Abstract
Rosacea is a common chronic inflammatory skin condition. It mainly affects the cheeks, nose, chin, and forehead, causing flushing or transient erythema, persistent erythema, phymatous changes, papules, pustules, and telangiectasias, and the eyes may also be affected by rosacea. Rosacea is more common in women than in men and can start at any age. Rosacea affects both fair-skinned and darker-skinned people. Physical changes in the face due to rosacea can cause embarrassment, leading to reduced quality of life and self-esteem. Rosacea has several triggers, and its pathogenesis involves multiple factors, which means there are several treatment options, and these options can be combined. A patient’s clinical findings and symptoms will help a doctor to diagnose and classify the condition. Treatment options may include lifestyle changes, topical medications, systemic antibiotics and light-based therapy. The best approach is to tailor the treatment to the individual’s condition and preferences. The aim of treatment is to manage symptoms and prevent the progression of the disease.
1. Introduction
Rosacea is a chronic inflammatory skin condition characterized by skin sensitivity, flushing, centrofacial erythema, papules, and pustules. The condition has been classified into four subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea [1]. Rosacea mainly affects the cheeks, nose, chin, and forehead. It causes flushing or transient erythema, persistent erythema, phymatous changes, papules, pustules, and telangiectasias. The eyes can also be affected by rosacea, causing conditions such as conjunctivitis, blepharitis, and, rarely, keratitis [2]. Physical changes in the face due to rosacea can cause embarrassment, leading to reduced quality of life and self-esteem [1,3].
2. Epidemiology of Rosacea
Rosacea is one of the most common chronic inflammatory skin conditions for which patients consult a dermatologist. The prevalence of rosacea in Swedish population is 10%, while a systematic review found a prevalence of 5.46% in the general adult population [2,4]. It has also been reported that rosacea affects around 16 million Americans [1].
Age-, Gender-, and Race-Specific Differentiations of Rosacea
Rosacea is more common in women than in men and can occur at any age, although it usually starts after the age of 30 [2]. A systematic review also found that the prevalence of rosacea was similar in men and women, and predominantly in the 45–60-year age group [5]. Moreover, phymatous changes, primarily localized in the nose, and referred to as ‘rhinophyma’, occur more frequently in men [1,3].
Rosacea is observed in fair-skinned patients, but it has also been diagnosed in patients with skin type IV and above, such as Asian, Latin American, African American, and African patients [2]. It is possible that darker-skinned patients with skin phototypes V and VI may not have erythema or telangiectasia, which may remain an underdiagnosed condition because pigmentation may mask the skin findings [6].
3. Pathogenesis of Rosacea
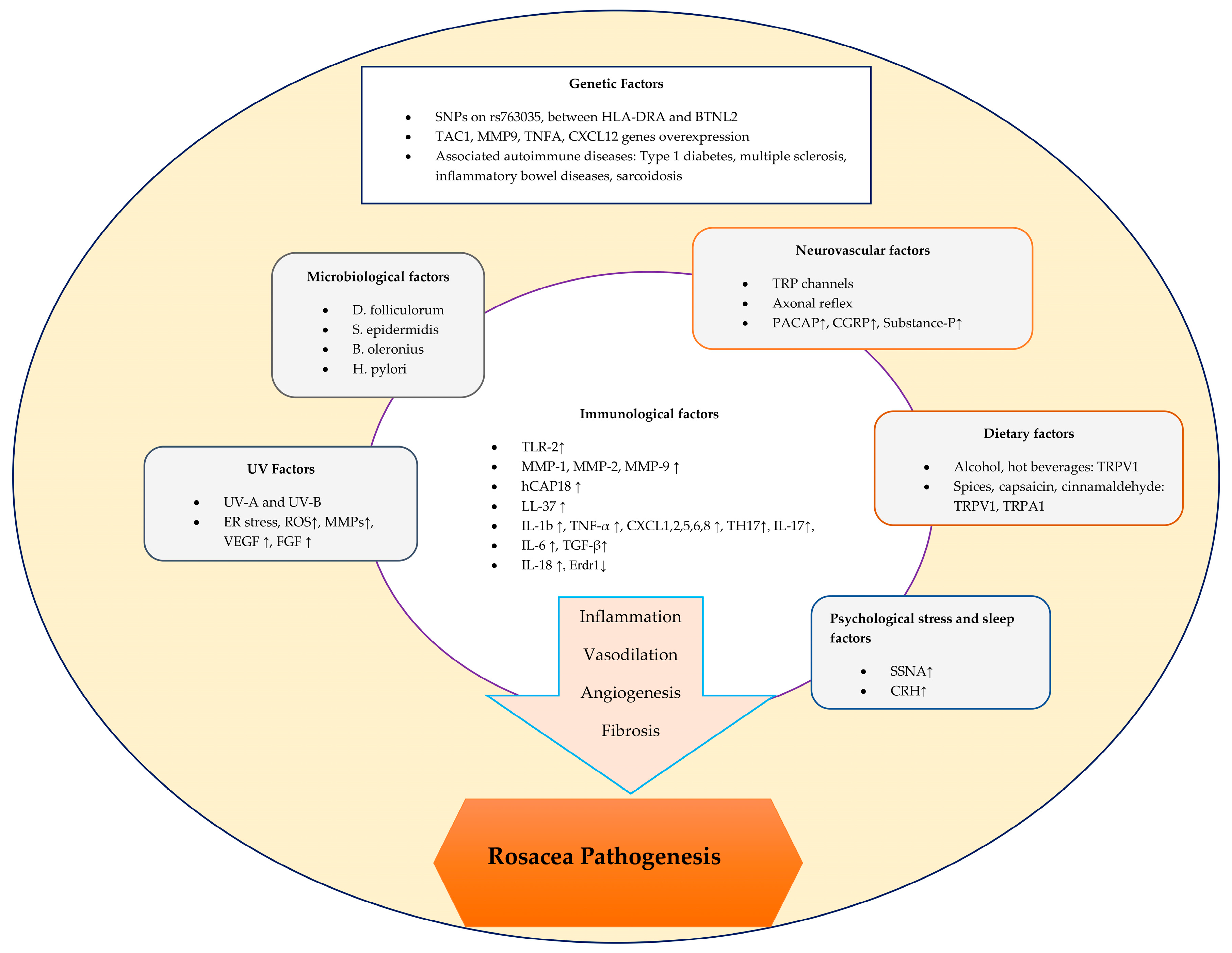
The pathogenesis of rosacea is complex and involves a variety of factors that can trigger both inflammatory and vascular responses [7] (Figure 1).
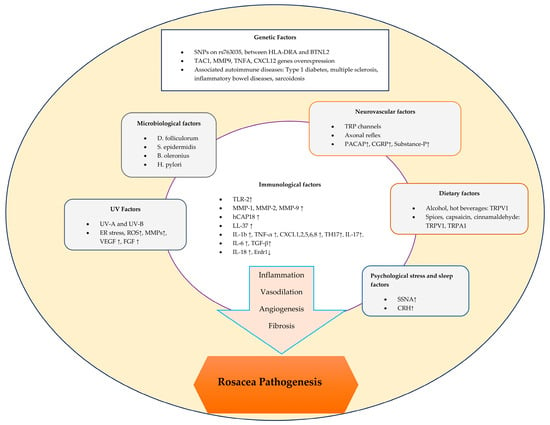
Figure 1.
Schematic representation of factors contributing to the pathogenesis of rosacea.
3.1. Immunological Factors in Rosacea
3.1.1. Dysregulation of the Innate Immune System
Rosacea skin has higher levels of TLR-2, KLK-5, cathelicidin, and matrix metalloproteinases (MMPs) than healthy skin [4]. Toll-like receptors (TLRs) are a subset of pattern recognition receptors (PRRs) that recognize damage-associated or pathogen-associated molecular patterns in the skin’s immune system. In particular, TLR-2 is abundant in rosacea skin and has been found in higher levels than in healthy skin [4,8]. In rosacea patients, keratinocytes produce pro-inflammatory cytokines and chemokines when TLR-2 is activated by triggering factors, leading to the increased expression of pro-inflammatory cytokine genes such as IL (interleukin)-8, IL-1b, and TNF (tumor necrosis factor)-α [9]. For instance, as an exogenous factor, ultraviolet radiation (UV) activates TLR-2 by inducing endoplasmic reticulum stress. This leads to the production of inflammatory cytokines, which, in turn, trigger inflammatory responses [4]. Moreover, TLR-2 was found to positively mediate a pro-inflammatory response following UV-B irradiation in an in vivo study [10].
IL-8 induces neutrophil chemotaxis in the skin, resulting in the release of proteases such as cathepsin G, elastase, and protease-3 [8]. In addition, IL-b and TNF-α also act as the angiogenic factor and vascular endothelial growth factor (VEGF) and may cause the vascular changes seen in rosacea [8,11]. In addition, a study has shown that TLR-2 expression in keratinocytes or TLR-2 ligand exposure induces the production and protease activity of the serine protease KLK (kallikrein)-5, resulting in cathelicidin activation [4,12]. Cathelicidin, an antimicrobial protein, is found in the lamellar body of keratinocytes and the granules of neutrophils. Human-specific cathelicidin is called human cationic antibacterial protein of 18 kDa (hCAP18), which is the inactive form, and it is cleaved by KLK-5 to the active peptide form called LL-37 [1,13,14]. LL-37 is an antimicrobial peptide that modifies host immunity and growth responses by promoting leukocyte chemotaxis and angiogenesis to rearrange extracellular matrix components. The LL-37 peptide in rosacea skin differs from healthy skin by having shorter fragment forms [1,14]. These fragments have been implicated in symptoms characteristic of rosacea, including erythema, vasodilatation, flushing, and telangiectasia, based on observations in studies of injected mice [15].
MMPs indirectly influence the pathogenesis of rosacea by activating the preproenzyme form of KLK5 after cleavage with MMP-9. Specifically, MMP-2 and MMP-9 are increased in the skin of rosacea patients, which could activate KLK5 more and increase LL-37 expression [1]. A study also suggested that mast cell degranulation in individuals with rosacea results in increased levels of MMP-9 and LL-37 [14].
3.1.2. Dysregulation of the Adaptive Immune System in Rosacea
T-Cell Mediated Dysregulation
In the pathogenesis of rosacea, pro-inflammatory cytokines and chemokines play dominant roles in the infiltration of inflammatory cells and trigger immune responses [8]. Among the total T cells in rosacea skin, an increase in CD4+ over CD8+ T cells has been demonstrated. There was also upregulation in the polarizing Th1 and Th17 gene sets, as well as the interferon gamma (IFN-γ) and IL-17A cytokines [16]. Th17 could be responsible for an effect on LL-37 via the expression of abnormal forms that are seen in rosacea [17]. IL-17 induces angiogenesis through the VEGF pathway and has an effect on the expression of LL-37 [11,17]. Thus, the connection between cytokines and chemokines with the Th1/Th17 pathways and rosacea supports the notion of their association [8].
One study found that the levels of regulatory T cells in rosacea, which are CD4+CD25+ regulatory T cells, were higher in rosacea than in lupus erythematosus. This was a supportive finding, as immune cells in rosacea are more likely to maintain immunological tolerance than in other autoimmune diseases [8,18]. A neutrophil chemotactic factor, CXCL8, was found to be increased in rosacea via the upregulation of its mRNA gene modification. CXCL1, CXCL2, CXCL5, and CXCL6 were also upregulated in rosacea patients. These chemokines in rosacea have angiogenic properties that also attract neutrophils and TH17 cells [16].
