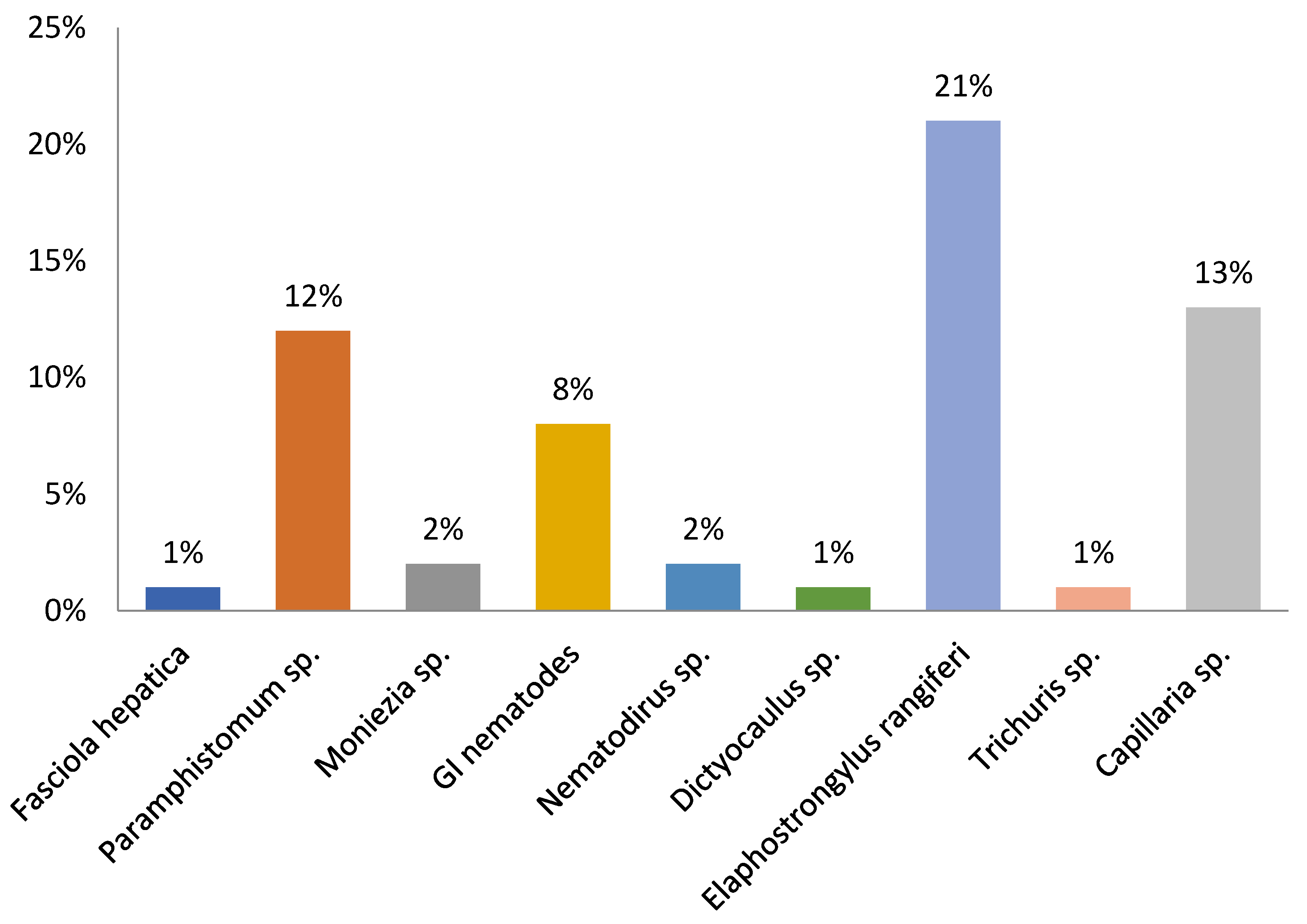Abstract
Zoo conditions are unique for reindeer, since domestic reindeer are not kept in captive facilities like cattle. In the zoo, reindeer are usually surrounded by many different animals that they would never encounter naturally. Thus, they might be infected with new helminths. Numerous petting zoos raise concerns about the safety of tactile interactions for human visitors. Our study is the first large-scale one. Qualitative and quantitative fecal analyses were carried out for 233 reindeer distributed over 50 Russian zoos according to the National Standard of the Russian Federation (GOST R 54627-2011) Ruminant animals—Methods of Laboratory Helminthological Diagnostics. Where possible, DNA analyses of helminths were performed targeting internal transcribed spacer region. As a result, F. hepatica, Paramphistomum sp., Moniezia sp. (including M. expansa), gastrointestinal strongylids (including Nematodirus spp.), Dictyocaulus sp., E. rangiferi, Trichuris sp., and Capillaria sp. were found in 106 (45%) zoo reindeer. All these helminths were previously reported in reindeer and pose no direct danger for humans. The intensity of invasions was mostly low. Fecal examination might be considered as an indirect method for mange diagnostics, as Chorioptes and Demodex mites were found in reindeer fecal samples. The latter may represent a novel species of mite specific for reindeer.
1. Introduction
Helminths in reindeer (Rangifer tarandus) have already been studied quite thoroughly, and the knowledge about them was summarized in thematic books [1,2,3] and chapters [4,5,6,7,8]. Ongoing studies expand our views on many aspects of reindeer helminths [9,10,11,12,13], and sometimes even add new species to the checklist of parasitic worms of Rangifer [14,15]. Helminths may cause nutritional deficiency in reindeer, decrease their physical condition, reduce their reproductive success, weaken immune system, cause anemia and blood loss, behavioral changes, damage to organs and tissues, and contribute to secondary infections [3,4,5,6,7,8,16].
Reindeer exist in domestic and wild forms, totaling more than 4 million animals worldwide [17]. Their popularity as zoo cervids in the 21st century is unprecedentedly growing. According to Species360 ZIMS database, there are more than 500 Rangifer spp. distributed in more than 150 zoos around the world [18]. Some research studies on reindeer helminths in captive facilities were published [3,16,19,20,21], but they are still too rare and fragmentary.
Meanwhile, zoo conditions are unique for reindeer because even domestic reindeer are not typically kept in farms or in captive facilities like cattle. In the zoo, reindeer are usually surrounded by many different animals that they would never encounter naturally, which might pose a risk of infection by new parasites [22,23]. Numerous petting zoos raise concerns about another issue: the safety of tactile interactions for human visitors.
Therefore, the aim of our study was to conduct a large-scale survey of reindeer helminths in Russian zoos. Fecal examination is a non-invasive method (an essential trait for zoo animals) that offers wide diagnostic capabilities, scoping gastrointestinal, lung, brain, and muscle parasitic worms. This study was carried out to find out what kinds of helminths inhabit zoo reindeer across Russia, how widespread they are, how typical they are, and how dangerous they are for both reindeer and humans.
