Rosacea: An Overview of Its Etiological Factors, Pathogenesis, Classification and Therapy Options
Abstract
:1. Introduction
2. Epidemiology of Rosacea
Age-, Gender-, and Race-Specific Differentiations of Rosacea
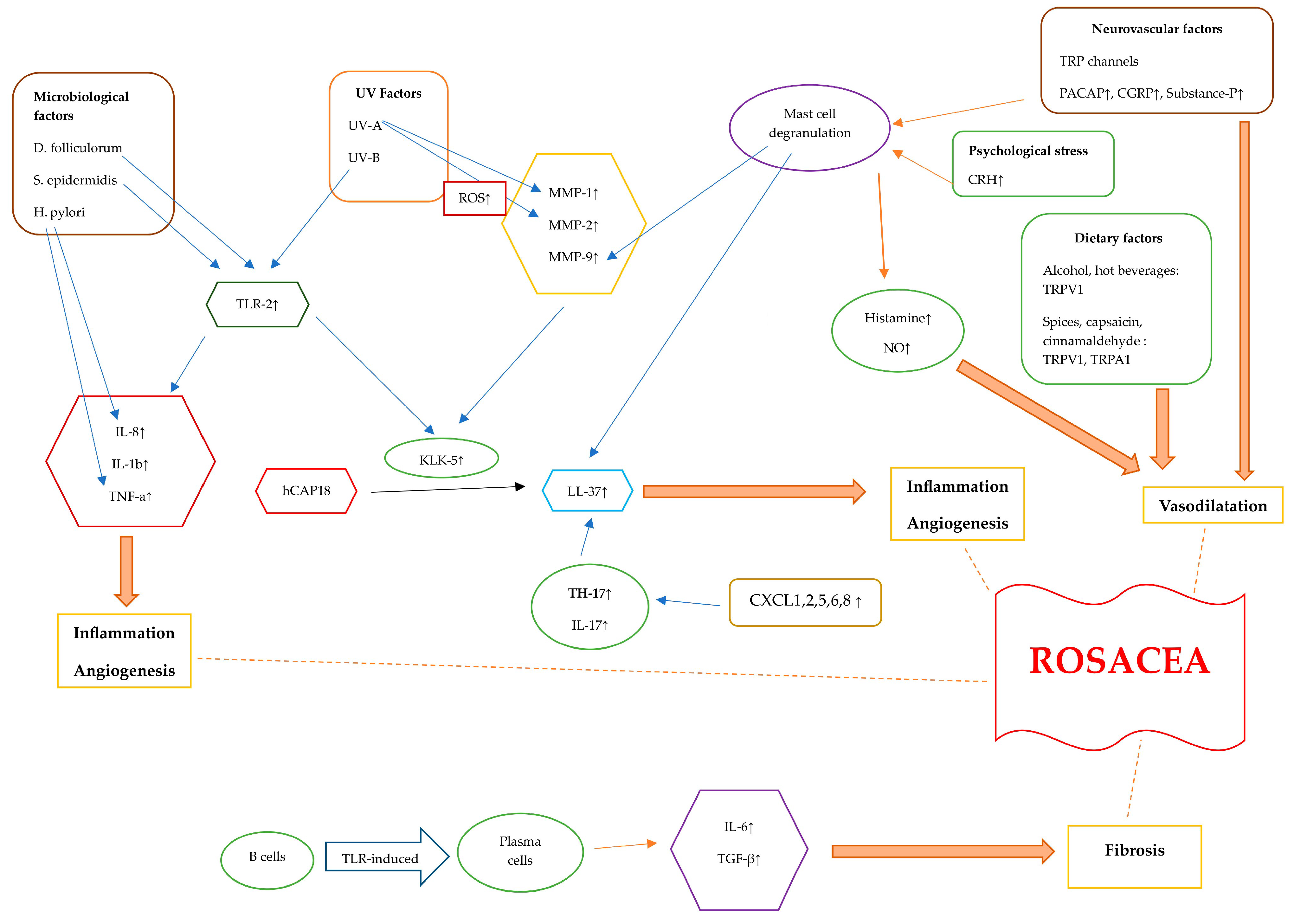
3. Pathogenesis of Rosacea
3.1. Immunological Factors in Rosacea
3.1.1. Dysregulation of the Innate Immune System
3.1.2. Dysregulation of the Adaptive Immune System in Rosacea
T-Cell Mediated Dysregulation
B Cell-Mediated Dysregulation
3.2. Microbiological Factors in Rosacea
3.3. Genetic Factors in Rosacea
3.4. Neurovascular Factors in Rosacea
3.5. Dietary Factors in Rosacea
3.6. UV Irradiation Factors in Rosacea
3.7. Psychological Stress and Sleep Factors in Rosacea
4. Rosacea Classification
4.1. Erythematotelangiectatic Rosacea (Subtype 1)
4.2. Papulopustular Rosacea (Subtype 2)
4.3. Phymatous Rosacea (Subtype 3)
4.4. Ocular Rosacea (Subtype 4)
4.5. Granulomatous Rosacea (Variant)
4.6. Rosacea Fulminans (Variant)
4.7. Neurogenic Rosacea (Subtype)
5. Rosacea Treatment
5.1. Skin Care and General Recommendations
5.2. Treatments for Erythematotelangiectatic Rosacea
5.2.1. Topical Treatment for Erythematotelangiectatic Rosacea
5.2.2. Systemic Treatment for Erythematotelangiectatic Rosacea
5.2.3. Instrumental Treatment for Erythematotelangiectatic Rosacea
5.2.4. Injection Treatment for Erythematotelangiectatic Rosacea
5.3. Treatments for Papulopustular Rosacea
5.3.1. Topical Treatment for Papulopustular Rosacea
5.3.2. Systemic Treatment for Papulopustular Rosacea
5.3.3. Instrumental Treatment for Papulopustular Rosacea
5.3.4. Injection Treatment for Papulopustular Rosacea
5.4. Treatments for Phymatous Rosacea
5.5. Treatments for Ocular Rosacea
5.6. Treatments for Granulomatous Rosacea
5.7. Treatments for Rosacea Fulminans
5.8. Treatments for Neurogenic Rosacea
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, categorization, histology, pathogenesis, and risk factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef]
- Gallo, R.L.; Granstein, R.D.; Kang, S.; Mannis, M.; Steinhoff, M.; Tan, J.; Thiboutot, D. Standard classification and pathophysiology of rosacea: The 2017 update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2018, 78, 148–155. [Google Scholar] [CrossRef] [PubMed]
- van Zuuren, E.J.; Arents, B.W.M.; van der Linden, M.M.D.; Vermeulen, S.; Fedorowicz, Z.; Tan, J. Rosacea: New concepts in classification and treatment. Am. J. Clin. Dermatol. 2021, 22, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.S.; Huang, W.W. Rosacea pathogenesis. Dermatol. Clin. 2018, 36, 81–86. [Google Scholar] [CrossRef]
- Gether, L.; Overgaard, L.K.; Egeberg, A.; Thyssen, J.P. Incidence and prevalence of rosacea: A systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Dlova, N.C.; Mosam, A. Rosacea in black South Africans with skin phototypes V and VI. Clin. Exp. Dermatol. 2017, 42, 670–673. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Woo, Y.R.; Lim, J.H.; Cho, D.H.; Park, H.J. Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int. J. Mol. Sci. 2016, 17, 1562. [Google Scholar] [CrossRef]
- Casas, C.; Paul, C.; Lahfa, M.; Livideanu, B.; Lejeune, O.; Alvarez-Georges, S.; Saint-Martory, C.; Degouy, A.; Mengeaud, V.; Ginisty, H.; et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp. Dermatol. 2012, 21, 906–910. [Google Scholar] [CrossRef]
- Park, H.S.; Jin, S.P.; Lee, Y.; Oh, I.G.; Lee, S.; Kim, J.H.; Cho, K.H.; Chung, J.H. Toll-like receptor 2 mediates a cutaneous reaction induced by repetitive ultraviolet B irradiation in C57/BL6 mice in vivo. Exp. Dermatol. 2014, 23, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Buhren, B.A.; Steinhoff, M.; Homey, B. Rosacea: The cytokine and chemokine network. J. Investig. Dermatol. Symp. Proc. 2011, 15, 40–47. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kanada, K.; Macleod, D.T.; Borkowski, A.W.; Morizane, S.; Nakatsuji, T.; Cogen, A.L.; Gallo, R.L. TLR-2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Investig. Dermatol. 2011, 131, 688–697. [Google Scholar] [CrossRef]