A recent case report of rosacea-like erythematous papulopustules on the face associated with the COVID-19 vaccine also supports the idea that the adaptive immune system is involved in rosacea. In this report, a single case was associated with the Vaxzevria (AstraZeneca) COVID-19 vaccine, while the other was related to the Pfizer-BioNTech COVID-19 vaccine. It has been suggested that the pathogenesis could involve elevated levels of cutaneous chemokines like CCL2, CCL5, and CXCL10, inducing chemotaxis and the infiltration of CD4+ T cells, monocytes/macrophages, and polymorphonuclear cells into the skin [19]. In addition, both vaccines were shown to induce, through different mechanisms, neutralizing antibodies against the SARS-CoV-2 spike protein and specific T-cell expansion with the secretion of cytokines such as IFN-γ, IL-2, and IL-10 [19]. Furthermore, the SARS-CoV-2 spike protein encoded by COVID-19 mRNA vaccines induces IL-1b secretion in macrophages. Moreover, elevated levels of IL-1 and IL-36 trigger an inflammatory response, leading to pustules in pustular psoriasis [20].
In the study by Salzer et al., erythroid differentiation regulator 1 (Erdr1) was found to be decreased in rosacea patients compared to the controls, whereas IL-18 was found to be increased. It was also found that Erdr1 levels were decreased by IL-18 in rosacea [21]. Erdr1 is expressed in normal skin epithelium and may suppress UV-induced oxidative stress in rosacea by reducing reactive oxygen species (ROS) levels by blocking heat shock protein 90 [8]. IL-18 regulates the immune response by activating Th1-mediated responses, as in many other chronic inflammatory diseases, such as psoriasis, atopic dermatitis, and contact dermatitis, and also downregulates the Erdr1 levels in rosacea [8,21]. Recombinant Erdr1 may be beneficial for rosacea patients by reducing UV-induced oxidative stress. In a mouse model of rosacea, recombinant Erdr1 also suppressed the expression of VEGF and reduced angiogenesis, giving hope for its use in human rosacea patients [21].
B Cell-Mediated Dysregulation
The relationship between TLRs and B cells involves mechanisms where TLR activation is necessary for certain antigen-specific antibody responses in B cells, and TLR agonists stimulate the differentiation of plasma cells from B cells [22]. As in phymatous rosacea, fibrotic changes in the skin can be stimulated by B cells through the TLR-inducing effect on fibrogenic cytokines such as IL-6 and TGF-β (transforming growth factor beta) [8]. Furthermore, elevated antinuclear antibody titers are frequently detected in patients with rosacea, indicating that the involvement of B cells in the pathogenesis of rosacea cannot be underestimated [8,23].
3.2. Microbiological Factors in Rosacea
Demodex folliculorum is the predominant microbial agent within the skin that is commonly associated with the development of rosacea. This saprophytic mite specifically targets the sebaceous gland area of healthy skin, where it can use epidermal cells and sebum components as nutrients. Facial skin is particularly rich in sebaceous glands, especially the nose, cheeks, forehead, and chin [24], and rosacea skin has a higher density of Demodex mites compared to normal facial skin [25].
High densities of D. folliculorum lead to the release of pro-inflammatory mediators such as TNF-α, IL-1b, IL-8, and LL-37 into the skin. Specifically, the exoskeleton of Demodex contains chitin, a type of polysaccharide that causes the activation of TLR-2 on keratinocytes, leading to protease activity [26]. In addition, D. folliculorum was found to be associated with IL-17, which plays a role in papulopustular rosacea by inducing angiogenesis, telangiectasia, inflammation, and pustules [25]. NLRP3 gene expression is also stimulated by mite colonization, leading to the production of IL-1b and the initiation of the inflammatory process [25,26]. Moreover, Demodex mites at high densities have also been shown to affect tissue compatibility. These are HLA Cw2 and Cw4 and cause leukocytes to undergo apoptosis. Immunosuppression in the mite’s microenvironment allows it to survive easily in the skin [27].
Staphylococcus epidermidis is the predominant bacteria that is commensal on the skin [4]. It may play an important role in the pathogenesis of pustular and ocular rosacea, as suggested by a study that isolated S. epidermidis from the pustules and eyelid margins of rosacea patients [28]. In addition, S. epidermidis antigens are recognized by TLR-2, and the interaction between S. epidermidis and TLR-2 leads to the initiation of an immune response. Both S. epidermidis and TLR-2 induce the expression of AMPs [1].
Another bacterial pathogen associated with rosacea, isolated from the Demodex mite, is Bacillus oleronius. It is a Gram-negative, endospore-forming, non-motile bacteria. One study found that patients with erythematous rosacea had higher levels of mononuclear cells to B. oleronius bacterial antigens in their peripheral blood [29].
Helicobacter pylori, a Gram-negative bacteria found in the gastric mucosa, is the other microbiological agent that causes rosacea symptoms [30]. H. pylori may have an effect on increasing ROS, leading to inflammation in the gut. Among the ROS, nitric oxide (NO) can specifically induce intestinal mucosal inflammation and alter physiological processes in the skin, including vasodilation, inflammation, and immunomodulation, leading to the clinical manifestations of flushing and erythema associated with rosacea [31,32]. Through its cytotoxin-associated gene-A (cagA)-encoded cytotoxin, H. pylori is thought to play a role in inducing the production of pro-inflammatory cytokines such as TNF- α and IL-8, leading to gastric mucosal inflammation and the clinical manifestation of rosacea [31,32].
It is demonstrated in the literature that the intestinal microbial population may have an immunomodulatory effect upon non-gastrointestinal systems including the skin. Changes in diet, such as the consumption of alcohol or high glycemic index foods, may cause a shift in the microbiome from a healthy to an unhealthy population [33,34]. The prevalence of small intestinal bacterial overgrowth (SIBO) was found to be significantly higher in rosacea patients than in the controls [35]. SIBO may also be linked to the activation of TNF-α, leading to inflammatory symptoms of rosacea [36].
3.3. Genetic Factors in Rosacea
Genetic factors are implicated in the manifestation of rosacea based on familial studies. In a cohort study evaluating the severity of rosacea between heterozygous and monozygous twins, monozygous twins showed greater severity and a higher correlation with clinical rosacea scores than heterozygous twins [37].
Fifteen genes were found to be over-expressed in the erythematotelangiectatic rosacea group compared to the healthy control group. The genes involved in neuropeptides are CALCA, CALCB, and TAC1. The genes involved in matrix remodeling are COL1, COL3, CYR61, DCN, MMP1, MMP3, and MMP9. Innate Immunity-Related Genes are DEFA1, CXCL12, and CXCR4. The genes involved in inflammatory markers are IL-12 B and TNFA [38]. Mainly, the TAC1, MMP9, TNFA, and CXCL12 genes have been identified as significant, as they encode substances such as P, MMPs, TNF-α, and chemoattractants for mast cells [39].
A single nucleotide polymorphism (SNP) that is significantly associated with rosacea was identified on chromosome 6 (rs763035), which is intergenic and located upstream of the HLA class II histocompatibility antigen, DR alpha chain (HLA-DRA), and is located downstream of butyrophilin-like 2 (BTNL2), which is mainly associated with histocompatibility complex class I. Both genes were linked through the coexpression of HLA-DRB5 and CIITA (class II, major histocompatibility complex, transactivator) [40]. In papulopustular rosacea, skin immunohistochemistry and an HLA allele analysis demonstrated the presence of HLA-DRA in epidermal Langerhans cells, the presence of BTNL2 in keratinocytes, and the presence both genes in the perifollicular inflammatory infiltrate and endothelial cells. A further genetic analysis of HLA alleles defined three significant replication groups related to rosacea: HLA-DRB1*03:01, HLA-DQB1*02:01, and HLADQA1*05:01. Both of these replication groups and HLA-DRA play roles in antigen presentation from extracellular sources, supporting the effects of microbial agents in the pathogenesis of rosacea [40]. In addition, thymic stromal lymphopoietin (TSLP) gene expression was found to be significantly decreased in papulopustular rosacea, resulting in the recruitment of dendritic cells and pathogenic Th17 cells with an inflammatory microenvironment of IL-17 and IFN-γ [39,41].
Rosacea has been shown to have several risk gene loci associated with autoimmune diseases. The HLA allele of the DRB1*03:01-DQB1*02:01-DQA1*05:01 haplotype is linked to rosacea [39]. Such an association between rosacea and these HLA alleles, along with HLA-DRA, supports the idea that extracellular antigen presentation plays a role in the etiology of the condition, suggesting a link with various microbiological agents. Moreover, HLADRB1*03:01 is specifically associated with retinopathy in type 1 diabetes and may be related to the abnormal proliferation of blood vessels observed in the ocular findings of rosacea [39,40]. Other genetic loci of shared autoimmune diseases include HLA-DRA, which is associated with rosacea and multiple sclerosis, and BTNL2, which is associated with rosacea, inflammatory bowel disease, and sarcoidosis [40,42,43].
3.4. Neurovascular Factors in Rosacea
Facial skin vascular activity is controlled by neuronal (sympathetic, parasympathetic, and nociceptive) and non-neuronal (local inflammatory) mechanisms [44]. Transient receptor potential (TRP) channels activate sensory nerve endings to release vasoactive neuropeptides. There are two calcium channel members of the TRP family called transient receptor potential vanilloid 1 (TRPV1) and ankyrin 1 (TRPA1). TRPV1 and TRPA1 are triggered by spices, alcohol, and temperature changes. In rosacea, these two channels coordinate the inflammatory response by inducing depolarization, which leads to the release of neuropeptides such as the pituitary adenylate cyclase-activated polypeptide (PACAP), substance P, and the calcitonin gene-related peptide (CGRP) [44]. In addition, local mediators trigger the release of vasodilator neuropeptides, CGRP, and substance P by activating skin nociceptors through an axon reflex effect [45]. Moreover, PACAP, substance P, and CGRP cause inflammatory responses by activating mast cells, macrophages, neutrophils, and T cells [44]. Heat can also activate both TRPV1 and TRPA1 in individuals suffering from rosacea. An increased and prolonged expression of TRPs in rosacea patients results in facial flushing, the dysregulation of the vascularity, and neurogenic leukocyte inflammation [8].
3.5. Dietary Factors in Rosacea
Keratinocytes and sensory nerves have TRPV1 receptors that are activated by substances such as alcohol, spicy foods, hot beverages, vanilla, cinnamon, caffeine, and UV radiation. These channels release substance P and CGRP, resulting in an inflammatory response, dilated arterioles, flushing, and edema in rosacea patients [46]. Alcohol is also converted into metabolites such as acetaldehyde and acetone. These compounds generate histamine, which is recognized to affect the dermal vasomotor system, resulting in flushing [47,48].
It is therefore important to note that the excessive consumption of spices could potentially lead to rosacea flares. Capsaicin from chili peppers and cinnamaldehyde from cinnamon can activate TRPV1 and TRPA1 receptors, respectively, causing an intense burning sensation. It has been suggested that spices may activate TRPA1 and TRPV1, resulting in flushing or burning sensations in rosacea patients. According to studies, the dilated microvascular structure and increased blood flow intensity in rosacea patients with papules and pustules may be due to TRPA1 and TRPV1 receptor activation [49].
