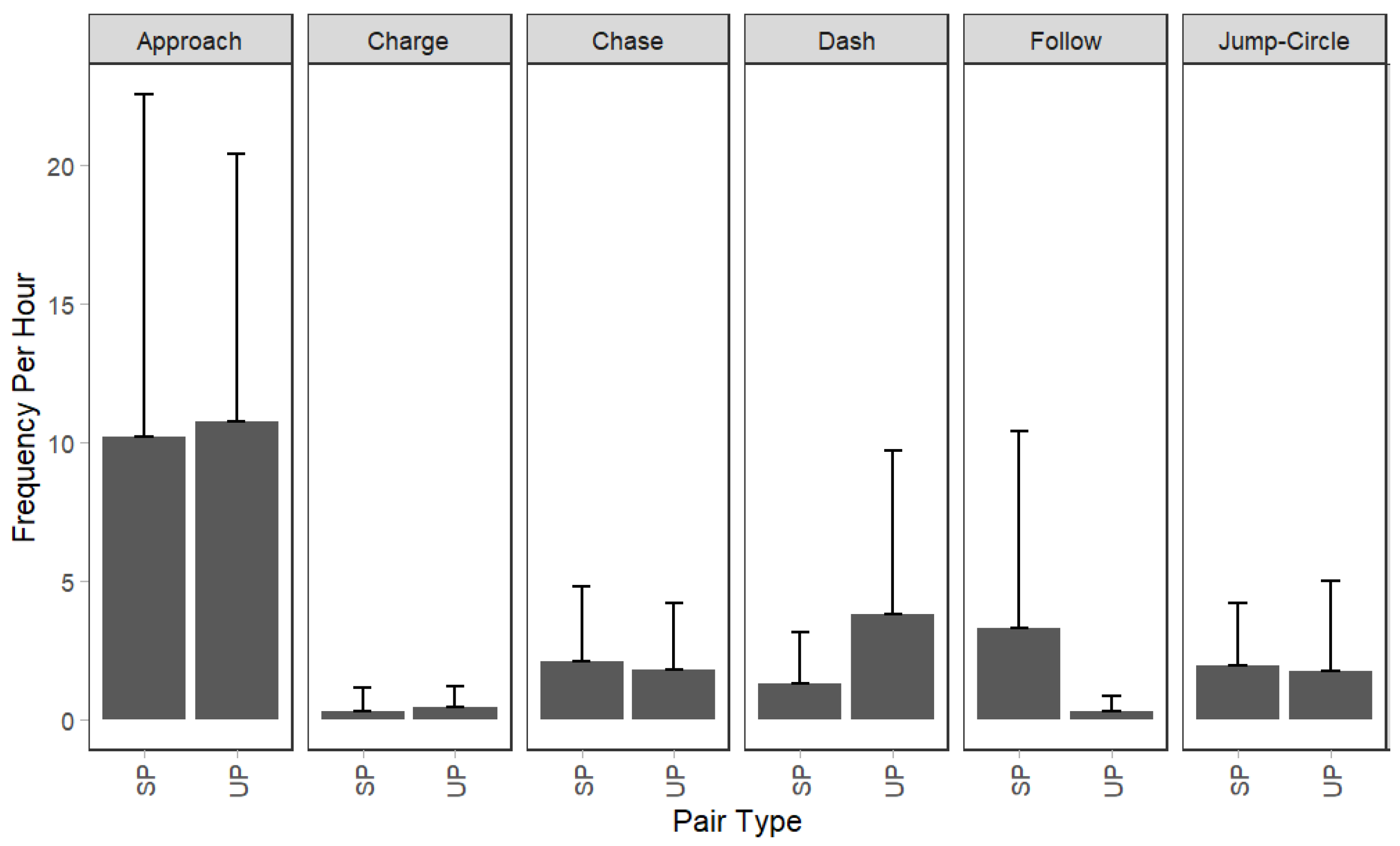
3.1. Behavioral Differences between SPs and UPs
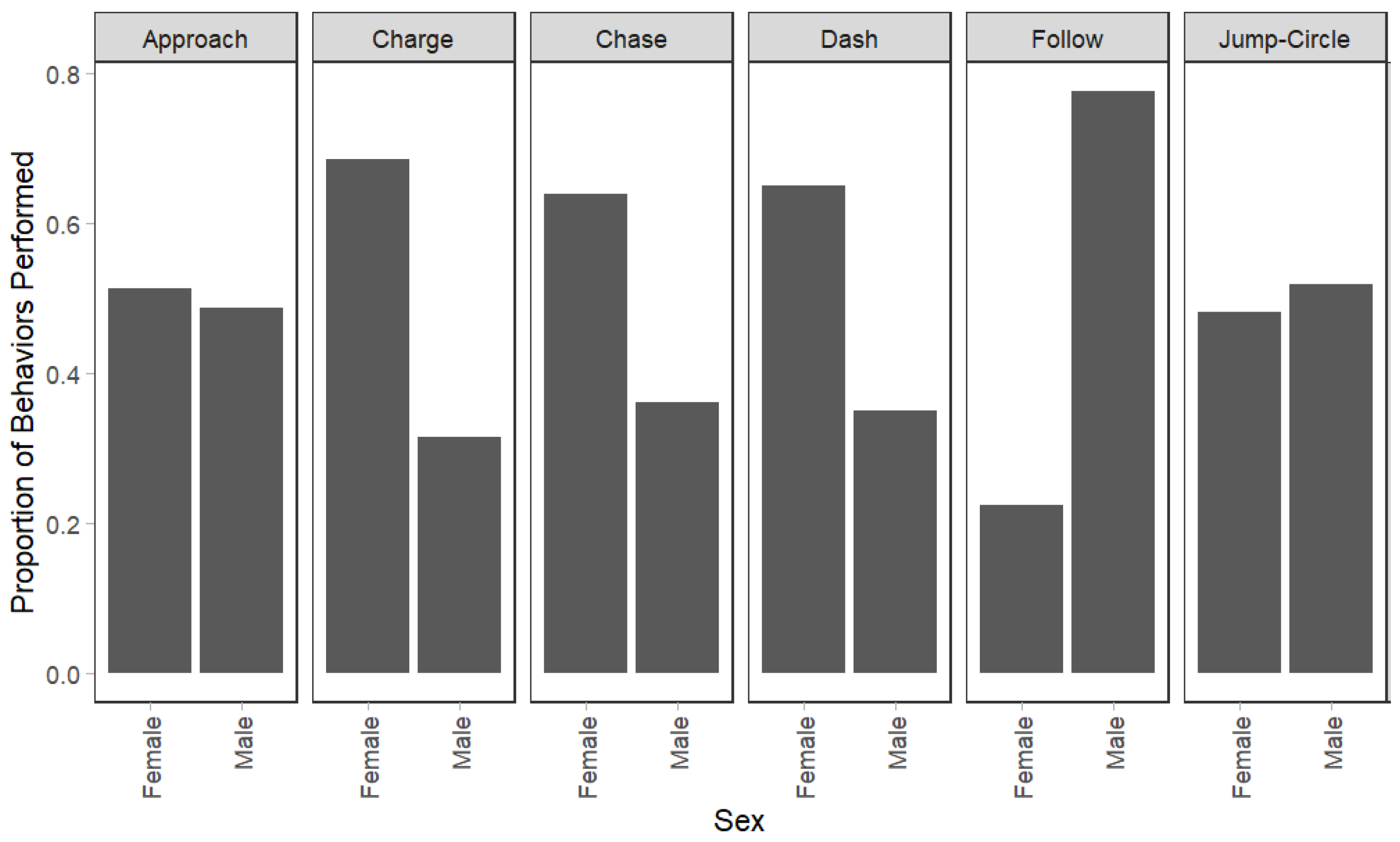
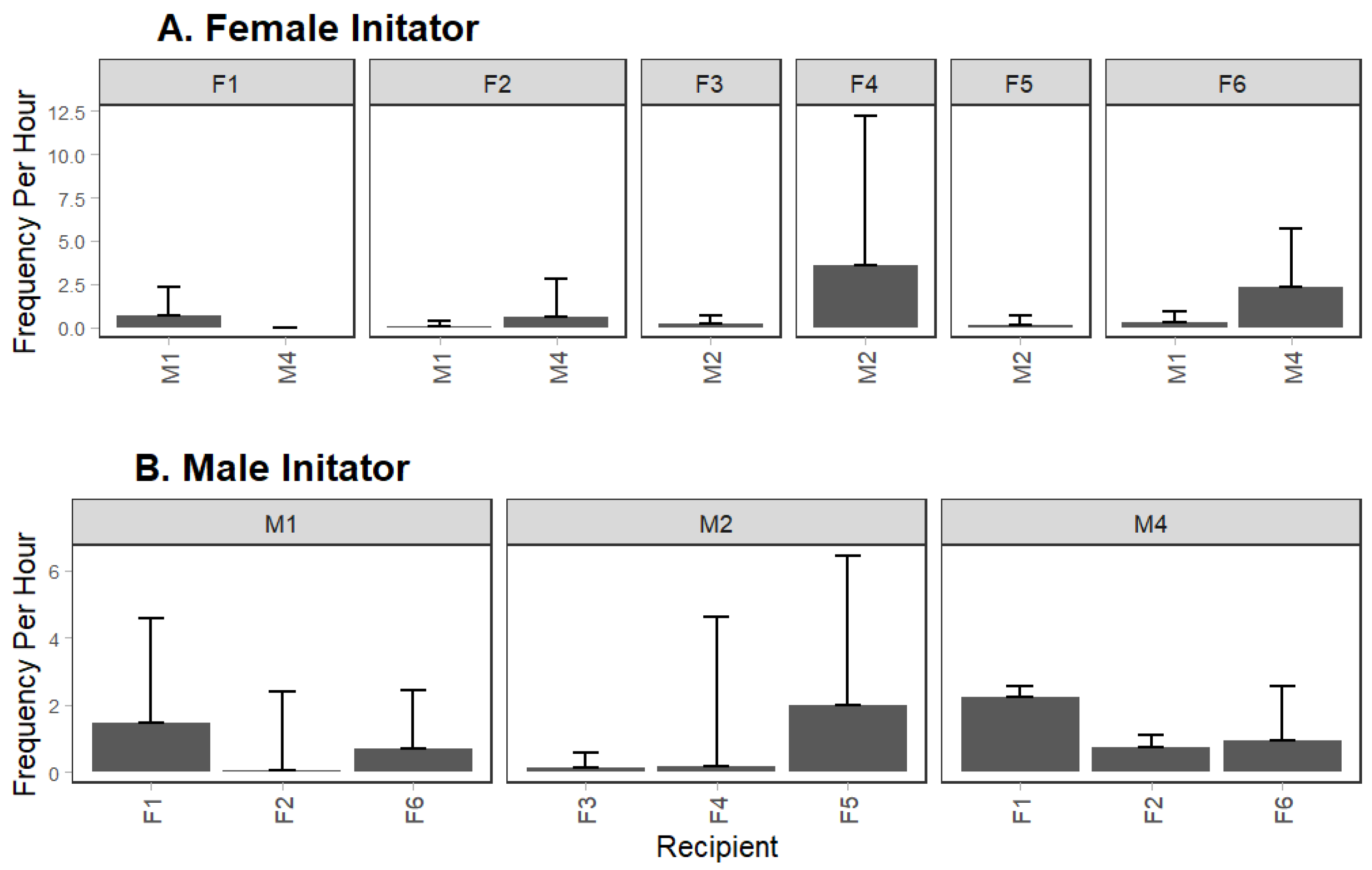
3.2. Variation in Behavioral Frequency by Sex and Individuals
4. Discussion
4.1. Study Design and Diel Activity
4.2. Gestation Length
4.3. Behavioral Differences between SPs and UPs
4.4. Ecology and Mate Choice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, J.A.; Flux, J.E. Introduction to the Lagomorpha. In Lagomorph Biology: Evolution, Ecology, and Conservation; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–9. [Google Scholar]
- Litvaitis, J.; Lanier, H.C. Sylvilagus transitionalis. In The IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 2019. [Google Scholar]
- Fenderson, L.E.; Kovach, A.I.; Litvaitis, J.A.; O’Brien, K.M.; Boland, K.M.; Jakubas, W.J. A multiscale analysis of gene flow for the New England cottontail, an imperiled habitat specialist in a fragmented landscape. Ecol. Evol. 2014, 4, 1853–1875. [Google Scholar] [CrossRef] [PubMed]