- Larrick, J.W.; Hirata, M.; Balint, R.F.; Lee, J.; Zhong, J.; Wright, S.C. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 1995, 63, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Wang, Z.; Vanderberghe, M.; Two, A.; Gallo, R.L.; Di Nardo, A. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J. Investig. Dermatol. 2014, 134, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Buhl, T.; Sulk, M.; Nowak, P.; Buddenkotte, J.; McDonald, I.; Aubert, J.; Carlavan, I.; Deret, S.; Reiniche, P.; Rivier, M.; et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J. Investig. Dermatol. 2015, 135, 2198–2208. [Google Scholar] [CrossRef]
- Sakabe, J.; Umayahara, T.; Hiroike, M.; Shimauchi, T.; Ito, T.; Tokura, Y. Calcipotriol increases hCAP18 mRNA expression but inhibits extracellular LL37 peptide production in IL-17/IL-22-stimulated normal human epidermal keratinocytes. Acta Derm.-Venereol. 2014, 94, 512–516. [Google Scholar] [CrossRef]
- Brown, T.T.; Choi, E.-Y.K.; Thomas, D.G.; Hristov, A.C.; Chan, M.P. Comparative analysis of rosacea and cutaneous lupus erythematosus: Histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J. Am. Acad. Dermatol. 2014, 71, 100–107. [Google Scholar] [CrossRef]
- Ciccarese, G.; Drago, F.; Rebora, A.; Parodi, A. Two cases of papulo-pustular rosacea-like eruptions following COVID-19 vaccinations. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e868–e870. [Google Scholar] [CrossRef]
- Karampinis, E.; Gravani, A.; Gidarokosta, P.; Bogdanos, D.P.; Roussaki-Schulze, A.V.; Zafiriou, E. Pustular Eruption following COVID-19 Vaccination: A Narrative Case-Based Review. Vaccines 2023, 11, 1298. [Google Scholar] [CrossRef]
- Salzer, S.; Ruzicka, T.; Schauber, J. Face-to-face with anti-inflammatory therapy for rosacea. Exp. Dermatol. 2014, 23, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Pasare, C.; Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 2005, 438, 364–368. [Google Scholar] [CrossRef]
- Woźniacka, A.; Salamon, M.; McCauliffe, D.; Sysa-Jędrzejowska, A. Antinuclear antibodies in rosacea patients. Postepy Dermatol. Alergol. 2013, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jarmuda, S.; O’Reilly, N.; Żaba, R.; Jakubowicz, O.; Szkaradkiewicz, A.; Kavanagh, K. Potential role of Demodex mites and bacteria in the induction of rosacea. J. Med. Microbiol. 2012, 61, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Amir Ali, A.; Vender, R.; Vender, R. The Role of IL-17 in Papulopustular Rosacea and Future Directions. J. Cutan. Med. Surg. 2019, 23, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Margalit, A.; Kowalczyk, M.J.; Żaba, R.; Kavanagh, K. The role of altered cutaneous immune responses in the induction and persistence of rosacea. J. Dermatol. Sci. 2016, 82, 3–8. [Google Scholar] [CrossRef]
- Akilov, O.E.; Mumcuoglu, K.Y. Association between human demodicosis and HLA class I. Clin. Exp. Dermatol. 2003, 28, 70–73. [Google Scholar] [CrossRef]
- Whitfeld, M.; Gunasingam, N.; Leow, L.J.; Shirato, K.; Preda, V. Staphylococcus epidermidis: A possible role in the pustules of rosacea. J. Am. Acad. Dermatol. 2011, 64, 49–52. [Google Scholar] [CrossRef]
- O’Reilly, N.; Menezes, N.; Kavanagh, K. Positive correlation between serum immuno reactivity to Demodex-associated Bacillus proteins and erythematotelangiectatic rosacea. Br. J. Dermatol. 2012, 167, 1032–1036. [Google Scholar] [CrossRef]
- Yang, X. Relationship between Helicobacter pylori and rosacea: Review and discussion. BMC Infect. Dis. 2018, 18, 318. [Google Scholar] [CrossRef]
- Holmes, A.D. Potential role of microorganisms in thepathogenesis of rosacea. J. Am. Acad. Dermatol. 2013, 69, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Joura, M.I.; Brunner, A.; Nemes-Nikodém, V.; Sárdy, M.; Ostorházi, E. Interactions between immune system and the microbiome of skin, blood and gut in pathogenesis of rosacea. Acta Microbiol. Immunol. Hung. 2021, 68, 1–6. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Carolides, S.; Al-Niaimi, F. Rosacea and the gastrointestinal system. Australas. J. Dermatol. 2020, 61, 307–311. [Google Scholar] [CrossRef]