2. Materials and Methods
2.1. Study Area
Our study collected fecal samples from 233 reindeer kept in 50 selected zoos and menageries located across Russia (Figure A1 and Table A1). Those include: all the state zoos in which collections reindeer are present (17); other state organizations that have live reindeer for exhibition (Children’s ecological station, Natural-ethnographic complex, and Arctic Tourism Center); private organizations embers of the Union of Zoos and Aquariums of Russia (5 private zoos and an urban farm); and 25 private menageries. The latter constitute “clubs”, “parks” (“recreation“, “ethnic“, “fauna“, and “safari park”), “reserve” (self-proclaimed), “mini zoo”, “ranch”, “farm”, “farmstead”, “pets’ corner” and unnamed menageries. Many of these organizations participated in our research anonymously; therefore, for uniformity, we do not provide the actual names of all the 50 of them. However, their location combined with the type of ownership makes the data quite transparent. For brevity, all of these organizations will be referred to as zoos.
2.2. Fecal Analysis
All fecal samples were processed according to the methods described in the National Standard of the Russian Federation (GOST R 54627-2011) Ruminant animals—Methods of Laboratory Helminthological Diagnostics [24]. Namely, they were the larvoscopic Vajda’s method, combined ovoscopic Darling’s method, and sedimentation ovoscopic method. The Vajda’s method involves placing a few fecal pellets on a slide; to pour approximately 1 mL of 40 °C tap water on the pellets; to expose it for 30 min; to put away the pellets; and to examine the liquid microscopically. Flotation with Darling’s solution involves mixing 3 g of feces with 60 mL of tap water; to filter and centrifuge the mixture; to pour off supernatant; to add the 1:1 mixture of glycerin and saturated sodium chloride solution (Darling’s solution); to centrifuge the mixture; and to examine the supernatant microscopically. Sedimentation involves mixing 3 g of feces with 60 mL of tap water; to filter the mixture; to let it settle for 5 min; to pour off sediment; to resuspend it with water; to repeat until transparent supernatant; and to examine the sediment microscopically.
2.3. Helminths Identification
Primary identification of helminths at the stage of egg or larva was based on their morphology and morphometric data. The morphology of eggs and larvae derived from zoo reindeer feces was examined via light microscopy (LM) using the optical microscope Micmed-6 (LOMO-MA, Saint Petersburg, Russia) using the lenses of 4× (to navigate the slide), 10×, 20×, 40× magnification. The parasites were mounted between the slide and coverslip (24 × 24 mm). The larvae were in tap water. Eggs were either in tap water (once obtained via sedimentation) or in Darling’s solution (derived via Darling’s method). No specific dye to visualize the internal structures of parasites was used. Micrographs were taken using the digital photo camera 5D Mark II (Canon, Tokyo, Japan) connected to the microscope with the C-mount adapter (LOMO-MA, Russia). Morphometry was based on the obtained micrographs using Fiji/ImageJ version 1.2.4 RRID:SCR_003070 software (National Institutes of Health, Bethesda, MD, USA) set using the microscope calibration slide (transmitted light object micrometer) OMP (LOMO-MA, Russia). The Straight Line mode was used to measure eggs, and the Segmented Line mode was used to measure larvae. Reference literature was used to identify the obtained helminths [1,2,3,4,5,6,25]. Where possible, morphological identification was supported by DNA analysis.
2.4. DNA Analysis
For trematodes, the QIAamp DNA Accessory Set, Micro kit (Quiagen, Venlo, the Netherlands) was used to extract DNA from embryonated eggs as described by Loginova et al. [26]. For nematodes, DNA was extracted either via digestion of first larval stages (L1) in presence of Proteinase K [27] as described by Loginova et al. [28], or by using the QIAamp DNA Accessory Set, Micro kit (Quiagen, Venlo, Netherlands). We targeted the region of the internal transcribed spacer (ITS rDNA). For trematodes, we used BD1, BD2 primers [29] and corresponding protocols [30]. For nematodes we used either NC1, NC2 primers and protocols as described by Gasser et al. [31], or 18S, 26S primers [32] and protocols as described by Loginova et al. [28]. Visual check of PCR, cleaning of the PCR products, sequencing, and analysis of obtained chromatograms were performed as described by Loginova et al. [33].
2.5. Statistical Analysis
In this study, the statistical sample equals population, because all possible zoo reindeer were examined. A total of 233 zoo reindeer may not be representative of a larger group of reindeer. There are four breeds of domestic reindeer and few ecotypes or subspecies of wild reindeer in Russia. All these kinds of reindeer differ between each other, in particular in terms of helminth resistance. Zoo reindeer are heterogeneous. Some of them are known to originate from wild forest ecotype reindeer, others originated from domestic animals (but it is hard to identify their breed), and some have no remaining origin information. Regular reindeer exchanges between zoos make it even more complicated. Moreover, the above mentioned GOST R 54627-2011 prescribes to examine no less than 10% of animals. For approximately 2 million reindeer in Russia, the statistical sample must be around 200 thousand reindeer, which is almost 900 times larger than the sample size in our study. Thus, a 95% confidence interval (CI) to express the uncertainty of sample prevalence as an estimate of population prevalence is not applicable here. Therefore, to analyze the obtained data, the index method was used. Namely, it was prevalence and intensity. Sample prevalence was calculated as the proportion of positive samples (i.e., infected individuals) within the sample set (i.e., host sample) and expressed as a percentage (from 0–100%). The intensity for every reindeer was calculated as the number of parasites (eggs or larvae) per 1 g of their feces. The VIGIS chamber (analogue of the McMaster device) of the Diapar kit (VIGIS, Moscow, Russia) was used to calculate the intensity.
3. Results
Flukes, tapeworms and round worms were found at the stage of egg or larva in the feces of 106 reindeer (45%) kept in 31 zoos (62%). Voucher and GenBank accession numbers of helminths recovered from zoo reindeer feces are presented in Table 1.

Table 1.
Species of helminths recovered from the feces of zoo reindeer and identified genetically.
