Influence of Ethylene-1-Alkene Copolymers Microstructure on Thermo-Rheological Behavior of Model Blends for Enhanced Recycling
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Dynamic Rheology
2.2.2. Thermal Analysis
3. Results and Discussions
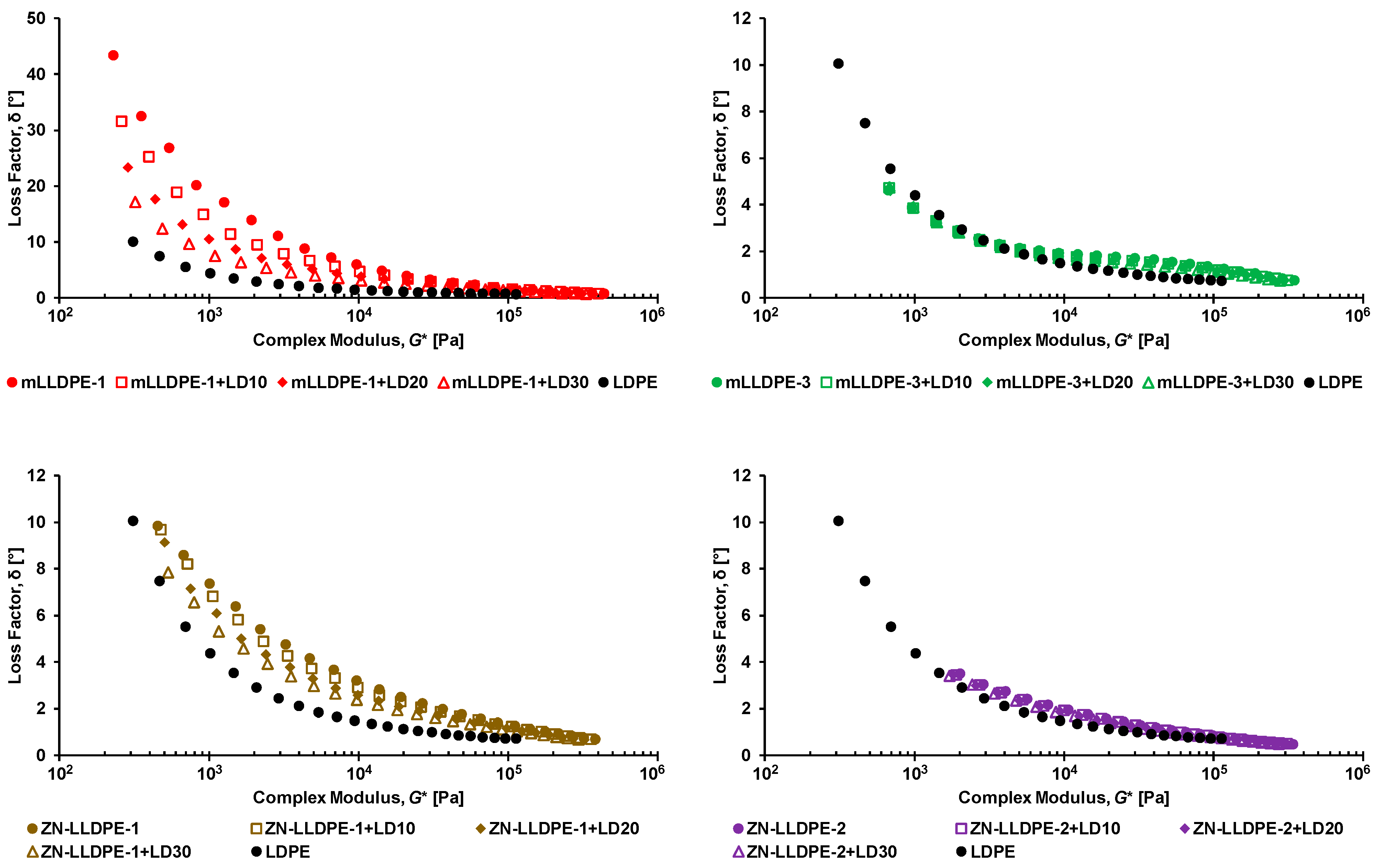
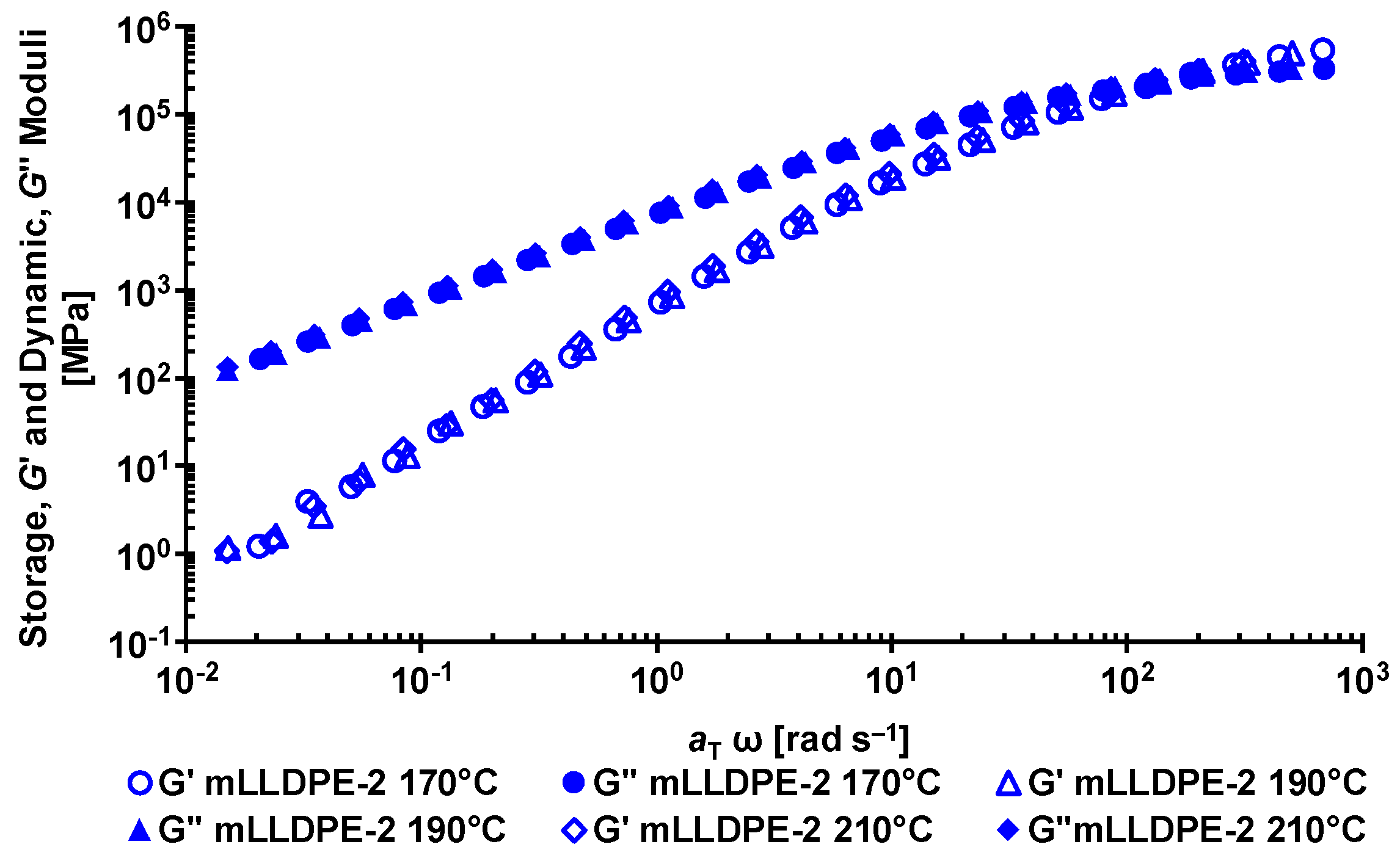
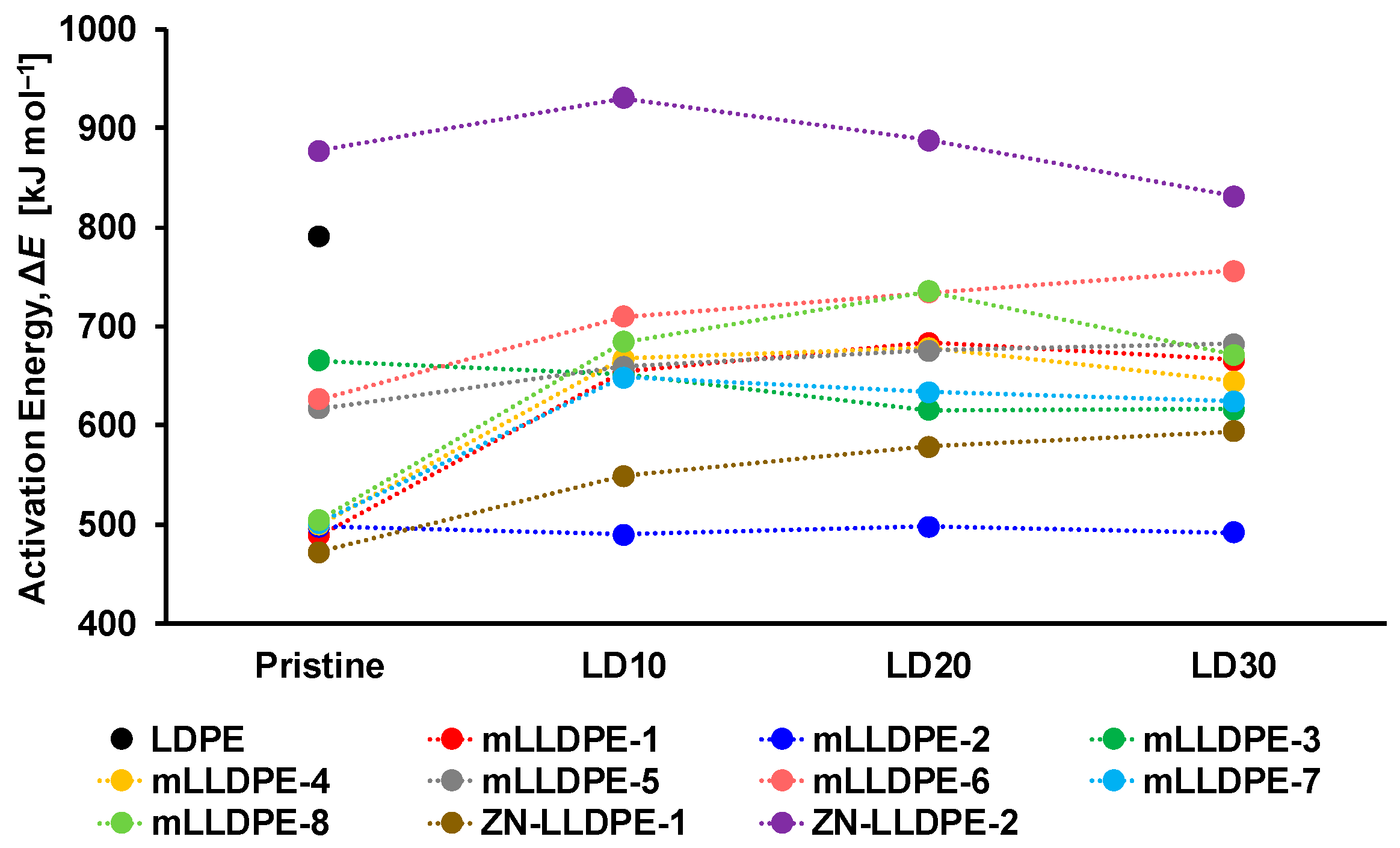
3.1. Rheological Behavior of LLDPE/LDPE Blends
