Low-Dose Radiation Therapy for COVID-19: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
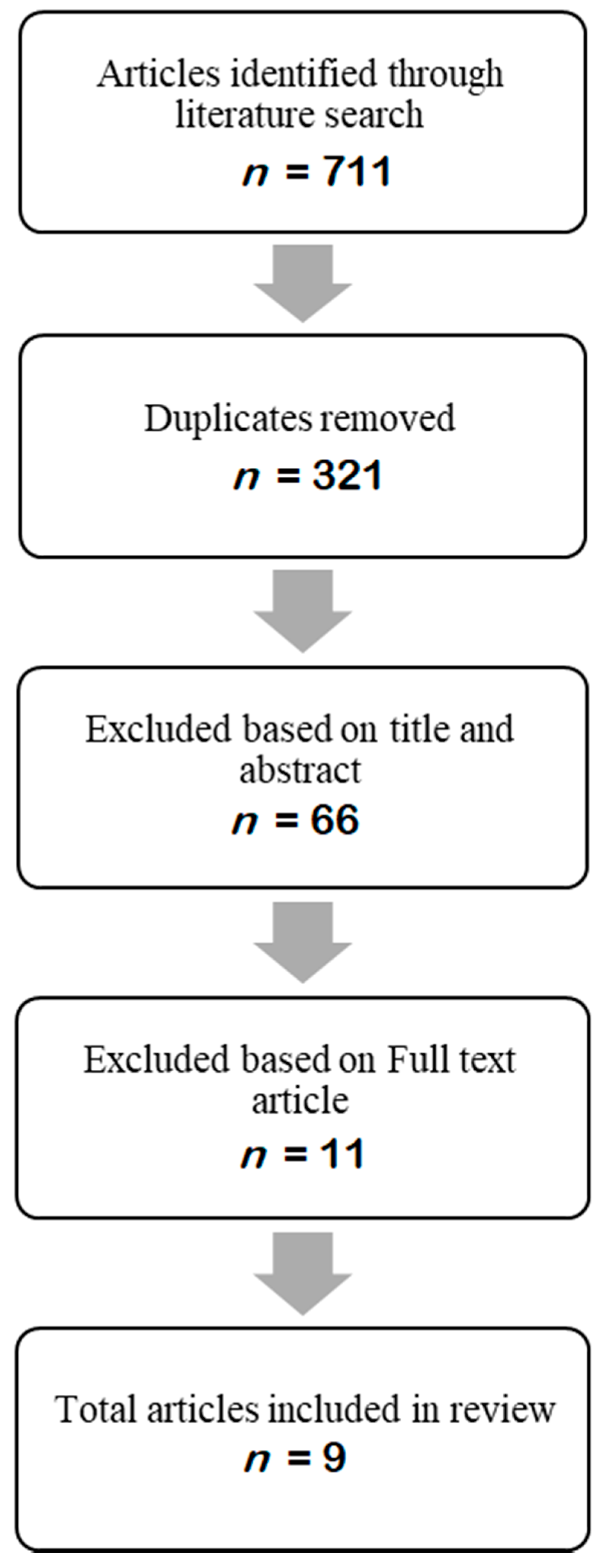
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
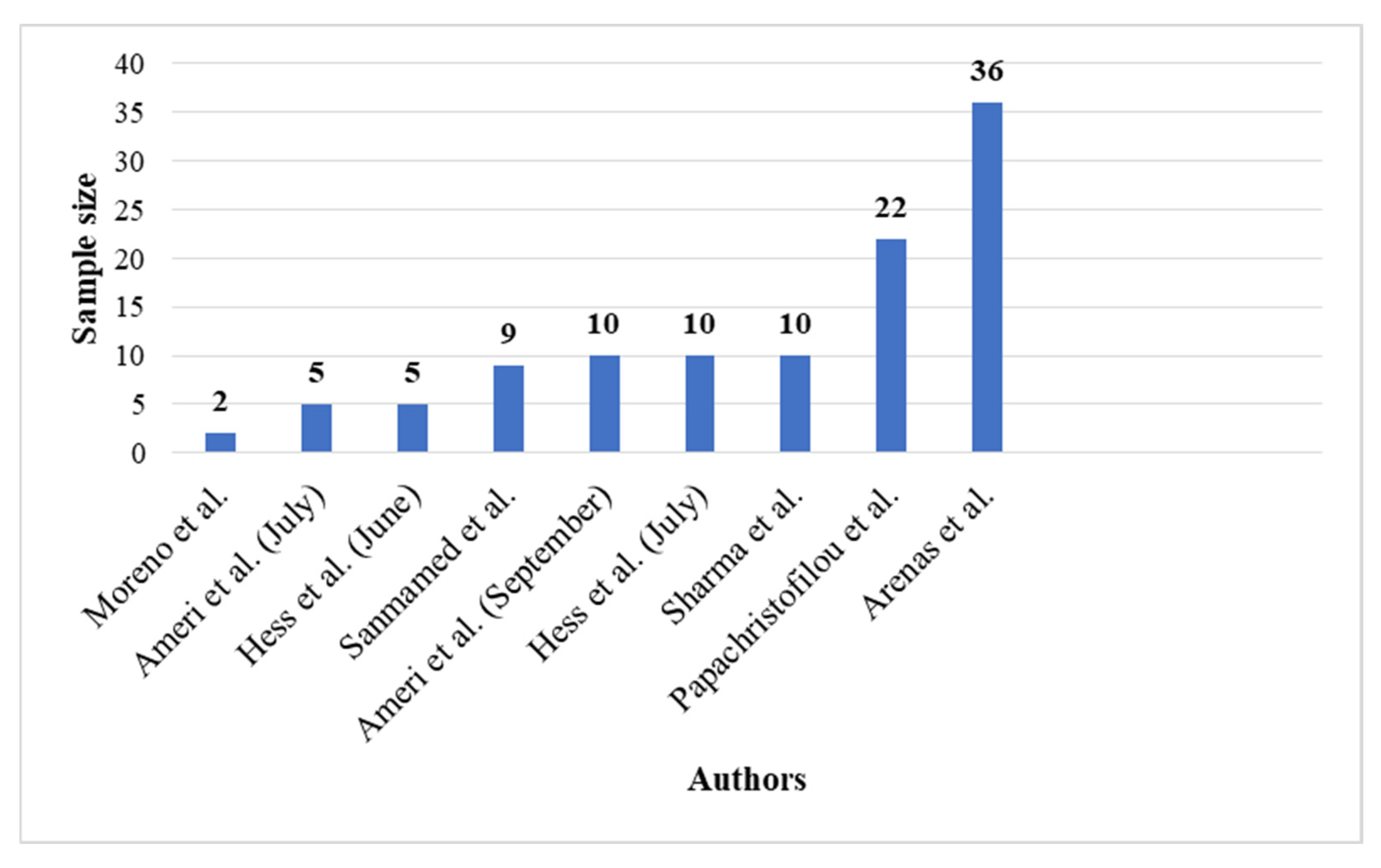
2.3. Sample Size
2.4. Low-Dose Radiation Therapy
2.5. Studied Parameters
3. Results
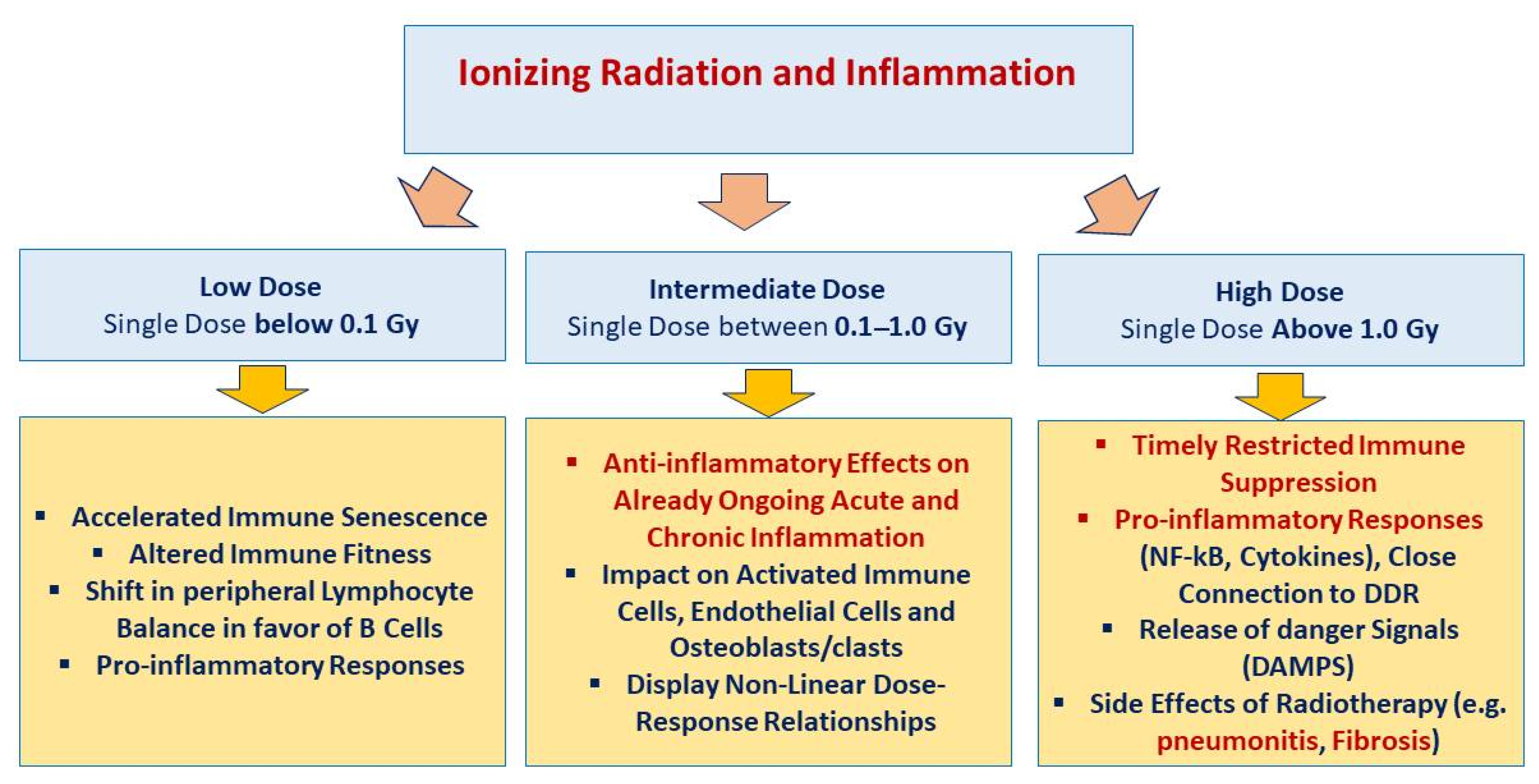
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Measured Parameters | Ameri et al. (July) [30] | Ameri et al. (September) [29] | Arenas et al. [27] | Hess et al. [33] | Hess et al. [32] | Moreno et al. [28] | Sanmamed et al. [35] | Sharma et al. [35] | Papachristofilou et al. [25] | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CRP | * | * | * | * | * | * | * | * | |
| 2 | Lactate dehydrogenase | * | ||||||||
| 3 | Creatine kinase | * | ||||||||
| 4 | D-dimer | * | * | * | * | * | * | |||
| 5 | troponin | * | ||||||||
| 6 | Aspartate aminotransferase (AST) | * | ||||||||
| 7 | Alanine aminotransferase (ALT) | * | ||||||||
| 8 | White blood cell count | * | ||||||||
| 9 | Creatinine | * | ||||||||
| 10 | Interleukin-6 | * | * | * | * | * | * | |||
| 11 | Myoglobin | * | ||||||||
| 12 | Fibrinogen | * | ||||||||
| 13 | Erythrocyte sedimentation rate | * | ||||||||
| 14 | Ferritin | * | * | * | * | * | * | * | ||
| 15 | Procalcitonin | * | * | |||||||
| 16 | LDH | * | * | * | ||||||
| 17 | Leucocyte | * | ||||||||
| 18 | Glutamate pyruvate transaminase (GPT) | |||||||||
| 19 | Hemoglobin | * | ||||||||
| 20 | Lymphocyte | * | * | * | ||||||
| 21 | Platelet | * | ||||||||
| 22 | Fibrinogen | * | ||||||||
| 23 | SatO2/FiO2 index (SAFI) | * | * | * | ||||||
| 24 | SpO2 | * | * | * | * | * | ||||
| 25 | Temperature | * | * | * |