In contrast to other inflammatory skin diseases such as atopic dermatitis and chronic urticaria [50,51], the serum vitamin D levels were found to be higher in rosacea patients compared to the controls [52]. In addition, vitamin D levels are associated with sun exposure, which is a triggering factor in rosacea [53]. However, another study showed that the serum vitamin D levels were significantly lower in rosacea patients than in the controls [54]. Although vitamin D has been demonstrated to enhance TLR-2 and KLK5 mRNA expressions [55] and influence LL-37 pathways that play a role in rosacea [8], larger epidemiologic studies are needed to prove the association between serum vitamin D levels and the presence of rosacea [53]. Also, vitamin B12 (riboflavin) was thought to be a potential critical factor in B complex deficiency, and this deficiency was thought to be associated with rosacea [56]. Zinc is a known antioxidant and anti-inflammatory trace element that may play a role in reducing Demodex mites or cutaneous bacteria, which play roles in the pathogenesis of rosacea [57,58].
In the literature, explanations of caffeine intake and rosacea exacerbation relationships are limited [56]. In a study, regular coffee consumption was associated with a reduction in the occurrence of rosacea. This reduction is proportional to the amount of coffee that is consumed on a daily basis [59]. However, it is thought that the consumption of hot coffee and hot tea, depending on the heat, may increase vasodilation and sympathetic activation, resulting in flushing and telangiectasia [56].
3.6. UV Irradiation Factors in Rosacea
The exacerbation of rosacea by sunlight has been linked to the inflammatory effects of UV radiation on keratinocytes. UV radiation stimulates ROS production in keratinocytes and activates cellular signaling pathways. UV-A radiation increases MMP-1 levels, and ROS also increase MMP-2 mRNA levels and suppress the tissue inhibitor of metalloproteinase-1 (TIMP-1) in human dermal fibroblasts. Thus, ROS cause vascular and dermal matrix damage by upregulating matrix metalloproteinases [60]. Furthermore, UV radiation induces endoplasmic reticulum stress, leading to the activation of TLR-2 [4]. Specifically, a study demonstrated the activation of TLR-2 by UV-B radiation [10]. Additionally, another study suggested that UV-B increases the VEGF and fibroblast growth factor (FGF) levels, leading to cutaneous angiogenesis and resulting in telangiectasia in rosacea patients [61]. Consequently, UV-induced increases in ROS and VEGF activity in the skin leads to enhanced inflammatory responses and contributes to the degeneration of collagens and matrix structures in the dermis [60].
3.7. Psychological Stress and Sleep Factors in Rosacea
Psychological factors play roles in rosacea, where emotions of embarrassment or laughter can initiate flushing [62]. It was shown that rosacea patients had a significantly higher intensity of stress from critical life situations than the control group, and that the emotional status of rosacea patients has an impact on their symptoms [63]. Mental stress induces vasodilatory activities via sympathetic nerve activity (SSNA) in the skin [64,65]. An elevated SSNA response was noted in the supraorbital skin of individuals with rosacea following mental stress [66]. In addition, stress induces cortisol-releasing hormone (CRH) that affects pro-inflammatory cytokines such as IL-6, IL-8, and IL-18, which regulate mitogen-activated protein kinase and the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and induce facial erythema. Additionally, CRH type 2 receptors are primarily expressed in blood vessels, acting as a direct vasodilator that is observable in rosacea skin [67]. Through CRH type 1 receptors, CRH induces mast cell degranulation, leading to the release of multiple vasodilatory mediators including histamine and NO [68]. Psychological stress in rosacea patients may modulate TLR signaling, induce ROS, and increase antimicrobial peptide and neuropeptide production, as well as increase other triggering factors such as UV radiation, heat, cold, spicy foods, and microbial factors [69].
In rosacea patients, the impact on appearance can cause a lack of self-confidence, anxiety, and depression, which can lead to psychological stress and affect sleep quality [70,71]. Poor sleep quality has been linked to the worsening of chronic inflammatory conditions, including psoriasis [72,73]. Regarding these data in the literature, one study found an association between poor sleep quality and rosacea, as well as the severity of rosacea [74]. This is because a lack of sleep can increase the expressions of MMP-9, TLR-2, and CAMP, affecting regional inflammatory factors in the skin [75,76,77]. In addition, the vascular dysregulation of the skin via increased VEGF expression and angiogenesis occurs in response to poor sleep [74]. Additionally, the investigation of genetic predisposition revealed that single nucleotide polymorphisms (SNPs) in the HTR2A and ADRB1 genes were correlated with sleep regulation in individuals affected by rosacea [74].
4. Rosacea Classification
Diagnostic criteria for rosacea have been described as primary and secondary features. Primary features include flushing (transient erythema), non-transient erythema, papules and pustules, and telangiectasias. In addition, secondary features may include burning or stinging, plaques, a dry appearance, edema, ocular manifestations, peripheral location, and phymatous changes. The presence of ≥1 primary feature is diagnostic, whereas secondary features are not necessarily present [78].
4.1. Erythematotelangiectatic Rosacea (Subtype 1)
The main features of this subtype are non-transient flushing and central facial edema. Telengiectasia may be accompanied by primary features, but these are not essential. In addition, flushing may be seen over the entire face, sparing the periocular and perioral areas, and the ears, neck, and upper chest may be affected [1,79].
4.2. Papulopustular Rosacea (Subtype 2)
On the central face, there are transient papules and pustules in addition to the ETR features. These papules and pustules, together with the ETR features, give a characteristic presentation of the condition [1].
4.3. Phymatous Rosacea (Subtype 3)
The skin becomes thicker and rougher due to phymatous changes, which mainly affect the nose. Rhinophyma is caused by an overgrowth of sebaceous glands in the skin of the nose, resulting in the thickening of the skin and an enlargement of the pores. This makes the skin appear rougher and thicker than it would normally be. These features can be seen in any sebaceous region. Phymatous changes can also be caused by a long-term exposure to ultraviolet radiation, which can stimulate oil production in the skin and further contribute to skin thickening [1].
4.4. Ocular Rosacea (Subtype 4)
The diagnosis of ocular rosacea depends on the presence of one or more of the following symptoms and findings in a patient: a watery or bloodshot appearance (interpalpebral conjunctival hyperemia), a foreign body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectasias of the conjunctiva and lid margin, or lid and periocular erythema. Blepharitis, conjunctivitis, and irregular lid margins may also occur. Chalasia and sties are also symptoms of ocular rosacea [78].
4.5. Granulomatous Rosacea (Variant)
Granulomatous rosacea is the only type of rosacea that does not have the features of the other rosacea subtypes. Typical findings in granulomatous rosacea are dense, yellow, brown, or red papules or nodules on the skin. These lesions may be large and scarring. Typically, lesions on the cheeks and periorificial areas may also occur on the phyma. The presence of other rosacea findings is not essential for the diagnosis of granulomatous rosacea [78].
4.6. Rosacea Fulminans (Variant)
Rosacea fulminans, also referred to as ‘pyoderma faciale’ or ‘rosacea conglobata’, is a rare disorder that is distinguished by the sudden appearance of inflamed papules, pustules, nodules, and cysts on the facial area [80]. Rosacea fulminans may have a potential association with both inflammatory bowel disease and pregnancy [81].
4.7. Neurogenic Rosacea (Subtype)
The etiopathogenesis of rosacea has revealed its association with neurovascular components, which have been elucidated in recent years [44]. The term ‘neurogenic rosacea’ refers to an uncommon form that typically presents as intense redness with burning sensations limited to the cheeks, which worsen with the exposure to heat and stress [82,83]. Clinically, patients have reported experiencing burning or tingling sensations, as well as occasional intense pain. Furthermore, certain patients may encounter exceedingly severe symptoms that are unresponsive to traditional medication therapies, whether taken orally or applied topically. For instance, flushing can cause a sensation of warmth and discomfort. This can evoke adverse connotations for numerous patients, resulting in erythrophobia and avoidant behaviors [82].
5. Rosacea Treatment
There are many treatment options for rosacea, as well as new emerging modalities for treatment. Avoiding trigger factors for rosacea can have a beneficial effect. In addition, the treatment approach should be based on the clinical type and severity of the disease (Table 1).

Table 1.
Algorithm of treatment options for rosacea types.
5.1. Skin Care and General Recommendations
Dermatologists have an important role in educating patients about the importance of skin care and the avoidance of triggers [84,85]. Protecting and supporting the epidermal barrier function is critical in rosacea patients. Gentle cleansing and the use of moisturizers strengthen the epidermal barrier function, and sunscreens provide UV protection [79,86,87]. Despite the lack of randomized trial data, clinical experience supports a number of common skin care practices [88]. Alcohol, hot beverages, and sun exposure are the main triggers of rosacea and affect the pathogenesis. The avoidance of triggers and irritant cosmetics and the usage of sunscreens, especially to prevent UVB, are recommended for rosacea patients [79,89].
5.2. Treatments for Erythematotelangiectatic Rosacea
5.2.1. Topical Treatment for Erythematotelangiectatic Rosacea
The selective alpha-2 adrenergic agonist 0.5% brimonidine topical gel has significant vasoconstrictive activity. In a Cochrane meta-analysis, 41% of patients treated with topical gel 0.5% brimonidine had a significant reduction in erythema compared to 20% of patients in the placebo control group [90]. After the application of brimonidine to the face, a reduction in erythema is observed within 30 min, with peaks between 3 and 6 h, and then the benefit diminishes. The use of brimonidine may also cause immediate erythema, pruritus, burning, and flushing, as well as rebound erythema [88]. Oxymetazoline hydrochloride 1% cream, an alpha-1 adrenergic agonist and partial alpha-2 adrenergic agonist, approved by the Food and Drug Administration (FDA) for the treatment of persistent erythema, was found to be significantly effective in reducing erythema in two randomized controlled trials [86,90]. Both drugs provide local vasoconstriction but do not reduce telangiectasias or other symptoms of the disease. Brimonidine also has additional mast cell-mediated anti-inflammatory properties and acts as a barrier [91,92]. In addition, a study showed that brimonidine was beneficial against alcohol-induced flushing and provided satisfaction in rosacea patients [93]. Both topical drugs could be used with topical, systemic, or instrumental therapies for rosacea. Although they are not sufficient for reducing persistent erythema, vasoconstrictors are a useful adjunctive therapy option in rosacea [79]. In a single anecdotal study, the topical application of the beta blocker drug timolol displayed a degree of improvement in erythema. However, the improvement was not statistically significant [94]. Topical tranexamic acid, a conventional treatment for melasma, may improve lesions in rosacea by modifying the immune system and angionesis [81]. Two studies of topical trenexamic acid for the treatment of erythema and telangiectasia in rosacea patients showed promising therapeutic effects with a good safety profile [95,96]. In one study, topical 1-methylnicotinamide, a metabolite of nicotinamide (vitamin B3), 0.25% gel was used to treat 34 patients with rosacea, including both erythematotelangiectatic and papulopustular subtypes. Improvement, defined as good or moderate, was seen in 26 patients. One patient reported skin irritation and withdrew from the study [97].