3.2. Thermal Characterization of LLDPE/LDPE Blends
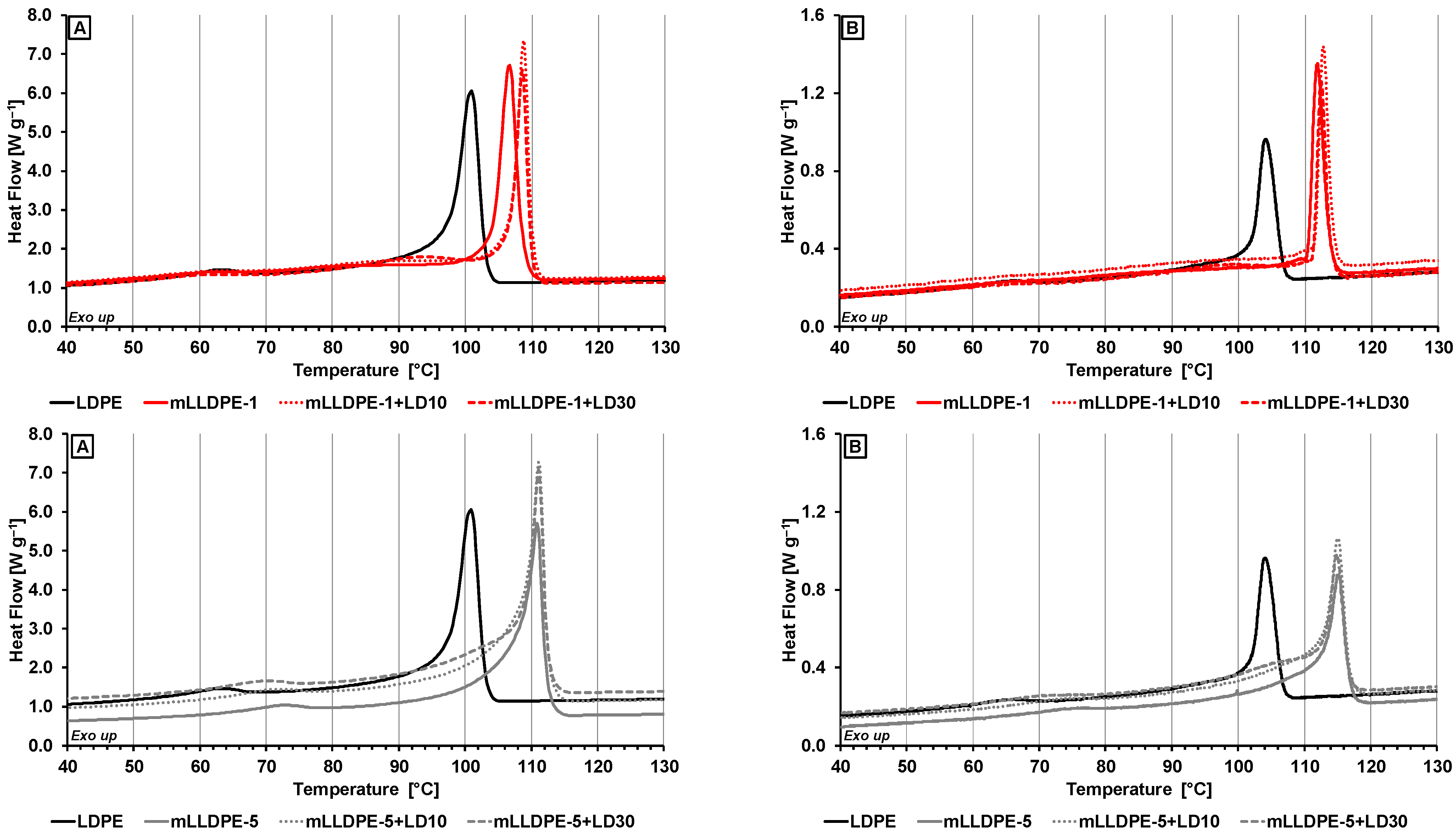
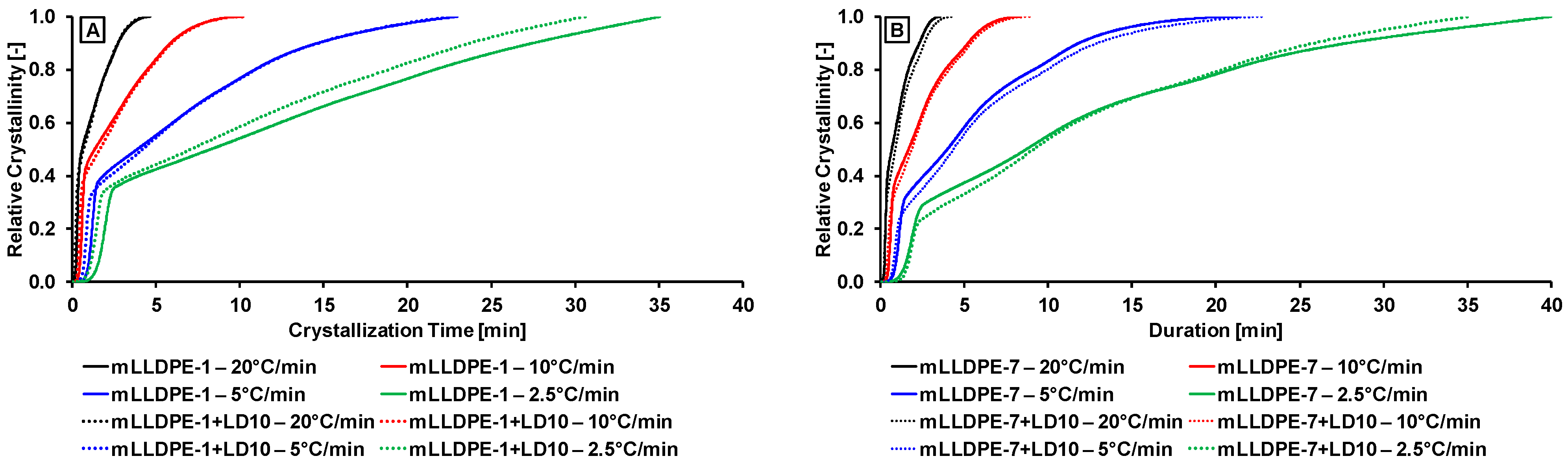
3.2.1. Effect of Cooling Rate
3.2.2. Effect of Blending LDPE to LLDPE
