Cutaneous Squamous Cell Carcinoma: An Up-to-Date Comprehensive Review with a Focus on Contemporary Optical Imaging Diagnostic Modalities
Abstract
1. Introduction
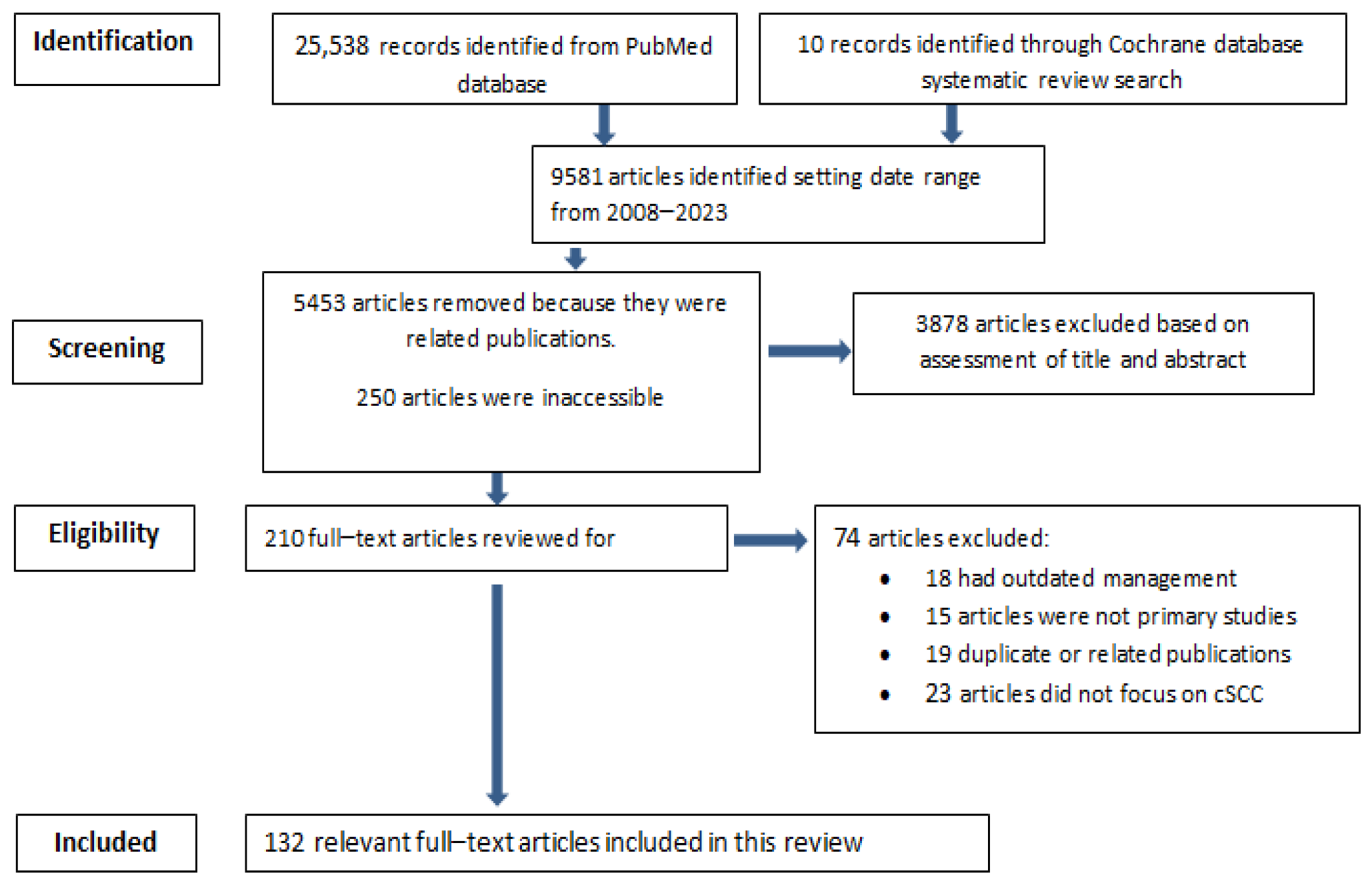
2. Materials and Methods
3. Epidemiology
4. Pathogenesis
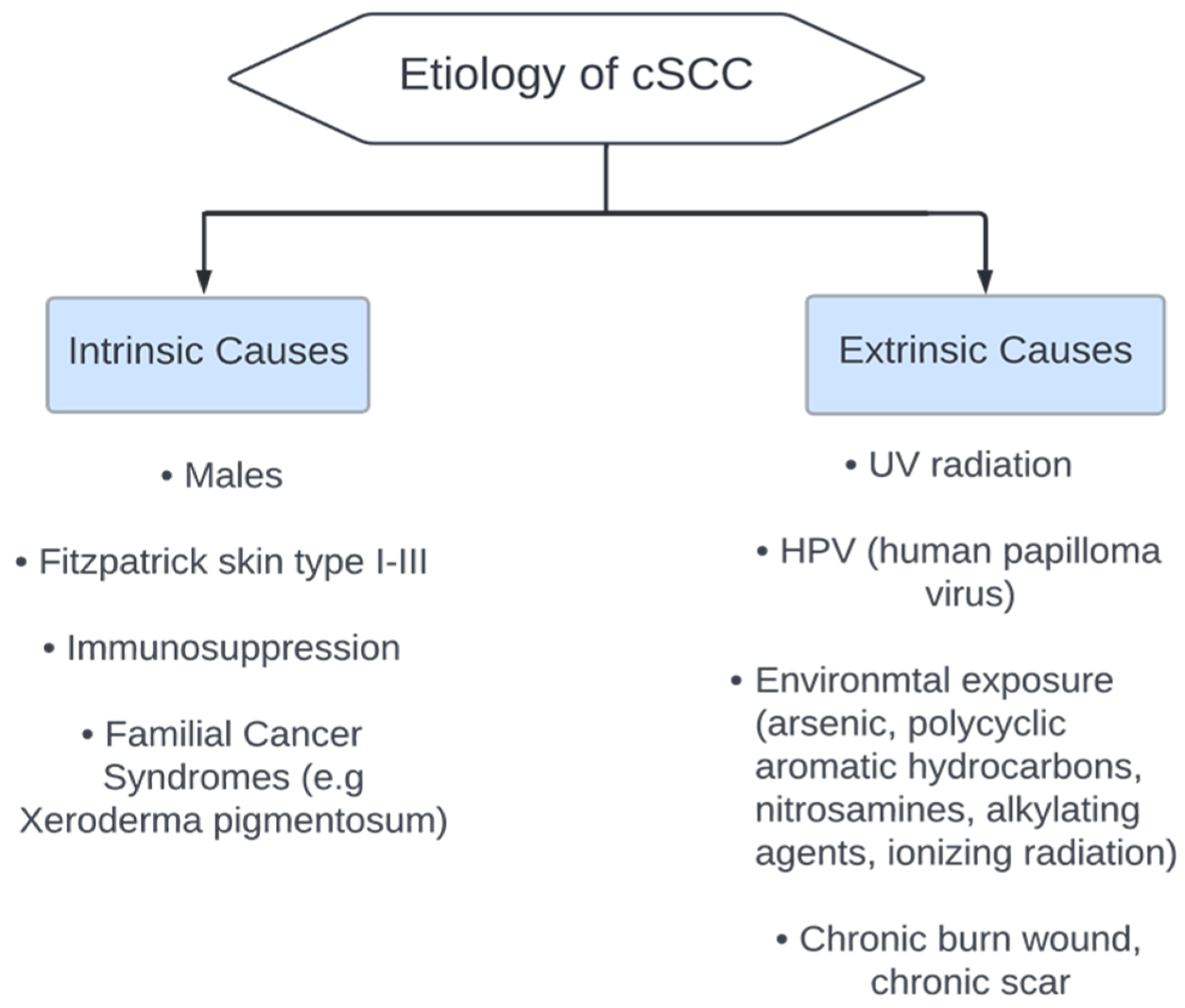
5. Etiology
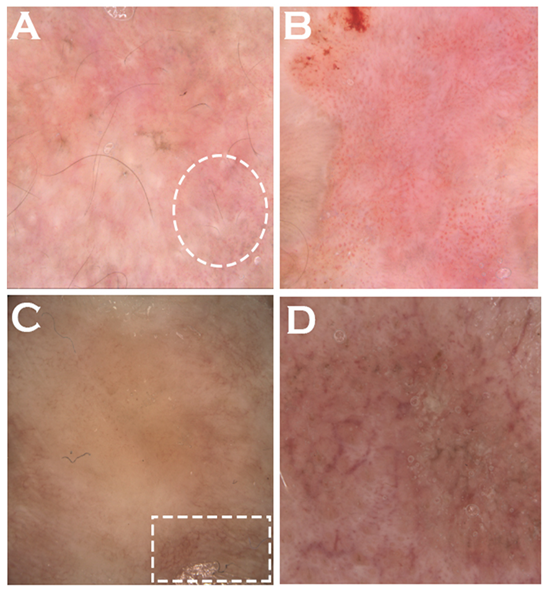
6. Clinical Presentation
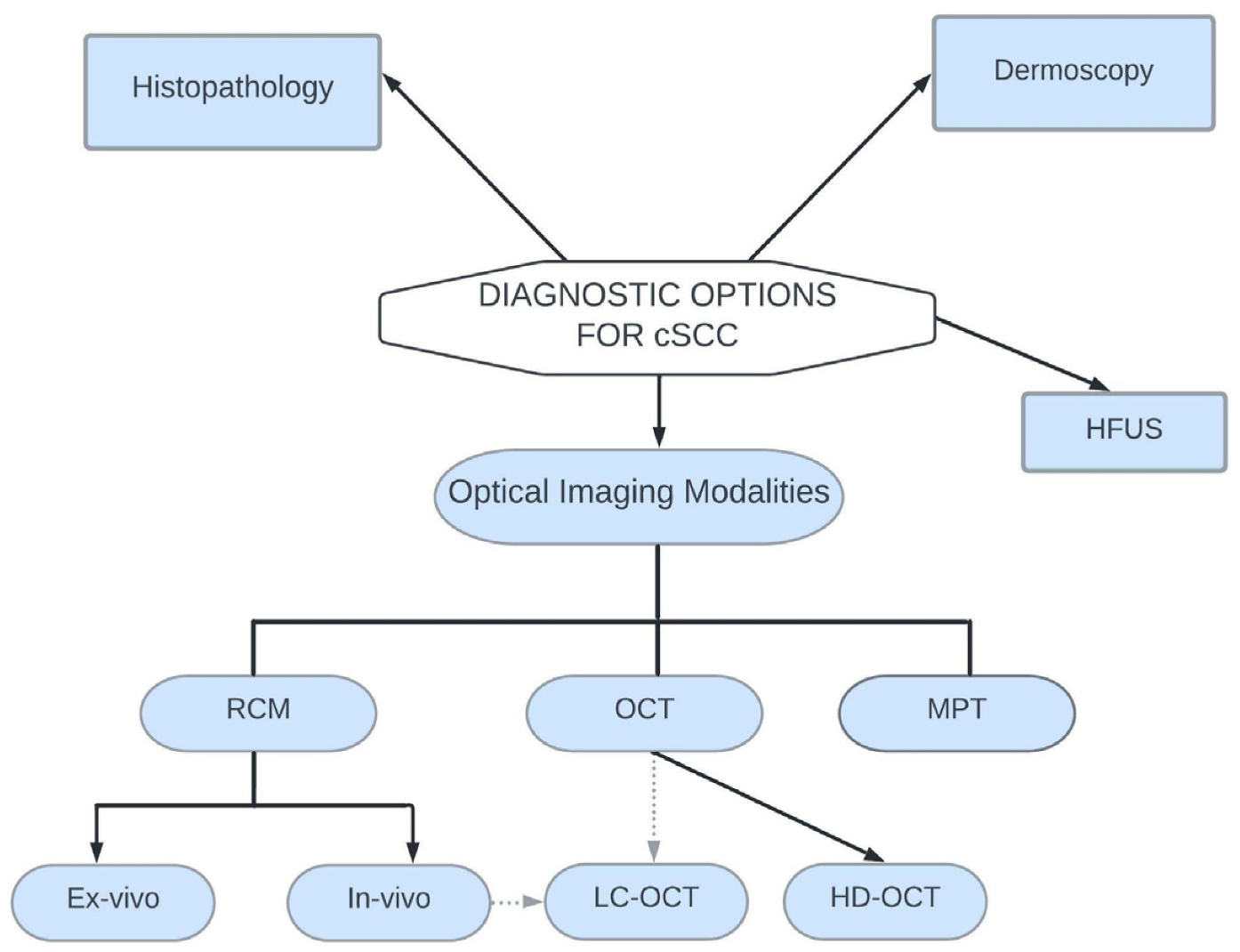
7. Diagnostic Options for Cutaneous Squamous Cell Carcinoma
7.1. Histopathology
7.2. Dermoscopy
7.3. High Frequency Ultrasonography
7.4. Optical Imaging Diagnostic Modalities
7.4.1. Reflectance Confocal Microscopy
A. In Vivo Reflectance Confocal Microscopy
B. Ex Vivo Confocal Microscopy
7.4.2. Optical Coherence Tomography
A. High-Definition Optical Coherence Tomography
7.4.3. Line-Field Confocal Optical Coherence Tomography
7.4.4. Multiphoton Tomography
8. Staging and Gene Expression Profiling
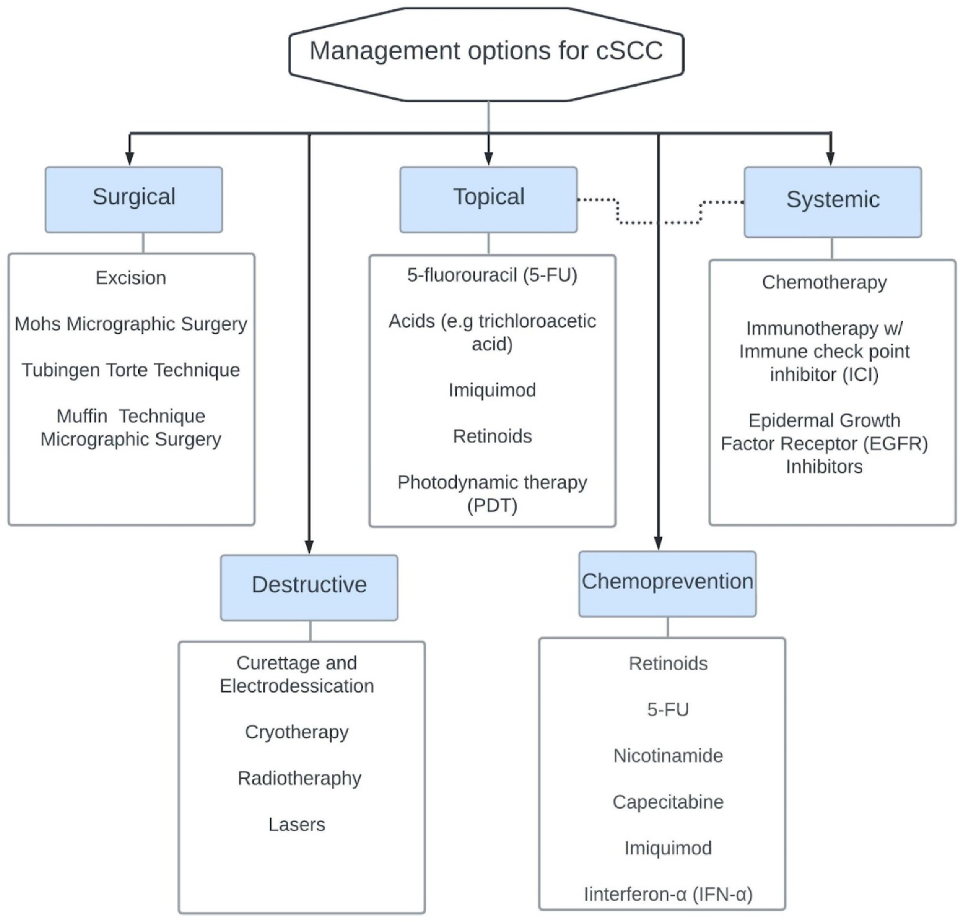
9. Management
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.Y.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P.; Alam, M.; Armstrong, A.; Baum, C.; Bordeaux, J.S.; et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous cell carcinoma of the skin: Epidemiology, classification, management, and novel trends. Int. J. Dermatol. 2015, 54, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Warszawik-Hendzel, O.; Olszewska, M.; Maj, M.; Rakowska, A.; Czuwara, J.; Rudnicka, L. Non-invasive diagnostic techniques in the diagnosis of squamous cell carcinoma. J. Dermatol. Case Rep. 2015, 9, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D. Cutaneous Malignant Melanoma: Body Site, Sun Exposure, Genetic Factors and Prognosis; Ka-Rolinska Institutet: Solna, Sweden, 2017. [Google Scholar]
- Gavioli, C.F.B.; Neto, C.F.; Tyring, S.K.; Silva, L.L.D.C.; De Oliveira, W.R.P. High-risk mucosal HPV types associated with squamous cell carcinoma on the nose tip in an immunocompetent young man. An. Bras. Dermatol. 2018, 93, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Combalia, A.; Carrera, C. Squamous Cell Carcinoma: An Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020066. [Google Scholar] [CrossRef]
- Larese Filon, F.; Buric, M.; Fluehler, C. UV exposure, preventive habits, risk perception, and occupation in NMSC patients: A case-control study in Trieste (NE Italy). Photodermatol. Photoimmunol. Photomed. 2019, 35, 24–30. [Google Scholar] [CrossRef]
- Muzic, J.G.; Schmitt, A.R.; Wright, A.C.; Alniemi, D.T.; Zubair, A.S.; Lourido, J.M.O.; Baum, C.L. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: A popula-tion-based study in Olmsted County, Minnesota, 2000 to 2010. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Garcovich, S.; Colloca, G.; Sollena, P.; Andrea, B.; Balducci, L.; Cho, W.C.; Bernabei, R.; Peris, K. Skin Cancer Epidemics in the Elderly as An Emerging Issue in Geriatric Oncology. Aging Dis. 2017, 8, 643–661. [Google Scholar] [CrossRef]
