Advanced Artificial Intelligence Techniques for Comprehensive Dermatological Image Analysis and Diagnosis
Abstract
1. Introduction
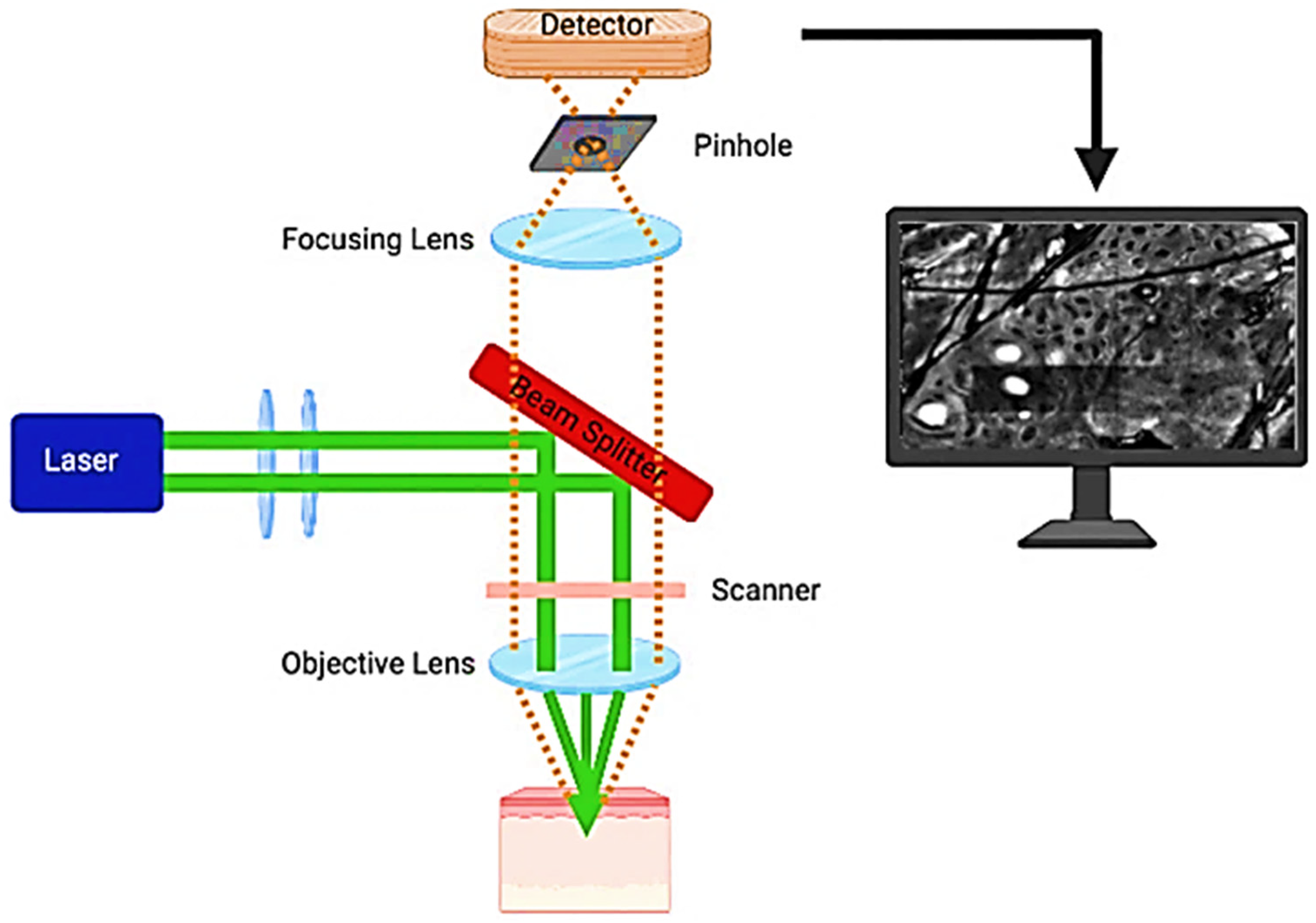
2. The Technical Foundations of Reflectance Confocal Microscopy
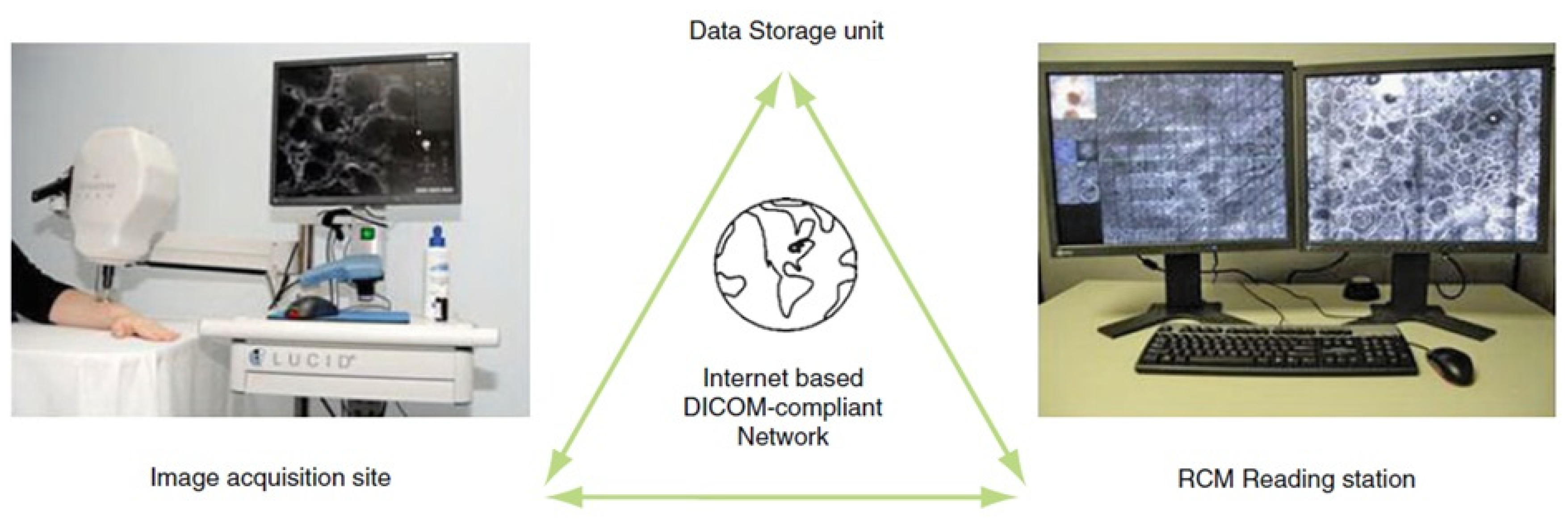
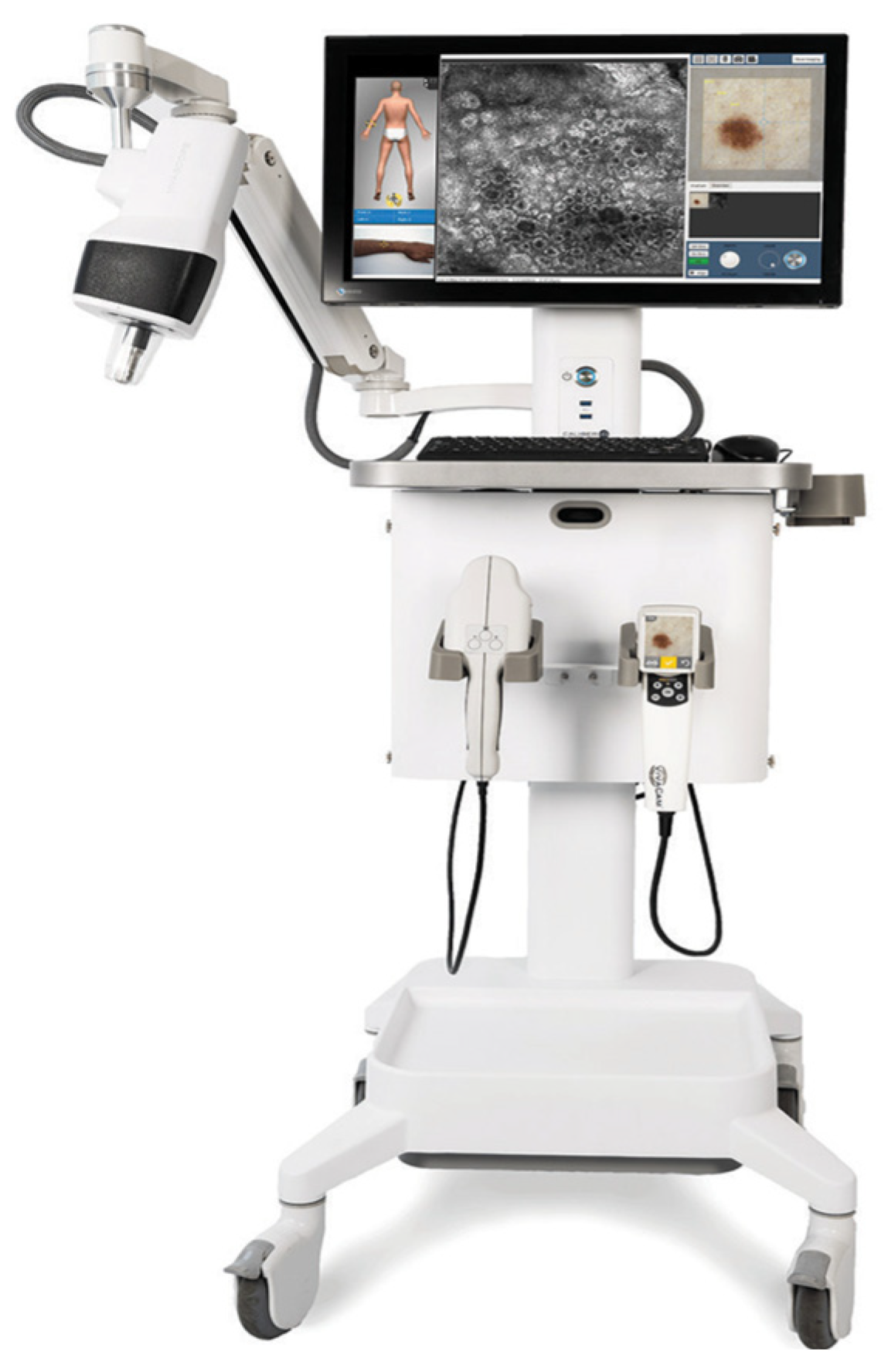
3. Tele-Reflectance Confocal Microscopy
4. Artificial Intelligence in Dermatology
5. Dermoscopic Image Datasets
6. Publicly Available Dermoscopic Image Datasets
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, H.-J.; Keum, C.; Witkowski, A.; Ludzik, J.; Petrie, T.; Hanson, H.A.; Leachman, S.A. Enhancing Diagnosis through AI-Driven Analysis of Reflectance Confocal Microscopy. arXiv 2024, arXiv:2404.16080. [Google Scholar]
- Malciu, A.M.; Lupu, M.; Voiculescu, V.M. Artificial Intelligence-Based Approaches to Reflectance Confocal Microscopy Image Analysis in Dermatology. J. Clin. Med. 2022, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Koller, S.; Wiltgen, M.; Ahlgrimm-Siess, V.; Weger, W.; Hofmann-Wellenhof, R.; Richtig, E.; Smolle, J.; Gerger, A. In Vivo Reflectance Confocal Microscopy: Automated Diagnostic Image Analysis of Melanocytic Skin Tumours. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Rajadhyaksha, M.; González, S.; Zavislan, J.M.; Rox Anderson, R.; Webb, R.H. In Vivo Confocal Scanning Laser Microscopy of Human Skin II: Advances in Instrumentation and Comparison With Histology11The Authors Have Declared Conflict of Interest. J. Investig. Dermatol. 1999, 113, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Robic, J.; Nkengne, A.; Perret, B.; Couprie, M.; Talbot, H.; Pellacani, G.; Vie, K. Clinical Validation of a Computer-based Approach for the Quantification of the Skin Ageing Process of Women Using in Vivo Confocal Microscopy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e68–e70. [Google Scholar] [CrossRef]