- Parodi, A.; Paolino, S.; Greco, A.; Drago, F.; Mansi, C.; Rebora, A.; Parodi, A.; Savarino, V. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clin. Gastroenterol. Hepatol. 2008, 6, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Katta, R. Diet and rosacea: The role of dietary change in the management of rosacea. Dermatol. Pract. Concept. 2017, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, N.; Gerstenblith, M.; Fu, P.; Tuttle, M.S.; Varma, P.; Gotow, E.; Cooper, K.D.; Mann, M.; Popkin, D.L. Genetic vs environmental factors that correlate with rosacea: A cohort-based survey of twins. JAMA Dermatol. 2015, 151, 1213–1219. [Google Scholar] [CrossRef]
- Helfrich, Y.R.; Maier, L.E.; Cui, Y.; Fisher, G.J.; Chubb, H.; Fligiel, S.; Sachs, D.; Varani, J.; Voorhees, J. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol. 2015, 151, 825–836. [Google Scholar] [CrossRef]
- Awosika, O.; Oussedik, E. Genetic predisposition to rosacea. Dermatol. Clin. 2018, 36, 87–92. [Google Scholar] [CrossRef]
- Chang, A.L.S.; Raber, I.; Xu, J.; Li, R.; Spitale, R.; Chen, J.; Kiefer, A.K.; Tian, C.; Eriksson, N.K.; Hinds, D.A.; et al. Assessment of the genetic basis of rosacea by genome-wide association study. J. Investig. Dermatol. 2015, 135, 1548–1555. [Google Scholar] [CrossRef]
- Dajnoki, Z.; Béke, G.; Kapitány, A.; Mócsai, G.; Gáspár, K.; Rühl, R.; Hendrik, Z.; Juhász, I.; Zouboulis, C.C.; Bácsi, A.; et al. Sebaceous Gland-Rich Skin Is Characterized by TSLP Expression and Distinct Immune Surveillance Which Is Disturbed in Rosacea. J. Investig. Dermatol. 2017, 137, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Boucher, G.; Lees, C.W.; Franke, A.; D’Amato, M.; Taylor, K.D.; Lee, J.C.; Goyette, P.; Imielinski, M.; Latiano, A.; et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Valentonyte, R.; Hampe, J.; Huse, K.; Rosenstiel, P.; Albrecht, M.; Stenzel, A.; Nagy, M.; Gaede, K.I.; Franke, A.; Haesler, R.; et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat. Genet. 2005, 37, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Schmelz, M.; Schauber, J. Facial erythema of rosacea—Aetiology, different pathophysiologies and treatment options. Acta Derm.-Venereol. 2016, 96, 579–586. [Google Scholar] [CrossRef]
- Albrecht, P.J.; Hou, Q.; Argoff, C.E.; Storey, J.R.; Wymer, J.P.; Rice, F.L. Excessive peptidergic sensory innervation of cutaneous arteriole-venules hunts (AVS) in the palmar glabrous skin of fibromyalgia patients: Implications for widespread deep tissue pain and fatigue. Pain. Med. 2013, 14, 895–915. [Google Scholar] [CrossRef]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Investig. Dermatol. 2012, 132, 1253–1262. [Google Scholar] [CrossRef]
- Buddenkotte, J.; Steinhoff, M. Recent advances in understanding and managing rosacea. F1000Research 2018, 3, 1885. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Brain, S.D. Neurovascular aspects of skin neurogenic inflammation. J. Investig. Dermatol. Symp. Proc. 2011, 15, 33–39. [Google Scholar] [CrossRef]
- Fioranelli, M.; Roccia, M.G.; Di Nardo, V.; Aracena, C.J.; Lotti, T. Vitamin D supplementation for childhood atopic dermatitis. Dermatol. Ther. 2016, 29, 303. [Google Scholar] [CrossRef]
- Woo, Y.R.; Jung, K.E.; Koo, D.W.; Lee, J.S. Vitamin D as a marker for disease severity in chronic urticaria and its possible role in pathogenesis. Ann. Dermatol. 2015, 2, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, O.; Balta, I.; Sen, B.B.; Dikilitaş, M.C.; Ozuğuz, P.; Rifaioğlu, E.N. Vitamin D status in patients with rosacea. Cutan. Ocul. Toxicol. 2014, 33, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Jamgochian, M.; Alamgir, M.; Rao, B. Diet in Dermatology: Review of Diet’s Influence on the Conditions of Rosacea, Hidradenitis Suppurativa, Herpes Labialis, and Vitiligo. Am. J. Lifestyle Med. 2021, 17, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Park, B.W.; Ha, J.M.; Cho, E.B.; Jin, J.K.; Park, E.J.; Park, H.R.; Kang, H.J.; Ko, S.H.; Kim, K.H.; Kim, K.J. A study on vitamin D and cathelicidin status in patients with rosacea: Serum level and tissue expression. Ann. Dermatol. 