- Laprise, C.; Cahoon, E.K.; Lynch, C.F.; Kahn, A.R.; Copeland, G.; Gonsalves, L.; Madeleine, M.M.; Pfeiffer, R.M.; Engels, E.A. Risk of lip cancer after solid organ transplantation in the United States. Am. J. Transplant. 2018, 19, 227–237. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef]
- Kueder-Pajares, T.; Descalzo, M.; García-Doval, I.; Ríos-Buceta, L.; Moreno-Ramírez, D. Evaluation of Structure Indicators for Assessing Skin Cancer Quality of Care in Dermatology Departments. Actas Dermo-Sifiliográficas (Engl. Ed.) 2018, 109, 807–812. [Google Scholar] [CrossRef]
- Raone, B.; Patrizi, A.; Gurioli, C.; Gazzola, A.; Ravaioli, G.M. Cutaneous carcinogenic risk evaluation in 375 patients treated with narrowband-UVB phototherapy: A 15-year experience from our Institute. Photodermatol. Photoimmunol. Photomed. 2018, 34, 302–306. [Google Scholar] [CrossRef]
- Govindan, R.; Hammerman, P.S.; Hayes, D.N.; Wilkerson, M.D.; Baylin, S.; Meyerson, M.; on behalf of the Cancer Genome Atlas (TCGA) Group. Comprehensive genomic characterization of squamous cell carcinoma of the lung. Am. Soc. Clin. Oncol. 2012, 30, 7006. [Google Scholar] [CrossRef]
- Wang, N.J.; Sanborn, Z.; Arnett, K.L.; Bayston, L.J.; Liao, W.; Proby, C.M.; Leigh, I.M.; Collisson, E.A.; Gordon, P.B.; Jakkula, L.; et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17761–17766. [Google Scholar] [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.-M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 Mutations in Human Cancers: Origins, Consequences, and Clinical Use. Cold Spring Harb. Perspect. Biol. 2009, 2, a001008. [Google Scholar] [CrossRef]
- Feldman, S.R.; Fleischer, A.B. Progression of actinic keratosis to squamous cell carcinoma revisited: Clinical and treatment implications. Cutis 2011, 87, 201–207. [Google Scholar]
- Dotto, G.P.; Rustgi, A.K. Squamous Cell Cancers: A Unified Perspective on Biology and Genetics. Cancer Cell 2016, 29, 622–637. [Google Scholar] [CrossRef]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; Breems, N.Y.D.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 Mutations Occur Early during Cutaneous Squamous Cell Carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef]
- Takács, T.; Kudlik, G.; Kurilla, A.; Szeder, B.; Buday, L.; Vas, V. The effects of mutant Ras proteins on the cell signalome. Cancer Metastasis Rev. 2020, 39, 1051–1065. [Google Scholar] [CrossRef]
- Mohan, S.V.; Chang, J.; Li, S.; Henry, A.S.; Wood, D.J.; Chang, A.L.S. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016, 152, 527–532. [Google Scholar] [CrossRef]