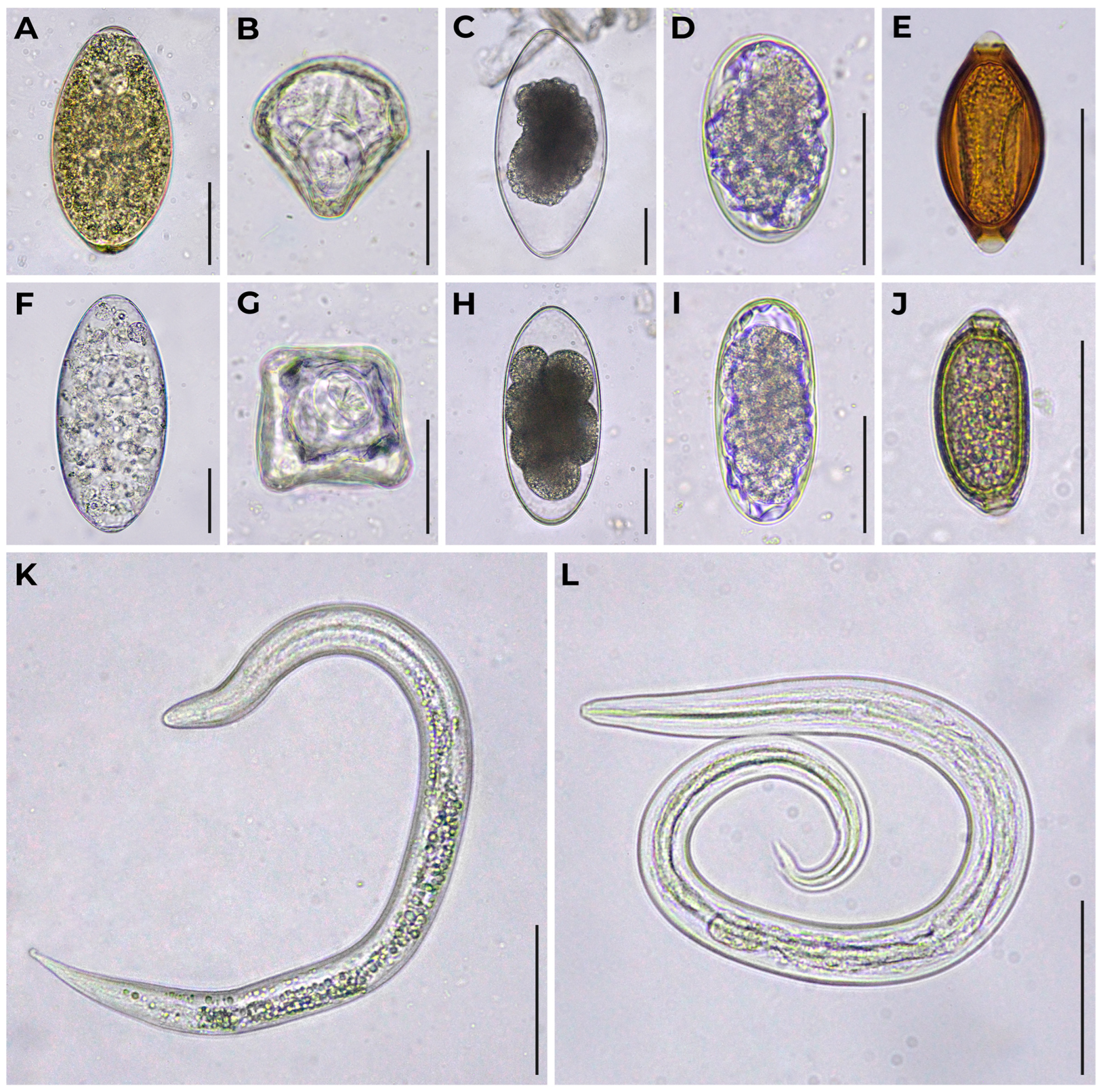
The appearance of eggs and larvae of helminths obtained from zoo reindeer feces is shown in Figure 1.
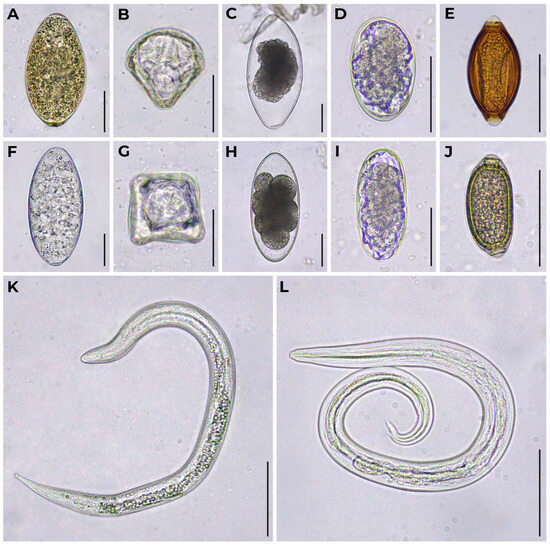
Figure 1.
Diagnostic stages of helminths obtained from feces of zoo reindeer. (A) Fasciola hepatica egg; (B) Moniezia expansa egg; (C) Nematodirus sp. egg at late stage of embryonic development; (D) rounded strongyle-type egg; (E) Trichuris sp. egg; (F) Paramphistomum sp. egg; (G) Moniezia sp. egg; (H) Nematodirus sp. egg at early stage of embryonic development; (I) elongated strongyle-type egg; (J) Capillaria sp. egg; (K) Dictyocaulus sp. L1; (L) Elaphostrongylus rangiferi L1. Bright field microscopy, 400× magnification. Scale bar equals 50 μm.
Prevalence rates and distribution of helminths obtained from zoo reindeer feces are summarized in Table 2. For brevity, only helminth-positive zoos are included.

Table 2.
Helminths found in the feces of zoo reindeer. Prevalence was calculated for each zoo.
Prevalence rates for all 233 reindeer examined are shown in Figure 2.
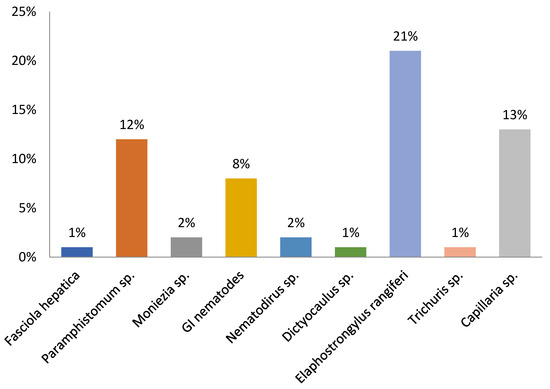
Figure 2.
Prevalence rates (%) of helminths found in feces of zoo reindeer.
Comparative intensity of infestation in the different zoos is presented in Table 3.

Table 3.
Intensity of infestation with helminths in zoo reindeer according to GOST R 54627-2011.
4. Discussion
Our study revealed the presence of trematodes (Fasciola hepatica and Paramphistomum sp.), cestodes (Moniezia expansa and Moniezia sp.), and nematodes (so-called small strongylids, Nematodirus spp., Dictyocaulus sp., Elaphostrongylus rangiferi, Trichuris sp., and Capillaria sp.) in zoo reindeer via fecal examination. All of these helminths were previously reported in reindeer [3,8].
In many samples, only one kind of helminth was found. However, we have also discovered singular combinations of two or three kinds of helminths (Table 4).

Table 4.
Combinations of helminths found in the feces of zoo reindeer.
Common liver fluke, F. hepatica, inhabits the bile ducts of its host. Previously, it was reported in reindeer only in Eurasia, with one of the cases involving zoo reindeer [3]. This liver fluke seldom parasitizes reindeer, which is considered an accidental host. Infestation of reindeer with F. hepatica is usually associated with contact with other infected ruminants in captive facilities, where definitive hosts shed F. hepatica eggs that eventually develop into metacercaria [3]. In the two private zoos where F. hepatica was found in reindeer, sheep, goats and sika deer (Cervus nippon) were also infected.