- Litvaitis, J.A.; Tash, T.P.; Litvaitis, M.K.; Marchand, M.N.; Kovach, A.I.; Innes, R. A Range-Wide Survey to Determine the Current Distribution of New England Cottontails Range-Wide Survey to Determine the Current Distribution of New England Cottontails. Wildl. Soc. Bull. 2006, 34, 1190–1197. [Google Scholar] [CrossRef]
- Brown, J.; Puccia, L. Ex situ breeding program with wild-caught founders provides the source for a collaborative effort to augment threatened New England cottontail populations. J. Zool. Bot. Gard. 2022, 3, 573–580. [Google Scholar] [CrossRef]
- Casteel, D.A. Timing of Ovulation and Implantation in the Cottontail Rabbit. J. Wildl. Manag. 1967, 31, 194–197. [Google Scholar] [CrossRef]
- Swihart, R.K. Body size, breeding season length, and life history tactics of lagomorphs. Oikos 1984, 43, 282–290. [Google Scholar] [CrossRef]
- Chapman, J.A.; Harman, A.L.; Samuel, D.E. Reproductive and physiological cycles in the cottontail complex in western Maryland and nearby West Virginia. Wildl. Monogr. 1977, 56, 3–73. [Google Scholar]
- Hawkins, M.; Battaglia, A. Breeding behavior of the platypus (Ornithorhynchus anatinus) in captivity. Aust. J. Zool. 2009, 57, 283–293. [Google Scholar] [CrossRef]