| Study Author(s) | Start Date | Location | Number of Patients | Mean Age | Interventions | Dose Radiation | Total Oxygen Supplementation Duration | Discharge | Criteria for Efficiency of LDRT | Potential Biases | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ameri et al. | 21 May 2020 and July 2020 | Imam Hossein Hospital, Tehran, Iran | 10 | 75 | (1) Standard national guideline for the management of COVID-19: (1) Supplemental oxygen (preferably) via high-flow nasal cannula, (2) unfractionated heparin 5000 units subcutaneously every 8 h or enoxaparin 40 mg subcutaneously once daily, (3) antibiotics (if clinically indicated; e.g., community-acquired pneumonia), (4) basic supportive care, (5) careful monitoring of patients for clinical indices, and (6) dexamethasone 8 mg daily for up to 10 days (at the physician’s discretion) | 0.5 or 1 Gy | All patients received O2 supplementation mainly (60%) via facial masks with reservoir bags | Median: 6th day; range: 2nd–14th days | Primary endpoints: improvement in SpO2, the number of hospital/intensive care unit (ICU) stay days, and the number of intubations performed after RT secondary endpoints: changes in laboratory test results (including CRP, IL-6, ferritin, procalcitonin, and D-dimer) following RT | 0.5 Gy LDRT: rise in SpO2: 80% clinical recovery (included patients who were discharged from the hospital or acquired SpO2 ≥93% on room air): 75% 1 Gy LDRT: rise in SpO2: 40% clinical recovery: 40% | |
| (2) Single-fraction whole-lung radiotherapy | |||||||||||