2018, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Yamasaki, K.; Kabigting, F.; Gallo, R.L. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D3, and retinoic acid. J. Investig. Dermatol. 2010, 130, 1297–1306. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Carolides, S.; Al-Niaimi, F. Rosacea and Diet: What is New in 2021? J. Clin. Aesthet. Dermatol. 2021, 14, 49–54. [Google Scholar]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.L.; Drucker, A.M.; Cho, E.; Geng, H.; Qureshi, A.A.; Li, W.Q. Association of caffeine intake and caffeinated coffee consumption with risk of incident rosacea in women. JAMA Dermatol. 2018, 154, 1394–1400. [Google Scholar] [CrossRef]
- Yamasaki, K.; Gallo, R.L. The molecular pathology of rosacea. J. Dermatol. Sci. 2009, 55, 77–81. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Bucana, C.D.; Sanchez, R.; Donawho, C.K.; Kripke, M.L.; Fidler, I.J. Molecular regulation of UVB-induced cutaneous angiogenesis. J. Investig. Dermatol. 1998, 111, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Orion, E.; Wolf, R. Psychologic factors in the development of facial dermatoses. Clin. Dermatol. 2014, 32, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Sowinska-Glugiewicz, I.; Ratajczak-Stefanska, V.; Maleszka, R. Role of psychological factors in course of rosacea. Rocz. Akad. Med. Bialyms. 2005, 50, 49–53. [Google Scholar]
- Muller, M.D.; Sauder, C.L.; Ray, C.A. Mental Stress Elicits Sustained and Reproducible Increases in Skin Sympathetic Nerve Activity. Physiol. Rep. 2013, 1, e00002. [Google Scholar] [CrossRef]
- Black, P.H. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav. Immun. 2002, 16, 622–653. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Wilson, K.; Toma, K.; Sammons, D.L.; Mann, S.; Jurovcik, A.J.; Demidova, O.; Wilson, T.E. Augmented supraorbital skin sympathetic nerve activity responses to symptom trigger events in rosacea patients. J. Neurophysiol. 2015, 114, 1530–1537. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Pisarchik, A.; Zbytek, B.; Linton, E.A.; Mazurkiewicz, J.E.; Wei, E.T. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001, 15, 1678–1693. [Google Scholar] [CrossRef]
- Hall, J.M.; Cruser, D.; Podawiltz, A.; Mummert, D.I.; Jones, H.; Mummert, M.E. Psychological Stress and the Cutaneous Immune Response: Roles of the HPA Axis and the Sympathetic Nervous System in Atopic Dermatitis and Psoriasis. Dermatol. Res. Pract. 2012, 2012, 403908. [Google Scholar] [CrossRef]
- Passeron, T.; Zouboulis, C.C.; Tan, J.; Andersen, M.L.; Katta, R.; Lyu, X.; Aguilar, L.; Kerob, D.; Morita, A.; Krutmann, J.; et al. Adult skin acute stress responses to short-term environmental and internal aggression from exposome factors. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1963–1975. [Google Scholar] [CrossRef]
- Thorburn, P.T.; Riha, R.L. Skin disorders and sleep in adults: Where is the evidence? Sleep. Med. Rev. 2010, 14, 351–358. [Google Scholar] [CrossRef]
- Egeberg, A.; Hansen, P.R.; Gislason, G.H.; Thyssen, J.P. Patients with rosacea have increased risk of depression and anxiety disorders: A Danish nation wide cohort study. Dermatology 2016, 232, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Mostaghimi, L. Prevalence of mood and sleep problems in chronic skin diseases: A pilot study. Cutis 2008, 81, 398–402. [Google Scholar] [PubMed]
- Wong, I.T.Y.; Chandran, V.; Li, S.; Gladman, D.D. Sleep disturbance in psoriatic disease: Prevalence and associated factors. J. Rheumatol. 2017, 44, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, H.; Gong, Y.; Ouyang, Y.; Deng, F.; Tang, Y.; Li, J. Relationship between rosacea and sleep. J. Dermatol. 2020, 47, 592–600. [Google Scholar] [CrossRef]
- Sa-Nunes, A.; Bizzarro, B.; Egydio, F.; Barros, M.S.; Sesti-Costa, R.; Soares, E.M.; Pina, A.; Russo, M.; Faccioli, L.H.; Tufik, S.; et al. The dual effect of paradoxical sleep deprivation on murine immune functions. J. Neuroimmunol. 2016, 290, 9–14. [Google Scholar] [CrossRef]