- Settle, R.A.; Ettling, J.A.; Wanner, M.D.; Schuette, C.D.; Briggler, J.T.; Mathis, A. Quantitative behavioral analysis of first successful captive breeding of endangered Ozark hellbenders. Front. Ecol. Evol. 2018, 6, 205. [Google Scholar] [CrossRef]
- Wielebnowski, N.C. Behavioral differences as predictors of breeding status in captive cheetahs. Zoo Biol. 1999, 18, 335–349. [Google Scholar] [CrossRef]
- Martin-Wintle, M.S.; Shepherdson, D.; Zhang, G.; Huang, Y.; Luo, B.; Swaisgood, R.R. Do opposites attract? Effects of personality matching in breeding pairs of captive giant pandas on reproductive success. Biol. Conserv. 2017, 207, 27–37. [Google Scholar] [CrossRef]
- Perrotti, L. (Roger Williams Park Zoo, Providence, RI, USA). Personal Communication, 2024.
- Poisson, M.K.; Butler, A.R.; Tate, P.; Bergeron, D.H.; Moll, R.J. Species-specific responses of mammal activity to exurbanization in New Hampshire, USA. J. Urban Ecol. 2023, 9, juad010. [Google Scholar] [CrossRef]
- Chapman, J.A.; Cramer, K.L.; Dippenaar, N.J.; Robinson, T.J. Systematics and geography of the New England cottontail, Sylvilagus transitionalis (Bangs, 1985), with the description of a new species from the Appalachian Mountains. Proc. Biol. Soc. Wash. 1992, 105, 841–866. [Google Scholar]
- Tefft, B.C.; Chapman, J.A. Social behavior of the New England cottontail, Sylvilagus transitionalis (Bangs) with a review of social behavior in New World rabbits (Mammalia: Leporidae). Rev. d’Ecol. Terre Vie 1987, 42, 235–276. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations methods. Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Bateson, M.; Martin, P. Measuring Behavior: An Introductory Guide; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Kaufman, A.B.; Rosenthal, R. Can you believe my eyes? The importance of interobserver reliability statistics in observations of animal behavior. Anim. Behav. 2009, 78, 1487. [Google Scholar] [CrossRef]
- Macirone, C.; Walton, A. Fecundity of male rabbits as determined by “dummy matings”. J. Agric. Sci. 1938, 28, 122–134. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 September 2021).