| Ameri et al. | 21 May 2020 and 24 June 2020 | Imam Hossein Hospital, Tehran, Iran | 5 | 71.8 | Single-fraction whole-lung radiotherapy | 0.5 Gy | Four of the patients recovered rapidly and were weaned from supplemental oxygen at a mean time of 1.5 days | 7 days | Vital signs (including blood oxygenation and body temperature) and laboratory findings (interleukin-6 and C-reactive peptide) | Clinical and paraclinical findings of 4 of the 5 patients improved on the first day of irradiation | |
| Arenas et al. | - Between June and November 2020 | Spain | 36 | 84 | Dexamethasone treatment | 0.5 Gy | Primary endpoints: increasing in the ratio of arterial oxygen partial pressure (PaO2) or the pulse oximetry saturation (SpO2) to fractional inspired oxygen (FiO2) ratio of at least 20% at 24 h with respect to the preirradiation value | Mean SpO2 pretreatment value was 94.28% and the SpO2/FiO2 ratio varied from 255 mm Hg to 283 mm Hg at 24 h and to 381 mm Hg at 1 week | |||
| Single-fraction whole-lung radiotherapy | |||||||||||
| Moreno-Olmedo et al. | April, 2020 | La MilagrosaHospital (Madrid, Spain) | 2 | 72.5 | (1) The medical therapy administered to both patients consisted of lopinavir/ritonavir, hydroxychloroquine, azithromycin, piperacillin/tazobactam, prophylactic doses of low-molecular-weight heparins (LMWHs), corticosteroids (methylprednisolone 250 mg × 3 boluses) and tocilizumab (single dose) | 0.8 Gy | (1) Patient 1 showed an improvement on his O2-Sat and PaFi02 (>300) two days after the treatment (2) Patient 2 showed a slower recovery, achieving less need for oxygen support 2, 5, and 7 days after the treatment | 8 and 14 days | Primary endpoints: achieving hospital discharge Radiological improvement secondary endpoints: SatO2 | Radiological improvement, achieving hospital discharge after 1 radiotherapy session over a period of 8 and 14 days SatO2 > 93% | |
| (2) Single-fraction whole-lung radiotherapy | |||||||||||