- Suurmond, D. Section 11. Precancerous lesions and cutaneous carcinomas. Fitzpatrick’s Color Atlas Synop. Clin. Dermatol. 2009, 6e, 232–236. [Google Scholar]
- Mayer, J.E.; Goldman, R.H. Arsenic and skin cancer in the USA: The current evidence regarding arsenic-contaminated drinking water. Int. J. Dermatol. 2016, 55, e585–e591. [Google Scholar] [CrossRef] [PubMed]
- Maner, B.S.; Dupuis, L.; Su, A.; Jueng, J.J.; Harding, T.P.; Meisenheimer, J.; Siddiqui, F.S.; Hardack, M.R.; Aneja, S.; Solomon, J.A. Overview of genetic signaling pathway interactions within cutaneous malignancies. J. Cancer Metastasis Treat. 2020, 6, 37–39. [Google Scholar] [CrossRef]
- Carøe, T.K.; Ebbehøj, N.E.; Wulf, H.C.; Agner, T. Occupational skin cancer may be underreported. Dan. Med. J. 2013, 60, A4624. [Google Scholar] [PubMed]
- Wessely, A.; Steeb, T.; Leiter, U.; Garbe, C.; Berking, C.; Heppt, M.V. Immune Checkpoint Blockade in Advanced Cutaneous Squamous Cell Carcinoma: What Do We Currently Know in 2020? Int. J. Mol. Sci. 2020, 21, 9300. [Google Scholar] [CrossRef]
- Jennings, L.; Schmults, C.D. Management of High-Risk Cutaneous Squamous Cell Carcinoma. J. Clin. Aesthet. Dermatol. 2010, 3, 39–48. [Google Scholar]
- Varra, V.; Smile, T.D.; Geiger, J.L.; Koyfman, S.A. Recent and Emerging Therapies for Cutaneous Squamous Cell Carcinomas of the Head and Neck. Curr. Treat. Options Oncol. 2020, 21, 37. [Google Scholar] [CrossRef]
- Rizvi, S.M.H.; Aagnes, B.; Holdaas, H.; Gude, E.; Boberg, K.M.; Bjørtuft, Ø.; Helsing, P.; Leivestad, T.; Møller, B.; Gjersvik, P. Long-term Change in the Risk of Skin Cancer after Organ Transplantation: A Population-Based Nationwide Cohort Study. JAMA Dermatol. 2017, 153, 1270–1277. [Google Scholar] [CrossRef]
- Plasmeijer, E.; Sachse, M.; Gebhardt, C.; Geusau, A.; Bavinck, J.B. Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance—The impact of immunosuppression on frequency of cSCC. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 33–37. [Google Scholar] [CrossRef]
- Inman, G.J.; Wang, J.; Nagano, A.; Alexandrov, L.B.; Purdie, K.J.; Taylor, R.G.; Sherwood, V.; Thomson, J.; Hogan, S.; Spender, L.C.; et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nat. Commun. 2018, 9, 3667. [Google Scholar] [CrossRef]
- Zwald, F.O.; Brown, M. Skin cancer in solid organ transplant recipients: Advances in therapy and management: Part II. Management of skin cancer in solid organ transplant recipients. J. Am. Acad. Dermatol. 2011, 65, 263–279. [Google Scholar] [CrossRef]
- Omland, S.H.; Gniadecki, R.; Hædersdal, M.; Helweg-Larsen, J.; Omland, L.H. Skin cancer risk in hematopoietic stem-cell transplant recipients compared with background population and renal transplant recipients: A population-based cohort study. JAMA Dermatol. 2016, 152, 177–183. [Google Scholar] [CrossRef]