5.2.2. Systemic Treatment for Erythematotelangiectatic Rosacea
Despite the lack of randomized trials, antihypertensive drugs are the only potentially effective therapy options for erythema and flushing [90]. Therapeutic options entail the use of alpha-2 adrenergic agonists, such as clonidine, and beta blockers like propranolol and carvedilol. Clonidine showed promising potential as a modifier of malar temperature and circulation, but a small placebo-controlled study failed to show clinical efficacy, also limited by its side effects [78,88,98]. Rilmenidine, a partial agonist of alpha-1 and alpha-2 adrenergic receptors, has been tried in a single clinical trial, it presented to be active in some cases of erythema, but the result was not significant [99].
Propranolol, a non-selective beta blocker, has been shown to reduce flushing by blocking beta receptors in cutaneous blood vessels. However, it is not recommended for rosacea because of adverse effects such as hypotension and bradycardia. In one case report, carvedilol therapy resulted in significant resolution of facial flushing within 2 weeks without side effects such as hypotension and bradycardia [100]. There are no placebo-controlled trials in the literature demonstrating the efficacy of beta blocker treatment in rosacea, yet it remains the most widely used treatment. Beta blocker therapy requires evaluation by a cardiologist with the performance of an electrocardiogram especially in patients over 50 years of age. Consequently, combination therapies with beta blockers accompanied by laser techniques or topical vasoconstrictive therapies may lead to more long-lasting and significant effect in rosacea [79].
5.2.3. Instrumental Treatment for Erythematotelangiectatic Rosacea
Light therapies and laser treatments, including intense pulsed light (IPL) 500–1200 nm, pulsed dye laser (PDL) 585–595 nm and long-pulsed neodymium:yttrium-aluminum-garnet laser (Nd:YAG) 1064 nm are effective treatment modalities for rosacea [101]. IPL generates a non-coherent light beam within the wavelength range of 500–1200 nm by employing cut-off filters for vascular lesions, specifically at 515, 550, 560, 570, and 590 nm [101]. In a study of 102 patients with mild to severe rosacea, IPL therapy was utilized with a 530 nm filter administered at intervals of one to three weeks and fluences varying from 10–30 J/cm2. For patients with acneiform breakouts in addition to telangiectasias, a 420 nm filter was used with fluences of 10–20 J/cm2. The study found that 80% of patients experienced a reduction in redness, 78% reported an improvement in flushing and skin texture, and 72% experienced a decrease in acneiform breakouts. No complications or adverse effects were identified [102]. In a separate study, thirty-four patients underwent treatment using IPL 515–1200 nm along with a 560 nm cut-off filter. The fluence range utilized was 24–32 J/cm2, and four treatments were administered to the face at 3-week intervals. The study observed reductions of 39% in erythema on the cheeks and 22% on the chin. The photographic improvement in redness and visible blood vessels was 46% and 55%, respectively. These results were maintained for 6 months with only minor side effects [103]. PDL emits light at a wavelength of 585–595 nm, which corresponds with the absorption peak of oxyhemoglobin that targets the superficial vessels [101]. In a study, PDL efficiently decreased telangiectasia in all 11 patients following a solitary treatment, with noteworthy outcomes evident after 6 weeks. Telangiectasia ratings decreased from 2.7 to 2.4 at lower fluences without purpura, while purpuragenic treatments led to a further decrease to 1.4 from the baseline [104]. Also, PDL has demonstrated comparable efficacy in decreasing symptoms and signs of rosacea, as observed in a controlled study comparing it to IPL. PDL (non-purpuragenic fluences: 7 J/cm2, 10 mm spot size, 6-ms pulse duration) and IPL (560 nm filter, pulse train: 2.4 and 6.0 ms, pulse delay: 15 ms, fluence: 25 J/cm2) were able to significantly and equally reduce the symptoms as erythema and telangiectasia when compared to the control group [105]. Although infrequently employed in rosacea treatment, the Nd:YAG laser (1064 nm) seems to be a secure and efficacious method for dealing with ETR [101]. In two split-face studies involving 14 patients, the non-purpuragenic 595 nm PDL exhibited superior efficacy in reducing facial redness in erythematotelangiectatic rosacea patients compared to the Nd:YAG laser. Nevertheless, Nd:YAG was determined to be a reliable and harmless treatment for rosacea. There were no noteworthy adverse effects noted. Additionally, the study has indicated that NdYAG laser is less uncomfortable than PDL and may offer advantages for patients with darker skin [106].
Another treatment modality for rosacea is photodynamic therapy (PDT), which has been shown to improve erythema and telangiectasia in small series when used in conjunction with lasers and light therapies [107]. A total of 30 patients diagnosed with erythematotelangiectatic and papulopustular rosacea were included in a retrospective study. These patients underwent a total of 39 treatments as part of various combinations involving blue light + PDL, blue light + IPL, blue light + PDL + IPL, or blue light + red light + PDL + IPL. Laser and light sources were utilized in a sequential manner with ALA (aminolevulinic acid) 20% serving as the photosensitizer. Treatment consisted of red light (630 nm, 37 J/cm2, 8 min), blue light (417 nm, 10 J/cm2, 15 min), PDL (5–15 J/cm2, 0.5–40 ms), and IPL (560 nm cutoff filter, 15–22 J/cm2, 3.5–4 ms). The improvement of rosacea was mild to moderate, while that of skin quality was moderate to good. The authors observed no noteworthy difference in improvement among patient groups [108]. Even the clear effect of PDT on reducing telengiectasia, results are shown to be transient [79]. On the other hand, it is crucial to select the proper parameters in studies of laser and light-based therapies, as high levels of photodynamic therapy may induce a worsening of rosacea [109].
Nevertheless, based primarily on observational studies, laser treatments are practically effective on erythema and telangiectasia, and no other laser method seems to be superior to another. Although a reduction in flushing in rosacea patients has been observed by clinicians, there is no significant result of reducing the number and duration of flushing by using laser or IPL methods. However, the shrinkage of the vessels by these treatment methods led us to assume that they are beneficial for the symptoms of flushing by reducing the number of attacks [79].
5.2.4. Injection Treatment for Erythematotelangiectatic Rosacea
Botulinum toxin appears to be an injectable method for rosacea patients, but studies are small groups and case reports. A double-blind, placebo-controlled, split-face study showed a significant decrease in the erythema index in the group treated with botulinum toxin. In this study, fifteen units of botulinum toxin were intradermally injected into one cheek, while the other cheek received a placebo injection with normal saline. Injections were administered at 1 cm intervals, with a volume of 0.05 mL at every point (30 points) within a 5 cm wide and 6 cm long section of the cheek, following a grid pattern [110]. A case report also demonstrated that intradermal botulinum toxin injections significantly reduced erythema, edema, telangiectasias, and flushing. In this report, botulinum toxin was diluted to a concentration of 10 units/mL and administered intradermally in the hypervascular and telangiectatic centrofacial area as 0.05 mL microdroplet injections, spaced 0.5 cm apart [111].
Erenumab represents a promising therapeutic alternative as it is a human monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) receptor. An open-label phase II study is underway to assess the effectiveness and tolerability of subcutaneously administering 140 mg of erenumab (AMG 334) every 4 weeks for persistent flushing and redness [112].
5.3. Treatments for Papulopustular Rosacea
5.3.1. Topical Treatment for Papulopustular Rosacea
For the treatment of papules and pustules, recommended therapies include topical azelaic acid and topical ivermectin (high-certainty evidence), and topical metronidazole and topical minocycline (moderate-certainty evidence) [90]. In addition, a systematic review and meta-analysis demonstrated that topical ivermectin is the most effective topical treatment for papulopustular rosacea and provides the greatest psychological benefit to these patients [113]. Topical ivermectin is effective in clearing almost all papules and pustules, especially in moderate to severe rosacea [114].
Two phase 3 randomized clinical trials involving 751 and 771 participants, respectively, evaluated the use of minocycline foam for the treatment of moderate to severe papulopustular rosacea. The trials found the foam to be effective and safe [115]. In addition, topical minocycline 3% gel was shown to significantly reduce inflammatory lesions compared with the placebo in patients with papulopustular rosacea in a prospective, 12-week, double-blind study involving 270 patients [116].
Topical treatment with benzyl benzoate (+crotamiton) normalized Demodex densities in a study of 344 patients, with symptoms disappearing in more than 80%, particularly in patients with good compliance [117]. In a retrospective study of 394 patients, consisting of 117 patients with rosacea and 277 patients with demodicosis, topical treatment with benzyl benzoate (+crotamiton) yielded similar results across all patient cohorts. The treatment proved to be effective in 46% of patients, with a curative rate of 20%. Higher dose regimens exhibited greater effectiveness compared to lower dose ones [118].
A study involving six women diagnosed with papulopustular rosacea or rosacea with irritant contact dermatitis found that soaking in a solution of tranexamic acid significantly reduced erythema, as well as decreased itching, flushing, and burning, without any reported side effects [119].
5.3.2. Systemic Treatment for Papulopustular Rosacea
Systemic oral treatments are recommended in the management of moderate to severe papulopustular rosacea [120]. Doxycycline modified-release treatment at 40 mg, as well as isotretinoin and minocycline, which have demonstrated moderate-certainty evidence, should be considered as potential interventions for reducing papules and pustules, according to the updated systematic review on treatments for rosacea [90]. Doxycycline is an effective drug in the tetracycline antibacterial group for papulopustular rosacea. It can be given as a low dose of 40 mg daily for prolonged periods. In severe cases, doxycycline could be used at 200 mg/day for 4 weeks with a tapering regimen [121,122]. Isotretinoin at a low dose of 0.3 mg/kg reduces inflammation in rosacea subtype 2 [123].
Metronidazole, azithromycin, and clarithromycin are other systemic antibiotic options. In addition, ivermectin may be considered as an antiparasitic therapy for papulopustular rosacea [120]. Studies on the efficacy of zinc sulfate treatment for rosacea have yielded conflicting results. One study conducted on 25 patients indicated that taking 100 mg of zinc sulfate three times a day is a highly effective treatment, as it led to significant improvements in rosacea severity scores [124]. However, a randomized double-blind study found no significant difference in rosacea improvement between those who were administered oral zinc sulfate at a dosage of 220 mg twice a day and those who were given a placebo [125].
Hydroxychloroquine (HCQ) displays potential as a viable treatment alternative for patients affected by rosacea [81]. In a study, the results indicated that HCQ can partially reduce the mast cell-associated inflammatory response in a rosacea-like mouse model, suggesting its potential as a therapeutic mechanism for rosacea. Furthermore, patients who underwent HCQ treatment for a duration of eight weeks experienced a decrease in erythema and inflammation, resulting in positive outcomes [126]. In an 8-week period, a randomized, double-blind, double-dummy study was conducted with 66 patients. The total score changes in the Rosacea-Specific Quality of Life instrument in the HCQ group were found to be noninferior in comparison to the doxycycline group. In addition, the HCQ group reported excellent improvement rates in the Clinician’s Erythema Assessment scores of ‘clear’ or ‘almost clear’, which were nearly equal to those in the doxycycline group [127].
Eliminating small intestine bacterial overgrowth (SIBO) using rifaximin resulted in an almost complete or complete control of papulopustular rosacea, and these outcomes remained consistent for a minimum of 9 months. Objective assessments suggest a substantial improvement [35]. In a separate study, patients with rosacea and SIBO who underwent successful eradication therapy were able to remain symptom-free from rosacea for up to three years during the follow-up [128]. In a clinical trial, individuals with rosacea and H. pylori infection were administered H. pylori eradication therapy. Subsequently, the grading score for rosacea decreased dramatically, indicating that the elimination of H. pylori in individuals with rosacea can alleviate symptoms [129]. One review suggested that all patients with rosacea should be tested for H. pylori and treated if positive, as this may lead to improved outcomes [30].