| Hess et al. | 23 April to 24 May 2020 | - | 10 | 78 | (1) Patients received best supportive care plus single-fraction whole-lung radiotherapy | 1.5 Gy | Median total time requiring oxygen supplementation was 10 days | Median time to hospital discharge: 20 and 12 days | Efficacy endpoints: time to clinical recovery, radiographic improvement, and serologic responses | Clinical recovery: 3 days for LDRT Median time to hospital discharge: 12 days, intubation rates: 10%, The LDRT cohort had faster radiographic improvement | |
| (2) Patients in the control cohort received best supportive care with or without COVID-directed therapies (i.e., remdesivir, hydroxychloroquine, glucocorticosteroids, etc.) per protocol or physician discretion | |||||||||||
| Hess et al. | 24 and 28 April 2020 | Emory University, Atlanta, U.S. | 5 | 90 | (1) Single-fraction whole-lung radiotherapy | 1.5 Gy | - | 12 days | Efficacy endpoints: time to clinical recovery, radiographic improvement, and serologic responses | Mean time to clinical recovery: 35 h | |
| (2) 3 patients received azithromycin 1, 2, and 3 days before LDRT | |||||||||||
| Sharma et al. | June to August 2020 | India | 10 | 51 | Single-fraction whole-lung radiotherapy | 0.7 Gy | No patient required RT interruption due to deterioration of vitals or oxygen saturation | 15 days | Clinical recovery, death, intubation | Nine patients survived One patient died Clinical recovery: ranging from 3 to 7 days | |
| Sanmamed et al. | April to June 2020 | 9 | 66 | Single-fraction whole-lung radiotherapy | 1 Gy | Oxygen requirements using SatO2/FiO2 index (SAFI) at Days 3 and 7 after LDRT | 34 days | Primary outcome: radiological response using severity and extension score on baseline