- Gerger, A.; Wiltgen, M.; Langsenlehner, U.; Richtig, E.; Horn, M.; Weger, W.; Ahlgrimm-Siess, V.; Hofmann-Wellenhof, R.; Samonigg, H.; Smolle, J. Diagnostic Image Analysis of Malignant Melanoma in in Vivo Confocal Laser-scanning Microscopy: A Preliminary Study. Ski. Res. Technol. 2008, 14, 359–363. [Google Scholar] [CrossRef]
- Kittler, H.; Pehamberger, H.; Wolff, K.; Binder, M. Diagnostic Accuracy of Dermoscopy. Lancet Oncol. 2002, 3, 159–165. [Google Scholar] [CrossRef]
- Gerger, A.; Koller, S.; Weger, W.; Richtig, E.; Kerl, H.; Samonigg, H.; Krippl, P.; Smolle, J. Sensitivity and Specificity of Confocal Laser-scanning Microscopy for in Vivo Diagnosis of Malignant Skin Tumors. Cancer 2006, 107, 193–200. [Google Scholar] [CrossRef]
- Pellacani, G.; Cesinaro, A.M.; Seidenari, S. Reflectance-Mode Confocal Microscopy of Pigmented Skin Lesions–Improvement in Melanoma Diagnostic Specificity. J. Am. Acad. Dermatol. 2005, 53, 979–985. [Google Scholar] [CrossRef]
- Mehrabi, J.N.; Baugh, E.G.; Fast, A.; Lentsch, G.; Balu, M.; Lee, B.A.; Kelly, K.M. A Clinical Perspective on the Automated Analysis of Reflectance Confocal Microscopy in Dermatology. Lasers Surg. Med. 2021, 53, 1011–1019. [Google Scholar] [CrossRef]
- Nori, S.; Rius-Díaz, F.; Cuevas, J.; Goldgeier, M.; Jaen, P.; Torres, A.; González, S. Sensitivity and Specificity of Reflectance-Mode Confocal Microscopy for in Vivo Diagnosis of Basal Cell Carcinoma: A Multicenter Study. J. Am. Acad. Dermatol. 