Abstract
This study aims to evaluate the antinociceptive effect of the C. rhamnifolioides leaf essential oil (OEFC) and the β-cyclodextrin inclusion complex (COEFC) and investigate the pain signaling pathways involved in the antinociceptive response. The effects of the OEFC and COEFC on the central nervous system (CNS) were determined by open field and rota-rod assays, and the antinociceptive effect was evaluated via the acetic acid-induced abdominal contortions, formalin, and hot plate models. Swiss (Mus musculus) male mice (20–30 g) were used in both trials. The OEFC (200 mg/kg/v.o-orally) and COEFC (83.5 mg/kg/v.o.) did not present alterations in the CNS. The OEFC (25, 50, 100, and 200 mg/kg/vo.) and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) demonstrated antinociceptive effects in the abdominal contortions, formalin, and hot plate tests. The OEFC (25 mg/kg/v.o.) and COEFC (8.35 mg/kg/v.o.) doses showed that the antinociceptive effect involves the activation of the opioid, cholinergic, and vanilloid systems, as well as the L-arginine/NO and α-2 adrenergic receptor pathways. The antinociceptive potential the OEFC and COEFC demonstrate possible alternatives for the therapy of pain. However, the COEFC presented more significant effects at lower doses than the isolated OEFC, where this action may be justified by the properties and advantages of the complexation.
1. Introduction
The Croton rhamnifolioides species belonging to the Croton genus and Euphorbiaceae family is known in popular medicine as “quebra-faca” or “caatinga-branca”, where the first name refers to the stiffness of its trunk, while the second refers to its aroma and stem color. This species is used for the treatment of stomachaches, gastric upset, vomiting, bloody diarrhea, and to reduce fever [1]. Chemically, terpenes predominate in the C. rhamnifolioides roots and leaves [2], with the monoterpene 1,8-cineole being the major constituent of the leaf essential oil [3]. Studies addressing the biological activities the C. rhamnifolioides essential oil are scarce [4]; however, some effects have already been described in the literature such as anti-inflammatory [3], gastroprotective [5], and larvicide [4].
Essential oils complexed with cyclodextrins are considered as important in medicinal chemistry due to changes in physicochemical properties, which allow the administration of lower doses and, consequently, a reduction in side effects [6]. Cyclodextrins possess unique physicochemical properties, including the ability to solubilize in aqueous media and simultaneously encapsulate hydrophobic molecules within their cavity [7], facilitating the transfer of hydrophobic guest molecules from a solution to lipophilic cell membranes, thus promoting absorption [8], in addition to having the ability to improve the bioavailability of some substances [9], which is associated with solubility, dissolution, and permeability that is promoted indirectly to the guest drug [10].
With respect to the antinociceptive effect of substances, the term nociception (in Latin, nocere, “to hurt”) refers to a stimulated sensory process, while pain refers to the perception of a describable sensation, which may range from irritative, painful, persistent, pulsatile, or intolerable sensations. Therefore, it is important to emphasize that nociception and pain are distinct in aspects, such that a person with tissue injuries may or may not exhibit the behavior of pain, whereas nociception may trigger pain, apparent or otherwise. In animal nociceptive experimental models, animals are exposed to a nociceptive stimulus developing behavioral, motor, and physiological responses, with which the nociceptive response is evaluated since animals do not have the ability to verbally express pain, which is a characteristic attributed only to humans [11].
Pain and inflammation are important in the body’s physiology, acting as a signal for the need to repair tissue damage, since changes associated with the inflammatory process usually result in peripheral sensitization [12]. Pain is a frequent manifestation in patients seeking different treatments. Thus, a variety of drugs are used for pain therapy, which may or may not be associated with the inflammatory process [13]. In this sense, several studies involving medicinal plants are becoming fundamental in the discovery of new molecules that may be associated with other pain signaling pathways with fewer adverse effects [14].
The present study suggests that essential oils complexed with cyclodextrins can be considered as a new therapeutic option in treatments involving painful processes and in the development of new antinociceptive agents, due to an improving bioavailability associated with solubility, dissolution, and permeability, as well as the reduction of doses and the side effects. Thus, in view of the advantages of complexing substances with cybclodextrins and their use in popular medicine, we take the perspective of evaluating the antinociceptive effect of the Croton rhamnifolioides Pax. & K. Hoffm leaf essential oil and the inclusion complex (OEFC/β-CD) in nociceptive animal models.
2. Materials and Methods
2.1. Chemical Substances
The essential oil was extracted from fresh Croton rhamnifolioides Pax. & K. Hoffm leaves (OEFC) using a hydrodistillation system [3], and the β-cyclodextrin OEFC inclusion complex (COEFC) was obtained using the coevaporation technique [15]. The physicochemical characterization the COEFC occurred through differential exploratory calorimetry, thermogravimetry/derived thermogravimetry, scanning electron microscopy, and Karl Fischer techniques [16]. Other substances such as β-cyclodextrin, acetic acid, phenylephrine, Prazosine, Yohimbine, Clonidine, Acetylcholine, PCPA, L-Arginine, L-NOARG, Caffeine, Glutamate, Capsaicin, Ruthenium Red, Diazepam, and Indomethacin were purchased from Sigma-Aldrich Corporation® (St. Louis, MO, USA), while atropine, ascorbic acid, formaldehyde, morphine, naloxone, and haloperidol were obtained from Fluka, Dinâmica, Vetec, Hypolabor, Hypolabor, and Crystal, respectively.
2.2. Animals
Swiss (Mus musculus) male mice with body mass (20–30 g) were used, kept in polypropylene cages, and maintained in an environment temperature of 23 ± 2 °C, using a light/dark cycle of 12 h and having free access to potable water and rodent-specific food (Presence, Purina®); the animals were fasted (8–10 h) of solids before testing. All the experimental procedures followed the norms of animal use, with the research being submitted and approved by the Animal Research Ethics Committee of the Regional University of Cariri (CEUA/URCA—n 43/2015.1).
2.3. Assays
The following protocols were performed to evaluate the antinociceptive effect: acetic acid-induced abdominal contortions, 2.5% formalin test, and hot plate. The animals were divided into groups (n = 6): negative control (H2O-0.1 mL/10 g/v.o.-orally), OEFC (25, 50, 100 and 200 mg/kg/v.o.), COEFC (8.35; 41.75 and 83.5 mg/kg/v.o.), the corresponding amount of the complex (oil + CD); and positive controls: indomethacin (10 mg/kg/s.c.) or morphine (5 mg/kg/s.c.). Pain signaling pathways (opioid, cholinergic, α1 and α2 adrenergic, serotonergic, nitric oxide, adenosinergic, dopaminergic, glutamatergic, and vanilloid) involved in the antinociceptive response of the OEFC (25 mg/kg/v.o.) and COEFC (8.35 mg/kg/v.o.) were investigated using the lowest effective dose defined in the previous protocols.
2.4. Effect of the OEFC and COEFC on the Central Nervous System
2.4.1. Open Field
Swiss mice (n = 6) were treated as follows: H2O control (0.1 mL/10 g/v.o.), diazepam (5 mg/kg/i.p-intraperitoneally), OEFC (200 mg/kg/v.o.), and COEFC (83.5 mg/kg/v.o.) diluted in water and Tween 80. After 1 h (v.o.) or 30 min (i.p.), the animals were placed individually in an open field for a period of 5 min, where their horizontal exploration (number of crossings), grooming, and rearing behaviors were recorded [17].
2.4.2. Rota-Rod
The animals (Swiss mice/n = 6) were selected and pre-trained with up to 3 sessions (1 min) 24 h before treatment. The selected animals were divided into groups and treated, respectively: H2O control (0.1 mL/10 g/v.o.), diazepam (5 mg/kg/i.p.), OEFC (200 mg/kg/v.o.), and COEFC (83.5 mg/kg/v.o.) diluted in water and Tween 80, where after 1 h (v.o.) or 30 min (i.p.), the animals were placed on the rota rod for 1 min (16 rpm), and the number of falls was recorded [18].