Rumen fluke, Paramphistomum sp., is widespread in Russian reindeer. When numerous, they cause atrophy of the rumen’s papillae and may lead to death [3,8]. Reindeer from the private zoo #30, which had a medium level of invasion with rumen flukes, died within 6 months.
The tapeworms found were present with M. expansa (easily recognizable by its triangular eggs) [25] and Moniezia sp. These helminths inhabit the small intestine and may be quite pathogenic, especially for calves [3,8]. However, in our study, it was only found in adults. The reindeer is considered an accidental host for M. expansa, and its infestation is also associated with sympatric livestock and captive facilities [3].
Supposedly, the small strongylids found in zoo reindeer belong to two different genera (Figure 1D,I) which may belong to fam. Chabertiidae, Cooperiidae, Haemonchidae or Trichostrongylidae. Coproculture is needed to obtain larvae and clarify the diagnosis. In any case, small strongylids inhabit the gastrointestinal tract, and their impact depends on many circumstances [8,10].
The eggs of Nematodirus obtained from the reindeer fecal sample from zoo #40 certainly belonged to two different species. Apart from their differences in size and shape, their development rates were also different. Figure 1H shows Nematodirus sp. with eight blastomeres, whereas Figure 1C shows Nematodirus sp. with gastrulated embryo. These two types of eggs were found in the same fecal sample. In total, four eggs (two per type) were obtained from that sample. We attempted to obtain third larval stages (L3s) for DNA analysis but did not succeed. However, light micrographs (taken daily for three weeks after the finding of these eggs) showed their embryonic development and are available as Supplementary Materials. Helminths of Nematodirus sp. inhabit the small intestine. In reindeer, they are often accompanied by Nematodirella longissimespiculata [3], but in this study the latter was not found.
The lung worm Dictyocaulus sp. is the most concerning finding, because its presence may be life threatening. In the only zoo (#30) where it was found, there was also a sika deer infected with Dictyocaulus sp. It is hard to tell which of the animals was the source of invasion, or if it was it a coincidental infestation.
Brain worm, E. rangiferi, was the most widespread helminth in our research. Its pathogenicity is tricky because it may cause lethal epizootics or have no manifestation of its presence [3,8,34]. Due to the localization of adult worms in the nervous system or between the muscles, there is no effective treatment. On the other hand, authors observed a few reindeer that were severely infected in 2018 that eventually outlived their brain worms. At least no E. rangiferi larvae have been found in their fecal samples since 2023. Probably, it would be impossible in the wild, but in human care, reindeer can live much longer and, apparently, rid themselves of some parasites naturally.
Whipworms, Trichuris sp., inhabit the large intestine and can cause hemorrhagic diarrhea [8]. They are more frequent in captive animals. There are reindeer specific species and those common to ruminants that can also infect reindeer [3]. Egg morphology is not sufficient for the identification up to the species level.
Roundworm, Capillaria sp., inhabits the intestine and is often associated with young animals [3,8]. However, the impact of this parasite is not known. Infection with Capillaria sp. was previously reported in zoo reindeer as well [3].
Thus, via fecal examination, we managed to find liver and rumen flukes, GINs, lung worms, brain worms, whipworms, and capillarids. However, this method is not suitable for those helminths that inhabit ligaments (like Onchocerca), abdominal cavity (like Setaria), muscles, lungs, liver and brain (like larval stages of Taeniidae). Therefore, despite the large scale of our study, the helminth status of zoo reindeer is still incomplete.
Another bias of this work concerns different seasons of fecal sampling (which probably affected the reproductive activity of the helminths) and differences in age and sex of the studied animals [3,8]. These circumstances should be kept in mind when comparing the obtained results between zoos.
As we said earlier, the popularity of reindeer as zoo animals in the 21st century is outstanding. Only one zoo (#17) has a 100-year history of keeping reindeer in captivity. It is not surprising that fecal samples from their reindeer were helminth negative not only in this study, but years prior to it. Many other zoos obtained their first reindeer only in 2023. The majority of private zoos purchase domestic reindeer from agricultural husbandries. These husbandries are all private and run by families of indigenous people from the north. Zoos are able to buy only those reindeer that indigenous people are willing to sell. Reindeer herders want to keep the best individuals in the herd, and reindeer for sale may be less resistant to helminths.
The domestic reindeer, as opposed to wild reindeer, can be pure white (leucism, not albinism) throughout the year [35,36]. White reindeer attract most people [37], and numerous private zoos tend to have exactly the white reindeer. However, reindeer herders noticed that white reindeer are not good survivors [37,38]. It might add another disadvantage to such zoo reindeer in terms of helminth resistance and requires special research.
Apart from husbandry “heritage”, zoo reindeer can become infected with helminths due to birds. Helminths eggs were reported to survive the sparrow digestive system and remain invasive [39]. Thus, birds can be vectors for helminths within the zoo and outside of its boundaries.
It is noteworthy that even in helminth-positive zoos, about 30% (from 0 to 83%) of reindeer fecal samples were helminth free, probably due to the different immune status of the reindeer.
Besides helminths, we found mites in reindeer fecal samples (Figure A2). They were either ingested during grooming and passed through the digestive tract or contaminated the fecal samples afterwards. Having found a mite egg in the sample from zoo #30, we contacted the zoo and shared our considerations. It turned out that the zoo indeed had problems with mange caused by mites (Figure A2E). One adult mite found in a sample from zoo #40 was identified as Chorioptes sp. (Figure A2D). Chorioptes mites were reported in reindeer in Canada, Norway, and Finland [8]. Another adult mite found in a reindeer fecal sample (zoo #12) met the morphological criteria for Demodex sp. To the authors’ knowledge, Demodex has never been reported in reindeer [3,8,40]. This finding also requires additional research, which may result in the description of a new species.
Among the helminths found in the zoo reindeer in our research, there are a few genera reported in humans. Those are Fasciola, GINs (Trichostrongylus, Oesophagostomum), Trichuris, and Capillaria [41,42,43,44]. However, infected reindeer pose no direct threat for human visitors or zoo employees for the following reasons: the trematode F. hepatica is indeed a species that can infect both cervids and humans, but its life cycle requires an intermediate host (freshwater mollusk) [3]. The eggs shed by reindeer are not at infective stage for any vertebrate host. Moreover, eggs are excreted without miracidium, which takes time to develop (and is possible only in water). Species of Trichostrongylus, Oesophagostomum, Trichuris, and Capillaria that can infect humans are not the same species that infect reindeer [3,8,42,44]. Furthermore, all these nematodes shed eggs that are not embryonated by the time they are excreted in feces. Larvae development also requires a few days or weeks. Thus, if reindeer dung is regularly removed, even reindeer infected with helminths are safe for humans.