2004, 51, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The Impact of In Vivo Reflectance Confocal Microscopy for the Diagnostic Accuracy of Melanoma and Equivocal Melanocytic Lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Popa, I.; Voiculescu, V.; Boda, D.; Caruntu, C.; Zurac, S.; Giurcaneanu, C. A Retrospective Study of the Diagnostic Accuracy of In Vivo Reflectance Confocal Microscopy for Basal Cell Carcinoma Diagnosis and Subtyping. J. Clin. Med. 2019, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Caruntu, A.; Boda, D.; Caruntu, C. In Vivo Reflectance Confocal Microscopy-Diagnostic Criteria for Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. J. Clin. Med. 2020, 9, 1987. [Google Scholar] [CrossRef] [PubMed]
- Lupu, M.; Popa, I.M.; Voiculescu, V.M.; Caruntu, A.; Caruntu, C. A Systematic Review and Meta-Analysis of the Accuracy of in VivoReflectance Confocal Microscopy for the Diagnosis of Primary Basal Cell Carcinoma. J. Clin. Med. 2019, 8, 1462. [Google Scholar] [CrossRef]
- Lupu, M.; Tebeica, T.; Voiculescu, V.M.; Ardigo, M. Tubular Apocrine Adenoma: Dermoscopic and in Vivo Reflectance Confocal Microscopic Aspects. Int. J. Dermatol. 2019, 58, e210–e211. [Google Scholar] [CrossRef]
- Lupu, M.; Caruntu, A.; Caruntu, C.; Boda, D.; Moraru, L.; Voiculescu, V.; Bastian, A. Non-Invasive Imaging of Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. Mol. Clin. Oncol. 2018, 8, 640–646. [Google Scholar] [CrossRef]
- Lupu, M.; Voiculescu, V.M.; Vajaitu, C.; Orzan, O.A. In Vivo Reflectance Confocal Microscopy for the Diagnosis of Scabies. BMJ Case Rep. 2021, 14, e240507. [Google Scholar] [CrossRef]
- Lupu, M.; Voiculescu, V.M.; Caruntu, A.; Tebeica, T.; Caruntu, C. Preoperative Evaluation through Dermoscopy and Reflectance Confocal Microscopy of the Lateral Excision Margins for Primary Basal Cell Carcinoma. Diagnostics 2021, 11, 120. [Google Scholar] [CrossRef]
- Wodzinski, M.; Skalski, A.; Witkowski, A.; Pellacani, G.; Ludzik, J. Convolutional Neural Network Approach to Classify Skin Lesions Using Reflectance Confocal Microscopy. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4754–4757. [Google Scholar]