- Kohl, M. MKinfer: Inferential Statistics. R Package Version 1.1. 2023. Available online: https://www.stamats.de (accessed on 1 April 2024).
- Adams, C.E. Stimulation of reproduction in captivity of the wild rabbit, Oryctolagus cuniculus. Reproduction 1974, 43, 97–102. [Google Scholar] [CrossRef]
- Murphy, W.F., Jr.; Scanlon, P.F.; Kirkpatrick, R.L. Induction of ovulation and pregnancy in captive cottontall rabbits by exogenous gonadotrophin treatment and artificial insemination. Theriogenology 1975, 4, 101–109. [Google Scholar] [CrossRef]
- O’Connor, K.M.; Rittenhouse, T.A. Temporal activity levels of mammals in patches of early successional and mature forest habitat in eastern Connecticut. Am. Midl. Nat. 2017, 177, 15–28. [Google Scholar] [CrossRef]
- Holler, N.R.; Marsden, H.M. Onset of evening activity of swamp rabbits and cottontails in relation to sunset. J. Wildl. Manag. 1970, 34, 349–353. [Google Scholar] [CrossRef]
- Villafuerte, R.; Kufner, M.B.; Delibes, M.; Moreno, S. Environmental factors influencing the seasonal daily activity of the European rabbit (Oryctolagus cuniculus) in a Mediterranean area. Mammalia 1993, 57, 341–348. [Google Scholar] [CrossRef]
- Dalke, P. The Cottontail Rabbits in Connecticut. In State Geological and Natural History Survey; Generic: Homebush, Australia, 1942; Volume 65, pp. 60–81. [Google Scholar]
- Scarlata, C.D.; Elias, B.A.; Godwin, J.R.; Powell, R.A.; Shepherdson, D.; Shipley, L.A.; Brown, J.L. Relationship between fecal hormone concentrations and reproductive success in captive pygmy rabbits (Brachylagus idahoensis). J. Mammal. 2012, 93, 759–770. [Google Scholar] [CrossRef]
- Marsden, H.M.; Conaway, C.H. Behavior and the reproductive cycle in the cottontail. J. Wildl. Manag. 1963, 27, 161–170. [Google Scholar] [CrossRef]
- Elias, B.A.; Shipley, L.A.; Sayler, R.D.; Lamson, R.S. Mating and parental care in captive pygmy rabbits. J. Mammal. 2006, 87, 921–928. [Google Scholar] [CrossRef]
- Stumpf, R.M.; Boesch, C. The efficacy of female choice in chimpanzees of the Taï Forest, Côte d’Ivoire. Behav. Ecol. Sociobiol. 2006, 60, 749–765. [Google Scholar] [CrossRef]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994; Volume 72. [Google Scholar]
- Hamilton, W.D.; Zuk, M. Heritable true fitness and bright birds: A role for parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.C. Mate choice and reproductive success of pikas. Anim. Behav. 1989, 37, 118–132. [Google Scholar] [CrossRef]
- Hohmann, G.; Fruth, B. Intra-and inter-sexual aggression by bonobos in the context of mating. Behaviour 2003, 1, 1389–1413. [Google Scholar]
- Bishop, C.R.; Burgess, M.E. Reproductive physiology, normal neonatology, and neonatal disorders of rabbits. In Management of Pregnant and Neonatal Dogs, Cats, and Exotic Pets; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 217–238. [Google Scholar]
- Dougherty, L.R. Designing mate choice experiments. Biol. Rev. 2020, 95, 759–781. [Google Scholar] [CrossRef]
- Kilpatrick, H.J.; Goodie, T.J. Spatial use and survival of sympatric populations of New England and eastern cottontails in Connecticut. J. Fish Wildl. Manag. 2020, 11, 3–10. [Google Scholar] [CrossRef]