5.3.3. Instrumental Treatment for Papulopustular Rosacea
IPL treatment, using a 420 nm filter and fluences ranging from 10 to 20 J/cm2, effectively reduced acneiform breakouts by 72% [102]. An Nd: YAG (1064 nm) laser has proven to be effective in treating papulopustular lesions of rosacea in an open clinical trial, especially in cases where traditional therapy has failed [120]. PDT, such as in the case of erythematotelangiectatic rosacea, has demonstrated a decrease in symptoms for papulopustular rosacea [108].
5.3.4. Injection Treatment for Papulopustular Rosacea
Injection therapy using IL-17 inhibitors has recently been utilized in subcutaneous injections for the treatment of rosacea. In a preliminary non-blinded study initiated by the investigator in which 20 patients were given secukinumab (300 mg per week for 5 weeks and then once a month for 2 months), noteworthy decreases in both the papules and the global severity score were observed. However, at least one infection was experienced by 39% of the patients in this study who received a minimum of one dose [130].
5.4. Treatments for Phymatous Rosacea
Before selecting the phyma treatment, it is necessary to evaluate whether the rosacea is inflamed or noninflamed, according to the latest update for rosacea treatment issued by the global Rosacea Consensus (ROSCO) panel. For inflamed phyma, it is recommended to undergo treatment with topical retinoids, oral doxycycline, and oral isotretinoin. On the other hand, for noninflamed phyma or fibrotic phyma, the recommended methods are instrumental or surgical therapies [85]. A recent report in the literature showcased the efficacy of CO2 laser ablation in treating rhinophyma in a patient, yielding positive results. In a recent retrospective study involving 28 patients, it was recommended that after-shave excision of rhinophyma be complemented with the use of a porcine extracellular matrix. This treatment was found to decrease the number of dressing changes needed and reduce the time to achieve re-epithelialization [131].
When medical treatments prove to be unsatisfactory, a range of surgical techniques can be helpful in treating phymatous rosacea. Scalpel or razor blade excision, in the form of a partial excision (superficial decortication) of hypertrophic tissue to allow for the re-epithelialization of pilosebaceous tissue, is a fast and effective cosmetic procedure with satisfactory results. Additionally, dermabrasion is primarily employed as a supplementary treatment to other modalities. Also, electrosurgery and electrocautery are surgical techniques, in which an electrosurgical current is applied, that could be used for phymatous rosacea [132].
5.5. Treatments for Ocular Rosacea
Recommendations for the treatment of ocular rosacea include a combination of eyelid hygiene and the systemic administration of tetracycline drugs, as suggested in an interdisciplinary review [133]. Therapeutic options comprise oral omega-3 fatty acids with moderate-certainty evidence, cyclosporin ophthalmic emulsion, and doxycycline with low-certainty evidence [90]. Pediatric patients with ocular rosacea received treatment consisting of warm compresses, eyelid scrubbing, preservative-free artificial tears, topical antibiotics, topical steroids, topical cyclosporine, oral doxycycline, azithromycin, and erythromycin suspension in a published study [134]. During a clinical observation, the utilization of intense pulsed light (IPL) with proper eye shield usage to protect the eyes aided in the reduction in dry eye symptoms for patients with rosacea after undergoing treatment for periocular facial skin [135]. An enhanced water-soluble silver (I) compound of metronidazole was found to relieve ocular rosacea symptoms in three patients experiencing severe cases [136]. Surgery, such as blepharoplasty, may be required in severe cases [120].
5.6. Treatments for Granulomatous Rosacea
Granulomatous rosacea has a chronic and challenging course to treat. It is difficult to manage with currently available therapeutic agents, making it a continuing challenge for dermatologists. Treatment modalities are presented in case reports and case series within the literature [81]. Topical treatments like pimecrolimus cream, azelaic acid gel, and topical ivermectin have been proven to be effective. Systemic oral therapies like minocycline, dapsone, doxycycline, metronidazole, steroids, and isotretinoin have also shown efficacy in treating granulomatous rosacea. The treatment for papulopustular rosacea can be used for granulomatous rosacea in practice. Moreover, chromophore gel-assisted phototherapy and intense pulsed light (IPL) treatments have been successful [81,120].
5.7. Treatments for Rosacea Fulminans
A comprehensive review indicates that rosacea fulminans would benefit from systemic corticosteroids and/or isotretinoin treatment to enhance symptoms and reduce scarring [80]. During systemic oral steroid treatment, to prevent relapses following steroid treatment cessation, it is recommended to undergo a regimen of oral prednisone at a dose of 40–60 mg/day, accompanied by a low-dose of isotretinoin 2 weeks later. The steroid taper should be slow while continuing isotretinoin until a cumulative dosage of 150 mg/kg is reached. Isotretinoin dosages ranged between 0.2 and 1.0 mg/kg/day, with 0.5 mg/kg/day being the most commonly used dosage [80].
5.8. Treatments for Neurogenic Rosacea
Clinical trials have not shown any therapeutic approaches to be effective in the treatment of neurogenic rosacea. Depending on specific symptoms, individualized treatment options may be necessary, such as beta blockers, neuropathic pain medication, laser treatments, or medical treatments for rosacea [79]. There are also unconventional treatments available for neuropathic pain, including gabapentin, pregabalin, and pain-modifying antidepressants such as duloxetine for neurogenic rosacea [82,83]. Psychiatric care or psychotherapy may be beneficial in certain cases. Dermatologists should refer patients who are unresponsive to conventional treatments to pain specialists or psychiatrists [79].
6. Conclusions
Rosacea is a chronic and multifactorial disease with various factors contributing to its pathogenesis. Genetic factors, along with other triggering factors such as microbial elements, UV exposure, diet, neurovascular factors, and stress, as well as immune dysregulation, have been linked to rosacea. In addition to triggering factors, the dysfunctionality of TLR-2, KLK-5, and cathelicidine results in the dysregulation of the adaptive and innate immune system. Induced cytokines and signaling factors culminate in inflammation, angiogenesis, vasodilation, and fibrotic changes (Figure 2). The clinical findings and symptoms of patients help the clinician to diagnose and classify the disease. While conventional therapies and the avoidance of triggers can help to reduce the symptoms of rosacea, there are also new treatment options that aim to target the pathogenesis of the disease. It is crucial for clinicians to establish measurable clinical outcomes for patients with rosacea to administer suitable treatments.
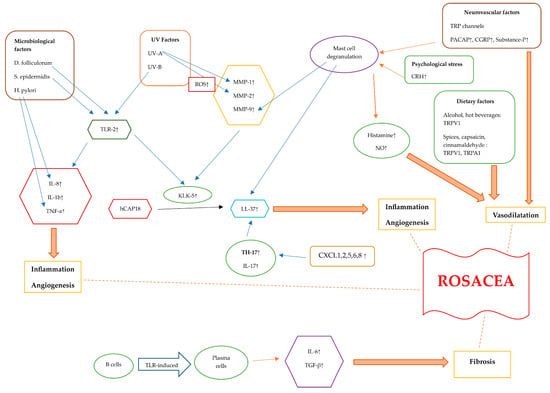
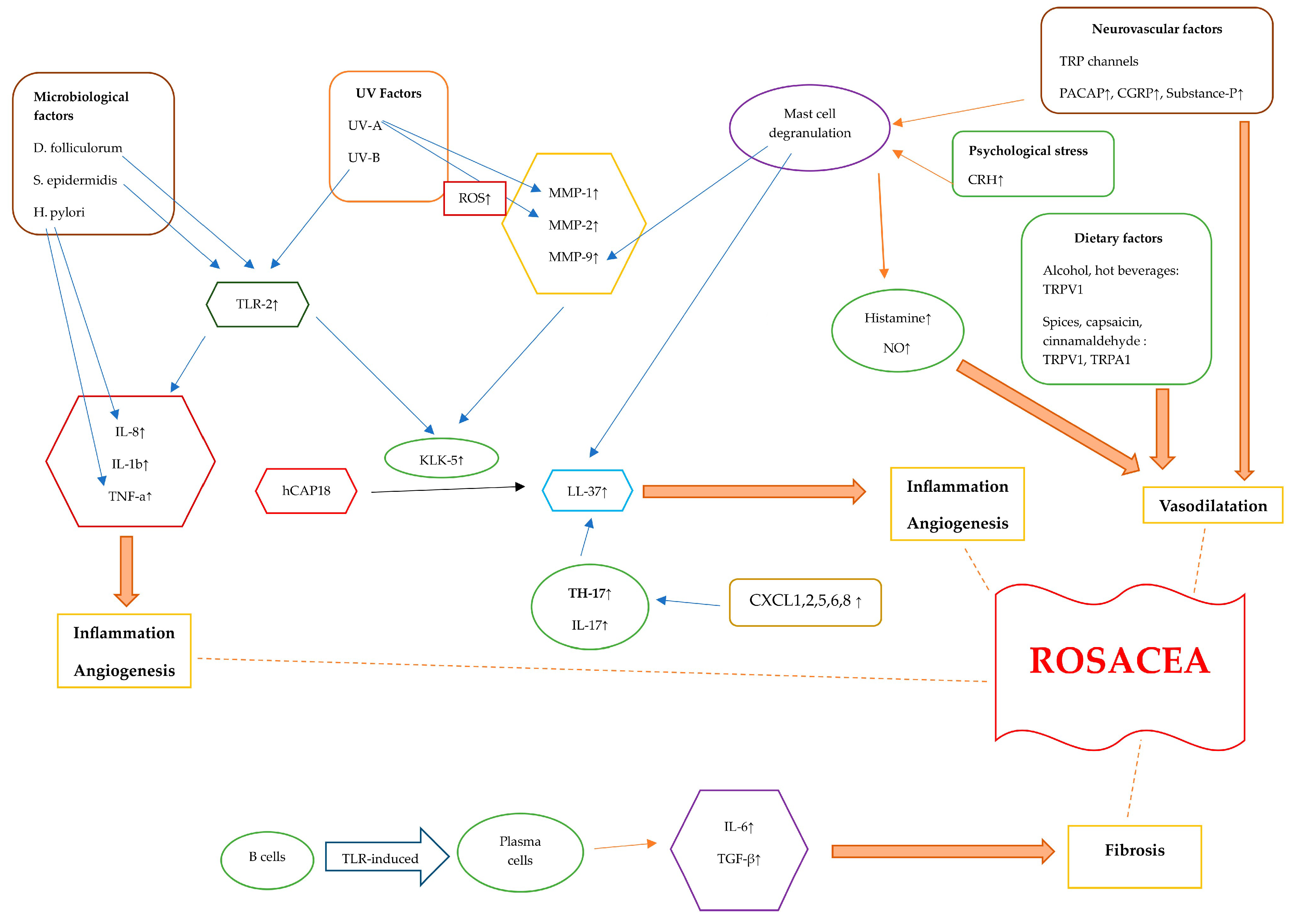
Figure 2.