- Sikorska, M.; Skalski, A.; Wodzinski, M.; Witkowski, A.; Pellacani, G.; Ludzik, J. Learning-Based Local Quality Assessment of Reflectance Confocal Microscopy Images for Dermatology Applications. Biocybern. Biomed. Eng. 2021, 41, 880–890. [Google Scholar] [CrossRef]
- Campanella, G.; Navarrete-Dechent, C.; Liopyris, K.; Monnier, J.; Aleissa, S.; Minhas, B.; Scope, A.; Longo, C.; Guitera, P.; Pellacani, G.; et al. Deep Learning for Basal Cell Carcinoma Detection for Reflectance Confocal Microscopy. J. Investig. Dermatol. 2022, 142, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.H.; Foltz, E.A.; Witkowski, A.; Ludzik, J. Analysis of Artificial Intelligence-Based Approaches Applied to Non-Invasive Imaging for Early Detection of Melanoma: A Systematic Review. Cancers 2023, 15, 4694. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, M.; Bozkurt, A.; Alessi-Fox, C.; Gill, M.; Brooks, D.H.; Rajadhyaksha, M.; Kose, K.; Dy, J.G. Semantic Segmentation of Reflectance Confocal Microscopy Mosaics of Pigmented Lesions Using Weak Labels. Sci. Rep. 2021, 11, 3679. [Google Scholar] [CrossRef] [PubMed]
- ISO 21073:2019; Microscopes—Confocal Microscopes—Optical Data of Fluorescence Confocal Microscopes for Biological Imaging. International Organization for Standardization (ISO): Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/69820.html (accessed on 31 August 2024).
- ISO 25178-607:2019; Geometrical Product Specifications (GPS)—Surface Texture: Areal Part 607: Nominal Characteristics of Non-Contact (Confocal Microscopy) Instruments. International Organization for Standardization (ISO): Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/67652.html (accessed on 31 August 2024).