- Martin, M.S.; Shepherdson, D.J. Role of familiarity and preference in reproductive success in ex-situ breeding programs. Conserv. Biol. 2021, 26, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Lacey, E.A.; Wieczorek, J.R.; Tucker, P.K. Male mating behaviour and patterns of sperm precedence in Arctic ground squirrels. Anim. Behav. 1997, 53, 767–779. [Google Scholar] [CrossRef]
- Soulsbury, C.D. Ovulation mode modifies paternity monopolization in mammals. Biol. Lett. 2010, 6, 39–41. [Google Scholar] [CrossRef] [PubMed]

| Behavior | Definition |
|---|---|
| Approach | One cottontail moves toward a recipient cottontail to a distance within three body lengths or less. |
| Dash | One cottontail moves towards a recipient. The trajectory of the approaching individual leads them past the approached individual within a distance of three body lengths or less from the recipient. |
| Charge | Following an approach, one of the individuals rapidly moves toward the other. |
| Follow | Results when an individual is approached, moves away, and is followed at slow speed by the approaching individual. |
| Chase | Results when an individual is approached, moves away rapidly, and is followed at high speed by the approaching individual. |
| Jump-Circle | After an approach, an individual rapidly exchanges places one or more times with the recipient; this may include low jumping over the other individual or continuous place exchange. |
| Attempted Mount | An individual climbs onto the back of the other while stationary or while moving; the individual may or may not hold on to the recipient’s sides with forelimbs. Pelvic thrusting is absent. |
| Mount (presumed copulation) Ejaculatory Fall-Off | An individual climbs onto the recipient’s back while stationary or while moving and holds the recipient’s sides with forelimbs; pelvic thrusting occurs. During a mount, the mounting cottontail’s hindlegs are thrown forward and the mounter falls backward off the recipient [19]. |
| Pair ID | Date of First Copulation | Time of First Copulation | Time till First Copulation (min) | Mount | Attempted Mounts | Gestation (Days) | Kits Born | Kits Released |
|---|---|---|---|---|---|---|---|---|
| M3F3 | 24 March 2019 | 03:46 | 2236 | 1 | 1 | 32 | 5 | 2 |
| M3F4 | 30 March 2019 | 03:23 | 2149 | 2 | 0 | 31 | 4 | 0 |
| M4F6 | 31 March 2019 | 23:48 | 494 | 19 | 171 | 32 | 5 | 3 |
| Avg. | 31.67 | 4.67 | 1.67 | |||||
| Sd. | ±0.57 | ±0.57 | ±1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petit, H.; Perrotti, L.; Richard, J.T. Behavioral Interactions and Mate Compatibility Influence the Reproductive Success of New England Cottontails (Sylvilagus transitionalis) in a Conservation Breeding Program. J. Zool. Bot. Gard. 2024, 5, 507-519. https://doi.org/10.3390/jzbg5030034
Petit H, Perrotti L, Richard JT. Behavioral Interactions and Mate Compatibility Influence the Reproductive Success of New England Cottontails (Sylvilagus transitionalis) in a Conservation Breeding Program. Journal of Zoological and Botanical Gardens. 2024; 5(3):507-519. https://doi.org/10.3390/jzbg5030034
Chicago/Turabian StylePetit, Hannah, Louis Perrotti, and Justin T. Richard. 2024. "Behavioral Interactions and Mate Compatibility Influence the Reproductive Success of New England Cottontails (Sylvilagus transitionalis) in a Conservation Breeding Program" Journal of Zoological and Botanical Gardens 5, no. 3: 507-519. https://doi.org/10.3390/jzbg5030034