Diagram illustrating the pathogenetic mechanisms of rosacea. Rosacea skin exhibits elevated levels of TLR-2, KLK-5, cathelicidin, and MMPs. The activation of TLR-2 by Demodex, as well as the interaction between S. epidermidis and TLR-2, initiates an immune response. Additionally, exposure to UV-B radiation can activate TLR-2, which then triggers inflammatory responses. TLR-2 induces the production and protease activity of serine protease KLK-5, which results in cathelicidin activation. The inactive form of human-specific cathelicidine is hCAP18, which is cleaved by KLK-5 to form the active peptide, LL-37. LL-37 promotes erythema, vasodilation, flushing, and telangiectasia. CXCL1, CXCL2, CXCL5, CXCL6, and CXCL8 are chemotactic factors that are upregulated in rosacea, attracting TH17 cells. Additionally, TH17 and IL-17 play roles in inducing the expression of LL-37. When triggered by external stimuli, TLR-2 activation enhances the expression of pro-inflammatory cytokine genes like IL-8, IL-1b, and TNF-α. Helicobacter pylori induces the production of pro-inflammatory cytokines such as TNF-α and IL-8, leading to inflammation. TLRs lead to the differentiation of B cells into plasma cells that generate fibrogenic cytokines, including IL-6 and TGF-β. UV radiation stimulates the production of ROS. UV-A radiation increases MMP-1 levels, and ROS also increase MMP-2 levels. Alcohol, hot beverages, capsaicin, cinnamaldehyde, and spices trigger TRPV1 and TRPA1 receptors. These channels release substance P and CGRP, leading to an inflammatory response and vasodilatation. PACAP, substance P, and CGRP initiate an inflammatory response through the activation of mast cells. Mast cell degranulation leads to elevated MMP-9 and LL-37 levels. Stress causes the release of CRH, which, in turn, causes mast cell degranulation. This leads to the release of several vasodilatory mediators such as histamine and NO. Abbreviations: TLR, Toll-like receptor; KLK-5, kallikrein-5; MMP, matrix metalloproteinase; TNF-a, tumor necrosis factor alfa; TRPV1, transient receptor potential vanilloid 1; TRPA1, transient receptor potential ankyrin 1; PACAP, pituitary adenylate cyclase-activated polypeptide; CGRP, calcitonin gene-related peptide; TGF-β, transforming growth factor beta; ROS, reactive oxygen species; CRH, corticotropin-releasing hormone; NO, nitric oxide.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the research is indicated in the references.
Conflicts of Interest
The author declares no conflict of interest.
References
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef]
- Gallo, R.L.; Granstein, R.D.; Kang, S.; Mannis, M.; Steinhoff, M.; Tan, J.; Thiboutot, D. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2018, 78, 148–155. [Google Scholar] [CrossRef] [PubMed]
- van Zuuren, E.J.; Arents, B.W.M.; van der Linden, M.M.D.; Vermeulen, S.; Fedorowicz, Z.; Tan, J. Rosacea: New concepts in classification and treatment. Am. J. Clin. Dermatol. 2021, 22, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.S.; Huang, W.W. Rosacea pathogenesis. Dermatol. Clin. 2018, 36, 81–86. [Google Scholar] [CrossRef]
- Gether, L.; Overgaard, L.K.; Egeberg, A.; Thyssen, J.P. Incidence and prevalence of rosacea: A systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Dlova, N.C.; Mosam, A. Rosacea in black South Africans with skin phototypes V and VI. Clin. Exp. Dermatol. 2017, 42, 670–673. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Woo, Y.R.; Lim, J.H.; Cho, D.H.; Park, H.J. Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int. J. Mol. Sci. 2016, 17, 1562. [Google Scholar] [CrossRef]
- Casas, C.; Paul, C.; Lahfa, M.; Livideanu, B.; Lejeune, O.; Alvarez-Georges, S.; Saint-Martory, C.; Degouy, A.; Mengeaud, V.; Ginisty, H.; et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp. Dermatol. 2012, 21, 906–910. [Google Scholar] [CrossRef]
- Park, H.S.; Jin, S.P.; Lee, Y.; Oh, I.G.; Lee, S.; Kim, J.H.; Cho, K.H.; Chung, J.H. Toll-like receptor 2 mediates a cutaneous reaction induced by repetitive ultraviolet B irradiation in C57/BL6 mice in vivo. Exp. Dermatol. 2014, 23, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Buhren, B.A.; Steinhoff, M.; Homey, B. Rosacea: The cytokine and chemokine network. J. Investig. Dermatol. Symp. Proc. 2011, 15, 40–47. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kanada, K.; Macleod, D.T.; Borkowski, A.W.; Morizane, S.; Nakatsuji, T.; Cogen, A.L.; Gallo, R.L. TLR-2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Investig. Dermatol. 2011, 131, 688–697. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Wang, Z.; Vanderberghe, M.; Two, A.; Gallo, R.L.; Di Nardo, A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J. Investig. Dermatol. 2014, 134, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Buhl, T.; Sulk, M.; Nowak, P.; Buddenkotte, J.; McDonald, I.; Aubert, J.; Carlavan, I.; Deret, S.; Reiniche, P.; Rivier, M.; et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J. Investig. Dermatol. 2015, 135, 2198–2208. [Google Scholar] [CrossRef]
- Sakabe, J.; Umayahara, T.; Hiroike, M.; Shimauchi, T.; Ito, T.; Tokura, Y. Calcipotriol increases hCAP18 mRNA expression but inhibits extracellular LL37 peptide production in IL-17/IL-22-stimulated normal human epidermal keratinocytes. Acta Derm.-Venereol. 2014, 94, 512–516. [Google Scholar] [CrossRef]
- Brown, T.T.; Choi, E.-Y.K.; Thomas, D.G.; Hristov, A.C.; Chan, M.P. Comparative analysis of rosacea and cutaneous lupus erythematosus: Histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J. Am. Acad. Dermatol. 2014, 71, 100–107. [Google Scholar] [CrossRef]
- Ciccarese, G.; Drago, F.; Rebora, A.; Parodi, A. Two cases of papulo-pustular rosacea-like eruptions following COVID-19 vaccinations. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e868–e870. [Google Scholar] [CrossRef]
- Karampinis, E.; Gravani, A.; Gidarokosta, P.; Bogdanos, D.P.; Roussaki-Schulze, A.V.; Zafiriou, E. Pustular Eruption following COVID-19 Vaccination: A Narrative Case-Based Review. Vaccines 2023, 11, 1298. [Google Scholar] [CrossRef]
- Salzer, S.; Ruzicka, T.; Schauber, J. Face-to-face with anti-inflammatory therapy for rosacea. Exp. Dermatol. 2014, 23, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Pasare, C.; Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 2005, 438, 364–368. [Google Scholar] [CrossRef]
- Woźniacka, A.; Salamon, M.; McCauliffe, D.; Sysa-Jędrzejowska, A. Antinuclear antibodies in rosacea patients. Postepy Dermatol. Alergol. 2013, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jarmuda, S.; O’Reilly, N.; Żaba, R.; Jakubowicz, O.; Szkaradkiewicz, A.; Kavanagh, K. Potential role of Demodex mites and bacteria in the induction of rosacea. J. Med. Microbiol. 2012, 61, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Amir Ali, A.; Vender, R.; Vender, R. The Role of IL-17 in Papulopustular Rosacea and Future Directions. J. Cutan. Med. Surg. 2019, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Margalit, A.; Kowalczyk, M.J.; Żaba, R.; Kavanagh, K. The role of altered cutaneous immune responses in the induction and persistence of rosacea. J. Dermatol. Sci. 2016, 82, 3–8. [Google Scholar] [CrossRef]
- Akilov, O.E.; Mumcuoglu, K.Y. Association between human demodicosis and HLA class I. Clin. Exp. Dermatol. 2003, 28, 70–73. [Google Scholar] [CrossRef]
- Whitfeld, M.; Gunasingam, N.; Leow, L.J.; Shirato, K.; Preda, V. Staphylococcus epidermidis: A possible role in the pustules of rosacea. J. Am. Acad. Dermatol. 2011, 64, 49–52. [Google Scholar] [CrossRef]
- O’Reilly, N.; Menezes, N.; Kavanagh, K. Positive correlation between serum immuno reactivity to Demodex-associated Bacillus proteins and erythematotelangiectatic rosacea. Br. J. Dermatol. 2012, 167, 1032–1036. [Google Scholar] [CrossRef]
- Yang, X. Relationship between Helicobacter pylori and rosacea: Review and discussion. BMC Infect. Dis. 2018, 18, 318. [Google Scholar] [CrossRef]
- Holmes, A.D. Potential role of microorganisms in thepathogenesis of rosacea. J. Am. Acad. Dermatol. 2013, 69, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Joura, M.I.; Brunner, A.; Nemes-Nikodém, V.; Sárdy, M.; Ostorházi, E. Interactions between immune system and the microbiome of skin, blood and gut in pathogenesis of rosacea. Acta Microbiol. Immunol. Hung. 2021, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Carolides, S.; Al-Niaimi, F. Rosacea and the gastrointestinal system. Australas. J. Dermatol. 2020, 61, 307–311. [Google Scholar] [CrossRef]
- Parodi, A.; Paolino, S.; Greco, A.; Drago, F.; Mansi, C.; Rebora, A.; Parodi, A.; Savarino, V. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clin. Gastroenterol. Hepatol. 2008, 6, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Katta, R. Diet and rosacea: The role of dietary change in the management of rosacea. Dermatol. Pract. Concept. 2017, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, N.; Gerstenblith, M.; Fu, P.; Tuttle, M.S.; Varma, P.; Gotow, E.; Cooper, K.D.; Mann, M.; Popkin, D.L. Genetic vs environmental factors that correlate with rosacea: A cohort-based survey of twins. JAMA Dermatol. 2015, 151, 1213–1219. [Google Scholar] [CrossRef]
- Helfrich, Y.R.; Maier, L.E.; Cui, Y.; Fisher, G.J.; Chubb, H.; Fligiel, S.; Sachs, D.; Varani, J.; Voorhees, J. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol. 2015, 151, 825–836. [Google Scholar] [CrossRef]
- Awosika, O.; Oussedik, E. Genetic predisposition to rosacea. Dermatol. Clin. 2018, 36, 87–92. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Raber, I.; Xu, J.; Li, R.; Spitale, R.; Chen, J.; Kiefer, A.K.; Tian, C.; Eriksson, N.K.; Hinds, D.A.; et al. Assessment of the genetic basis of rosacea by genome-wide association study. J. Investig. Dermatol. 2015, 135, 1548–1555. [Google Scholar] [CrossRef]