- De Lorenzo, B.H.; de Oliveira Marchioro, L.; Greco, C.R.; Suchecki, D. Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology 2015, 57, 134–143. [Google Scholar] [CrossRef]
- Kalinchuk, A.V.; McCarley, R.W.; Porkka-Heiskanen, T.; Basheer, R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J. Neurosci. 2010, 30, 13254–13264. [Google Scholar] [CrossRef]
- Wilkin, J.; Dahl, M.; Detmar, M.; Drake, L.; Feinstein, A.; Odom, R.; Powell, F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J. Am. Acad. Dermatol. 2002, 46, 584–587. [Google Scholar] [CrossRef]
- Cribier, B. Rosacea: Treatment targets based on new physiopathology data. Ann. Dermatol. Venereol. 2022, 149, 99–107. [Google Scholar] [CrossRef]
- Walsh, R.K.; Endicott, A.A.; Shinkai, K. Diagnosis and Treatment of Rosacea Fulminans: A Comprehensive Review. Am. J. Clin. Dermatol. 2018, 19, 79–86. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, K.; Wang, Y.; Fang, R.; Sun, Q. Rosacea treatment: Review and update. Dermatol. Ther. 2021, 11, 13–24. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Yost, J.M.; Truong, S.V.; Steinhoff, M.; Wang, K.C.; Berger, T.G. Neurogenic rosacea: A distinct clinical subtype requiring a modified approach to treatment. Arch. Dermatol. 2011, 147, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Kang, S.Y.; Kim, K.E.; Cho, S.Y.; Kim, K.H.; Kim, I.H. Neurogenic rosacea in Korea. J. Dermatol. 2021, 48, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Berg, A.; Barr, C. Managing rosacea in the clinic: From pathophysiology to treatment-a review of the literature. J. Clin. Aesth. Dermatol. 2020, 13, S17–S22. [Google Scholar]
- Schaller, M.; Almeida, L.M.; Bewley, A.; Cribier, B.; Dlova, N.C.; Kautz, G.; Mannis, M.; Oon, H.H.; Rajagopalan, M.; Steinhoff, M.; et al. Rosacea treatment update: Recommendations from the global ROSacea COnsensus (ROSCO) panel. Br. J. Dermatol. 2017, 176, 465–471. [Google Scholar] [CrossRef]
- Thiboutot, D.; Anderson, R.; Cook-Bolden, F.; Draelos, Z.; Gallo, R.L.; Granstein, R.D.; Kang, S.; Macsai, M.; Gold, L.S.; Tan, J. Standard management options for rosacea: The 2019 update by the National Rosacea Society Expert Committee. J. Am. Acad. Dermatol. 2020, 82, 1501–1510. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Gunt, H.; Levy, S.B. Natural skin care products as adjunctive to prescription therapy in moderate to severe Rosacea. J. Drugs Dermatol. 2019, 18, 141–146. [Google Scholar] [PubMed]
- van Zuuren, E.J. Rosacea. N. Engl. J. Med. 2017, 377, 1754–1764. [Google Scholar] [CrossRef]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part II. Topical and systemic therapies in the treatment of rosacea. J. Am. Acad. Dermatol. 2015, 72, 761–770. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Carter, B.; van der Linden, M.M.; Charland, L. Interventions for rosacea. Cochrane Database Syst. Rev. 2015, 2015, CD003262. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Jeong, S.W.; Jo, H.; Woo, Y.R.; Park, H.J. Inhibition of mast cell infiltration in an LL-37-induced rosacea mouse model using topical brimonidine tartrate 0.33% gel. Exp. Dermatol. 2017, 26, 1143–1145. [Google Scholar] [CrossRef] [PubMed]
- Bertino, B.; Blanchet-Réthoré, S.; Thibaut de Ménonville, S.; Reynier, P.; Méhul, B.; Bogouch, A.; Gamboa, B.; Dugaret, A.S.; Zugaj, D.; Petit, L.; et al. Brimonidine displays anti-inflammatory properties in the skin through the modulation of the vascular barrier function. Exp. Dermatol. 2018, 27, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Y.; Lu, B.; Tan, D.; Aroyan, C.; Shinkai, K.; Leslie, K.S.; Fox, L.P.; Yu, S.; Neuhaus, I.M.; Grekin, R.C.; et al. Effect of Topical Brimonidine on Alcohol-Induced Flushing in Asian Individuals: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Al Mokadem, S.M.; Ibrahim, A.M.; El Sayed, A.M. Efficacy of Topical Timolol 0.5% in the Treatment of Acne and Rosacea: A Multicentric Study. J. Clin. Aesthet. Dermatol. 2020, 13, 22–27. [Google Scholar] [PubMed]