CTat Days 3 and 7 after LDRT Secondary outcomes: toxicity using CTCAE v5, duration of hospitalization, blood work evolution and oxygen requirements using SatO2/FiO2 index (SAFI) at Days 3 and 7 after LDRT | Significant changes in the extension score (p = 0.03) SAFI index significantly improved 72 h and 1 week after LDRT (p = 0.01) Inflammatory blood parameters decreased | ||
| Papachristofilou et al. | November and December 2020 | University Hospital Basel, Basel, Switzerland | 22 | 75 | Whole-lung low-dose radiation therapy (LDRT) | 1 Gy | - | - | Primary endpoint: ventilator-free days (VFDs) at Day 15 postintervention Secondary endpoints included overall survival, changes in oxygenation, and inflammatory markers | Whole-lung LDRT failed to improve clinical outcomes in critically ill patients requiring mechanical ventilation for COVID-19 pneumonia |
References
- Abdollahi, H.; Shiri, I.; Bevelacqua, J.; Jafarzadeh, A.; Rahmim, A.; Zaidi, H.; Mortazavi, S.; Mortazavi, S. Low dose radiation therapy and convalescent plasma: How a hybrid method may maximize benefits for COVID-19 patients. J. Biomed. Phys. Eng. 2020, 10, 387. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wilson, G.D.; Mehta, M.P.; Welsh, J.S.; Chakravarti, A.; Rogers, C.L.; Fontanesi, J. Investigating low-dose thoracic radiation as a treatment for COVID-19 patients to prevent respiratory failure. Radiat. Res. 2020, 194, 1–8. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Matteo, L.D. Global Storm-The Effects of the COVID-19 Pandemic and Responses around the World. Available online: https://www.fraserinstitute.org/studies/global-storm-the-effects-of-the-covid-19-pandemic-and-responses-around-the-world (accessed on 18 June 2021).