- Dajnoki, Z.; Béke, G.; Kapitány, A.; Mócsai, G.; Gáspár, K.; Rühl, R.; Hendrik, Z.; Juhász, I.; Zouboulis, C.C.; Bácsi, A.; et al. Sebaceous Gland-Rich Skin Is Characterized by TSLP Expression and Distinct Immune Surveillance Which Is Disturbed in Rosacea. J. Investig. Dermatol. 2017, 137, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Boucher, G.; Lees, C.W.; Franke, A.; D’Amato, M.; Taylor, K.D.; Lee, J.C.; Goyette, P.; Imielinski, M.; Latiano, A.; et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Valentonyte, R.; Hampe, J.; Huse, K.; Rosenstiel, P.; Albrecht, M.; Stenzel, A.; Nagy, M.; Gaede, K.I.; Franke, A.; Haesler, R.; et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat. Genet. 2005, 37, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Schmelz, M.; Schauber, J. Facial erythema of rosacea—Aetiology, different pathophysiologies and treatment options. Acta Derm.-Venereol. 2016, 96, 579–586. [Google Scholar] [CrossRef]
- Albrecht, P.J.; Hou, Q.; Argoff, C.E.; Storey, J.R.; Wymer, J.P.; Rice, F.L. Excessive peptidergic sensory innervation of cutaneous arteriole-venules hunts (AVS) in the palmar glabrous skin of fibromyalgia patients: Implications for widespread deep tissue pain and fatigue. Pain. Med. 2013, 14, 895–915. [Google Scholar] [CrossRef]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Investig. Dermatol. 2012, 132, 1253–1262. [Google Scholar] [CrossRef]
- Buddenkotte, J.; Steinhoff, M. Recent advances in understanding and managing rosacea. F1000Research 2018, 3, 1885. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Brain, S.D. Neurovascular aspects of skin neurogenic inflammation. J. Investig. Dermatol. Symp. Proc. 2011, 15, 33–39. [Google Scholar] [CrossRef]
- Fioranelli, M.; Roccia, M.G.; Di Nardo, V.; Aracena, C.J.; Lotti, T. Vitamin D supplementation for childhood atopic dermatitis. Dermatol. Ther. 2016, 29, 303. [Google Scholar] [CrossRef]
- Woo, Y.R.; Jung, K.E.; Koo, D.W.; Lee, J.S. Vitamin D as a marker for disease severity in chronic urticaria and its possible role in pathogenesis. Ann. Dermatol. 2015, 2, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, O.; Balta, I.; Sen, B.B.; Dikilitaş, M.C.; Ozuğuz, P.; Rifaioğlu, E.N. Vitamin D status in patients with rosacea. Cutan. Ocul. Toxicol. 2014, 33, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Jamgochian, M.; Alamgir, M.; Rao, B. Diet in Dermatology: Review of Diet’s Influence on the Conditions of Rosacea, Hidradenitis Suppurativa, Herpes Labialis, and Vitiligo. Am. J. Lifestyle Med. 2021, 17, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Park, B.W.; Ha, J.M.; Cho, E.B.; Jin, J.K.; Park, E.J.; Park, H.R.; Kang, H.J.; Ko, S.H.; Kim, K.H.; Kim, K.J. A study on vitamin D and cathelicidin status in patients with rosacea: Serum level and tissue expression. Ann. Dermatol. 2018, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Yamasaki, K.; Kabigting, F.; Gallo, R.L. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D3, and retinoic acid. J. Investig. Dermatol. 2010, 130, 1297–1306. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Carolides, S.; Al-Niaimi, F. Rosacea and Diet: What is New in 2021? J. Clin. Aesthet. Dermatol. 2021, 14, 49–54. [Google Scholar]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.L.; Drucker, A.M.; Cho, E.; Geng, H.; Qureshi, A.A.; Li, W.Q. Association of caffeine intake and caffeinated coffee consumption with risk of incident rosacea in women. JAMA Dermatol. 2018, 154, 1394–1400. [Google Scholar] [CrossRef]
- Yamasaki, K.; Gallo, R.L. The molecular pathology of rosacea. J. Dermatol. Sci. 2009, 55, 77–81. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Bucana, C.D.; Sanchez, R.; Donawho, C.K.; Kripke, M.L.; Fidler, I.J. Molecular regulation of UVB-induced cutaneous angiogenesis. J. Investig. Dermatol. 1998, 111, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Orion, E.; Wolf, R. Psychologic factors in the development of facial dermatoses. Clin. Dermatol. 2014, 32, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Sowinska-Glugiewicz, I.; Ratajczak-Stefanska, V.; Maleszka, R. Role of psychological factors in course of rosacea. Rocz. Akad. Med. Bialyms. 2005, 50, 49–53. [Google Scholar]
- Muller, M.D.; Sauder, C.L.; Ray, C.A. Mental Stress Elicits Sustained and Reproducible Increases in Skin Sympathetic Nerve Activity. Physiol. Rep. 2013, 1, e00002. [Google Scholar] [CrossRef]
- Black, P.H. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav. Immun. 2002, 16, 622–653. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Wilson, K.; Toma, K.; Sammons, D.L.; Mann, S.; Jurovcik, A.J.; Demidova, O.; Wilson, T.E. Augmented supraorbital skin sympathetic nerve activity responses to symptom trigger events in rosacea patients. J. Neurophysiol. 2015, 114, 1530–1537. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Pisarchik, A.; Zbytek, B.; Linton, E.A.; Mazurkiewicz, J.E.; Wei, E.T. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001, 15, 1678–1693. [Google Scholar] [CrossRef]
- Hall, J.M.; Cruser, D.; Podawiltz, A.; Mummert, D.I.; Jones, H.; Mummert, M.E. Psychological Stress and the Cutaneous Immune Response: Roles of the HPA Axis and the Sympathetic Nervous System in Atopic Dermatitis and Psoriasis. Dermatol. Res. Pract. 2012, 2012, 403908. [Google Scholar] [CrossRef]
- Passeron, T.; Zouboulis, C.C.; Tan, J.; Andersen, M.L.; Katta, R.; Lyu, X.; Aguilar, L.; Kerob, D.; Morita, A.; Krutmann, J.; et al. Adult skin acute stress responses to short-term environmental and internal aggression from exposome factors. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1963–1975. [Google Scholar] [CrossRef]
- Thorburn, P.T.; Riha, R.L. Skin disorders and sleep in adults: Where is the evidence? Sleep. Med. Rev. 2010, 14, 351–358. [Google Scholar] [CrossRef]
- Egeberg, A.; Hansen, P.R.; Gislason, G.H.; Thyssen, J.P. Patients with rosacea have increased risk of depression and anxiety disorders: A Danish nation wide cohort study. Dermatology 2016, 232, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Mostaghimi, L. Prevalence of mood and sleep problems in chronic skin diseases: A pilot study. Cutis 2008, 81, 398–402. [Google Scholar] [PubMed]
- Wong, I.T.Y.; Chandran, V.; Li, S.; Gladman, D.D. Sleep disturbance in psoriatic disease: Prevalence and associated factors. J. Rheumatol. 2017, 44, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, H.; Gong, Y.; Ouyang, Y.; Deng, F.; Tang, Y.; Li, J. Relationship between rosacea and sleep. J. Dermatol. 2020, 47, 592–600. [Google Scholar] [CrossRef]
- Sa-Nunes, A.; Bizzarro, B.; Egydio, F.; Barros, M.S.; Sesti-Costa, R.; Soares, E.M.; Pina, A.; Russo, M.; Faccioli, L.H.; Tufik, S.; et al. The dual effect of paradoxical sleep deprivation on murine immune functions. J. Neuroimmunol. 2016, 290, 9–14. [Google Scholar] [CrossRef]
- De Lorenzo, B.H.; de Oliveira Marchioro, L.; Greco, C.R.; Suchecki, D. Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology 2015, 57, 134–143. [Google Scholar] [CrossRef]
- Kalinchuk, A.V.; McCarley, R.W.; Porkka-Heiskanen, T.; Basheer, R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J. Neurosci. 2010, 30, 13254–13264. [Google Scholar] [CrossRef]
- Wilkin, J.; Dahl, M.; Detmar, M.; Drake, L.; Feinstein, A.; Odom, R.; Powell, F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J. Am. Acad. Dermatol. 2002, 46, 584–587. [Google Scholar] [CrossRef]
- Cribier, B. Rosacea: Treatment targets based on new physiopathology data. Ann. Dermatol. Venereol. 2022, 149, 99–107. [Google Scholar] [CrossRef]
- Walsh, R.K.; Endicott, A.A.; Shinkai, K. Diagnosis and Treatment of Rosacea Fulminans: A Comprehensive Review. Am. J. Clin. Dermatol. 2018, 19, 79–86. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, K.; Wang, Y.; Fang, R.; Sun, Q. Rosacea treatment: Review and update. Dermatol. Ther. 2021, 11, 13–24. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Yost, J.M.; Truong, S.V.; Steinhoff, M.; Wang, K.C.; Berger, T.G. Neurogenic rosacea: A distinct clinical subtype requiring a modified approach to treatment. Arch. Dermatol. 2011, 147, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Kang, S.Y.; Kim, K.E.; Cho, S.Y.; Kim, K.H.; Kim, I.H. Neurogenic rosacea in Korea. J. Dermatol. 2021, 48, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Berg, A.; Barr, C. Managing rosacea in the clinic: From pathophysiology to treatment-a review of the literature. J. Clin. Aesth. Dermatol. 2020, 13, S17–S22. [Google Scholar]
- Schaller, M.; Almeida, L.M.; Bewley, A.; Cribier, B.; Dlova, N.C.; Kautz, G.; Mannis, M.; Oon, H.H.; Rajagopalan, M.; Steinhoff, M.; et al. Rosacea treatment update: Recommendations from the global ROSacea COnsensus (ROSCO) panel. Br. J. Dermatol. 2017, 176, 465–471. [Google Scholar] [CrossRef]
- Thiboutot, D.; Anderson, R.; Cook-Bolden, F.; Draelos, Z.; Gallo, R.L.; Granstein, R.D.; Kang, S.; Macsai, M.; Gold, L.S.; Tan, J. Standard management options for rosacea: The 2019 update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2020, 82, 1501–1510. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Gunt, H.; Levy, S.B. Natural skin care products as adjunctive to prescription therapy in moderate to severe Rosacea. J. Drugs Dermatol. 2019, 18, 141–146. [Google Scholar] [PubMed]
- van Zuuren, E.J. Rosacea. N. Engl. J. Med. 2017, 377, 1754–1764. [Google Scholar] [CrossRef]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part II. Topical and systemic therapies in the treatment of rosacea. J. Am. Acad. Dermatol. 2015, 72, 761–770. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Carter, B.; van der Linden, M.M.; Charland, L. Interventions for rosacea. Cochrane Database Syst. Rev. 2015, 2015, CD003262. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Jeong, S.W.; Jo, H.; Woo, Y.R.; Park, H.J. Inhibition of mast cell infiltration in an LL-37-induced rosacea mouse model using topical brimonidine tartrate 0.33% gel. Exp. Dermatol. 2017, 26, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Bertino, B.; Blanchet-Réthoré, S.; Thibaut de Ménonville, S.; Reynier, P.; Méhul, B.; Bogouch, A.; Gamboa, B.; Dugaret, A.S.; Zugaj, D.; Petit, L.; et al. Brimonidine displays anti-inflammatory properties in the skin through the modulation of the vascular barrier function. Exp. Dermatol. 2018, 27, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Y.; Lu, B.; Tan, D.; Aroyan, C.; Shinkai, K.; Leslie, K.S.; Fox, L.P.; Yu, S.; Neuhaus, I.M.; Grekin, R.C.; et al. Effect of Topical Brimonidine on Alcohol-Induced Flushing in Asian Individuals: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Al Mokadem, S.M.; Ibrahim, A.M.; El Sayed, A.M. Efficacy of Topical Timolol 0.5% in the Treatment of Acne and Rosacea: A Multicentric Study. J. Clin. Aesthet. Dermatol. 2020, 13, 22–27. [Google Scholar] [PubMed]