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance Confocal Microscopy. J. Am. Acad. Dermatol. 2021, 84, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.; Alessi-Fox, C.; Kose, K. Artifacts and Landmarks: Pearls and Pitfalls for in Vivo Reflectance Confocal Microscopy of the Skin Using the Tissue-Coupled Device. Dermatol. Online J. 2019, 25, 1. [Google Scholar] [CrossRef]
- Longo, C.; Farnetani, F.; Ciardo, S.; Cesinaro, A.M.; Moscarella, E.; Ponti, G.; Zalaudek, I.; Argenziano, G.; Pellacani, G. Is Confocal Microscopy a Valuable Tool in Diagnosing Nodular Lesions? A Study of 140 Cases. Br. J. Derm. 2013, 169, 58–67. [Google Scholar] [CrossRef]
- Que, S.K.T. Research Techniques Made Simple: Noninvasive Imaging Technologies for the Delineation of Basal Cell Carcinomas. J. Investig. Dermatol. 2016, 136, e33–e38. [Google Scholar] [CrossRef]
- Alawi, S.A.; Kuck, M.; Wahrlich, C.; Batz, S.; McKenzie, G.; Fluhr, J.W.; Lademann, J.; Ulrich, M. Optical Coherence Tomography for Presurgical Margin Assessment of Non-melanoma Skin Cancer—A Practical Approach. Exp. Dermatol. 2013, 22, 547–551. [Google Scholar] [CrossRef]
- Wurm, E.M.T.; Hofmann-Wellenhof, R.; Wurm, R.; Soyer, H.P. Telemedicine and Teledermatology: Past, Present and Future. J. Dtsch. Derma Gesell 2008, 6, 106–112. [Google Scholar] [CrossRef]
- Wurm, E.M.T.; Campbell, T.M.; Soyer, H.P. Teledermatology: How to Start a New Teaching and Diagnostic Era in Medicine. Dermatol. Clin. 2008, 26, 295–300. [Google Scholar] [CrossRef]
- Gronbeck, C.; Grant-Kels, J.M.; Fox, C.; Feng, H. Trends in Utilization of Reflectance Confocal Microscopy in the United States, 2017–2019. J. Am. Acad. Dermatol. 2022, 86, 1395–1398. [Google Scholar] [CrossRef]
- Longo, C.; Hemmer, P.; Pellacani, G. Tele-Reflectance Confocal Microscopy. In Telemedicine in Dermatology; Soyer, H.P., Binder, M., Smith, A.C., Wurm, E.M.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 73–77. ISBN 978-3-642-20800-3. [Google Scholar]
- Wurm, E.M.T.; Longo, C.; Hemmer, P.; Pellacani, G. Tele-Reflectance Confocal Microscopy. In Reflectance Confocal Microscopy for Skin Diseases; Hofmann-Wellenhof, R., Pellacani, G., Malvehy, J., Soyer, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Jayamohanan, R. Confocal Microscopy—Working Principle and Applications in Dermatology. J. Ski. Sex. Transm. Dis. 2022, 5, 81–89. [Google Scholar] [CrossRef]
- Liopyris, K.; Gregoriou, S.; Dias, J.; Stratigos, A.J. Artificial Intelligence in Dermatology: Challenges and Perspectives. Dermatol. Ther. 2022, 12, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.A. Artificial Intelligence, Machine Learning, Deep Learning, and Cognitive Computing: What Do These Terms Mean and How Will They Impact Health Care? J. Arthroplast. 2018, 33, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Benjamens, S.; Dhunnoo, P.; Meskó, B. The State of Artificial Intelligence-Based FDA-Approved Medical Devices and Algorithms: An Online Database. Npj Digit. Med. 2020, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Haggenmüller, S.; Maron, R.C.; Hekler, A.; Utikal, J.S.; Barata, C.; Barnhill, R.L.; Beltraminelli, H.; Berking, C.; Betz-Stablein, B.; Blum, A.; et al. Skin Cancer Classification via Convolutional Neural Networks: Systematic Review of Studies Involving Human Experts. Eur. J. Cancer 2021, 156, 202–216. [Google Scholar] [CrossRef]
- Tschandl, P.; Rosendahl, C.; Akay, B.N.; Argenziano, G.; Blum, A.; Braun, R.P.; Cabo, H.; Gourhant, J.-Y.; Kreusch, J.; Lallas, A.; et al. Expert-Level Diagnosis of Nonpigmented Skin Cancer by Combined Convolutional Neural Networks. JAMA Derm. 2019, 155, 58. [Google Scholar] [CrossRef]