- Day, M. COVID-19: Four fifths of cases are asymptomatic, China figures indicate. BMJ 2020, 369, m1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020, 25, 2000180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, D.; Fuchs, K.; D’alton, M.; Goffman, D. Universal screening for SARS-CoV-2 in women admitted for delivery. N. Engl. J. Med. 2020, 382, 2163–2164. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi-Moghadam, A.; Haghani, M.; Bevelacqua, J.; Jafarzadeh, A.; Kaveh-Ahangar, A.; Mortazavi, S.; Ghadimi-Moghadam, A.; Mortazavi, S. COVID-19 tragic pandemic: Concerns over unintentional “directed accelerated evolution” of novel Coronavirus (SARS-CoV-2) and introducing a modified treatment method for ARDS. J. Biomed. Phys. Eng. 2020, 10, 241. [Google Scholar]
- Calabrese, E.J.; Dhawan, G. How radiotherapy was historically used to treat pneumonia: Could it be useful today? Yale J. Biol. Med. 2013, 86, 555. [Google Scholar]
- Oppenheimer, A. Roentgen therapy of “virus” pneumonia. Am. J. Roentgenol. Rad. Ther. 1943, 49, 635–638. [Google Scholar]
- Kirsch, D.G.; Diehn, M.; Cucinoata, F.A.; Weichselbaum, R. Lack of supporting data make the risks of a clinical trial of radiation therapy as a treatment for COVID-19 pneumonia unacceptable. Radiother. Oncol. 2020, 147, 217–220. [Google Scholar] [CrossRef]
- Correll, H.; Cowan, I. Primary atypical pneumonia; analysis of therapeutic results in 155 cases. US Nav. Med. Bull. 1943, 41, 980–987. [Google Scholar]
- Arenas, M.; Sabater, S.; Hernández, V.; Rovirosa, A.; Lara, P.; Biete, A.; Panes, J. Anti-inflammatory effects of low-dose radiotherapy. Strahlenther. Onkol. 2012, 188, 975–981. [Google Scholar] [CrossRef]
- Kirkby, C.; Mackenzie, M. Is low dose radiation therapy a potential treatment for COVID-19 pneumonia? Radiother. Oncol. 2020, 147, 221. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.; Dhawan, G.; Kapoor, R.; Kozumbo, W. Radiotherapy treatment of human inflammatory diseases and conditions: Optimal dose. Hum. Exp. Toxicol. 2019, 38, 888–898. [Google Scholar] [CrossRef]
- Rodel, F.; Frey, B.; Gaipl, U.; Keilholz, L.; Fournier, C.; Manda, K.; Schollnberger, H.; Hildebrandt, G.; Rodel, C. Modulation of inflammatory immune reactions by low-dose ionizing radiation: Molecular mechanisms and clinical application. Curr. Med. Chem. 2012, 19, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Rödel, F.; Keilholz, L.; Herrmann, M.; Sauer, R.; Hildebrandt, G. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int. J. Radiat. Biol. 2007, 83, 357–366. [Google Scholar] [CrossRef]
- Trott, K. Therapeutic effects of low radiation doses. Strahlenther. Onkol. 1994, 170, 1–12. [Google Scholar]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P. Platelet oxidative stress and thrombosis. Thromb. Res. 2012, 129, 378–381. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Bevelacqua, J.; Mortazavi, S.; Mortazavi, S. COVID-19: Introducing low dose radiation as an effective treatment for pneumonia that shouldn’t induce selective pressure and new mutations. J. Biomed. Phys. Eng. 2020, 10, 247. [Google Scholar]