5. Conclusions
In our study, F. hepatica, Paramphistomum sp., Moniezia spp. (including M. expansa), gastrointestinal strongylids (including Nematodirus spp.), Dictyocaulus sp., E. rangiferi, Trichuris sp., and Capillaria sp. were found in 106 Russian zoo reindeer (out of 233) via fecal examination. All these helminths were previously reported in reindeer and pose no direct danger for humans. The intensity of invasions was mostly low. Fecal examination might be considered as an indirect method for mange diagnostics, as Chorioptes and Demodex mites were found in reindeer fecal samples. The latter may represent a novel species of mite specific for reindeer.
Supplementary Materials
The following supporting information can be downloaded from: https://www.mdpi.com/article/10.3390/jzbg5030033/s1, Figures S1–S25: Light microscopic images of the two types of Nematodirus eggs showing the development of embryos from 13 December 2023 to 4 January 2024, respectively. Eggs were obtained from the same fecal sample from a reindeer from zoo #40. The eggs were placed in a watch glass filled with tap water. Pictures were taken via photo camera using the smart phone Xperia2 DS Black (H4113) (Sony, China) and the optical microscope Micmed-6 (LOMO-MA, Russia).
Author Contributions
Conceptualization, O.A.L. and S.V.A.; methodology, O.A.L., D.N.E., S.E.S. and L.M.B.; formal analysis, O.A.L.; investigation, O.A.L. and S.E.S.; resources, O.A.L., S.V.A., D.N.E., N.S.E., S.E.S., I.K.P., L.M.B., Y.E.K., D.I.C., A.A.K., I.V.V., D.A.G., Y.A.S., I.A.M., D.V.P., M.G.B. and T.P.S.; writing—original draft preparation, O.A.L.; writing—review and editing, S.V.A., D.N.E., N.S.E., S.E.S., I.K.P., L.M.B., Y.E.K., D.I.C., A.A.K., I.V.V., D.A.G., Y.A.S., I.A.M., D.V.P., M.G.B. and T.P.S.; visualization, O.A.L.; supervision, L.M.B. and S.E.S.; project administration, S.V.A.; funding acquisition, I.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this article was prepared within the framework of the State assignment for the N. Laverov Federal Center for Integrated Arctic Research of the Ural Branch of the Russian Academy of Sciences “Scientific foundations and social and cultural factors for the conservation and use of the potential of biological diversity in the European North and the Arctic” (registration number—122011400382-8)”. Another part of this study was performed under the State order of IB KRC RAS (project FMEN-2022–0003).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Authors are deeply grateful to all the zoo administrators, veterinarians, keepers, and workers (as well as those not involved in zoo work directly) who assisted with fecal sampling and made this assistance possible. In particular, we would like to thank Alexander Makhov (Moscow), Kakhramon Khuzhanov (Moscow), Nelly Khafizova (Moscow), Andrey Soludkov (Moscow), Igor Tsaryov (Moscow), Andrey Kajdalov (Saint Petersburg), Lyudmila Zakharova (Saint Petersburg), Kristina Zabarina (Samara), Nataliya Chervyakova (Moscow), Vera Shiryaeva (Saint Petersburg), Irina Pavlova (Saint Petersburg), Daria Zimina (Izhevsk), Elena Kolesnikova (Khabarovsk), Vitaliy Soroka (Saint Petersburg), Irina Chuprak (Saint Petersburg), Irina Suvorova (Moscow), Evgeniy Popov and Alexander Sokolov from the Veterinary service of the Yamalo-Nenets Autonomous Okrug (Salekhard), and Vladimir Vasilev from the Northern Forum (Yakutsk). We also extend our gratitude to our colleagues who assisted in mite identification: Sergey Mironov from the Zoological Institute of the Russian Academy of Sciences (Saint Petersburg) and Nadezhda Gavrilova from the Saint Petersburg State University of Veterinary Medicine (Saint Petersburg). Special thanks to Dmitry Kuznetsov for his suggestion to write this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
The study area is represented in Figure A1.
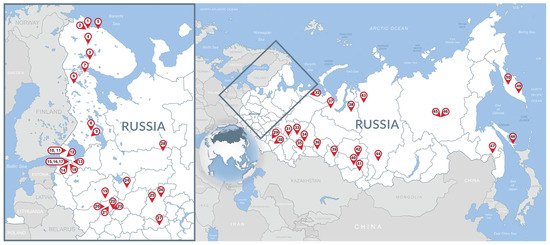
Figure A1.
Map of Russia indicating the zoos. Insert on the right represents magnified area marked with a rectangle. Numbers correspond to the zoo ID numbers in Table A1.
Figure A1.
Map of Russia indicating the zoos. Insert on the right represents magnified area marked with a rectangle. Numbers correspond to the zoo ID numbers in Table A1.
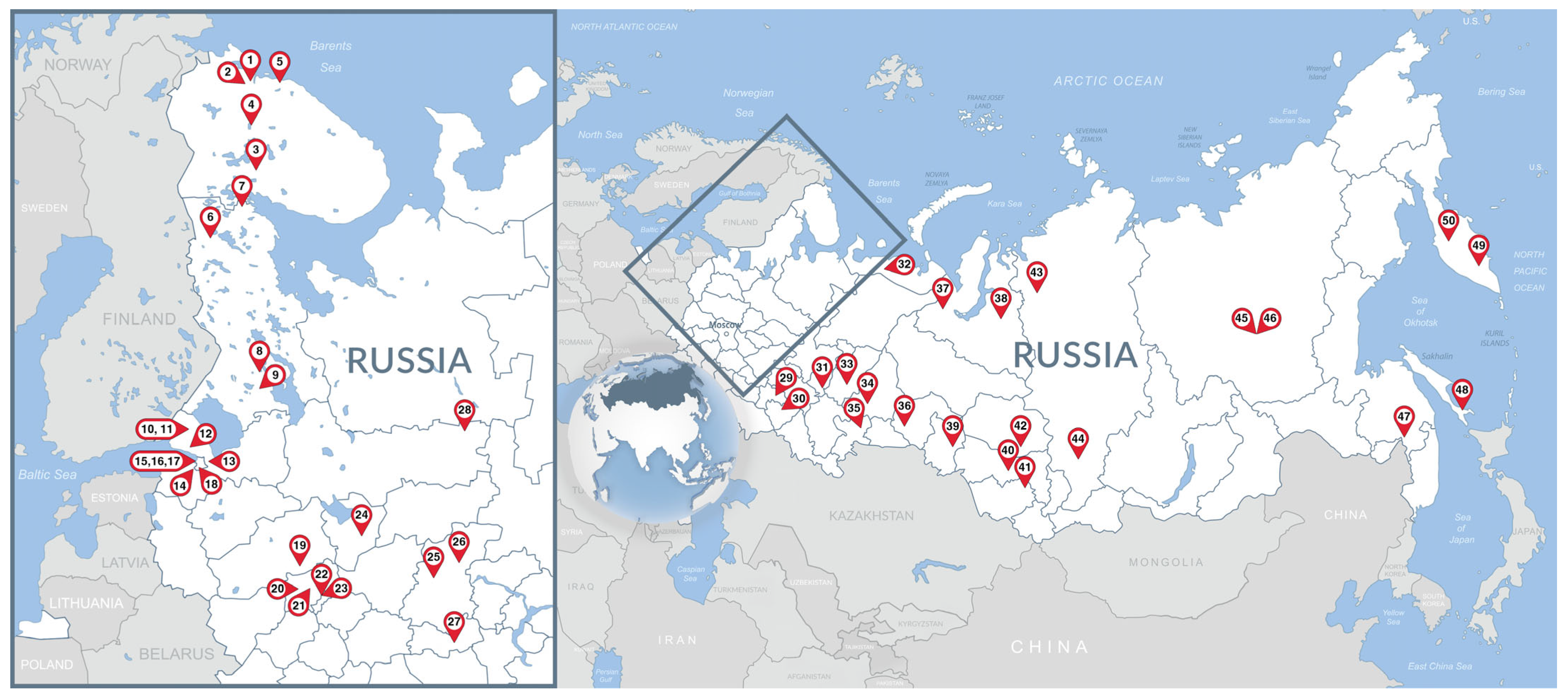

Table A1.