2.5. Antinociceptive Effect of the OEFC and COEFC
2.5.1. Acetic Acid-Induced Abdominal Contortions
The animals (Swiss mice/n = 6) were treated according to the groups: H2O control (0.1 mL/10 g/v.o.), indomethacin (10 mg/kg/s.c.), OEFC (25, 50, 100 and 200 mg/kg/v.o.), and COEFC (8.35; 41.75 and 83.5 mg/kg/v.o.) diluted in water and Tween 80. After 1 h (v.o.) or 30 min (s.c.) from the treatments, the animals received PA glacial acetic acid (0.6%/0.1 mL/10 g/i.p.) diluted in injection water. Following acetic acid administration, the animals were placed under individual transparent glass funnels for 30 min, and the number of abdominal contortions was quantified cumulatively and characterized by the contraction and rotation of the abdomen, followed by the extension of one or both hind paws [19].
2.5.2. Formalin Test (2.5%)
Swiss mice (n = 6) were treated: H2O control (0.1 mL/10 g/v.o.), morphine (5 mg/kg/s.c.), indomethacin (10 mg/kg/s.c.), OEFC (25, 50 100 and 200 mg/kg/v.o.), and COEFC (8.35, 41.75 and 83.5 mg/kg/v.o.) diluted in water and Tween 80. After 1 h (v.o.) or 30 min (s.c.), the animals were injected with 20 μL of formalin (2.5%) in the right paw (sub plantar space) and placed individually soon afterwards under an inverted glass funnel, next to a mirror for ease of observation. The time (seconds) in which the animal licked, continued licking, or bit the injected paw (“licking-time”) during the first phase, attributed to the neurogenic phase (0–5 min.), was recorded in addition to the second phase, which was characterized as the inflammatory phase (15–30 min) [20].
2.5.3. Hot Plate
Swiss mice (n = 6) were individually placed on a hot temperature plate (52–54 °C ± 0.5 °C). After two baseline values were obtained 24 h and 30 min before the test, the mice were treated according to the groups: H2O control (0.1 mL/10 g/v.o.), morphine (5 mg/kg/s.c.), OEFC (25, 50, 100, and 200 mg/kg/v.o.), and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) diluted in water and Tween 80. Then, their response was evaluated after 30, 60, 120, and 180 min following treatment administration with the maximum contact time of the animal with the hot plate being kept at 15 s (basal cut-off time) and 30 s (test cut-off time) to prevent foot injuries. The nociceptive response was characterized by agitation of their hind paws, licking their paws, raising their paw, or jumping from the plate [21]. To compare the effects over time, the percentage of each group was calculated using the average effect observed at all times.
2.6. Pain Signaling Pathways Involved in the OEFC and COEFC Antinociceptive Response (Opioid, Cholinergic, α1 and α2 Adrenergic, Serotonergic, Nitric Oxide, Adenosinergic, Dopaminergic, Glutamatergic, and Vanilloid)
2.6.1. Opioid System Participation
The animals (Swiss mice/n = 6) were divided into 8 groups, such that the first 4 groups were treated with H2O control (0.1 mL/10 g/v.o.), morphine–opioid agonist (5 mg/kg/s.c.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.) diluted in water and Tween 80, respectively, while the remaining 4 groups were given naloxone–opioid antagonist (4 mg/kg i.p.) 15 min prior to treatment. After 1 h (v.o.) or 30 min (s.c.) of treatments, the animals were evaluated with the 2.5% formalin-induced nociception test (first phase: 0–5 min.) [19].
2.6.2. Cholinergic System Participation
The animals (Swiss mice/n = 6) were divided into 8 groups, where the first 4 groups were treated with H2O control (0.1 mL/10 g/v.o.), acetylcholine (cholinergic agonist; 1 mg/kg/i.p.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.) diluted in water and Tween 80, while the other 4 groups were treated with atropine (non-selective cholinergic antagonist; 1 mg/kg/i.p.) 15 min before treatment with the OEFC or COEFC. Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were evaluated with respect to the 2.5% formalin test (first phase: 0–5 min) [22].
2.6.3. α-1. Adrenergic Receptor Participation
Swiss mice (n = 6) were divided into 8 groups, where the first 4 groups were treated with H2O control (0.1 mL/10 g/v.o.), phenylephrine (α1 agonist; 10 mg/kg/i.p.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.) diluted with water and Tween 80, while the other 4 groups were treated with Prazosin (α1 antagonist; 0.15mg/kg/i.p.) 15 min before treatment with the OEFC or COEFC. Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were evaluated with respect to the 2.5% formalin test (first phase: 0–5 min.) [23].
2.6.4. α-2. Adrenergic Receptor Participation
The animals (Swiss mice/n = 6) were divided into 8 groups, where the first 4 groups were treated with H2O control (0.1 mL/10 g/v.o.), clonidine (α2 agonist, 0.1 mg/kg/i.p.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.), while the other 4 groups were treated with yohimbine (α2 antagonist; 0.15 mg/kg/i.p.) 15 min before treatment with the OEFC or COEFC. Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were evaluated with respect to the 2.5% formalin test (first phase: 0–5 min.) [23].
2.6.5. L-arginine/Nitric Oxide/cGMP Pathway Participation
Swiss mice (n = 6) were divided into 8 groups, where the first 4 groups were treated with H2O control (0.1 mL/10 g/v.o.), L-NOARG (nitric oxide synthase-NOS inhibitor; 75 mg/kg, i.p.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.), while the other 4 groups were treated with L-Arginine (NOS substrate; 600 mg/kg/i.p.), 15 min before treatment with the OEFC or COEFC. Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were evaluated with respect to the 2.5% formalin test (first phase: 0–5 min) [24].
2.6.6. Participation of the Vanilloid System
The animals (Swiss mice/n = 6) were divided into 4 groups that were treated with H2O control (0.1 mL/10 g/v.o.), Ruthenium red (non-selective TRP antagonist; 3 mg/kg, i.p.) [25], OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o). Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were analyzed for 5 min with respect to the nociception induced by the intraplantar injection of 20 µL of capsaicin (TRPV1 receptor agonist) at 5.2 nmol/paw [26], where the time(s) in which the animal spent licking its paw was considered to suggest pain.
2.6.7. Participation of Serotonergic Pathways
The animals were divided into 6 groups (Swiss mice/n = 6), where the first 3 were treated with p-chlorophenylalanine–serotonin receptor antagonist (PCPA-100 mg/kg i.p., once daily/4 consecutive days), while the other 3 were treated with H2O control (0.1 mL/10 g/v.o./1 time per day/4 consecutive days). Then, 30 min after the last PCPA and control treatment, the animals received H2O control (0.1 mL/10 g/v.o.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.). Then, 1 h after the groups were treated, the animals were evaluated with respect to the 2.5% formalin test (first phase: 0–5 min.) [27].
2.6.8. Participation of the Dopaminergic System
The animals (Swiss mice/n = 6) were divided into 6 groups: the first 4 groups were treated with H2O control (0.1 mL/10 g/v.o.), haloperidol (non-selective dopamine receptor antagonist; 2 mg/kg i.p.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.), while the other 2 groups were treated with haloperidol 15 min before treatment with the OEFC or COEFC. Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were evaluated with respect to the 2.5% formalin test (first phase: 0–5 min) [28].
2.6.9. Participation of the Adenosinergic System
Swiss mice (n = 6) were divided into 6 groups; the first 4 groups were treated with H2O control (0.1 mL/10 g/v.o.), caffeine (10 mg/kg/i.p.), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.), while the other 2 groups were treated with caffeine 15 min prior to treatment with the OEFC or COEFC. Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were evaluated with respect to the 2.5% formalin test (first phase: 0–5 min) [29].