- Jackson, M.R.; Stevenson, K.; Chahal, S.K.; Curley, E.; Finney, G.; Gutierrez-Quintana, R.; Onwubiko, E.; Rupp, A.F.; Strathdee, K.; MacLeod, M.K. Low-dose lung radiotherapy for COVID-19 lung disease: A preclinical efficacy study in a bleomycin model of pneumonitis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Papachristofilou, A.; Finazzi, T.; Blum, A.; Zehnder, T.; Zellweger, N.; Lustenberger, J.; Bauer, T.; Dott, C.; Avcu, Y.; Kohler, G. Low dose radiation therapy for severe COVID-19 pneumonia: A randomized double-blind study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Meziani, L.; Robert, C.; Mordant, P.; Deutsch, E. Low doses of radiation therapy increase the immunosuppressive profile of lung macrophages via IL-10 production and IFNγ/IL-6 suppression: A therapeutic strategy to counteract lung inflammation? bioRxiv 2021. [Google Scholar] [CrossRef]
- Arenas, M.; Algara, M.; De Febrer, G.; Rubio, C.; Sanz, X.; de la Casa, M.A.; Vasco, C.; Marín, J.; Fernández-Letón, P.; Villar, J.; et al. Could pulmonary low-dose radiation therapy be an alternative treatment for patients with COVID-19 pneumonia? Preliminary results of a multicenter SEOR-GICOR nonrandomized prospective trial (IPACOVID trial). Strahlenther. Onkol. 2021, 1–11. [Google Scholar] [CrossRef]
- Moreno-Olmedo, E.; Suárez-Gironzini, V.; Pérez, M.; Filigheddu, T.; Sanjuan-Sanjuan, A.; González, J.A.; Rivas, D.; Gorospe, L.; Larrea, L.; López, E. Early results of COVID-19 pneumonia cases treated with Ultra-Low doses of Radiotherapy (ULTRA-COVID study). Strahlenther. Onkol. 2020, 197, 429–437. [Google Scholar] [CrossRef]
- Ameri, A.; Ameri, P.; Rahnama, N.; Mokhtari, M.; Sedaghat, M.; Hadavand, F.; Bozorgmehr, R.; Haghighi, M.; Taghizadeh-Hesary, F. Low-dose Whole-lung Irradiation for COVID-19 Pneumonia: What is the Optimal Dose? Final Results of a Pilot Study. Int. J. Radiat. Oncol Biol. Phys 2021, 109, 859–866. [Google Scholar] [CrossRef]
- Ameri, A.; Rahnama, N.; Bozorgmehr, R.; Mokhtari, M.; Farahbakhsh, M.; Nabavi, M.; Shoaei, S.D.; Izadi, H.; Kashi, A.S.Y.; Dehbaneh, H.S. Low-dose whole-lung irradiation for COVID-19 pneumonia: Short course results. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1134–1139. [Google Scholar] [CrossRef]
- Del Castillo, R.; Martinez, D.; Sarria, G.J.; Pinillos, L.; Garcia, B.; Castillo, L.; Carhuactocto, A.; Giordano, F.A.; Sarria, G.R. Low-dose radiotherapy for COVID-19 pneumonia treatment: Case report, procedure, and literature review. Strahlenther. Onkol. 2020, 196, 1086–1093. [Google Scholar] [CrossRef]
- Hess, C.B.; Buchwald, Z.S.; Stokes, W.; Nasti, T.H.; Switchenko, J.M.; Weinberg, B.D.; Rouphael, N.; Steinberg, J.P.; Godette, K.D.; Murphy, D.J. Low-dose whole-lung radiation for COVID-19 pneumonia. medRxiv 2020. [Google Scholar] [CrossRef]
- Hess, C.B.; Buchwald, Z.S.; Stokes, W.; Nasti, T.H.; Switchenko, J.M.; Weinberg, B.D.; Steinberg, J.P.; Godette, K.D.; Murphy, D.; Ahmed, R. Low-dose whole-lung radiation for COVID-19 pneumonia: Planned day 7 interim analysis of a registered clinical trial. Cancer 2020, 126, 5109–5113. [Google Scholar] [CrossRef]
- Sanmamed, N.; Alcantara, P.; Cerezo, E.; Gaztañaga, M.; Cabello, N.; Gómez, S.; Bustos, A.; Doval, A.; Corona, J.; Rodriguez, G. Low-Dose Radiation Therapy in the Management of Coronavirus Disease 2019 (COVID-19) Pneumonia (LOWRAD-Cov19): Preliminary Report. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 880–885. [Google Scholar] [CrossRef]
- Sharma, D.N.; Guleria, R.; Wig, N.; Mohan, A.; Rath, G.K.; Subramani, V.; Bhatnagar, S.; Mallick, S.; Sharma, A.; Patil, P. Low Dose Radiation Therapy for COVID-19 Pneumonia: A Pilot Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Ahmadikia, K.; Hashemi, S.J.; Khodavaisy, S.; Getso, M.I.; Alijani, N.; Badali, H.; Mirhendi, H.; Salehi, M.; Tabari, A.; Mohammadi Ardehali, M.; et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021, 64, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Mortazavi, S.M.J.; Sihver, L. Selective Pressure-Free Treatments for COVID-19. Radiation 2021, 1, 3. [Google Scholar] [CrossRef]