- Bageorgou, F.; Vasalou, V.; Tzanetakou, V.; Kontochristopoulos, G. The new therapeutic choice of tranexamic acid solution in treatment of erythematotelangiectatic rosacea. J. Cosmet. Dermatol. 2019, 18, 563–567. [Google Scholar] [CrossRef]
- Jakhar, D.; Kaur, I.; Misri, R. Topical 10% tranexamic acid for erythematotelangiectatic steriod- induced rosacea. J. Am. Acad. Dermatol. 2022, 86, e1–e2. [Google Scholar] [CrossRef]
- Wozniacka, A.; Wieczorkowska, M.; Gebicki, J.; Sysa-Jedrzejowska, A. Topical application of 1-methylnicotinamide in the treatment of rosacea: A pilot study. Clin. Exp. Dermatol. 2005, 30, 632–635. [Google Scholar] [CrossRef]
- Van Landuyt, H.; Joubert-Lequain, I.; Humbert, P.; Lucas, A.; Drobacheff, C.; Mercier, M.; Laurent, R. Treatment of rosacea. Clonidine (0.075 mg per day) versus placebo (initial results). Ann. Dermatol. Venereol. 1997, 124, 729. [Google Scholar] [PubMed]
- Grosshans, E.; Michel, C.; Arcade, B.; Cribier, B. Rilmenidine in rosacea: A double-blind study versus placebo. Ann. Dermatol. Venereol. 1997, 124, 687–691. [Google Scholar] [PubMed]
- Layton, A.; Thiboutot, D. Emerging therapies in rosacea. J. Am. Acad. Dermatol. 2013, 69, 57–65. [Google Scholar] [CrossRef]
- Sharma, A.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Goren, A.; Grabbe, S.; Goldust, M. Rosacea management: A comprehensive review. J. Cosmet. Dermatol. 2022, 21, 1895–1904. [Google Scholar] [CrossRef]
- Kassir, R.; Kolluru, A.; Kassir, M. Intense pulsed light for the treatment of rosacea and telangiectasias. J. Cosmet. Laser Ther. 2011, 13, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, P.; Clayton, W.; Norwood, S.; Chopra, S.; Rustin, M. Treatment of rosacea with intense pulsed light: Significant improvement and long-lasting results. Br. J. Dermatol. 2008, 159, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Dover, J.S.; Arndt, K.A. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: Comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol. Surg. 2003, 29, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, I.M.; Zane, L.T.; Tope, W.D. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol. Surg. 2009, 35, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Voravutinon, N.; Warycha, M.; Whiting, D.; Nodzenski, M.; Yoo, S.; West, D.P.; Veledar, E.; Poon, E. Comparative effectiveness of nonpurpuragenic 595-nm pulsed dye laser and microsecond 1064-nm neodymium:yttrium-aluminum-garnet laser for treatment of diffuse facial erythema: A double-blind randomized controlled trial. J Am Acad Dermatol. 2013, 69, 438–443. [Google Scholar] [CrossRef]
- Li, A.; Fang, R.; Mao, X.; Sun, Q. Photodynamic therapy in the treatment of rosacea: A systematic review. Photodiagnosis Photodyn. Ther. 2022, 38, 102875. [Google Scholar] [CrossRef]
- Friedmann, D.P.; Goldman, M.P.; Fabi, S.G.; Guiha, I. Multiple sequential light and laser sources to activate aminolevulinic acid for rosacea. J. Cosmet. Dermatol. 2016, 15, 407–412. [Google Scholar] [CrossRef]
- Wollina, U.; Bitel, A.; Vojvodic, A.; Lotti, T. Rosacea Flare—Up after Photodynamic Therapy (PDT) for Field Cancerization and a Review on Adverse Events with PDT in General. Open Access Maced. J. Med. Sci. 2019, 7, 2998–3001. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Cheon, H.I.; Hur, M.S.; Han, S.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Assessment of skin physiology change andsafetyafterintradermalinjectionswithbotulinumtoxin: A randomized, double-blind, placebo-controlled, split-face pilot study in rosaceapatientswithfacialerythema. Dermatol. Surg. 2019, 45, 1155–1162. [Google Scholar] [CrossRef]
- Bharti, J.; Sonthalia, S.; Jakhar, D. Mesotherapy with botulinum toxin for the treatment of refractory vascular and papulopustular rosacea. J. Am. Acad. Dermatol. 2023, 88, e295–e296. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Danish Headache Center. Efcacy and Tolerability of Erenumab in the Management of Persistent Redness and Fushing in Rosacea (STOP Ros). ClinicalTrials.gov. 2020; Identifer NCT04419259. Available online: https://clinicaltrials.gov/ct2/show/NCT04419259 (accessed on 16 September 2023).