- Sherston, S.N.; Vogt, K.; Schlickeiser, S.; Sawitzki, B.; Harden, P.N.; Wood, K.J. Demethylation of the TSDR Is a Marker of Squamous Cell Carcinoma in Transplant Recipients. Am. J. Transplant. 2014, 14, 2617–2622. [Google Scholar] [CrossRef]
- Velez, N.F.; Karia, P.S.; Vartanov, A.R.; Davids, M.S.; Brown, J.R.; Schmults, C.D. Association of advanced leukemic stage and skin cancer tumor stage with poor skin cancer out-comes in patients with chronic lymphocytic leukemia. JAMA Dermatol. 2014, 150, 280–287. [Google Scholar] [CrossRef]
- FDA. Commissioner O of the FDA Approves First Treatment for Advanced Form of the Second Most Common Skin Cancer. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-advanced-form-second-most-common-skin-cancer (accessed on 2 November 2022).
- Zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Efird, J.T.; Toland, A.E.; Lea, C.S.; Phillips, C.J. The Combined Influence of Oral Contraceptives and Human Papillomavirus Virus on Cutaneous Squamous Cell Carcinoma. Clin. Med. Insights: Oncol. 2011, 5, CMO-S6905. [Google Scholar] [CrossRef]
- Aldabagh, B.; Angeles, J.G.C.; Cardones, A.R.; Arron, S.T. Cutaneous squamous cell carcinoma and human papillomavirus: Is there an association? Dermatol. Surg. 2013, 39 Pt 1, 1–23. [Google Scholar] [CrossRef]
- Jaju, P.D.; Ransohoff, K.J.; Tang, J.Y.; Sarin, K.Y. Familial skin cancer syndromes: Increased risk of nonmelanotic skin cancers and extracutaneous tumors. J. Am. Acad. Dermatol. 2016, 74, 437–451. [Google Scholar] [CrossRef]
- Lekalakala, P.T.; Khammissa, R.A.G.; Kramer, B.; Ayo-Yusuf, O.A.; Lemmer, J.; Feller, L. Oculocutaneous Albinism and Squamous Cell Carcinoma of the Skin of the Head and Neck in Sub-Saharan Africa. J. Ski. Cancer 2015, 2015, 7847. [Google Scholar] [CrossRef]
- Black, J.O. Xeroderma Pigmentosum. Head Neck Pathol. 2016, 10, 139–144. [Google Scholar] [CrossRef]
- Tokez, S.; Wakkee, M.; Louwman, M.; Noels, E.; Nijsten, T.; Hollestein, L. Assessment of Cutaneous Squamous Cell Carcinoma (cSCC) In Situ Incidence and the Risk of Developing Invasive cSCC in Patients with Prior cSCC In Situ vs. the General Population in the Netherlands, 1989–2017. JAMA Dermatol. 2020, 156, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Sheff, E.K.; Nicholas, P.K.; Evans, L. Emerging Management Trends. Prim. Care E-Book Collab. Pract. 2019, 1, 403. [Google Scholar]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Agostini, T.; Spinelli, G.; Arcuri, F.; Perello, R. Metastatic Squamous Cell Carcinoma of the Lower Lip: Analysis of the 5-Year Survival Rate. Arch. Craniofacial Surg. 2017, 18, 105–111. [Google Scholar] [CrossRef] [PubMed]
- External Ear—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/external-ear (accessed on 2 October 2022).