- Tschandl, P.; Rinner, C.; Apalla, Z.; Argenziano, G.; Codella, N.; Halpern, A.; Janda, M.; Lallas, A.; Longo, C.; Malvehy, J.; et al. Human–Computer Collaboration for Skin Cancer Recognition. Nat. Med. 2020, 26, 1229–1234. [Google Scholar] [CrossRef]
- Pehamberger, H.; Steiner, A.; Wolff, K. In Vivo Epiluminescence Microscopy of Pigmented Skin Lesions. I. Pattern Analysis of Pigmented Skin Lesions. J. Am. Acad. Dermatol. 1987, 17, 571–583. [Google Scholar] [CrossRef]
- Jiang, A.; Jefferson, I.S.; Robinson, S.K.; Griffin, D.; Adams, W.; Speiser, J.; Winterfield, L.; Peterson, A.; Tung-Hahn, E.; Lee, K.; et al. Skin Cancer Discovery during Total Body Skin Examinations. Int. J. Women’s Dermatol. 2021, 7, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.A.; Saraiya, M.; Thompson, T.D.; King, S.C.; Guy, G.P. Total Body Skin Examination for Skin Cancer Screening among U.S. Adults from 2000 to 2010. Prev. Med. 2014, 61, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Izquierdo, J.A.; Molina-López, I.; Rodríguez-Lomba, E.; Marquez-Rodas, I.; Suarez-Fernandez, R.; Lazaro-Ochaita, P. Who Detects Melanoma? Impact of Detection Patterns on Characteristics and Prognosis of Patients with Melanoma. J. Am. Acad. Dermatol. 2016, 75, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Faldetta, C.; Kaleci, S.; Chester, J.; Ruini, C.; Ciardo, S.; Manfredini, M.; Guida, S.; Chello, C.; Cantisani, C.; Young, J.N.; et al. Melanoma Clinicopathological Groups Characterized and Compared with Dermoscopy and Reflectance Confocal Microscopy. J. Am. Acad. Dermatol. 2024, 90, 309–318. [Google Scholar] [CrossRef]
- De, A.; Sarda, A.; Gupta, S.; Das, S. Use of Artificial Intelligence in Dermatology. Indian J Derm. 2020, 65, 352. [Google Scholar] [CrossRef]
- Du-Harpur, X.; Watt, F.M.; Luscombe, N.M.; Lynch, M.D. What Is AI? Applications of Artificial Intelligence to Dermatology. Br. J. Derm. 2020, 183, 423–430. [Google Scholar] [CrossRef]
- De Guzman, L.C.; Maglaque, R.P.C.; Torres, V.M.B.; Zapido, S.P.A.; Cordel, M.O. Design and Evaluation of a Multi-Model, Multi-Level Artificial Neural Network for Eczema Skin Lesion Detection. In Proceedings of the 2015 3rd International Conference on Artificial Intelligence, Modelling and Simulation (AIMS), Kota Kinabalu, Malaysia, 2–4 December 2015; pp. 42–47. [Google Scholar]
- Aksoy, S.; Demircioglu, P.; Bogrekci, I. Enhancing Melanoma Diagnosis with Advanced Deep Learning Models Focusing on Vision Transformer, Swin Transformer, and ConvNeXt. Dermatopathology 2024, 11, 239–252. [Google Scholar] [CrossRef]
- Young, A.T.; Xiong, M.; Pfau, J.; Keiser, M.J.; Wei, M.L. Artificial Intelligence in Dermatology: A Primer. J. Investig. Dermatol. 2020, 140, 1504–1512. [Google Scholar] [CrossRef]
- Anzelc, M. Can Artificial Intelligence Technology Replace Human Scribes? Cutis 2021, 108, 310–311. [Google Scholar] [CrossRef]
- Pala, P.; Bergler-Czop, B.S.; Gwiżdż, J. Teledermatology: Idea, Benefits and Risks of Modern Age—a Systematic Review Based on Melanoma. Adv. Dermatol. Allergol. /Postępy Dermatol. I Alergol. 2020, 37, 159–167. [Google Scholar] [CrossRef]
- Rinkunas, S. This Terrifying App Shows You What Not Using Sunscreen Will Do to Your Face. 2018. Available online: https://www.vice.com/en/article/paw3mb/facial-aging-app-sunface-uv-skin-damage (accessed on 31 August 2024).
- Chan, S.; Reddy, V.; Myers, B.; Thibodeaux, Q.; Brownstone, N.; Liao, W. Machine Learning in Dermatology: Current Applications, Opportunities, and Limitations. Dermatol. Ther. 2020, 10, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Spatharou, A.; Hieronimus, S.; Jenkins, J. Transforming Healthcare with AI: The Impact on the Workforce and Organizations. 2020. Available online: https://www.mckinsey.com/industries/healthcare/our-insights/transforming-healthcare-with-ai#/ (accessed on 31 August 2024).