- Husein-ElAhmed, H.; Steinhoff, M. Efficacy of topical ivermectin and impact on quality of life in patients with papulopustular rosacea: A systematic review and meta-analysis. Dermatol. Ther. 2020, 33, e13203. [Google Scholar] [CrossRef] [PubMed]
- Trave, I.; Merlo, G.; Cozzani, E.; Parodi, A. Real-life experience on effectiveness and tolerability of topical ivermectin in papulopustular rosacea and antiparasitic effect on Demodex mites. Dermatol. Ther. 2019, 32, e13093. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.S.; Del Rosso, J.Q.; Kircik, L.; Bhatia, N.D.; Hooper, D.; Nahm, W.K.; Stuart, I. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: Results of 2 phase 3, randomized, clinical trials. J. Am. Acad. Dermatol. 2020, 82, 1166–1173. [Google Scholar] [CrossRef]
- Webster, G.; Draelos, Z.D.; Graber, E.; Lee, M.S.; Dhawan, S.; Salman, M.; Magrath, G.N. A multicentre, randomized, double-masked, parallel group, vehicle-controlled phase IIb study to evaluate the safety and efficacy of 1% and 3% topical minocycline gel in patients with papulopustular rosacea. Br. J. Dermatol. 2020, 183, 471–479. [Google Scholar] [CrossRef]
- Forton, F.M.N.; De Maertelaer, V. Effectiveness of benzyl benzoate treatment on clinical symptoms and Demodex density over time in patients with rosacea and demodicosis: A real life retrospective follow-up study comparing low- and high-dose regimens. J. Dermatol. Treat. 2022, 33, 456–465. [Google Scholar] [CrossRef]
- Forton, F.M.N.; De Maertelaer, V. Treatment of rosacea and demodicosis with benzyl benzoate: Effects of different doses on Demodex density and clinical symptoms. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 365–369. [Google Scholar] [CrossRef]
- Kim, M.S.; Chang, S.E.; Haw, S.; Bak, H.; Kim, Y.J.; Lee, M.W. Tranexamic acid solution soaking is an excellent approach for rosacea patients: A preliminary observation in six patients. J. Dermatol. 2013, 40, 70–71. [Google Scholar] [CrossRef]
- Anzengruber, F.; Czernielewski, J.; Conrad, C.; Feldmeyer, L.; Yawalkar, N.; Häusermann, P.; Cozzio, A.; Mainetti, C.; Goldblum, D.; Läuchli, S.; et al. Swiss S1 guideline for the treatment of rosacea. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1775–1791. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Schlessinger, J.; Werschler, P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J. Drugs Dermatol. 2008, 7, 573–576. [Google Scholar] [PubMed]
- Del Rosso, J.Q. Anti-inflamatory dose doxycycline in the treatment of rosacea. J. Drugs Dermatol. 2009, 8, 664–668. [Google Scholar] [PubMed]
- Gollnick, H.; Blume-Peytavi, U.; Szabo, E.L.; Meyer, K.G.; Hauptmann, P.; Popp, G.; Sebastian, M.; Zwingers, T.; Willers, C.; von der Weth, R. Systemic isotretinoin in the treatment of rosacea—Doxycycline- and placebo-controlled, randomized clinical study. J. Dtsch. Dermatol. Ges. 2010, 8, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Sharquie, K.E.; Najim, R.A.; Al-Salman, H.N. Oral zinc sulfate in the treatment of rosacea: A double-blind, placebo-controlled study. Int. J. Dermatol. 2006, 45, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Bamford, J.T.; Gessert, C.E.; Haller, I.V.; Kruger, K.; Johnson, B.P. Randomized, double-blind trial of 220 mg zinc sulfate twice daily in the treatment of rosacea. Int. J. Dermatol. 2012, 51, 459–462. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Tang, Y.; Wang, B.; Deng, Z.; Huang, Y.; Liu, F.; Zhao, Z.; Zhang, Y. Hydroxychloroquine is a novel therapeutic approach for rosacea. Int. Immunopharmacol. 2020, 79, 106178. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, X.; Huang, X.; Tang, Y.; Zhao, Z.; Yang, B.; Yang, B.; Zheng, Y.; Yuan, C.; Xie, H.; et al. Efficacy and safety of hydroxychloroquine for treatment of patients with rosacea: A multicenter, randomized, double-blind, double-dummy, pilot study. J. Am. Acad. Dermatol. 2021, 84, 543–545. [Google Scholar] [CrossRef]
- Drago, F.; De Col, E.; Agnoletti, A.F.; Schiavetti, I.; Savarino, V.; Rebora, A.; Paolino, S.; Cozzani, E.; Parodi, A. The role of small intestinal bacterial overgrowth in rosacea: A 3-year follow-up. J. Am. Acad. Dermatol. 2016, 75, e113–e115. [Google Scholar] [CrossRef]