- Rödel, F.; Arenas, M.; Ott, O.J.; Fournier, C.; Georgakilas, A.G.; Tapio, S.; Trott, K.-R.; Gaipl, U.S. Low-dose radiation therapy for COVID-19 pneumopathy: What is the evidence? Strahlenther. Onkol. 2020, 196, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.C.; Burgos, J.; Macias, D. Low dose lung radiotherapy for COVID-19 pneumonia. The rationale for a cost-effective anti-inflammatory treatment. Clin. Transl. Radiat. Oncol. 2020, 23, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Trott, K.-R.; Kamprad, F. Estimation of cancer risks from radiotherapy of benign diseases. Strahlenther. Onkol. 2006, 182, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Impens, N.; Armengol, G.; Candéias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rödel, F.; Schaue, D. Low dose ionizing radiation effects on the immune system. Environ. Int. 2020, 149, 106212. [Google Scholar] [CrossRef] [PubMed]
- Arruda, G.V.; Weber, R.R.D.S.; Bruno, A.C.; Pavoni, J.F. The risk of induced cancer and ischemic heart disease following low dose lung irradiation for COVID-19: Estimation based on a virtual case. Int. J. Radiat. Biol. 2021, 97, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Bevelacqua, J.J.; Welsh, J.S.; Mortazavi, S. Regarding: “The risk of induced cancer and ischemic heart disease following low dose lung irradiation for COVID-19: Estimation based on a virtual case”. Int. J. Radiat. Biol. 2021, 97, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Barabanova, A.; Bushmanov, A.; Kotenko, K. Acute Radiation Sickness from Chernobyl. In Encyclopedia of Environmental Health; Elsevier Science: Amsterdam, The Netherlands; Burlington, MA, USA, 2011; pp. 1–8. [Google Scholar]

| Management of Pneumonia, ARDS, and Other Fatal Changes Associated with COVID-19 | |
|---|---|
| Low-Dose Radiation (LDR) Triggers: | Low-Dose Radiation (LDR) Inhibits: |
| ○ Anti-inflammatory effects | ○ Cytokine-releasing cells |
| ○ Antithrombosis effects | ○ Selective pressure |
| ○ Immune system optimization and metabolic rewiring | ○ Adaptive mutations and viral evolution |
| ○ Alveolar acceleration ○ Mucus absorption | ○ Emergence of new variants with more virulence and transmissibility |
| Hess et al. | 1.5 Gy |
| Ameri et al. (2nd phase) | 1.0 Gy |
| Papachristofilou et al. | 1.0 Gy |
| Sanmamed et al. | 1.0 Gy |
| Minimal effect in bone marrow | 0.5–0.7 Gy |
| Clinically significant effect in bone marrow | >1 Gy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mortazavi, S.M.J.; Shams, S.F.; Mohammadi, S.; Mortazavi, S.A.R.; Sihver, L. Low-Dose Radiation Therapy for COVID-19: A Systematic Review. Radiation 2021, 1, 234-249. https://doi.org/10.3390/radiation1030020
Mortazavi SMJ, Shams SF, Mohammadi S, Mortazavi SAR, Sihver L. Low-Dose Radiation Therapy for COVID-19: A Systematic Review. Radiation. 2021; 1(3):234-249. https://doi.org/10.3390/radiation1030020
Chicago/Turabian StyleMortazavi, Seyed Mohammad Javad, Seyedeh Fatemeh Shams, Sahar Mohammadi, Seyed ALi Reza Mortazavi, and Lembit Sihver. 2021. "Low-Dose Radiation Therapy for COVID-19: A Systematic Review" Radiation 1, no. 3: 234-249. https://doi.org/10.3390/radiation1030020