2.6.10. Participation of the Glutamatergic System
The animals (Swiss mice/n = 6) were divided into 4 groups treated with H2O control (0.1 mL/10 g/v.o.), ascorbic acid (NMDA receptor antagonist, 100 mg/kg ip), OEFC (25 mg/kg/v.o.), and COEFC (8.35 mg/kg/v.o.). Then, 1 h (v.o.) or 30 min (i.p.) after treatment, the animals were analyzed for 15 min with respect to the nociception induced by the intraplantar injection of 20 μL of buffered glutamate at 20 μmoL/paw, where the time(s) the animal spent licking its paw was considered as a parameter suggestive of pain [30].
2.7. Data Expression and Statistical Analysis
The results are presented as the mean ± standard error of the mean (S.E.M), evaluated by a one-way and/or two-way analysis of variance (ANOVA), using Dunnett’s and Tukey’s multiple comparison tests (when necessary). The calculations were performed using the GraphPad Prism statistical software (version 6.0), according to the values obtained in the tests. For all analyzes, p < 0.05 was considered significant.
3. Results
The chemical fingerprint of the essential oil [5] and physicochemical characterization of the complex [16] were previously published. The essential oil was analyzed by gas-phase chromatography coupled to mass spectrometry (GC/MS) and the complexations were characterized by different physical methods as differential scanning calorimetry (DSC), TG/DTG (thermogravimetry/derivative thermogravimetry), Karl Fischer method, and electronic microscope.
3.1. Effect of the OEFC and COEFC on the Central Nervous System (CNS)
3.1.1. Open Field
Oral treatment with the OEFC (200 mg/kg) and COEFC (83.5 mg/kg) did not change the number of crossings (horizontal explorations) with percentages of 97.39% and 87.33%, respectively (Figure 1A). With respect to grooming (self-cleaning behavior), the percentages observed were 85% and 95.14% (Figure 1B), and for rearing (vertical exploratory behavior), these were 96.52% and 99.19% (Figure 1C), respectively, when compared to the control group. However, diazepam (5 mg/kg/i.p.) showed a significant reduction of 43.95% in the number of crossings, a 95.42% reduction in grooming and 98.19% reduction in rearing when compared to the control group.
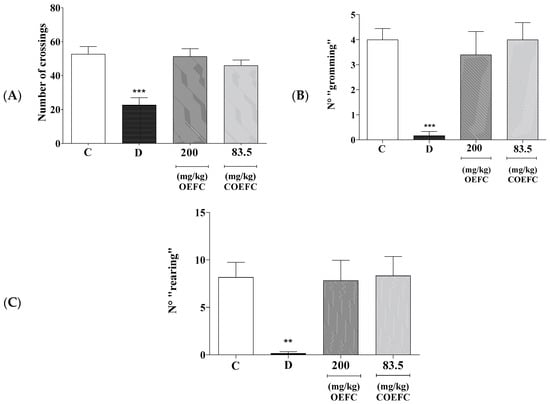
Figure 1.
Effect of the C. rhamnifolioides leaf essential oil (OEFC, 200 mg/kg/v.o.) and β-cyclodextrin inclusion complex (COEFC, 83.5 mg/kg/v.o.) on the number of crossings (A), grooming (B), and rearing (C) in the open field test. The effects of the OEFC (200 mg/kg/v.o.) and COEFC (83.5 mg/kg/v.o.) on the number of crossings (A), grooming (B), and rearing (C) in the open field test are outlined below. Groups: control-C (0.1 mL/10g/v.o.), Diazepam-D (5 mg/kg/v.o.), OEFC (200 mg/kg/v.o.) and COEFC (83.5 mg/kg/v.o). Values represent the mean ± S.E.M. (standard error of the mean) for groups of 6 animals with: ** p < 0.01; *** p < 0.001 when compared to the control group; statistical analysis: ANOVA followed by Dunnett’s Test with multiple comparisons.
3.1.2. Rota-Rod
Oral treatment with the OEFC (200 mg/kg) and COEFC (83.5 mg/kg) did not present significant differences regarding the number of falls when compared to the control (Figure 2). Moreover, the slight myorelaxant effect of the OEFC and COEFC (8%) probably did not interfere with the motor capacity of the animals.
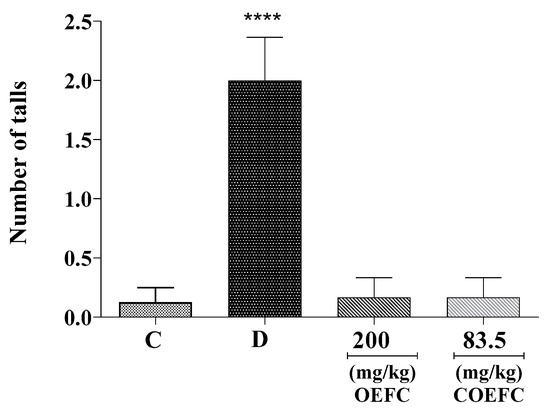
Figure 2.
Effect of the OEFC (200 mg/kg/v.o) and COEFC (83.5 mg/kg/v.o.) on the number of falls on the Rota-rod test. Effect of the OEFC (200 mg/kg/v.o) and COEFC (83.5 mg/kg/v.o.) on the number of falls on the Rota-rod test. Groups: control-C (0.1 mL/10 g/v.o.), diazepam-D (5 mg/kg/v.o.), OEFC (200 mg/kg/v.o.), and COEFC (83.5 mg/kg/v.o.). Values represent the mean ± S.E.M. (standard error of the mean) for groups of 6 animals, where **** p < 0.0001 when compared to the control group. Statistical analysis: ANOVA followed by Dunnett’s test with multiple comparisons.
3.2. Antinociceptive Effect of the OEFC and COEFC
3.2.1. Acetic Acid-Induced Abdominal Contortions
The OEFC at 25, 50, 100, and 200 mg/kg doses significantly reduced the number of abdominal contortions by 72.33, 68.13, 74.43, and 60.3%, respectively (Figure 3A), and the COEFC (8.35, 41.75, and 83.5 mg/kg) reduced the number of abdominal contortions by 90.1, 81.2, and 77.03% (Figure 3B) when compared to the control group. Indomethacin (10 mg/kg) (anti-inflammatory) presented a significant reduction of 85.40% (Figure 3A,B).
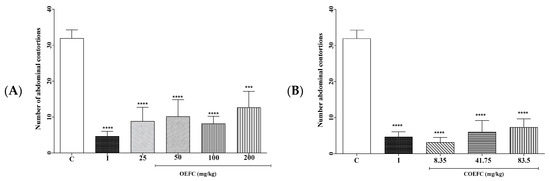
Figure 3.
Effect of the OEFC (25, 50, 100, and 200 mg/kg/v.o.) (A) and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) (B) on the number of acetic acid-induced abdominal contortions. Effect of the OEFC (25, 50, 100, and 200 mg/kg/v.o.) (A) and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) (B) on the number of acetic acid-induced abdominal contortions. Groups: control-C (0.1 mL/10 g/v.o.), indomethacin-I (10 mg/kg/s.c.), OEFC (25, 50, 100, and 200 mg/kg/v.o.) (A) and COEFC (8, 35, 41.75, and 83.5 mg/kg/v.o.) (B). Values represent the mean ± S.E.M. (standard error of the mean) for groups of 6 animals, where: *** p < 0.001; **** p < 0.0001 when compared to the control group; Statistical analysis: ANOVA followed by Dunnett’s Test with multiple comparisons.
3.2.2. Formalin Test (2.5%)
In the first phase (neurogenic/0–5 min), the OEFC (25, 50, 100, and 200 mg/kg/v.o.) significantly reduced the licking time of the formalin-injected paw by 59.1, 66.55, 69.55, and 66.86%, respectively (Figure 4A), when compared to the control, while the COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) reduced this by 67.45, 63.28, and 54.02%, respectively (Figure 4C). Indomethacin (10 mg/kg) and morphine (5 mg/kg), both administered subcutaneously, significantly reduced paw licking time by 43.57 and 91.34%, respectively (Figure 4A,C).