- Saleh, P.; Naghavi-Behzad, M.; Herizchi, H.; Mokhtari, F.; Mirza-Aghazadeh-Attari, M.; Piri, R. Effects of Helicobacter pylori treatment on rosacea: A single-arm clinical trial study. J. Dermatol. 2017, 44, 1033–1037. [Google Scholar] [CrossRef]
- Kumar, A.M.; Chiou, A.S.; Shih, Y.H.; Li, S.; Chang, A.L.S. An exploratory, open-label, investigator-initiated study of interleukin-17 blockade in patients with moderate-to-severe papulopustular rosacea. Br. J. Dermatol. 2020, 183, 942–943. [Google Scholar] [CrossRef]
- Schmitz, L.; Hessam, S.; Scholl, L.; Reitenbach, S.; Segert, M.H.; Bechara, F.G. Wound Care With a Porcine Extracellular Matrix After Surgical Treatment of Rhinophyma. J. Cutan. Med. Surg. 2020, 24, 253–258. [Google Scholar] [CrossRef]
- Sadick, H.; Goepel, B.; Bersch, C.; Goessler, U.; Hoermann, K.; Riedel, F. Rhinophyma: Diagnosis and treatment options for a disfiguring tumor of the nose. Ann. Plast. Surg. 2008, 61, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jabbehdari, S.; Memar, O.M.; Caughlin, B.; Djalilian, A.R. Update on the pathogenesis and management of ocular rosacea: An interdisciplinary review. Eur. J. Ophthalmol. 2021, 31, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Donmez, O.; Akova, Y.A. Pediatric Ocular Acne Rosacea: Clinical Features and Long Term Follow-Up of Sixteen Cases. Ocul. Immunol. Inflamm. 2021, 29, 57–65. [Google Scholar] [CrossRef]
- Vazirnia, A.; Wat, H.; Danesh, M.J.; Anderson, R.R. Intense pulsed light for improving dry eye disease in rosacea. J. Am. Acad. Dermatol. 2020, 83, e105. [Google Scholar] [CrossRef]
- Waszczykowska, A.; Żyro, D.; Jurowski, P.; Ochocki, J. Effect of treatment with silver(I) complex of metronidazole on ocular rosacea: Design and formulation of new silver drug with potent antimicrobial activity. J. Trace Elem. Med. Biol. 2020, 61, 126531. [Google Scholar] [CrossRef] [PubMed]

| Treatment Options/Types of Rosacea | Topical | Systemic (Oral) | Devices | Injection | Other | Prevention |
|---|---|---|---|---|---|---|
| Erythematelengiectatic Rosacea (subtype 1) | -Alpha-2 adrenergic agonists (brimonidine, oxymetazoline) | -Beta blockers | -IPL * -Lasers (Nd:YAG †, PDL ‡) -PDT § | -Botulinum toxin (intralesional) | -Sunscreen -Skin care | |
| Papulopustular Rosacea (subtype 2) | -Azelaic acid -Ivermectin -Metronidazole -Minocycline | -Tetracyclines -Macrolide -Isotretinoin -Metronidazole -H. pylori eradication | -Lasers (Nd:YAG) -IPL -PDT | -Sunscreen -Skin care | ||
| Phymatous Rosacea (subtype 3) | -Topical retinoids | -Doxycycline -Isotretinoin | -Lasers (CO2 Laser) ** | -Surgery | -Sunscreen -Skin care | |
| Ocular Rosacea (subtype 4) | -Topical antibiotics -Topical steroids -Cyclosporin ophthalmic solution -Warm compresses -Eyelid scrubbing -Preservative-free artificial tears | -Omega-3 fatty acids -Doxycycline -Macrolide | -Surgery | -Sunscreen -Skin care | ||
| Granulomatous Rosacea (variant) | -Azelaic acid -Ivermectin -Pimecrolimus | -Tetracyclines -Macrolide -İsotretinoin -Metronidazole -Dapsone -Steroids | -Chromophore gel-assisted phototherapy -IPL | -Sunscreen -Skin care | ||
| Rosacea Fulminans (variant) | -İsotretinoin -Steroids | -Sunscreen -Skin care | ||||
| Neurogenic Rosacea (subtype) | - Beta blockers -Neuropathic pain medication (gabapentin, pregabalin) -Antidepressants (duloxetine) | -Laser treatments | -Consultations with pain specialists or psychiatrists | -Sunscreen -Skin care |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maden, S. Rosacea: An Overview of Its Etiological Factors, Pathogenesis, Classification and Therapy Options. Dermato 2023, 3, 241-262. https://doi.org/10.3390/dermato3040019
Maden S. Rosacea: An Overview of Its Etiological Factors, Pathogenesis, Classification and Therapy Options. Dermato. 2023; 3(4):241-262. https://doi.org/10.3390/dermato3040019
Chicago/Turabian StyleMaden, Serap. 2023. "Rosacea: An Overview of Its Etiological Factors, Pathogenesis, Classification and Therapy Options" Dermato 3, no. 4: 241-262. https://doi.org/10.3390/dermato3040019
APA StyleMaden, S. (2023). Rosacea: An Overview of Its Etiological Factors, Pathogenesis, Classification and Therapy Options. Dermato, 3(4), 241-262. https://doi.org/10.3390/dermato3040019