- Bageorgou, F.; Vasalou, V.; Tzanetakou, V.; Kontochristopoulos, G. The new therapeutic choice of tranexamic acid solution in treatment of erythematotelangiectatic rosacea. J. Cosmet. Dermatol. 2019, 18, 563–567. [Google Scholar] [CrossRef]
- Jakhar, D.; Kaur, I.; Misri, R. Topical 10% tranexamic acid for erythematotelangiectatic steriod- induced rosacea. J. Am. Acad. Dermatol. 2022, 86, e1–e2. [Google Scholar] [CrossRef]
- Wozniacka, A.; Wieczorkowska, M.; Gebicki, J.; Sysa-Jedrzejowska, A. Topical application of 1-methylnicotinamide in the treatment of rosacea: A pilot study. Clin. Exp. Dermatol. 2005, 30, 632–635. [Google Scholar] [CrossRef]
- Van Landuyt, H.; Joubert-Lequain, I.; Humbert, P.; Lucas, A.; Drobacheff, C.; Mercier, M.; Laurent, R. Treatment of rosacea. Clonidine (0.075 mg per day) versus placebo (initial results). Ann. Dermatol. Venereol. 1997, 124, 729. [Google Scholar] [PubMed]
- Grosshans, E.; Michel, C.; Arcade, B.; Cribier, B. Rilmenidine in rosacea: A double-blind study versus placebo. Ann. Dermatol. Venereol. 1997, 124, 687–691. [Google Scholar] [PubMed]
- Layton, A.; Thiboutot, D. Emerging therapies in rosacea. J. Am. Acad. Dermatol. 2013, 69, 57–65. [Google Scholar] [CrossRef]
- Sharma, A.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Goren, A.; Grabbe, S.; Goldust, M. Rosacea management: A comprehensive review. J. Cosmet. Dermatol. 2022, 21, 1895–1904. [Google Scholar] [CrossRef]
- Kassir, R.; Kolluru, A.; Kassir, M. Intense pulsed light for the treatment of rosacea and telangiectasias. J. Cosmet. Laser Ther. 2011, 13, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, P.; Clayton, W.; Norwood, S.; Chopra, S.; Rustin, M. Treatment of rosacea with intense pulsed light: Significant improvement and long-lasting results. Br. J. Dermatol. 2008, 159, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Dover, J.S.; Arndt, K.A. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: Comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol. Surg. 2003, 29, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, I.M.; Zane, L.T.; Tope, W.D. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol. Surg. 2009, 35, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Voravutinon, N.; Warycha, M.; Whiting, D.; Nodzenski, M.; Yoo, S.; West, D.P.; Veledar, E.; Poon, E. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: A double-blind randomized controlled trial. J Am Acad Dermatol. 2013, 69, 438–443. [Google Scholar] [CrossRef]
- Li, A.; Fang, R.; Mao, X.; Sun, Q. Photodynamic therapy in the treatment of rosacea: A systematic review. Photodiagnosis Photodyn. Ther. 2022, 38, 102875. [Google Scholar] [CrossRef]
- Friedmann, D.P.; Goldman, M.P.; Fabi, S.G.; Guiha, I. Multiple sequential light and laser sources to activate aminolevulinic acid for rosacea. J. Cosmet. Dermatol. 2016, 15, 407–412. [Google Scholar] [CrossRef]
- Wollina, U.; Bitel, A.; Vojvodic, A.; Lotti, T. Rosacea Flare—Up after Photodynamic Therapy (PDT) for Field Cancerization and a Review on Adverse Events with PDT in General. Open Access Maced. J. Med. Sci. 2019, 7, 2998–3001. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Cheon, H.I.; Hur, M.S.; Han, S.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Assessment of skin physiology change andsafetyafterintradermalinjectionswithbotulinumtoxin: A randomized, double-blind, placebo-controlled, split-face pilot study in rosaceapatientswithfacialerythema. Dermatol. Surg. 2019, 45, 1155–1162. [Google Scholar] [CrossRef]
- Bharti, J.; Sonthalia, S.; Jakhar, D. Mesotherapy with botulinum toxin for the treatment of refractory vascular and papulopustular rosacea. J. Am. Acad. Dermatol. 2023, 88, e295–e296. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Danish Headache Center. Efcacy and Tolerability of Erenumab in the Management of Persistent Redness and Fushing in Rosacea (STOP Ros). ClinicalTrials.gov. 2020; Identifer NCT04419259. Available online: https://clinicaltrials.gov/ct2/show/NCT04419259 (accessed on 16 September 2023).
- Husein-ElAhmed, H.; Steinhoff, M. Efficacy of topical ivermectin and impact on quality of life in patients with papulopustular rosacea: A systematic review and meta-analysis. Dermatol. Ther. 2020, 33, e13203. [Google Scholar] [CrossRef] [PubMed]
- Trave, I.; Merlo, G.; Cozzani, E.; Parodi, A. Real-life experience on effectiveness and tolerability of topical ivermectin in papulopustular rosacea and antiparasitic effect on Demodex mites. Dermatol. Ther. 2019, 32, e13093. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.S.; Del Rosso, J.Q.; Kircik, L.; Bhatia, N.D.; Hooper, D.; Nahm, W.K.; Stuart, I. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: Results of 2 phase 3, randomized, clinical trials. J. Am. Acad. Dermatol. 2020, 82, 1166–1173. [Google Scholar] [CrossRef]
- Webster, G.; Draelos, Z.D.; Graber, E.; Lee, M.S.; Dhawan, S.; Salman, M.; Magrath, G.N. A multicentre, randomized, double-masked, parallel group, vehicle-controlled phase IIb study to evaluate the safety and efficacy of 1% and 3% topical minocycline gel in patients with papulopustular rosacea. Br. J. Dermatol. 2020, 183, 471–479. [Google Scholar] [CrossRef]
- Forton, F.M.N.; De Maertelaer, V. Effectiveness of benzyl benzoate treatment on clinical symptoms and Demodex density over time in patients with rosacea and demodicosis: A real life retrospective follow-up study comparing low- and high-dose regimens. J. Dermatol. Treat. 2022, 33, 456–465. [Google Scholar] [CrossRef]
- Forton, F.M.N.; De Maertelaer, V. Treatment of rosacea and demodicosis with benzyl benzoate: Effects of different doses on Demodex density and clinical symptoms. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 365–369. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, S.E.; Haw, S.; Bak, H.; Kim, Y.J.; Lee, M.W. Tranexamic acid solution soaking is an excellent approach for rosacea patients: A preliminary observation in six patients. J. Dermatol. 2013, 40, 70–71. [Google Scholar] [CrossRef]
- Anzengruber, F.; Czernielewski, J.; Conrad, C.; Feldmeyer, L.; Yawalkar, N.; Häusermann, P.; Cozzio, A.; Mainetti, C.; Goldblum, D.; Läuchli, S.; et al. Swiss S1 guideline for the treatment of rosacea. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1775–1791. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Schlessinger, J.; Werschler, P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J. Drugs Dermatol. 2008, 7, 573–576. [Google Scholar] [PubMed]
- Del Rosso, J.Q. Anti-inflamatory dose doxycycline in the treatment of rosacea. J. Drugs Dermatol. 2009, 8, 664–668. [Google Scholar] [PubMed]
- Gollnick, H.; Blume-Peytavi, U.; Szabo, E.L.; Meyer, K.G.; Hauptmann, P.; Popp, G.; Sebastian, M.; Zwingers, T.; Willers, C.; von der Weth, R. Systemic isotretinoin in the treatment of rosacea—Doxycycline- and placebo-controlled, randomized clinical study. J. Dtsch. Dermatol. Ges. 2010, 8, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Sharquie, K.E.; Najim, R.A.; Al-Salman, H.N. Oral zinc sulfate in the treatment of rosacea: A double-blind, placebo-controlled study. Int. J. Dermatol. 2006, 45, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Bamford, J.T.; Gessert, C.E.; Haller, I.V.; Kruger, K.; Johnson, B.P. Randomized, double-blind trial of 220 mg zinc sulfate twice daily in the treatment of rosacea. Int. J. Dermatol. 2012, 51, 459–462. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Tang, Y.; Wang, B.; Deng, Z.; Huang, Y.; Liu, F.; Zhao, Z.; Zhang, Y. Hydroxychloroquine is a novel therapeutic approach for rosacea. Int. Immunopharmacol. 2020, 79, 106178. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, X.; Huang, X.; Tang, Y.; Zhao, Z.; Yang, B.; Yang, B.; Zheng, Y.; Yuan, C.; Xie, H.; et al. Efficacy and safety of hydroxychloroquine for treatment of patients with rosacea: A multicenter, randomized, double-blind, double-dummy, pilot study. J. Am. Acad. Dermatol. 2021, 84, 543–545. [Google Scholar] [CrossRef]
- Drago, F.; De Col, E.; Agnoletti, A.F.; Schiavetti, I.; Savarino, V.; Rebora, A.; Paolino, S.; Cozzani, E.; Parodi, A. The role of small intestinal bacterial overgrowth in rosacea: A 3-year follow-up. J. Am. Acad. Dermatol. 2016, 75, e113–e115. [Google Scholar] [CrossRef]
- Saleh, P.; Naghavi-Behzad, M.; Herizchi, H.; Mokhtari, F.; Mirza-Aghazadeh-Attari, M.; Piri, R. Effects of Helicobacter pylori treatment on rosacea: A single-arm clinical trial study. J. Dermatol. 2017, 44, 1033–1037. [Google Scholar] [CrossRef]
- Kumar, A.M.; Chiou, A.S.; Shih, Y.H.; Li, S.; Chang, A.L.S. An exploratory, open-label, investigator-initiated study of interleukin-17 blockade in patients with moderate-to-severe papulopustular rosacea. Br. J. Dermatol. 2020, 183, 942–943. [Google Scholar] [CrossRef]
- Schmitz, L.; Hessam, S.; Scholl, L.; Reitenbach, S.; Segert, M.H.; Bechara, F.G. Wound Care With a Porcine Extracellular Matrix After Surgical Treatment of Rhinophyma. J. Cutan. Med. Surg. 2020, 24, 253–258. [Google Scholar] [CrossRef]
- Sadick, H.; Goepel, B.; Bersch, C.; Goessler, U.; Hoermann, K.; Riedel, F. Rhinophyma: Diagnosis and treatment options for a disfiguring tumor of the nose. Ann. Plast. Surg. 2008, 61, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jabbehdari, S.; Memar, O.M.; Caughlin, B.; Djalilian, A.R. Update on the pathogenesis and management of ocular rosacea: An interdisciplinary review. Eur. J. Ophthalmol. 2021, 31, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Donmez, O.; Akova, Y.A. Pediatric Ocular Acne Rosacea: Clinical Features and Long Term Follow-Up of Sixteen Cases. Ocul. Immunol. Inflamm. 2021, 29, 57–65. [Google Scholar] [CrossRef]
- Vazirnia, A.; Wat, H.; Danesh, M.J.; Anderson, R.R. Intense pulsed light for improving dry eye disease in rosacea. J. Am. Acad. Dermatol. 2020, 83, e105. [Google Scholar] [CrossRef]
- Waszczykowska, A.; Żyro, D.; Jurowski, P.; Ochocki, J. Effect of treatment with silver(I) complex of metronidazole on ocular rosacea: Design and formulation of new silver drug with potent antimicrobial activity. J. Trace Elem. Med. Biol. 2020, 61, 126531. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).