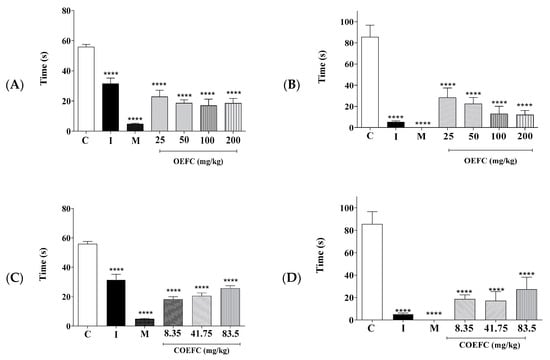
Figure 4.
Effect of the OEFC (25, 50, 100, and 200 mg/kg/v.o.) and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) on paw licking time in the formalin test in mice. First phase (A,C); second phase (B,D). The effects of the OEFC (25, 50, 100, and 200 mg/kg/v.o.) and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) on paw licking time in the formalin test in mice are outlined below. First phase (A,C); second phase (B,D). Groups: control-C (0.1 mL/10 g/v.o.), indomethacin-I (10 mg/kg/s.c.), morphine-M (5 mg/kg/s.c.), OEFC (25, 50, 100, and 200 mg/kg/v.o.) (A,B), and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) (C/D). Values represent the mean ± S.E.M. (standard error of the mean) for groups of 6 animals, where **** p < 0.0001 when compared to the control group; Statistical analysis: ANOVA followed by Dunnett’s Test with multiple comparisons.
With respect to the second phase (inflammatory/15–30 min), the OEFC (25, 50, 100, and 200 mg/kg/v.o.) produced a considerable reduction in licking time of 66.86, 73.68, 84.79, and 85.96%, respectively (Figure 4B), while the COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) reduced this by 78.16, 79.91, and 68.03%, respectively (Figure 4D). The positive controls, morphine (5 mg/kg) and indomethacin (10 mg/kg), both administered subcutaneously, were able to significantly decrease paw licking time by 100 and 93.96%, respectively (Figure 4B,D), when compared to the control.
3.2.3. Hot Plate
Treatment with the OEFC with the 25, 50, 100, and 200 mg/kg (v.o.) doses increased plate permanence time by 88.51, 47.65, 58.51, and 88.25%, respectively (Figure 5A), while the COEFC at 8.35, 41.75, and 83.5 mg/kg doses increased permanence time by 92.16, 68.27, and 79.5%, respectively (Figure 5B) when compared to the control group at the 30 to 180-min time intervals. Morphine (5 mg/kg/s.c.), used as a positive control, increased plate permanence time by 92.16% (Figure 5A,B).
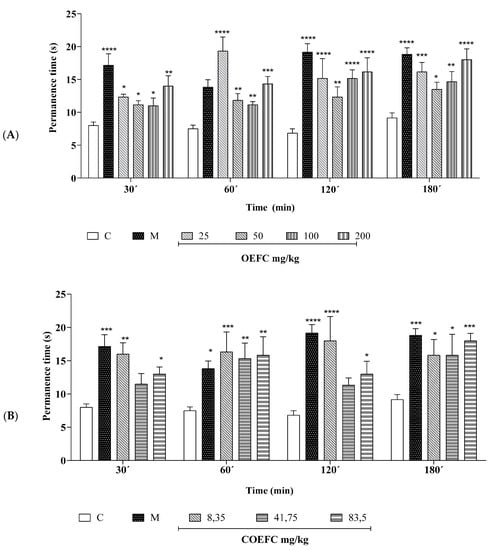
Figure 5.
Effect of the OEFC (25, 50, 100, and 200 mg/kg/v.o.) (A) and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) (B) on hot plate permanence time. The effects of the OEFC (25, 50, 100 and 200 mg/kg/v.o.) (A) and COEFC (8.35, 41.75 and 83.5 mg/kg/v.o.) (B) on Hot plate permanence time are outlined below. Groups: control-C (10 mg/kg/v.o.), morphine-M (6 mg/kg/s.c.), OEFC (25, 50, 100, and 200 mg/kg/v.o.) (A) and COEFC (8.35, 41.75, and 83.5 mg/kg/v.o.) (B). Values represent the mean ± S.E.M. (n = 6/group), where: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 when compared to the control group; Statistical analysis: ANOVA followed by Dunnett’s test with multiple comparisons.
3.3. Pain Signaling Pathways Involved in the OEFC and COEFC Antinociceptive Response (Opioid, Cholinergic, α1 and α2 Adrenergic, Serotonergic, Nitric Oxide, Adenosinergic, Dopaminergic, Glutamatergic and Vanilloid)
With respect to the participation of the opioid system, treatment with the OEFC (25 mg/kg/v.o.), COEFC (25 mg/kg/v.o.), and morphine (opioid agonist) significantly reduced the licking time of the formalin-injected paw compared to control by 60.3, 67.69, and 91.08%, respectively, demonstrating an antinociceptive action; moreover, when the animals were previously (15 min) treated with naloxone (opioid antagonist), the antinociceptive effects of the OEFC, COEFC, and morphine were reversed, proving this action involves the opioid system (Figure 6A).
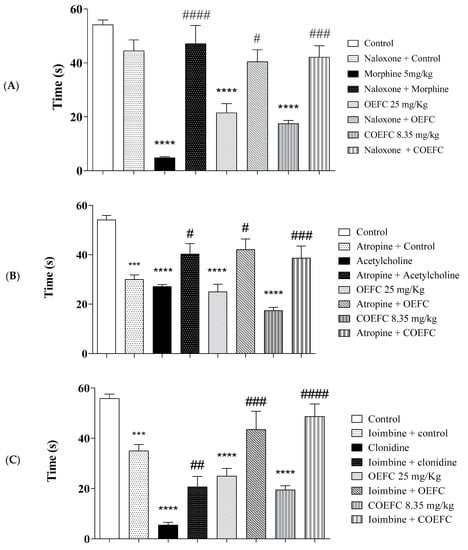
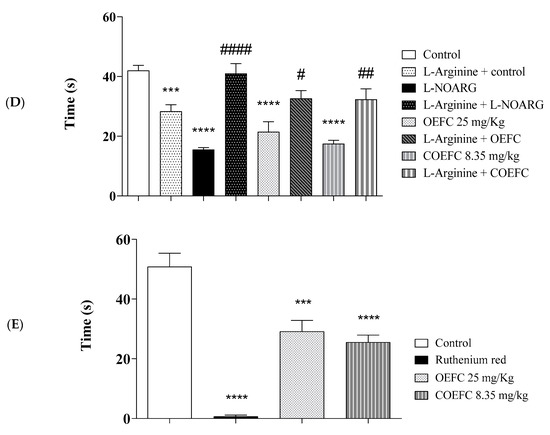
Figure 6.
Pain signaling pathways involved in the antinociceptive response of OEFC (25 mg/kg) and COEFC (8.35 mg/kg) (opioid, cholinergic, α2 adrenergic, nitric oxide, and vanilloid) (A–E). Involvement of the opioid, cholinergic, vanilloid, α-2 adrenergic receptor, and L-arginine/nitric oxide/cGMP pathway in the OEFC (25 mg/kg/v.o.) and COEFC (8.35 mg/kg/v.o.) antinociceptive activity in the formalin-induced paw nociception in mice. Values represent the mean ± S.E.M. (standard error of the mean) for groups of 6 animals, where * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 when compared to the control group; # p < 0.05, ## p < 0.01, ### p < 0.001, and #### p < 0.0001 when comparing the antagonist and agonist vs. agonist. Statistical analysis: ANOVA followed by Tukey´s test with multiple comparisons.