- Codella, N.; Rotemberg, V.; Tschandl, P.; Celebi, M.E.; Dusza, S.; Gutman, D.; Helba, B.; Kalloo, A.; Liopyris, K.; Marchetti, M.; et al. Skin Lesion Analysis Toward Melanoma Detection 2018: A Challenge Hosted by the International Skin Imaging Collaboration (ISIC). arXiv 2019, arXiv:1902.03368. [Google Scholar] [CrossRef]
- Codella, N.C.F.; Gutman, D.; Celebi, M.E.; Helba, B.; Marchetti, M.A.; Dusza, S.W.; Kalloo, A.; Liopyris, K.; Mishra, N.; Kittler, H.; et al. Skin Lesion Analysis toward Melanoma Detection: A Challenge at the 2017 International Symposium on Biomedical Imaging (ISBI), Hosted by the International Skin Imaging Collaboration (ISIC). In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; pp. 168–172. [Google Scholar]
- Gutman, D.; Codella, N.C.F.; Celebi, E.; Helba, B.; Marchetti, M.; Mishra, N.; Halpern, A. Skin Lesion Analysis toward Melanoma Detection: A Challenge at the International Symposium on Biomedical Imaging (ISBI) 2016, Hosted by the International Skin Imaging Collaboration (ISIC). arXiv 2016, arXiv:1605.01397. [Google Scholar] [CrossRef]
- Combalia, M.; Codella, N.; Rotemberg, V.; Carrera, C.; Dusza, S.; Gutman, D.; Helba, B.; Kittler, H.; Kurtansky, N.R.; Liopyris, K.; et al. Validation of Artificial Intelligence Prediction Models for Skin Cancer Diagnosis Using Dermoscopy Images: The 2019 International Skin Imaging Collaboration Grand Challenge. Lancet Digit. Health 2022, 4, e330–e339. [Google Scholar] [CrossRef] [PubMed]
- Senan, E.M.; Jadhav, M.E.; Kadam, A. Classification of PH2 Images for Early Detection of Skin Diseases. In Proceedings of the 2021 6th International Conference for Convergence in Technology (I2CT), Maharashtra, India, 2–4 April 2021; pp. 1–7. [Google Scholar]
- Latha, M.; Manjula, G.; Raghavendra, Y.M.; Keerthi Kumar, M.; Rashmi, H.C. Enhancing Skin Cancer Classification on the PH2 Dataset Through Transfer Learning Technique. Int. Res. J. Adv. Eng. Hub (IRJAEH) 2024, 2, 500–507. [Google Scholar] [CrossRef]
- Que, S.K.T.; Fraga-Braghiroli, N.; Grant-Kels, J.M.; Rabinovitz, H.S.; Oliviero, M.; Scope, A. Through the Looking Glass: Basics and Principles of Reflectance Confocal Microscopy. J. Am. Acad. Dermatol. 2015, 73, 276–284. [Google Scholar] [CrossRef]
- Massone, C.; Brunasso, A.M.G.; Campbell, T.M.; Soyer, H.P. Mobile Teledermoscopy—Melanoma Diagnosis by One Click? Semin. Cutan. Med. Surg. 2009, 28, 203–205. [Google Scholar] [CrossRef]
- Giotis, I.; Molders, N.; Land, S.; Biehl, M.; Jonkman, M.F.; Petkov, N. MED-NODE: A Computer-Assisted Melanoma Diagnosis System Using Non-Dermoscopic Images. Expert Syst. Appl. 2015, 42, 6578–6585. [Google Scholar] [CrossRef]
- DermIS Dermatology Information System. 2019. Available online: https://www.dermis.net/dermisroot/en/home/index.htm (accessed on 31 August 2024).
- Ashraf, R.; Afzal, S.; Rehman, A.U.; Gul, S.; Baber, J.; Bakhtyar, M.; Mehmood, I.; Song, O.-Y.; Maqsood, M. Region-of-Interest Based Transfer Learning Assisted Framework for Skin Cancer Detection. IEEE Access 2020, 8, 147858–147871. [Google Scholar] [CrossRef]
- Tschandl, P.; Rosendahl, C.; Kittler, H. The HAM10000 Dataset, a Large Collection of Multi-Source Dermatoscopic Images of Common Pigmented Skin Lesions. Sci. Data 2018, 5, 180161. [Google Scholar] [CrossRef]
- Faes, L.; Wagner, S.K.; Fu, D.J.; Liu, X.; Korot, E.; Ledsam, J.R.; Back, T.; Chopra, R.; Pontikos, N.; Kern, C.; et al. Feasibility of Automated Deep Learning Design for Medical Image Classification by Healthcare Professionals with Limited Coding Experience. bioRxiv 2019, 650366. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3402015 (accessed on 31 August 2024). [CrossRef]
- Dermatologist-Ai. Available online: https://github.com/udacity/dermatologist-ai (accessed on 31 August 2024).
- Derm7pt. Available online: https://github.com/jeremykawahara/derm7pt (accessed on 31 August 2024).
- MedAGI. Available online: https://github.com/JoshuaChou2018/MedAGI (accessed on 31 August 2024).
- Dermatology. Available online: https://github.com/datasets/dermatology (accessed on 31 August 2024).
- Deepskin. Available online: https://github.com/Nico-Curti/Deepskin (accessed on 31 August 2024).