Collection data for fecal samples from reindeer (Rangifer tarandus) in the Russian zoos (federal subjects arranged from west to east).
Table A1.
Collection data for fecal samples from reindeer (Rangifer tarandus) in the Russian zoos (federal subjects arranged from west to east).
| Zoo ID | Ownership Type (S/P) 1 | Location (Federal Subject of Russia) | Coordinates (Decimal Degrees) | Number of Fecal Samples 3 | Date Collected |
|---|---|---|---|---|---|
| 1 | P | Murmansk Oblast | 68.98716 33.07261 | 2 | November 2023 |
| 2 | P | Murmansk Oblast | 68.85722 33.19556 | 12 | November 2023 |
| 3 | P | Murmansk Oblast | 67.56317 33.36571 | 2 | November 2023 |
| 4 | P | Murmansk Oblast | 67.65276 33.66051 | 11 | November 2023 |
| 5 | P | Murmansk Oblast | 69.16835 35.13280 | 3 | November 2023 |
| 6 | P | Republic of Karelia | 65.76340 31.07419 | 1 | March 2024 |
| 7 | P | Republic of Karelia | 66.43669 32.85459 | 7 | June 2022 |
| 8 | P | Republic of Karelia | 62.33297 34.00604 | 3 | March 2024 |
| 9 | P | Republic of Karelia | 61.87838 34.07819 | 9 | March 2024 |
| 10 | P | Leningrad Oblast | 60.59067 30.00422 | 1 | July 2020 |
| 11 | P 2 | Leningrad Oblast | 60.59161 30.11204 | 8 | May 2019 |
| 12 | P | Leningrad Oblast | 60.14195 30.32823 | 11 | August 2018 |
| 13 | P | Leningrad Oblast | 59.94762 30.68122 | 3 | May 2023 |
| 14 | P | Saint Petersburg | 59.84108 30.06470 | 2 | June 2019 |
| 15 | P | Saint Petersburg | 59.98017 30.24438 | 3 | March 2024 |
| 16 | P | Saint Petersburg | 59.97054 30.25672 | 4 | January 2020 |
| 17 | S 2 | Saint Petersburg | 59.95210 30.30891 | 9 | March 2024 |
| 18 | P | Saint Petersburg | 59.67605 30.42401 | 2 | February 2018 |
| 19 | P | Tver Oblast | 56.75051 36.36719 | 1 | June 2020 |
| 20 | S 2 | Moscow Oblast | 55.94012 36.21055 | 7 | January 2024 |
| 21 | P | Moscow Oblast | 56.13343 36.50051 | 2 | March 2024 |
| 22 | S 2 | Moscow | 55.76347 37.57537 | 3 | December 2023 |
| 23 | P 2 | Moscow | 55.83306 37.62197 | 1 | October 2023 |
| 24 | S 2 | Yaroslavl Oblast | 57.67709 39.90005 | 6 | November 2023 |
| 25 | P 2 | Nizhny Novgorod Oblast | 56.33468 43.85420 | 6 | November 2023 |
| 26 | P | Nizhny Novgorod Oblast | 56.92384 45.40185 | 4 | August 2022 |
| 27 | S 2 | Republic of Mordovia | 54.17500 45.18599 | 3 | November 2023 |
| 28 | S 2 | Vologda Oblast | 60.74784 46.17739 | 5 | November 2023 |
| 29 | P 2 | Ulyanovsk Oblast | 54.35485 48.52409 | 3 | February 2024 |
| 30 | P | Samara Oblast | 53.34493 50.22240 | 21 | August 2021 |
| 31 | S 2 | Udmurt Republic | 56.86555 53.17413 | 2 | November 2023 |
| 32 | S | Nenets Autonomous Okrug | 67.63436 53.24135 | 1 | February 2024 |
| 33 | S 2 | Perm Krai | 58.01672 56.23728 | 4 | November 2023 |
| 34 | P | Sverdlovsk Oblast | 57.17585 60.65764 | 11 | February 2024 |
| 35 | S 2 | Chelyabinsk Oblast | 55.16894 61.36764 | 5 | November 2023 |
| 36 | P | Tyumen Oblast | 56.99540 65.73485 | 7 | February 2024 |
| 37 | S | Yamalo-Nenets Autonomous Okrug | 66.59257 66.85846 | 3 | January 2024 |
| 38 | S 2 | Yamalo-Nenets Autonomous Okrug | 66.07485 76.65427 | 2 | November 2023 |
| 39 | S 2 | Omsk Oblast | 56.08978 74.64219 | 5 | November 2023 |
| 40 | S 2 | Novosibirsk Oblast | 55.05612 82.88010 | 7 | December 2023 |
| 41 | P 2 | Altai Krai | 53.35593 83.68230 | 2 | November 2023 |
| 42 | S 2 | Tomsk Oblast | 56.60427 84.86807 | 1 | March 2024 |
| 43 | P | Krasnoyarsk Krai | 69.42091 88.26126 | 1 | February 2024 |
| 44 | S 2 | Krasnoyarsk Krai | 55.96669 92.73100 | 5 | November 2023 |
| 45 | S 2 | Republic of Sakha (Yakutia) | 61.67818 129.35184 | 5 | April 2024 |
| 46 | P | Republic of Sakha (Yakutia) | 62.03243 129.72416 | 5 | August 2020 |
| 47 | S 2 | Khabarovsk Krai | 48.62218 135.06819 | 6 | December 2019 |
| 48 | S 2 | Sakhalin Oblast | 46.96788 142.75403 | 2 | December 2019 |
| 49 | S 2 | Kamchatka Krai | 53.18850 158.38604 | 1 | April 2019 |
| 50 | P | Kamchatka Krai | 55.92095 158.69500 | 3 | March 2024 |
1 S is for State ownership, P is for Private ownership. 2 Member of the Union of Zoos and Aquariums of Russia. 3 Number of fecal samples equals the number of reindeer in the zoo.