In the cholinergic system investigation, pretreatment with atropine (non-selective antagonist) 15 min before administration of the OEFC (25 mg/kg/v.o.), COEFC (25 mg/kg/v.o.), and acetylcholine (cholinergic agonist) reversed the antinociceptive action of the treated groups. The OEFC (25 mg/kg/v.o.), COEFC (25 mg/kg/v.o.), and acetylcholine presented a significant reduction of 49.75, 64.6, and 48.6%, respectively, in the formalin-injected paw licking time when compared to the control group (Figure 6B). The data presented suggest that the OEFC and COEFC may also act through a potential Gq/Gi protein-coupled muscarinic receptor agonist, since their antinociceptive action reverses in the presence of the antagonist.
Regarding the α-2 adrenergic receptor, pretreatment with yohimbine (α-2 antagonist) 15 min before administration of the OEFC (25 mg/kg/v.o.), COEFC (8.35 mg/kg/v.o.), and clonidine (α2 agonist) reversed their antinociceptive action. The OEFC, COEFC, and clonidine presented a significant reduction in formalin-induced paw licking time by 55.22, 65.07, and 90.14%, respectively, when compared to the control group (Figure 6C). Reversal of the antinociceptive action of the OEFC and COEFC promoted by their association with yohimbine demonstrates a similar action when yohimbine was associated with the α-2 adrenergic receptor agonist (clonidine), which suggests a possible OEFC and COEFC agonistic action on α2-adrenergic receptors.
In Figure 6D, the participation of the L-arginine/Nitric Oxide/cGMP pathway can be observed where pretreatment with L-arginine (NOS substrate) 15 min before OEFC (25 mg/kg/v.o.), COEFC (25 mg/kg/v.o.), and L-NOARG (nitric oxide synthase inhibitor, NOS) reversed the antinociceptive effect. The OEFC, COEFC, and L-NOARG reduced the formalin-induced paw licking time by 47.72, 60.22, and 64.77%, respectively, when compared to the control group. The antinociceptive effect of the OEFC and COEFC involves the participation of the L-arginine/nitric oxide/cGMP pathway, since the OEFC and COEFC had their antinociceptive activity reversed by the action of L-arginine, which is a substrate that promotes the release and production of nitric oxide and, consequently, the painful stimulus. In Figure 6E, treatment with the OEFC (25 mg/kg/v.o.), COEFC (25 mg/kg/v.o.), and ruthenium red (non-selective TRP antagonist) significantly reduced the capsaicin-induced paw licking time by 42.61, 49.83, and 98.70%, when compared to the control group, certifying their participation in the vanilloid system.
Regarding the participation of α-1 adrenergic receptors, treatment with the OEFC (25 mg/kg/v.o.), COEFC (25 mg/kg/v.o.), and phenylephrine (α1 agonist) promoted antinociceptive effects by significantly reducing the licking time of paws injected with 2.5% formalin in the first phase by 51.94, 66.48, and 98.65%, respectively, when compared to the control group. However, previous treatment with the α1-adrenergic receptor antagonist (Prazosine) did not reverse the antinociceptive effect of the OEFC and COEFC; however, this was effective against the action of phenylephrine. Thus, the antinociceptive effect of the OEFC and COEFC does not involve α-1 adrenergic receptors.
When investigating the involvement of serotonergic pathways, treatment with the OEFC (25 mg/kg/v.o.) and COEFC (25 mg/kg/v.o.) demonstrated an antinociceptive effect of 46.35 and 58.06%. However, their antinociceptive action was not reversed when associated with the serotonin synthesis inhibitor, PCPA (p-chlorophenylalanine), i.e., the antinociceptive effect of the OEFC and COEFC did not show participation of the serotonergic pathway.
As for the dopaminergic system, treatment with the OEFC (25 mg/kg/v.o.) and the COEFC (25 mg/kg/v.o.) significantly reduced the licking time of formalin-injected paws by 58.38 and 66.13%, respectively, compared to the control. However, haloperidol (a non-selective dopamine receptor antagonist) did not modify the antinociceptive effect of the OEFC and COEFC, suggesting that the antinociceptive action of both the OEFC and COEFC did not involve the dopaminergic system.
In the adenosinergic system investigation, treatment with the OEFC (25 mg/kg/v.o.) and COEFC (25 mg/kg/v.o.) significantly reduced the formalin-induced licking time, both by 67.69%, when compared to the control group. However, caffeine pretreatment did not reverse the antinociceptive activity exerted by the OEFC and COEFC, demonstrating that their antinociceptive effect does not involve the adenosinergic system. While for the glutamatergic system, treatment with the OEFC (25 mg/kg/v.o.) and COEFC (25 mg/kg/v.o.) did not significantly reduce the licking time of the glutamate-injected paw when compared to the control group, demonstrating there was no participation of this system. However, ascorbic acid (NMDA receptor antagonist) significantly decreased paw licking time by 58.78%, demonstrating an effective action.
4. Discussion
This study describes for the first time the antinociceptive effect of the Croton rhamnifolioides Pax. & K. Hoffm leaf essential oil and the inclusion complex (OEFC/β-CD), as well as the pain signaling pathways involved in their antinociceptive effect using animal models. The physical–chemical analysis showed effective complexation using the coevaporation method; these results corroborate with data just published [16].
In the open field model, treatment with the OEFC and COEFC did not cause changes in the number of crossings, self-grooming, and vertical exploratory behaviors, demonstrating that OEFC and COEFC do not promote a CNS depressant activity, since this action could directly influence the antinociceptive effect. In the rota-rod model, the OEFC and COEFC did not alter the motor coordination of the animals, with these results corroborating with the OEFC and COEFC action in the open field test, where neither of these altered the number of crossings, grooming, or rearing. Thus, the data suggest that the OEFC and COEFC do present a sedative effect, which could mask the nociceptive response. Diazepam significantly increased the number of falls when compared to the control group, demonstrating its effectiveness in altering the motor coordination of animals [31].
Regarding the central nervous system evaluation, the open field test allows the stimulating or depressing effect of a given substance to be evaluated. The natural tendency of the animal is to explore the new environment, despite the confrontation hypothesis of the fear provoked when facing a different environment [11,17]. The rota-rod test allows the specificity of nociceptive action to be evaluated, verifying if drug treatments cause motor incoordination, either by sedation or muscle relaxation, by recording the number of falls in an established period [11,19]. With respect to the Croton genus, the species Croton cordiifolius Bail.l and Croton urucurana complement the results of this study for not altering motor mobility and behavior in the open field test.
Regarding the antinociceptive effect, treatment with the OEFC and COEFC significantly reduced the number of abdominal contortions. However, the COEFC presented a more significant action than the isolated OEFC when evaluated with respect to the number of contortions. These data suggest that lower inclusion complex doses present more significant results [32], which affirm the more robust antinociceptive effect of inclusion complexes with respect to isolated essential oils and monoterpenes. The literature data show that β-CD increases the bioavailability of analgesic and anti-inflammatory drugs used in human therapy, thereby improving their efficacy [9], which corroborates our data, since lower doses of COEFC compared to OEFC showed more significant results concerning the parameters evaluated.
The abdominal contortions model is used as a parameter to evaluate central and peripheral effects, since acetic acid induces pain sensitivity through the release of substances derived from mast cells and macrophages, as well as the sensitization of peripheral afferent sensory nerve endings [33].
Treatment with the OEFC and COEFC at all doses in the formalin test presented an antinociceptive effect in the first and second phases. However, no significant differences were observed between the tested doses. This effect may possibly be associated with the presence of 1,8-cineol, which has proven antinociceptive activity in the literature. Importantly, the antinociceptive effect of the OEFC and COEFC in the formalin test corroborates with the abdominal contortions test, where a more significant effect was observed with the COEFC at its lowest dose compared to the isolated OEFC. The antinociceptive effect of the OEFC and COEFC between the abdominal contortion and formalin tests differ only by local and chemical stimuli.