- Adams, C.C.; Thomas, B.; Bingham, J.L. Cutaneous squamous cell carcinoma with perineural invasion: A case report and review of the literature. Cutis 2014, 93, 141–144. [Google Scholar]
- Bradford, P.T. Skin cancer in skin of color. Derm. Nurs. 2009, 21, 170–177; discussion 206, quiz 178. [Google Scholar]
- Hogue, L.; Harvey, V.M. Basal Cell Carcinoma, Squamous Cell Carcinoma, and Cutaneous Melanoma in Skin of Color Patients. Dermatol. Clin. 2019, 37, 519–526. [Google Scholar] [CrossRef]
- Calonje, J.E.; Brenn, T.; Lazar, A.J.; Billings, S.D. Pathology of the Skin E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Caudill, J.; Thomas, J.E.; Burkhart, C.G. The risk of metastases from squamous cell carcinoma of the skin. Int. J. Dermatol. 2022, 62, 483–486. [Google Scholar] [CrossRef]
- Brantsch, K.D.; Meisner, C.; Schönfisch, B.; Trilling, B.; Wehner-Caroli, J.; Röcken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Brancaccio, G.; Fargnoli, M.C.; Briatico, G.; Pellegrini, C.; Rocco, T.; Moscarella, E. Risk Factors and Diagnosis of Advanced Cutaneous Squamous Cell Carcinoma. Dermatol. Pract. Concept. 2021, 11, e2021166S. [Google Scholar] [CrossRef]
- Lallas, A.; Pyne, J.; Kyrgidis, A.; Andreani, S.; Argenziano, G.; Cavaller, A.; Giacomel, J.; Longo, C.; Malvestiti, A.; Moscarella, E.; et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br. J. Dermatol. 2014, 172, 1308–1315. [Google Scholar] [CrossRef]
- Zalaudek, I.; Giacomel, J.; Schmid, K.; Bondino, S.; Rosendahl, C.; Cavicchini, S.; Tourlaki, A.; Gasparini, S.; Bourne, P.; Keir, J.; et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: A progression model. J. Am. Acad. Dermatol. 2012, 66, 589–597. [Google Scholar] [CrossRef]
- Gutiérrez-Mendoza, D.; Narro-Llorente, R.; Karam-Orantes, M.; Fonte-Avalos, V.; Martínez-Luna, E.; Toussaint-Caire, S.; Domíguez-Cherit, J. Dermoscopy Clues in Pigmented Bowen’s Disease. Dermatol. Res. Pract. 2010, 2010, 464821. [Google Scholar] [CrossRef]
- Jin, H.; Yang, M.-Y.; Kim, J.-M.; Kim, G.-W.; Kim, H.-S.; Ko, H.-C.; Kim, B.-S.; Kim, M.-B. Arborizing Vessels on Dermoscopy in Various Skin Diseases Other Than Basal Cell Carcinoma. Ann. Dermatol. 2017, 29, 288–294. [Google Scholar] [CrossRef]
- Wortsman, X.; Wortsman, J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J. Am. Acad. Dermatol. 2010, 62, 247–256. [Google Scholar] [CrossRef]
- Maj, M.; Warszawik-Hendzel, O.; Szymanska, E.; Walecka, I.; Rakowska, A.; Antczak-Marczak, M.; Kuna, P.; Kruszewski, J.; Nasierowska-Guttmejer, A.; Litniewski, J.; et al. High frequency ultrasonography: A complementary diagnostic method in evaluation of primary cutaneous melanoma. G. Ital. Derm. Venereol. 2015, 150, 595–601. [Google Scholar]
- Crisan, M.; Crisan, D.; Sannino, G.; Lupsor, M.; Badea, R.; Amzica, F.; Lupsor-Platon, M. Ultrasonographic staging of cutaneous malignant tumors: An ultrasonographic depth index. Arch. Dermatol. Res. 2013, 305, 305–313. [Google Scholar] [CrossRef]
- Guitera, P.; Li, L.; Crotty, K.; FitzGerald, P.; Mellenbergh, R.; Pellacani, G.; Menzies, S. Melanoma histological Breslow thickness predicted by 75-MHz ultrasonography. Br. J. Dermatol. 2008, 159, 364–369. [Google Scholar] [CrossRef]
- Jasaitiene, D.; Valiukeviciene, S.; Linkeviciute, G.; Raisutis, R.; Jasiuniene, E.; Kazys, R. Principles of high-frequency ultrasonography for investigation of skin pathology. J. Eur. Acad. Dermatol Venereol. 2011, 25, 375–382. [Google Scholar] [CrossRef]
- Zhu, A.Q.; Wang, L.F.; Li, X.L.; Wang, Q.; Li, M.X.; Ma, Y.Y.; Xiang, L.H.; Guo, L.H.; Xu, H.X. High-frequency ultrasound in the diagnosis of the spectrum of cutaneous squamous cell carcinoma: Noninvasively distinguishing actinic keratosis, Bowen’s Disease, and invasive squamous cell carcinoma. Skin Res. Technol. 2021, 27, 831–840. [Google Scholar] [CrossRef]
- Tambe, S.; Bhatt, K.; Jerajani, H.; Dhurat, R. Utility of high-frequency ultrasonography in the diagnosis of benign and malignant skin tumors. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 162–182. [Google Scholar] [CrossRef] [PubMed]
- Tokez, S.; Koekelkoren, F.H.J.; de Jong, R.J.B.; Grünhagen, D.J.; Mooyaart, A.L.; Nijsten, T.; van der Lugt, A.; Wakkee, M. Assessment of the Diagnostic Accuracy of Baseline Clinical Examination and Ultrasonographic Imaging for the Detection of Lymph Node Metastasis in Patients with High-risk Cutaneous Squamous Cell Carcinoma of the Head and Neck. JAMA Dermatol. 2022, 158, 151. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Ruini, C.; Sattler, E.; Welzel, J. Konfokale Line-Field-OCT. Hautarzt 2021, 72, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Arginelli, F.; Farnetani, F.; Ciardo, S.; Bertoni, L.; Manfredini, M.; Zerbinati, N.; Longo, C.; Pellacani, G. Clinical Applications of In Vivo and Ex Vivo Confocal Microscopy. Appl. Sci. 2021, 11, 1979. [Google Scholar] [CrossRef]
- Manfredini, M.; Longo, C.; Ferrari, B.; Piana, S.; Benati, E.; Casari, A.; Pellacani, G.; Moscarella, E. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1828–1833. [Google Scholar] [CrossRef]
- Titze, U.; Sievert, K.-D.; Titze, B.; Schulz, B.; Schlieker, H.; Madarasz, Z.; Weise, C.; Hansen, T. Ex Vivo Fluorescence Confocal Microscopy in Specimens of the Liver: A Proof-of-Concept Study. Cancers 2022, 14, 590. [Google Scholar] [CrossRef]
- Hartmann, D.; Krammer, S.; Bachmann, M.R.; Mathemeier, L.; Ruzicka, T.; Bagci, I.S.; Von Braunmühl, T. Ex vivo confocal microscopy features of cutaneous squamous cell carcinoma. J. Biophotonics 2018, 11, e201700318. [Google Scholar] [CrossRef]
- Longo, C.; Ragazzi, M.; Gardini, S.; Piana, S.; Moscarella, E.; Lallas, A.; Raucci, M.; Argenziano, G.; Pellacani, G. Ex vivo fluorescence confocal microscopy in conjunction with Mohs micrographic surgery for cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2015, 73, 321–322. [Google Scholar] [CrossRef]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Chapter 3; Springer: Cham, Switzerland, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554044/ (accessed on 22 December 2022).