Appendix B
Mites found in the feces of zoo reindeer and manifestation of mange caused by mites in reindeer is shown in Figure A2.
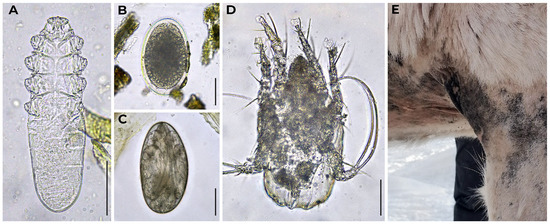
Figure A2.
Mites of reindeer. (A) Demodex sp. obtained from the feces of a reindeer from zoo #12; (B) egg of mite at early developmental stage obtained from the feces of a reindeer from zoo #30; (C) egg of mite at late developmental stage obtained from the feces of a reindeer from zoo #48; (D) Chorioptes sp. obtained from the feces of a reindeer from zoo #40; (E) manifestation of mange caused by mites in a reindeer from zoo #30 (furless patches in the front leg and chest are shown); photo courtesy: Kristina Zabarina. Light micrographs were made via bright field microscopy, 400× magnification. Scale bar equals 50 μm.
Figure A2.
Mites of reindeer. (A) Demodex sp. obtained from the feces of a reindeer from zoo #12; (B) egg of mite at early developmental stage obtained from the feces of a reindeer from zoo #30; (C) egg of mite at late developmental stage obtained from the feces of a reindeer from zoo #48; (D) Chorioptes sp. obtained from the feces of a reindeer from zoo #40; (E) manifestation of mange caused by mites in a reindeer from zoo #30 (furless patches in the front leg and chest are shown); photo courtesy: Kristina Zabarina. Light micrographs were made via bright field microscopy, 400× magnification. Scale bar equals 50 μm.
References
- Skrjabin, K.I. Glistnye Invazii Severnogo Olenya (Helminth Diseases of Reindeer); Selkhozgiz: Moscow, Russia, 1931; 88p. (In Russian) [Google Scholar]
- Polyanskaya, M.V. Gel’mintosy Severnykh Oleney (Helminthiases of Reindeer); Knizhnoe Izdatelstvo: Murmansk, Russia, 1963; 47p. (In Russian) [Google Scholar]
- Mizkewitsch, V.Y. Gel’minty Severnogo Olenya i Vyzyvayemyye imi Zabolevaniya (Reindeer Helminths and the Diseases They Cause); Kolos: Saint Petersburg, Russia, 1967; 308p. (In Russian) [Google Scholar]
- Golosov, I.M.; Mizkewitsch, V.Y. Helminthiases. In Parazitarnye Bolezni Severnykh Oleney (Parasitic Diseases of Reindeer); Lyzhin, K., Ed.; Krasnoyarskoe Knizhnoe Izdatelstvo: Krasnoyarsk, Russia, 1964; pp. 57–141. (In Russian) [Google Scholar]
- Laaksonen, S. Trematoda. Cestoda. Nematoda. In TUNNE PORO. Poron Sairaudet ja Terveydenhoito (FEEL THE REINDEER. Reindeer Diseases and Health Care); Livonia Print: Riga, Latvia, 2016; pp. 220–251. (In Finnish) [Google Scholar]
- Belova, L.M.; Loginova, O.A. Helminthiases. In Bolezni Severnykh Oleney (Diseases of Reindeer); Zabrodin, V.A., Laishev, K.A., Eds.; Knizhnoe Izdatelstvo: Saint Petersburg, Russia, 2019; pp. 94–113. (In Russian) [Google Scholar]
- Kutz, S.J.; Ducrocq, J.; Verocai, G.G.; Hoar, B.M.; Colwell, D.D.; Beckmen, K.B.; Polley, L.; Elkin, B.T.; Hoberg, E.P. Parasites in ungulates of Arctic North America and Greenland: A view of contemporary diversity, ecology, and impact in a world under change. Adv. Parasitol. 2012, 79, 99–252. [Google Scholar] [CrossRef] [PubMed]
- Kutz, S.J.; Laaksonen, S.; Asbakk, K.; Nilssen, A.C. Helminths. In Reindeer and Caribou. Health and Disease; Tryland, M., Kutz, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 180–205. [Google Scholar]
- Hrabok, J.T.; Oksanen, A.; Nieminen, M.; Waller, P.J. Population dynamics of nematode parasites of reindeer in the sub-arctic. Vet. Parasitol. 2006, 142, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Jokelainen, P.; Moroni, B.; Hoberg, E.; Oksanen, A.; Laaksonen, S. Gastrointestinal parasites in reindeer (Rangifer tarandus tarandus): A review focusing on Fennoscandia. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100317. [Google Scholar] [CrossRef] [PubMed]
- Emelyanova, A.; Savolainen, A.; Oksanen, A.; Nieminen, P.; Loginova, O.; Abass, K.; Rautio, A. Research on Selected Wildlife Infections in the Circumpolar Arctic—A Bibliometric Review. Int. J. Environ. Res. Public Health 2022, 19, 11260. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.R.; Orsel, K.; Cuyler, C.; Kutz, S.J. Life history matters: Differential effects of abomasal parasites on caribou fitness. Int. J. Parasitol. 2023, 53, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Andrew, C.L.; Wagner, B.; Harms, N.J.; Jenkins, E.J.; Jung, T.S. Comparative Prevalence and Intensity of Endoparasites in a Dynamic Boreal Ungulate Community. Diversity 2024, 16, 230. [Google Scholar] [CrossRef]
- Verocai, G.G.; Kutz, S.J.; Simard, M.; Hoberg, E.P. Varestrongylus eleguneniensis sp. n. (Nematoda: Protostrongylidae): A widespread, multi-host lungworm of wild North American ungulates, with an emended diagnosis for the genus and explorations of biogeography. Parasites Vectors 2014, 7, 1–22. [Google Scholar] [CrossRef]
- Loginova, O.A.; Kolpashchikov, L.A.; Spiridonov, S.E. First report of Orthostrongylus sp. (Nematoda: Protostrongylidae) in wild reindeer (Rangifer tarandus) from the Taimyr, Russia: Nearctic parasites in a Palearctic host. Parasitol. Res. 2023, 122, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Hoberg, E.P.; Kocan, A.A.; Rickard, L.G. Gastrointestinal strongyles in wild ruminants. In Parasitic Diseases of Wild Mammals; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Wiley: New York, NY, USA, 2001; pp. 193–227. [Google Scholar]
- Tryland, M.; Kutz, S.J. Reindeer and Caribou. Health and Disease; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2019; p. 3. ISBN 978-1-4822-5068-8. [Google Scholar]
- Species 360. Data Science for Zoos and Aquariums. Available online: https://species360.org/products-services/zoo-aquarium-animal-management-software-2/ (accessed on 31 May 2024).