The formalin-induced nociception test is considered an efficient, reliable, and sensitive method to investigate various analgesic substances, presenting two distinct nociception mechanistic phases. The first phase (0–5 min), characterized by neurogenic pain and associated with the direct chemical stimulation of type C and Aδ (in part) afferent fibers, is associated with the release of excitatory amino acids, substance P, nitric oxide, and others. The second phase (15–30 min) is characterized by inflammatory pain, during which the release of various pro-inflammatory mediators, including prostaglandins (PGs), bradykinin, histamine, and serotonin, occurs [34].
The centrally acting antinociceptive effect of the OEFC and COEFC in the hot plate test with all tested doses corroborates with the effect of the OEFC and COEFC on abdominal contortions and the formalin test, especially during the first formalin phase, suggesting that the OEFC and COEFC present centrally acting antinociceptive activity.
The hot plate test aims to evaluate the analgesic effect of substances mediated by central mechanisms, being used in the evaluation of opioid drugs, including others, with central effects such as sedatives and hypnotics [35].
The major constituent of the studied species, 1,8-cineol, presented antinociceptive activity by significantly inhibiting the response time (paw licking) in both phases [36] and by its action in the hot plate and tail flick models [37]. Recent studies with species belonging to the Croton genus presenting an antinociceptive action include Croton urucurana [38], Croton guatemalensis Lotsy [39], and Croton cordiifolius Baill. [40].
However, despite a proven antinociceptive action of the OEFC and COEFC, investigating the pain signaling pathways involved in both antinociceptive responses using the following systems was necessary: opioid, cholinergic, glutamatergic, adenosinergic, vanilloid, dopaminergic, α1 and α2 adrenergic receptors, serotoninergic pathways, and nitric oxide pathways, from which both the OEFC and COEFC demonstrated participation in the systems: opioid, cholinergic, vanilloid, α2 adrenergic receptors, and nitric oxide pathways.
In the opioid system, the reversal of the OEFC and COEFC antinociceptive effects promoted by the opioid receptor antagonist affirms their possible participation in this system. The opioid system presents analgesic action through mu, delta, and kappa receptors [41] coupled to G proteins, which act by inhibiting Ca2+ channels, blocking protein phosphorylation, reducing the release of excitatory neurotransmitters, activating K+ channels, and thereby decreasing nociceptive stimulation [42]. Morphine is a µ opioid receptor agonist which inhibits painful stimuli [42]. Additionally, naloxone (antagonist) promotes the blockade of opioid receptors [42], favoring the painful response.
With respect to the cholinergic system, the reversal of the OEFC and COEFC antinociceptive action suggests that both potentially act on Gq/Gi protein-coupled muscarinic receptors. According to recent studies, acetylcholine (agonist) acts on M1, M3, and M5 muscarinic receptors coupled to Gq proteins, as well as on M2 and M4 receptors coupled to Gi proteins, decreasing Ca2+ influx and the release of excitatory mediators, favoring K+ channel activation, inducing hyperpolarization and, consequently, favoring the antinociceptive effect [42].
Given the results presented in the present study, the OEFC and COEFC may act on α2-adrenergic receptors, given the reversal of their antinociceptive effect when these were associated with the selective antagonist; however, when the OEFC and COEFC were evaluated with respect to α1-adrenergic receptors, neither one presented an effect.
The activation of α-1 adrenergic receptors stimulates antinociceptive effects [43] acting especially on adrenergic analgesia in the formalin test [43], whereas the antagonist—prazosine—reduces these effects [44], promoting painful stimuli. The α2-adrenergic pathway acts on α2 receptors coupled to a G protein (Gi), which when activated, inhibits adenylate cyclase, reducing the level of intracellular cAMP [42] and blocking Ca2+ influx, promoting K+ channel activation and thereby inhibiting pain transmission [45].
As for the L-arginine/nitric oxide/cGMP pathway, the OEFC and COEFC had their antinociceptive effect reversed when associated with the antagonist (L-arginine/NOS substrate), demonstrating their possible participation in the pathway. Recent research states that nitric oxide is an indirect mediator of the inflammatory response, which aids in vascular and cellular events. Therefore, when iNOS (inductive nitric oxide synthase) inhibition occurs, the production of this mediator is reduced, possibly minimizing inflammatory effects, consequently also decreasing the indirect response to pain [46].
Regarding the vanilloid system, the OEFC and COEFC demonstrated an antinociceptive action by reducing the capsaicin-induced paw licking time, which suggests participation in the vanilloid system, which corroborates with the data obtained in the hot plate test since both models activate vanilloid receptors.
The data obtained in this study do not support participation of the serotoninergic pathway, the dopaminergic, adenosinergic, and glutamatergic systems, nor of α1-adrenergic receptors in the OEFC and COEFC antinociceptive effect. Serotoninergic pathways in the central nervous system are regulated by the release of 5-HT (serotonin) [47], which possesses an inhibitory effect on nociceptive neuron transmission via 5-HT1, 5-HT2, and 5-HT3 receptors [48], which when activated increase serotonin levels and promote an antinociceptive response [49]. In the dopaminergic system, dopamine promotes pain suppression through dopaminergic receptors [50], especially through D2 receptors, but also through Gi protein-coupled D3 and D4 receptors, inhibiting Ca2+ channels and increasing intracellular K+ levels [51]. However, its antinociceptive effect may be inhibited following the administration of its antagonist haloperidol [52].
In the adenosinergic system, it is noteworthy that adenosinergic receptors are coupled to Gi proteins and act by reducing intracellular cAMP, promoting the opening of potassium channels, which promotes hyperpolarization, inhibiting the opening of calcium channels, thus reducing the release of neurotransmitters and, consequently, the painful stimulus. Caffeine acts on the central nervous system by non-selectively antagonizing the adenosine receptors A1, A2a, and A3 [53], promoting the increase of 3,5-cyclic-AMP through phosphodiesterase inhibition and intracellular calcium release [54].
As for the glutamatergic system, glutamate is an excitatory neurotransmitter that stimulates nociception by activating AMPA (α-amino-3-hidorxy-5-methyl-4-isoxazole propionic acid) and NMDA (N-methyl-D-aspartic acid) receptors, allowing the passage of Na+ and Ca2+, promoting the action potential and, thereafter, a nociceptive response [42].
The major constituent of the species under study, 1,8-cineol, is considered a rare natural TRPA1 receptor antagonist since its mechanism of action involves activating TRPM8 receptors and inhibiting TRPA1 [55], where TRPM8 is considered a mediator that promotes analgesia in acute and inflammatory pain [56]. The aforementioned study corroborates the present study and suggests the action of the OEFC and COEFC, which involves the participation of the vanilloid system, is influenced by the action of its major constituent 1,8-cineol.
In a previous study conducted with the Croton rhamnifolioides species, this was shown to possess gastroprotective effects involving the opioid system and the nitric oxide pathway [5], which are results that corroborate with our study. Some studies investigating the possible mechanisms of action involved in the antinociceptive response with species from the Croton genus, such as those using the Croton conduplicatus [57] and Croton argyrophyllus Kunth [58] essential oils, also support our research. Moreover, a previous study described the anti-inflammatory effect of the OEFC [3] and COEFC [16], which are results that corroborate with the antinociceptive action observed in the present study, since pain is a classic sign of the inflammatory process.