- SkinGPT-4. Available online: https://github.com/JoshuaChou2018/SkinGPT-4 (accessed on 31 August 2024).
| Applications | AI Model | Function | Advantages | Refs. |
|---|---|---|---|---|
| RCM | Deep Learning Models | Automated detection and analysis of melanoma features. | Non-invasive, cellular-level imaging. | [49] |
| Skin Disease Identification | CNNs | Classifies various skin diseases based on RCM images. | Increases diagnostic accuracy; reduces subjectivity. | [2] |
| Basal Cell Carcinoma (BCC) Detection | CNNs | Automatically detects BCC in RCM images | Achieved high specificity; reduced number of biopsies needed. | [22] |
| Skin Cancer Analysis | CNNs | Analyzes images to detect skin cancer, including melanoma. | High sensitivity (95%) and specificity (82.5%) compared to dermatologists. | [41] |
| Ulcer Treatment | CNNs | Measures wound boundaries to assess ulcer impacts. | Accurate wound assessment for better treatment planning. | [50,51] |
| Eczema Diagnosis | Artificial Neural Networks (ANNs) | Multi-model, multi-level architecture for detecting eczema by analyzing patient data and classifying skin lesions. | Higher confidence in diagnosis and personalized treatment recommendations. | [52] |
| F65Melanoma Classification | ConvNeXt, ViT Base-16, and Swin V2 S | Classifies benign and malignant melanoma using dermoscopic images. | Achieved highest diagnostic accuracy among tested models. | [53] |
| Personalized Treatment Planning | Deep Neural Network Algorithm | Distinguishes between different skin diseases and suggests treatments. | Improved accuracy in diagnosis and treatment recommendations, including rare skin conditions. | [52] |
| General Dermatological Imaging | CNNs | Utilizes imaging to analyze various skin conditions like psoriasis and ulcers. | Enhanced diagnostic accuracy and efficiency. | [51,54] |
| Teledermatology | Various AI Models | Allows remote analysis of skin disorders through imaging. | Increased accessibility to dermatological care, especially for remote or underserved populations. | [55,56] |
| Appointment and Case Management | Various AI Models | Automates administrative tasks such as scheduling appointments, managing case files, and generating referral letters. | Reduces workload for healthcare professionals, increasing efficiency and patient throughput. | [55,56] |
| Patient–Physician Interaction | AI Programs (e.g., Hello Rache) | Analyzes and transcribes patient–physician interactions during appointments. | Saves time for healthcare professionals by automating documentation tasks. | [55] |
| Public Health and Education | Mobile Applications (e.g., Sunface) | Assesses user’s skin to recommend skincare products and daily reminders for sunscreen application. | Improves public health through personalized skincare advice and preventative measures. | [57] |
| Research and Simulation | Various AI Models | Uses AI to simulate testing for research purposes. | More effective research with lower costs and higher efficiency. | [58] |
| General Dermatological Practice | Various AI Models | Enhances practice efficiency by reducing errors, personalizing care, and improving diagnostic turnaround times. | Better patient outcomes and streamlined care processes. | [58,59] |
| Dataset Name | # of Images | Lesion Types |
|---|---|---|
| ISIC (International Skin Imaging Collaboration) Dataset | 25,000+ | Melanoma, nevi, and basal cell carcinoma |
| HAM10000 (The Human against Machine with 10,000) | 10,015 | Melanoma and nevi |
| PH2 Dataset | 200 | Melanoma, nevi, and seborrheic keratosis |
| DermQuest Dataset | 1500 | Melanoma and basal cell carcinoma |
| Repository Name | Description | Access/Link |
|---|---|---|
| dermatologist-ai | A machine learning project focused on building an AI model to classify skin lesions. | dermatologist-ai [73] |
| derm7pt | Tools and resources for the Derm7PT assessment—a checklist for evaluating skin lesions. | derm7pt [74] |
| MedAGI | Integrating AI with medical applications, particularly in dermatology, including datasets and algorithms. | MedAGI [75] |
| dermatology | A collection of datasets related to dermatology for the research and analysis of skin diseases. | dermatology [76] |
| DeepSkin | Deep learning models and techniques for skin lesion classification. | Deepskin [77] |
| SkinGPT-4 | AI model tailored for dermatology using GPT-4 for skin condition analysis and diagnosis. | SkinGPT-4 [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksoy, S.; Demircioglu, P.; Bogrekci, I. Advanced Artificial Intelligence Techniques for Comprehensive Dermatological Image Analysis and Diagnosis. Dermato 2024, 4, 173-186. https://doi.org/10.3390/dermato4040015
Aksoy S, Demircioglu P, Bogrekci I. Advanced Artificial Intelligence Techniques for Comprehensive Dermatological Image Analysis and Diagnosis. Dermato. 2024; 4(4):173-186. https://doi.org/10.3390/dermato4040015
Chicago/Turabian StyleAksoy, Serra, Pinar Demircioglu, and Ismail Bogrekci. 2024. "Advanced Artificial Intelligence Techniques for Comprehensive Dermatological Image Analysis and Diagnosis" Dermato 4, no. 4: 173-186. https://doi.org/10.3390/dermato4040015
APA StyleAksoy, S., Demircioglu, P., & Bogrekci, I. (2024). Advanced Artificial Intelligence Techniques for Comprehensive Dermatological Image Analysis and Diagnosis. Dermato, 4(4), 173-186. https://doi.org/10.3390/dermato4040015