- Haigh, J.C.; Mackintosh, C.; Griffin, F. Viral, parasitic and prion diseases of farmed deer and bison. Rev. Sci. Tech. 2002, 21, 219–248. [Google Scholar] [CrossRef]
- Flach, E. Cervidae and Tragulidae. In Zoo and Wild Animal Medicine, 5th ed.; Fowler, M.E., Miller, E.R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 634–649. [Google Scholar]
- Goossens, E.; Vercruysse, J.; Boomker, J.; Vercammen, F.; Dorny, P. A 12-month survey of gastrointestinal helminth infections of cervids kept in two zoos in Belgium. J. Zoo Wildl. Med. 2005, 36, 470–478. [Google Scholar] [CrossRef]
- Manninen, S.M.; Thamsborg, S.M.; Laaksonen, S.; Oksanen, A. The reindeer abomasal nematode (Ostertagia gruehneri) is naturally transmitted to sheep when sharing pastures. Parasitol. Res. 2014, 113, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Utaaker, K.S.; Ytrehus, B.; Davey, M.L.; Fossøy, F.; Davidson, R.K.; Miller, A.L.; Robertsen, P.-A.; Strand, O.; Rauset, G.R. Parasite spillover from domestic sheep to wild reindeer—The role of salt licks. Pathogens 2023, 12, 186. [Google Scholar] [CrossRef]
- Latypov, D.G.; Timerbayeva, R.R.; Kirillov, E.G. Parazitologiya i Invazionnyye Bolezni Zhvachnykh Zhivotnykh (Parasitology and Invasive Diseases of Ruminants); Lan: Saint Petersburg, Russia, 2019; pp. 59–67. ISBN 978-5-8114-3561-6. (In Russian) [Google Scholar]
- Verocai, G.G.; Chaudhry, U.N.; Lejeune, M. Diagnostic Methods for Detecting Internal Parasites of Livestock. Vet. Clin. Food. Anim. 2020, 36, 125–143. [Google Scholar] [CrossRef]
- Loginova, O.; Efeykin, B.; Krutikova, A.; Mizin, I.; Spiridonov, S. Fasciola hepatica: Updates on egg morphology, host range, and distribution. Food Waterborne Parasitol. 2024, 36, e00237. [Google Scholar] [CrossRef]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-Wide Analysis of SSU rDNA Reveals Deep Phylogenetic Relationships among Nematodes and Accelerated Evolution toward Crown Clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Loginova, O.A.; Belova, L.M.; Spiridonov, S.E. The First Report on Elaphostrongylus rangiferi (Reindeer Invasive Parasite) in Leningrad Oblast. Russ. J. Biol. Invasions 2022, 13, 232–244. [Google Scholar] [CrossRef]
- Luton, K.; Walker, D.; Blair, D. Comparisons of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea). Mol. Biochem. Parasitol. 1992, 56, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Mahami-Oskouei, M.; Dalimi, A.; Forouzandeh-Moghadam, M.; Rokni, M.B. Molecular identification and differentiation of Fasciola isolates using PCR-RFLP method based on internal transcribed spacer (ITS1, 5.8 S rDNA, ITS2). Iranian J. Parasitol. 2011, 6, 35–42. [Google Scholar]
- Gasser, R.B.; Chilton, N.B.; Hoste, H.; Beveridge, I. Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Res. 1993, 21, 2525–2526. [Google Scholar] [CrossRef]
- Vrain, T.C.; Wakarchuk, D.A.; Levesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Loginova, O.A.; Rozenfeld, S.B.; Sipko, T.P.; Mizin, I.A.; Panchenko, D.V.; Laishev, K.A.; Bondar, M.G.; Kolpashchikov, L.A.; Gruzdev, A.R.; Kulemeev, P.S.; et al. Diversity and Distribution of Helminths in Wild Ruminants of the Russian Arctic: Reindeer (Rangifer tarandus), Muskoxen (Ovibos moschatus), and Snow Sheep (Ovis nivicola). Diversity 2023, 15, 672. [Google Scholar] [CrossRef]
- Handeland, K.; Slettbakk, T. Outbreaks of Clinical Cerebrospinal Elaphostrongylosis in Reindeer (Rangifer tarandus tarandus) in Finnmark, Norway, and their Relation to Climatic Conditions. J. Vet. Med. Ser. B 1994, 41, 407–410. [Google Scholar] [CrossRef]
- Baskin, L.M. Odomashnivaniye Severnogo Olenya. Ot Okhotnika do Pastukha i Ranchevoda (Reindeer Domestication. From Hunter to Herdsman and Rancher); Tovarishchestvo Nauchnykh Izdaniy KMK: Moscow, Russia, 2021; p. 280. ISBN 978-5-907372-89-4. (In Russian) [Google Scholar]
- Holand, Ø.; Mizin, I.; Weladji, R.B. Reindeer Rangifer tarandus (Linnaeus, 1758). In Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer: Cham, Switzerland, 2022; pp. 247–277. ISBN 978-3-030-24474-3. [Google Scholar] [CrossRef]
- Smith, T. Velvet Antlers, Velvet Nose. The Story of a Reindeer Family; Coronet Books, Hodder and Stoughton: London, UK, 1995; p. 90. ISBN 0-340-66003-1. [Google Scholar]
- Wella, Y. Azbuka Olenevoda (Reindeer Herder’s ABC); Studiya O.K.: Surgut, Russia, 2011; 43p. (In Russian) [Google Scholar]
- Mosgovoy, A.A. Sparrows as agent distributing helminthic infections among domestic animals. In Papers on Helminthology; Schultz, R.-E.S., Gnedina, M.P., Eds.; All-Union Lenin Academy of Agricultural Sciences: Moscow, Russia, 1937; pp. 398–402. (In Russian) [Google Scholar]
- Izdebska, J.N.; Fryderyk, S. Demodex acutipes Bukva et Preisler, 1988 (Acari, Demodecidae)—A rare parasite of red deer (Cervus elaphus L.). Ann. Parasitol. 2012, 58, 161–166. [Google Scholar] [PubMed]
- Vasilevich, F.I.; Belova, L.M.; Burmistrova, M.I. Parazitarnye Zoonozy (Parasitic Zoonoses); ZooVetKniga: Moscow, Russia, 2020; pp. 21–28. ISBN 978-5-6045650-5-6. (In Russian) [Google Scholar]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018, 145, 1665–1699. [Google Scholar] [CrossRef]
- Lattes, S.; Ferte, H.; Delaunay, P.; Depaquit, J.; Vassallo, M.; Vittier, M.; Kokcha, S.; Coulibaly, E.; Marty, P. Trichostrongylus colubriformis Nematode Infections in Humans, France. Emerg. Inf. Dis. 2011, 17, 1301–1302. [Google Scholar] [CrossRef]
- Dhaliwal, B.B.S.; Juyal, P.D. Textbook of Parasitic Zoonoses; Springer Nature: Singapore, 2022; pp. 46–110. ISBN 978-81-322-1550-9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).