5. Conclusions
The present study showed that with respect to an activity over the central nervous system, the C. rhamnifolioides essential oil (OEFC) and β-cyclodextrin complex (COEFC) did not alter exploratory activity or motor coordination, suggesting these do not present a depressant or excitatory action on the CNS. As for the antinociceptive effect, the OEFC and COEFC demonstrated central and peripheral antinociceptive activity in the acetic acid-induced abdominal contortions model, the 2.5% formalin and hot plate tests, the actions of which involved the participation of the opioid, cholinergic, and vanilloid systems, the L-arginine/NO pathway, and α-2 adrenergic receptors. Importantly, the COEFC presented more significant effects at lower doses than the isolated OEFC, where this action may be justified by the properties and advantages of the complexation of substances with cyclodextrin. The data herein suggest that the OEFC and COEFC may be considered as new therapeutic options for treatments involving painful processes and in the development of new antinociceptive agents.
Author Contributions
A.G.W., I.R.A.d.M., L.J.Q.J., H.D.M.C. and A.A.d.S.A. proposed the experimental design; A.O.B.P.B.M., L.B.R., F.R.A.S.C., M.R.C.d.O., I.S.A., M.S.A.d.S., F.F.eC., E.P.d.N., T.R.d.A. performed the in vivo experiments involved in the evaluation of the antinociceptive effect of C. rhamnifolioides essential oil (OEFC) and inclusion complex (OEFC/β-CD). A.G.W. and I.R.A.d.M. provided the facilities and reagents for the work. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the financial support provided by the FACEPE, CAPES, CNPq and FUNCAP institutions.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Federal University of Pernambuco (UFPE), Regional University of Cariri (URCA) and Federal University of Sergipe (UFS).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ribeiro, D.A.; Macêdo, D.G.; Oliveira, L.G.S.; Saraiva, M.E.; Oliveira, S.F.; Souza, M.M.A.; Menezes, I.R. Therapeutic potential and use of medicinal plants in an area of the Caatinga in the state of Ceará, northeastern Brazil. Rev. Bras. Pl. Med. 2014, 16, 912–930. [Google Scholar] [CrossRef]
- Randau, K.P.; Florêncio, D.C.; Ferreira, C.P.; Xavier, H.S. Pharmacognostic study of Croton rhamnifolius HBK and Croton rhamnifolioides Pax & Hoffm.(Euphorbiaceae). Rev. Bras. Farmacogn. 2004, 14, 89–96. [Google Scholar]
- Martins, A.O.B.P.B.; Rodrigues, L.B.; Cesário, F.R.A.S.; de Oliveira, M.R.C.; Tintino, C.D.M.; e Castro, F.F.; Alcântara, I.S.; Fernandes, M.N.M.; de Albuquerque, T.R.; da Silva, M.S.A. Anti-edematogenic and anti-inflammatory activity of the essential oil from Croton rhamnifolioides leaves and its major constituent 1, 8-cineole (eucalyptol). Biomed. Pharmacother. 2017, 96, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.K.N.; Dutra, K.A.; Lira, C.S.; Lima, B.N.; Napoleão, T.H.; Paiva, P.M.G.; Maranhão, C.A.; Brandão, S.S.F.; Navarro, D.M.A.F. Effects of Croton rhamnifolioides essential oil on Aedes aegypti oviposition, larval toxicity and trypsin Activity. Molecules 2014, 19, 16573–16587. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.S.; Martins, A.O.B.P.B.; de Alencar Silva, A.; de Oliveira, M.R.C.; Ribeiro-Filho, J.; de Albuquerque, T.R.; Coutinho, H.D.M.; da Silva Almeida, J.R.G.; Quintans, L.J.; de Menezes, I.R.A. Gastroprotective effect and mechanism of action of Croton rhamnifolioides essential oil in mice. Biomed. Pharmacother. 2017, 89, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, U.N.; de Lima, S.G.; Moura, L.C.B.; de Almeida, L.T.G.; Oliveira, T.M.; de Freitas, R.M.; Silva, R.M.; Rocha, M.S. Preparação e caracterização do complexo de inclusão do óleo essencial de Croton zehntneri com β-Ciclodextrina. Quim. Nova 2014, 37, 50–55. [Google Scholar] [CrossRef]
- CUNHA-FILHO, M.S.S.; Sá-BARRETO, L.C.L. Utilização de ciclodextrinas na formação de complexos de inclusão de interesse farmacêutico. Rev. Ciências Farm. Básica Apl. 2009, 28, 1–9. [Google Scholar]
- de Lima Guedes, F.; Alves, G.M.C.; dos Santos, F.L.A.; de Lima, L.F.; Rolim, L.A.; Neto, P.J.R. Ciclodextrinas: Como adjuvante tecnológico para melhorar a biodisponibilidade de fármacos. Rev. Bras. Farm 2008, 89, 3. [Google Scholar]
- Sithole, M.N.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Development of a Novel Polymeric Nanocomposite Complex for Drugs with Low Bioavailability. AAPS PharmSciTech. 2018, 19, 303–314. [Google Scholar] [CrossRef]
- Brito, M.; JÚNIOR, C.S.N.; SANTOS, H.F. Análise estrutural de ciclodextrinas: Um estudo comparativo entre métodos teóricos clássicos e quânticos. Química. Nov. 2004, 6, 882–888. [Google Scholar] [CrossRef]
- Lapa, A.J.; Souccar, C.; Lima-Landman, M.T.R.; de Castro, M.S.A.; Lima, T.C.M. Métodos de avaliação da atividade farmacológica de plantas medicinais. Soc. Bras. Plantas Med. 2003, 64, 66. [Google Scholar]
- WOOLFE, G.; MacDonald, A.D. The evaluation of the analgesic action of pethidine hydrochloride (Demerol). J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar]
- Peura, D.A.; Goldkind, L. Balancing the gastrointestinal benefits and risks of nonselective NSAIDs. Arthritis Res. Ther. 2005, 7, S7. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, C.R.; Scully, S.S. Analgesic substances derived from natural products (natureceuticals). Life Sci. 2005, 78, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.P.; Serafini, M.R.; Quintans-Júnior, L.J.; Silva, G.F.; Oliveira, J.F.; Carvalho, F.M.S.; Souza, J.C.C.; Matos, J.R.; Alves, P.B.; Matos, I.L. Inclusion complex of (−)-linalool and β-cyclodextrin. J. Therm. Anal. Calorim. 2014, 115, 2429–2437. [Google Scholar] [CrossRef]
- Martins, A.O.B.P.B.; Wanderley, A.G.; Alcântara, I.S.; Rodrigues, L.B.; Cesário, F.R.A.; Oliveira, M.R.C.; Castro, F.F.; Albuquerque, T.R.; Silva, M.S.A.; Ribeiro-Filho, J.; et al. Anti-Inflammatory and Physicochemical Characterization of the Croton Rhamnifolioides Essential Oil Inclusion Complex in β-Cyclodextrin. Biology 2020, 9, 114. [Google Scholar]
- Archer, J. Tests for emotionality in rats and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Dunham, N.W.; Miya, T.S. A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, G.W.; Bilsky, E.J.; Negus, S.S. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: Effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J. Pain 2006, 7, 408–416. [Google Scholar] [CrossRef]
- Tjølsen, A.; Berge, O.-G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Jacob, J.J.C.; Ramabadran, K. Enhancement of a nociceptive reaction by opioid antagonists in mice. Br. J. Pharmacol. 1978, 64, 91–98. [Google Scholar] [CrossRef]