- Boone, M.A.L.M.; Norrenberg, S.; Jemec, G.B.E.; Del Marmol, V. Imaging actinic keratosis by high-definition optical coherence tomography. Histomorphologic correlation: A pilot study. Exp. Dermatol. 2013, 22, 93–97. [Google Scholar] [CrossRef]
- Maier, T.; Braun-Falco, M.; Laubender, R.P.; Ruzicka, T.; Berking, C. Actinic keratosis in the en face and slice imaging mode of high-definition optical coherence tomography and comparison with histopathology. Br. J. Dermatol 2013, 168, 120–128.e20. [Google Scholar] [CrossRef]
- Boone, M.; Jemec, G.B.E.; Del Marmol, V. High-definition optical coherence tomography enables visualization of individual cells in healthy skin: Comparison to reflectance confocal microscopy. Exp. Dermatol. 2012, 21, 740–744. [Google Scholar] [CrossRef]
- Boone, M.; Marneffe, A.; Suppa, M.; Miyamoto, M.; Alarcon, I.I.; Hofmann-Wellenhof, R.R.; Malvehy, J.; Pellacani, G.; Del Marmol, V. High-definition optical coherence tomography algorithm for the discrimination of actinic keratosis from normal skin and from squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1606–1615. [Google Scholar] [CrossRef]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography—Practical applications in dermatology and comparison with established imaging methods. Ski. Res. Technol. 2020, 27, 340–352. [Google Scholar] [CrossRef]
- Cinotti, E.; Tognetti, L.; Cartocci, A.; Lamberti, A.; Gherbassi, S.; Cano, C.O.; Lenoir, C.; Dejonckheere, G.; Diet, G.; Fontaine, M.; et al. Line-field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: A descriptive study. Clin. Exp. Dermatol. 2021, 46, 1530–1541. [Google Scholar] [CrossRef]
- Weinigel, M.; Breunig, H.G.; Kellner-Höfer, M.; Buckle, R.; Darvin, M.E.; Klemp, M.; Lademann, J.; König, K. In vivohistology: Optical biopsies with chemical contrast using clinical multiphoton/coherent anti-Stokes Raman scattering tomography. Laser Phys. Lett. 2014, 11, 55601. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Choe, C.S.; Ahlberg, S.; Meinke, M.C.; Alexiev, U.; Lademann, J.; Darvin, M.E. Penetration of silver nanoparticles into porcine skin ex vivo using fluorescence lifetime imaging microscopy, Raman microscopy, and surface-enhanced Raman scattering microscopy. J. Biomed. Opt. 2015, 20, 051006. [Google Scholar] [CrossRef]
- Klemp, M.; Meinke, M.C.; Weinigel, M.; Röwert-Huber, H.-J.; König, K.; Ulrich, M.; Lademann, J.; Darvin, M.E. Comparison of morphologic criteria for actinic keratosis and squamous cell carcinoma using in vivo multiphoton tomography. Exp. Dermatol. 2016, 25, 218–222. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Laura, R.M., Ed.; American Joint Committee on Cancer, American Cancer Society, CAPM-Managing Editor; Springer: Chicago, IL, USA, 2017; ISBN 978-3-319-40617-6. [Google Scholar]
- Jambusaria-Pahlajani, A.; Kanetsky, P.A.; Karia, P.S.; Hwang, W.-T.; Gelfand, J.M.; Whalen, F.M.; Elenitsas, R.; Xu, X.; Schmults, C.D. Evaluation of AJCC Tumor Staging for Cutaneous Squamous Cell Carcinoma and a Proposed Alternative Tumor Staging System. JAMA Dermatol. 2013, 149, 402–410. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Karia, P.S.; Besaw, R.; Schmults, C.D. Performance of the American Joint Committee on Cancer Staging Manual, 8th Edition vs. the Brigham and Women’s Hospital Tumor Classification System for Cutaneous Squamous Cell Carcinoma. JAMA Dermatol. 2019, 155, 819–825. [Google Scholar] [CrossRef]
- Venables, Z.C.; Tokez, S.; Hollestein, L.M.; Mooyaart, A.L.; Bos, R.R.V.D.; Rous, B.; Leigh, I.M.; Nijsten, T.; Wakkee, M. Validation of four cutaneous squamous cell carcinoma staging systems using nationwide data. Br. J. Dermatol. 2021, 186, 835–842. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cherpelis, B.S.; Marcusen, C.; Lang, P.G. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol. Surg. 2002, 28, 268–273. [Google Scholar] [PubMed]
- Rowe, D.E.; Carroll, R.J.; Day, C.L., Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Rodolico, V.; Barresi, E.; Di Lorenzo, R.; Leonardi, V.; Napoli, P.; Rappa, F.; Di Bernardo, C. Lymph node metastasis in lower lip squamous cell carcinoma in relation to tumour size, histologic variables and p27Kip1 protein expression. Oral Oncol. 2003, 40, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Mullen, J.T.; Feng, L.; Xing, Y.; Mansfield, P.F.; Gershenwald, J.E.; Lee, J.E.; Ross, M.I.; Cormier, J.N. Invasive Squamous Cell Carcinoma of the Skin: Defining a High-Risk Group. Ann. Surg. Oncol. 2006, 13, 902–909. [Google Scholar] [CrossRef]
- Kraus, D.H.; Carew, J.F.; Harrison, L.B. Regional lymph node metastasis from cutaneous squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 582–587. [Google Scholar] [CrossRef]
- Ratner, D.; Ar, S.; Jd, B.; Js, B.; Cl, B. Faculty Opinions recommendation of Staging for cutaneous squamous cell carcinoma as a predictor of sentinel lymph node biopsy results: Meta-analysis of American Joint Committee on Cancer criteria and a proposed alternative system. JAMA Dermatol. 2014, 150, 19–24. [Google Scholar] [CrossRef]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Fargnoli, M.C.; Forsea, A.M.; Frenard, C.; et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur. J. Cancer 2020, 128, 60–82. [Google Scholar] [CrossRef]
- Farberg, A.S.; Hall, M.A.; Douglas, L.; Covington, K.R.; Kurley, S.; Cook, R.W.; Dinehart, S.M. Integrating gene expression profiling into NCCN high-risk cutaneous squamous cell carcinoma management recommendations: Impact on patient management. Curr. Med. Res. Opin. 2020, 36, 1301–1307. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 5.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf (accessed on 1 August 2022).
- Waldman, A.; Schmults, C. Cutaneous squamous cell carcinoma. Hematol. Oncol. Clin. 2019, 33, 1–12. [Google Scholar] [CrossRef]
- Peris, K.; Piccerillo, A.; Del Regno, L.; Di Stefani, A. Treatment approaches of advanced cutaneous squamous cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2021, 36, 19–22. [Google Scholar] [CrossRef]