- Schechtmann, G.; Song, Z.; Ultenius, C.; Meyerson, B.A.; Linderoth, B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain 2008, 139, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.S.; Gadotti, V.M.; Oliveira, G.L.; Tibola, D.; Paszcuk, A.F.; Neto, A.; Spindola, H.M.; Souza, M.M.; Rodrigues, A.L.S.; Calixto, J.B. Mechanisms involved in the antinociception caused by agmatine in mice. Neuropharmacology 2005, 48, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, A.; Tegeder, I.; Geisslinger, G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 2009, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Mantovani, M.; Kaster, M.P.; Pertile, R.; Calixto, J.B.; Rodrigues, A.L.S.; Santos, A.R.S. Mechanisms involved in the antinociception caused by melatonin in mice. J. Pineal. Res. 2006, 41, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Nayebi, A.M.; Garjani, A. Effects of central and peripheral depletion of serotonergic system on carrageenan-induced paw oedema. Int. Immunopharmacol. 2005, 5, 1723–1730. [Google Scholar] [CrossRef]
- Finan, P.H.; Smith, M.T. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Med. Rev. 2013, 17, 173–183. [Google Scholar] [CrossRef]
- Ferré, S.; Diamond, I.; Goldberg, S.R.; Yao, L.; Hourani, S.M.O.; Huang, Z.L.; Urade, Y.; Kitchen, I. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry: Implications for drug addiction, sleep and pain. Prog. Neurobiol. 2007, 83, 332–347. [Google Scholar] [CrossRef]
- Zeraati, F.; Araghchian, M.; Farjoo, M.H. Ascorbic Acid interaction with analgesic effect of morphine and tramadol in mice. Anesthesiol. Pain Med. 2014, 4, 5. [Google Scholar] [CrossRef]
- Talarek, S.; Orzelska, J.; Listos, J.; Fidecka, S. Effects of sildenafil treatment on the development of tolerance to diazepam-induced motor impairment and sedation in mice. Pharmacol. Rep. 2010, 62, 627–634. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Barreto, R.S.S.; Menezes, P.P.; Almeida, J.R.G.S.; Viana, A.F.S.C.; Oliveira, R.; Oliveira, A.P.; Gelain, D.P.; Lucca Júnior, W.; Araújo, A.A.S. β-Cyclodextrin-complexed (−)-linalool produces antinociceptive effect superior to that of (−)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 2013, 113, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.P.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.S. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. [Google Scholar] [CrossRef]
- Santos, F.A.A.; Rao, V.S.N. Antiinflammatory and antinociceptive effects of 1, 8-cineole a terpenoid oxide present in many plant essential oils. Phyther. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Liapi, C.; Anifantis, G.; Chinou, I.; Kourounakis, A.P.; Theodosopoulos, S.; Galanopoulou, P. Antinociceptive properties of 1, 8-cineole and β-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med. 2007, 73, 1247–1254. [Google Scholar] [CrossRef]
- Cordeiro, K.W.; Felipe, J.L.; Malange, K.F.; do Prado, P.R.; de Oliveira Figueiredo, P.; Garcez, F.R.; de Cássia Freitas, K.; Garcez, W.S.; Toffoli-Kadri, M.C. Anti-inflammatory and antinociceptive activities of Croton urucurana Baillon bark. J. Ethnopharmacol. 2016, 183, 128–135. [Google Scholar] [CrossRef]
- del Carmen, R.-O.J.; Willam, H.M.J.; del Carmen, G.M.A.; Nataly, J.-G.; Stefany, C.O.S.; Anahi, C.A.; Domingo, P.T.J.; Leonardo, G.P.; de la Mora Miguel, P. Antinociceptive effect of aqueous extracts from the bark of Croton guatemalensis Lotsy in mice. Res. Pharm. Sci. 2016, 11, 15. [Google Scholar] [PubMed]
- de Nogueira, L.M.; da Silva, M.R.; dos Santos, S.M.; de Albuquerque, J.F.C.; Ferraz, I.C.; de Albuquerque, T.T.; de Mota, C.R.F.C.; Araújo, R.M.; de Viana, G.S.B.; Martins, R.D. Antinociceptive effect of the essential oil obtained from the leaves of Croton cordiifolius Baill.(Euphorbiaceae) in mice. Evid. Based Complement Altern. Med. 2015, 2015, 620865. [Google Scholar] [CrossRef]
- Gavériaux-Ruff, C. Opiate-induced analgesia: Contributions from mu, delta and kappa opioid receptors mouse mutants. Curr. Pharm. Des. 2013, 19, 7373–7381. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Basbaum, A.I.; Way, W.L. Opioid Analgesics and Antagonists; Katzung, B.G., Masters, S.B., Trevor, A.J., Eds.; McGraw-Hill Educationpublisher: San Francisco, CA, USA, 2012. [Google Scholar]
- Tasker, R.A.R.; Connell, B.J.; Yole, M.J. Systemic injections of alpha-1 adrenergic agonists produce antinociception in the formalin test. Pain 1992, 49, 383–391. [Google Scholar] [CrossRef]
- Otsuka, N.; Kiuchi, Y.; Yokogawa, F.; Masuda, Y.; Oguchi, K.; Hosoyamada, A. Antinociceptive efficacy of antidepressants: Assessment of five antidepressants and four monoamine receptors in rats. J. Anesth. 2001, 15, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, W.A.; Lemônica, L. Mecanismos celulares e moleculares da dor inflamatória. Modulação periférica e avanços terapêuticos. Rev. Bras. Anestesiol. 1998, 48, 137–158. [Google Scholar]
- Shih, C.-C.; Hwang, H.-R.; Chang, C.-I.; Su, H.-M.; Chen, P.-C.; Kuo, H.-M.; Li, P.-J.; Wang, H.-M.D.; Tsui, K.-H.; Lin, Y.-C. Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia ceylanica. Int. J. Mol. Sci. 2017, 18, 2437. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.; Schloss, P. Differential regulation of serotonin transporter cell surface expression. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 259–268. [Google Scholar] [CrossRef]
- Millan, M.J.; Seguin, L.; Honoré, P.; Girardon, S.; Bervoets, K. Pro-and antinociceptive actions of serotonin (5-HT) 1A agonists and antagonists in rodents: Relationship to algesiometric paradigm. Behav. Brain Res. 1995, 73, 69–77. [Google Scholar] [CrossRef]
- Bobinski, F.; Ferreira, T.A.A.; Córdova, M.M.; Dombrowski, P.A.; da Cunha, C.; do Espírito Santo, C.C.; Poli, A.; Pires, R.G.W.; Martins-Silva, C.; Sluka, K.A. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain following sciatic nerve injury in mice. Pain 2015, 156, 2595. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 2008, 8, 781–797. [Google Scholar] [CrossRef]
- Kellendonk, C.; Simpson, E.H.; Polan, H.J.; Malleret, G.; Vronskaya, S.; Winiger, V.; Moore, H.; Kandel, E.R. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006, 49, 603–615. [Google Scholar] [CrossRef]
- Lin, M.T.; Wu, J.J.; Chandra, A.; Tsay, B.L. Activation of striatal dopamine receptors induces pain inhibition in rats. J. Neural Transm. 1981, 51, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Benitez, J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin. Pharmacokinet. 2000, 39, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Padgett, W.L.; Daly, J.W. Caffeine analogs: Effects on ryanodine-sensitive calcium-release channels and GABA A receptors. Cell. Mol. Neurobiol. 2003, 23, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Fujita, F.; Uchida, K.; Yamamoto, S.; Sawada, M.; Hatai, C.; Shimizu, M.; Tominaga, M. 1, 8-cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain 2012, 8, 86. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain® 2013, 154, 2169–2177. [Google Scholar] [CrossRef]
- de Oliveira Júnior, R.G.; Ferraz, C.A.A.; Silva, J.C.; de Oliveira, A.P.; Diniz, T.C.; Silva, M.G.; Quintans Júnior, L.J.; de Souza, A.V.V.; dos Santos, U.S.; Turatti, I.C.C. Antinociceptive Effect of the Essential Oil from Croton conduplicatus Kunth (Euphorbiaceae). Molecules 2017, 22, 900. [Google Scholar] [CrossRef]
- de Ramos, J.M.O.; dos Santos, C.A.; Santana, D.G.; Antoniolli, A.R.; de Santos, D.A.; Alves, P.B.; Thomazzi, S.M. Impact of Croton argyrophyllus essential oil on behavioural models of nociception. Flavour Fragr. J. 2017, 32, 40–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).