- Surmanowicz, P.; Sivanand, A.; Du, A.X.; Mahmood, M.N.; Gniadecki, R. Muffin Technique Micrographic Surgery for Non-melanoma Skin Cancer. Front. Med. 2021, 7, 1141. [Google Scholar] [CrossRef]
- Brodland, D.G.; Zitelli, J.A. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1992, 27, 241–248. [Google Scholar] [CrossRef]
- Mohs, F.E. Chemosurgical Treatment of Cancer of the Face and Lips: A Microscopically Controlled Method of Excision. Surg. Clin. N. Am. 1958, 38, 929–943. [Google Scholar] [CrossRef]
- Strock, D.M.; Militello, M.; Sivesind, T.E.; Matin, R.N.; Dellavalle, R.P. From the Cochrane Library: Non-surgical interventions for cutaneous Bowen’s disease. J. Am. Acad. Dermatol. 2022, 87, 494–495. [Google Scholar] [CrossRef]
- Lansbury, L.; Bath-Hextall, F.; Perkins, W.; Stanton, W.; Leonardi-Bee, J. Interventions for non-metastatic squamous cell carcinoma of the skin: Systematic review and pooled analysis of observational studies. BMJ 2013, 347, f6153. [Google Scholar] [CrossRef]
- Miller, S.J. The National Comprehensive Cancer Network (NCCN) guidelines of care for nonmelanoma skin cancers. Dermatol. Surg. 2000, 26, 289–292. [Google Scholar] [CrossRef]
- Locke, J.; Karimpour, S.; Young, G.; Lockett, M.A.; Perez, C.A. Radiotherapy for epithelial skin cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 748–755. [Google Scholar] [CrossRef]
- Inaba, K.; Ito, Y.; Suzuki, S.; Sekii, S.; Takahashi, K.; Kuroda, Y.; Murakami, N.; Morota, M.; Mayahara, H.; Sumi, M.; et al. Results of radical radiotherapy for squamous cell carcinoma of the eyelid. J. Radiat. Res. 2013, 54, 1131–1137. [Google Scholar] [CrossRef]
- Barrett, T.L.; Greenway, H.T.; Massullo, V.; Carlson, C. Treatment of basal cell carcinoma and squamous cell carcinoma with perineural invasion. Adv. Dermatol. 1993, 8, 277–304; discussion 305. [Google Scholar]
- Covadonga Martínez-González, M.; del Pozo, J.; Paradela, S.; Fernández-Jorge, B.; Fernández-Torres, R.; Fonseca, E. Bowen’s disease treated by carbon dioxide laser. A series of 44 patients. J. Dermatol. Treat. 2008, 19, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Van Egmond, S.; Wakkee, M.; Droger, M.; Bastiaens, M.; van Rengen, A.; de Roos, K.; Nijsten, T.; Lugtenberg, M. Needs and preferences of patients regarding basal cell carcinoma and cutaneous squamous cell carcinoma care: A qualitative focus group study. Br. J. Dermatol. 2018, 180, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chuang, G.S.; Lu, L.K.; Cummins, D.L.; Wu, H.; Finn, D.; Rogers, G.S.; Lee, D. Incidence of Invasive Squamous Cell Carcinomas in Biopsy-Proven Squamous Cell Carcinomas In Situ Sent for Mohs Micrographic Surgery. Dermatol. Surg. 2012, 38, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Stratigos, A.J. Recent Advances in the Diagnosis and Management of High-Risk Cutaneous Squamous Cell Carcinoma. Cancers 2022, 14, 3556. [Google Scholar] [CrossRef]
- Pentangelo, G.; Nisticò, S.P.; Provenzano, E.; Cisale, G.Y.; Bennardo, L. Topical 5% Imiquimod Sequential to Surgery for HPV-Related Squamous Cell Carcinoma of the Lip. Medicina 2021, 57, 563. [Google Scholar] [CrossRef]
- Hamouda, B.; Jamila, Z.; Najet, R.; Slim, T.; Rafiaa, N.; Noureddine, B.; Abderrahman, L. Topical 5-fluorouracil to treat multiple or unresectable facial squamous cell carcinomas in xeroderma pigmentosum. J. Am. Acad. Dermatol. 2001, 44, 1054. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R.; Capezzera, R.; Parrinello, G.; Specchia, C.; Zane, C. Methylaminolaevulinate-based photodynamic therapy of Bowen’s disease and squamous cell carcinoma. Br. J. Dermatol. 2008, 159, 137–144. [Google Scholar] [CrossRef]
- Stebbins, W.G.; Hanke, C.W. MAL-PDT for difficult to treat nonmelanoma skin cancer. Dermatol. Ther. 2011, 24, 82–93. [Google Scholar] [CrossRef]
- Levine, N.; Moon, T.E.; Cartmel, B.; Bangert, J.L.; Rodney, S.; Dong, Q.; Peng, Y.M.; Alberts, D.S. Trial of retinol and isotretinoin in skin cancer prevention: A randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol. Biomark. Prev. 1997, 6, 957–961. [Google Scholar]
- Moon, T.E.; Levine, N.; Cartmel, B.; Bangert, J.L.; Rodney, S.; Dong, Q.; Peng, Y.M.; Alberts, D.S. Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects: A randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol. Biomark. Prev. 1997, 6, 949–956. [Google Scholar]
- Weinstock, M.A.; Thwin, S.S.; Siegel, J.A.; Marcolivio, K.; Means, A.D.; Leader, N.F.; Shaw, F.M.; Hogan, D.; Eilers, D.; Swetter, S.M.; et al. Chemoprevention of Basal and Squamous Cell Carcinoma with a Single Course of Fluorouracil, 5%, Cream. JAMA Dermatol. 2018, 154, 167–174. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Brown, V.L.; Atkins, C.L.; Ghali, L.; Cerio, R.; Harwood, C.A.; Proby, C.M. Safety and Efficacy of 5% Imiquimod Cream for the Treatment of Skin Dysplasia in High-Risk Renal Transplant Recipients: Randomized, Double-blind, Placebo-Controlled Trial. Arch. Dermatol. 2005, 141, 985–993. [Google Scholar] [CrossRef]
- Hanlon, A.; Kim, J.; Leffell, D.J. Intralesional interferon alfa-2b for refractory, recurrent squamous cell carcinoma of the face. J. Am. Acad. Dermatol. 2013, 69, 1070–1072. [Google Scholar] [CrossRef]
- DeConti, R.C. Chemotherapy of Squamous Cell Carcinoma of the Skin. Semin. Oncol. 2012, 39, 145–149. [Google Scholar] [CrossRef]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef]
- Keeping, S.; Xu, Y.; Chen, C.-I.; Cope, S.; Mojebi, A.; Kuznik, A.; Konidaris, G.; Ayers, D.; Sasane, M.; Allen, R.; et al. Comparative efficacy of cemiplimab versus other systemic treatments for advanced cutaneous squamous cell carcinoma. Future Oncol. 2021, 17, 611–627. [Google Scholar] [CrossRef]
- Grob, J.-J.; Gonzalez, R.; Basset-Seguin, N.; Vornicova, O.; Schachter, J.; Joshi, A.; Meyer, N.; Grange, F.; Piulats, J.M.; Bauman, J.R.; et al. Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629). J. Clin. Oncol. 2020, 38, JCO1903054. [Google Scholar] [CrossRef]
- Maubec, E.; Boubaya, M.; Petrow, P.; Beylot-Barry, M.; Basset-Seguin, N.; Deschamps, L.; Grob, J.-J.; Dréno, B.; Scheer-Senyarich, I.; Bloch-Queyrat, C.; et al. Phase II Study of Pembrolizumab as First-Line, Single-Drug Therapy for Patients with Unresectable Cutaneous Squamous Cell Carcinomas. J. Clin. Oncol. 2020, 38, 3051–3061. [Google Scholar] [CrossRef]
- Phan, G.Q.; Yang, J.C.; Sherry, R.M.; Hwu, P.; Topalian, S.L.; Schwartzentruber, D.J.; Restifo, N.P.; Haworth, L.R.; Seipp, C.A.; Freezer, L.J.; et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 2003, 100, 8372–8377. [Google Scholar] [CrossRef]
- Van der Pols, J.C.; Williams, G.M.; Pandeya, N.; Logan, V.; Green, A.C. Prolonged Prevention of Squamous Cell Carcinoma of the Skin by Regular Sunscreen Use. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2546–2548. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Williams, G.; Logan, V.; Strutton, G.M. Reduced Melanoma after Regular Sunscreen Use: Randomized Trial Follow-up. J. Clin. Oncol. 2011, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Pazdrowski, J.; Golusiński, P.; Dańczak-Pazdrowska, A.; Pawlaczyk, M.; Sygut, J.; Marszałek, A.; Golusiński, W. Outdoor work as a risk factor for high-grade cutaneous squamous cell carcinoma of the head and neck. Adv. Dermatol. Allergol. 2018, 35, 408–412. [Google Scholar] [CrossRef]
- Robinson, J.K.; Wayne, J.D.; Martini, M.C.; Hultgren, B.A.; Mallett, K.A.; Turrisi, R. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: A randomized clinical trial. JAMA Dermatol. 2016, 152, 979–985. [Google Scholar] [CrossRef] [PubMed]
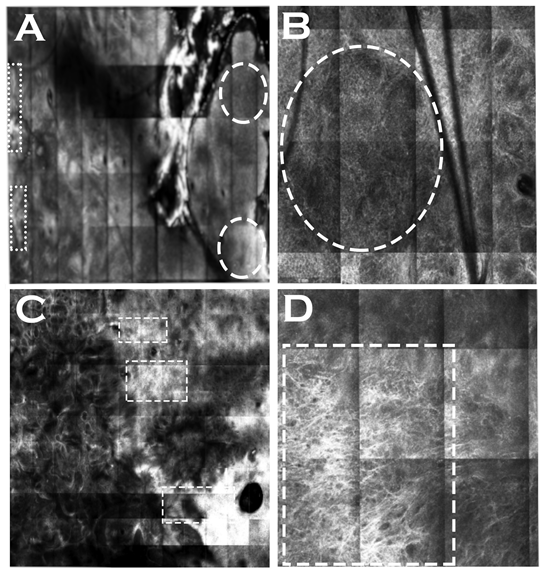
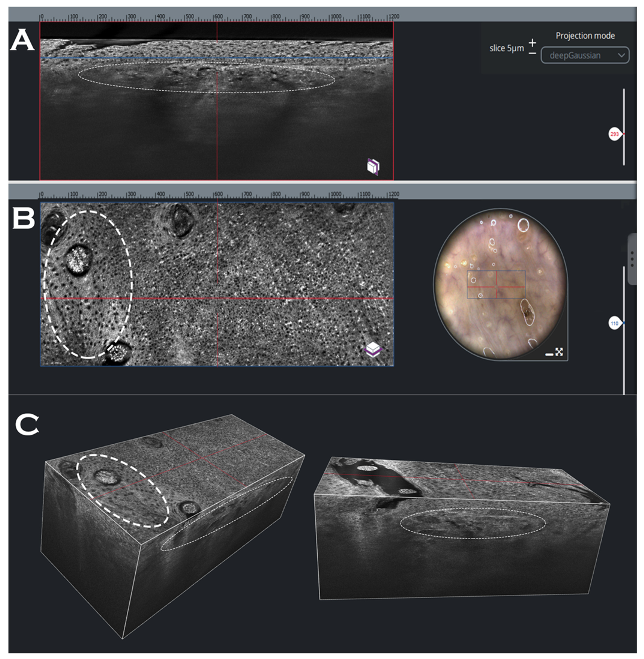
| Features of Squamous Cell Carcinoma | Definition | IVRCM | EVCM | OCT, HD-OCT | LC-OCT Prevalence in Studied Lesions (%) [80] | MPT |
|---|---|---|---|---|---|---|
| Disorganized/absent dermal–epidermal junction | Linear or jagged homogeneous hyporeflective bands separating the epidermis from the dermis. | X | X | X | Visible DEJ: 44% Outlined DEJ, if visible: 22% | |
| Hyperkeratosis, parakeratosis Hyperkeratosis of horned layer in MPT | Stratum corneum exceeding greater than 20 µm in thickness along with presence of retained nuclei in the stratum corneum | X | 77%, 52% | X | ||
| Absence of hyperkeratosis | X | X | X | |||
| Disorganized epidermal structure | Variation in reflectivity along with shape and size of epidermal nuclei of keratinocytes; the normal architecture of the epidermis is disrupted disarranged honeycomb pattern on RCM | X | X | 99% | ||
| “Cocarde image” around the hair follicle | Enlarged hair follicle epithelium with nuclei of irregular shape and size | X | 31% | |||
| Erosion, ulceration | Irregularly contoured dark areas with sharp borders with cellular debris and amorphous material | X | X | 66% | ||
| Acanthosis | Epidermal thickness greater than 60 µm | X | 77% | |||
| Dendritic cells in epidermis | Large elongated cells with clearly visible dendrites connected to the cell | X | ||||
| Keratinocytic atypia (Plump, bright, or speckled cells in the epidermis) | Hyper-reflective large, round cells within the epidermis Roundish to polygonal, slightly larger, bright cells with speckled appearance or indistinct borders in the epidermis | X | X | 73% | ||
| Atypical nuclei | Irregular nuclei in shape and size | X | X | 94% | X | |
| Tumor budding | Atypical keratinocytes with blurred outline forming a rounded projection | 31% | ||||
| Dilated linear vessels | Elongated areas in the dermis, well-defined, with blood cells. | X | X | 53% | ||
| Plump or bright speckled cells in the dermis | Roundish to polygonal, slightly larger, bright cells with speckled appearance or indistinct borders in the dermis | X | X | |||
| Keratin pearls | Whorl-shaped accumulation of keratin appearing as bright, lamellar, sometimes speckled aggregations in the dermis. Often appearance of black hole in the center of the structure is present | X | ||||
| Inflammatory infiltration | Tiny, regular, roundish to oval, bright dots in the dermis | X | X | |||
| Nest-like structures in the dermis | Dermal, irregular aggregates of cells that are larger than inflammatory cells | X | X | |||
| Dilated blood vessels | Dilated horizontal blood vessels in the dermis, with visible blood flow in their inside. | X | ||||
| Button-hole vessels | Dilated blood vessels within the dermal papillae that run perpendicular to the horizontal RCM plane of imaging | X |
| BWH Staging System | AJCC8 Staging System | ||
|---|---|---|---|
| Stage | Features | Stage | Features |
| T1 | No high-risk characteristics | T1 | Tumor diameter < 2 cm |
| T2a | One high-risk characteristic | T2 | Tumor diameter > 2 cm and <4 cm |
| T2b | 2–3 high-risk characteristics | T3 | Tumor diameter > 4 cm, or PNI, or minor bone erosion, or deep invasion. |
| T3 | All four high-risk characteristics or bone invasion | T4 | Tumor with gross cortical bone/marrow invasion |
| High-risk Factors | Tumor diameter ≥ 2 cm, poorly differentiated, invasion beyond fat, PNI ≥ 0.1 mm | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razi, S.; Khan, S.; Truong, T.M.; Zia, S.; Khan, F.F.; Uddin, K.M.; Rao, B.K. Cutaneous Squamous Cell Carcinoma: An Up-to-Date Comprehensive Review with a Focus on Contemporary Optical Imaging Diagnostic Modalities. Dermato 2023, 3, 161-181. https://doi.org/10.3390/dermato3020013
Razi S, Khan S, Truong TM, Zia S, Khan FF, Uddin KM, Rao BK. Cutaneous Squamous Cell Carcinoma: An Up-to-Date Comprehensive Review with a Focus on Contemporary Optical Imaging Diagnostic Modalities. Dermato. 2023; 3(2):161-181. https://doi.org/10.3390/dermato3020013
Chicago/Turabian StyleRazi, Shazli, Samavia Khan, Thu M. Truong, Shamail Zia, Farozaan Feroz Khan, Khalid Mahmood Uddin, and Babar K. Rao. 2023. "Cutaneous Squamous Cell Carcinoma: An Up-to-Date Comprehensive Review with a Focus on Contemporary Optical Imaging Diagnostic Modalities" Dermato 3, no. 2: 161-181. https://doi.org/10.3390/dermato3020013
APA StyleRazi, S., Khan, S., Truong, T. M., Zia, S., Khan, F. F., Uddin, K. M., & Rao, B. K. (2023). Cutaneous Squamous Cell Carcinoma: An Up-to-Date Comprehensive Review with a Focus on Contemporary Optical Imaging Diagnostic Modalities. Dermato, 3(2), 161-181. https://doi.org/10.3390/dermato3020013