- High-temperature crystallization region (100–120 °C) corresponding to crystallization behavior of pristine LLDPE and of LLDPE in the presence of LDPE;
- Medium-temperature crystallization region (90–110 °C) corresponding to crystallization behavior of LDPE in the presence of LLDPE;
- Low-temperature region (60–80 °C) corresponding to crystallization behavior of relatively highly branched polymer fraction.
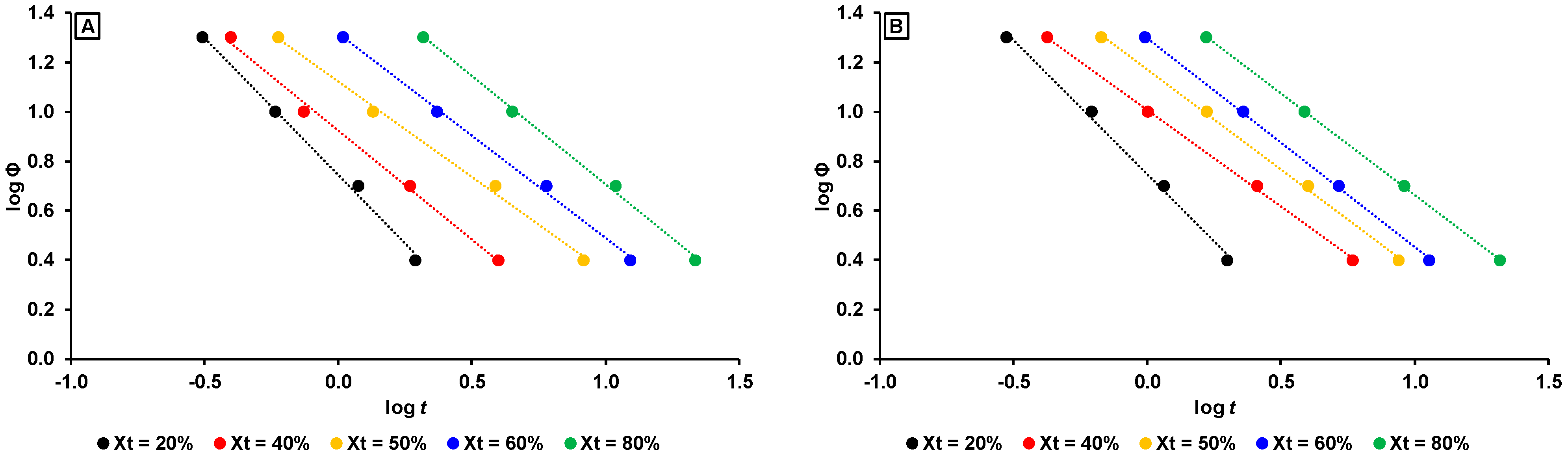
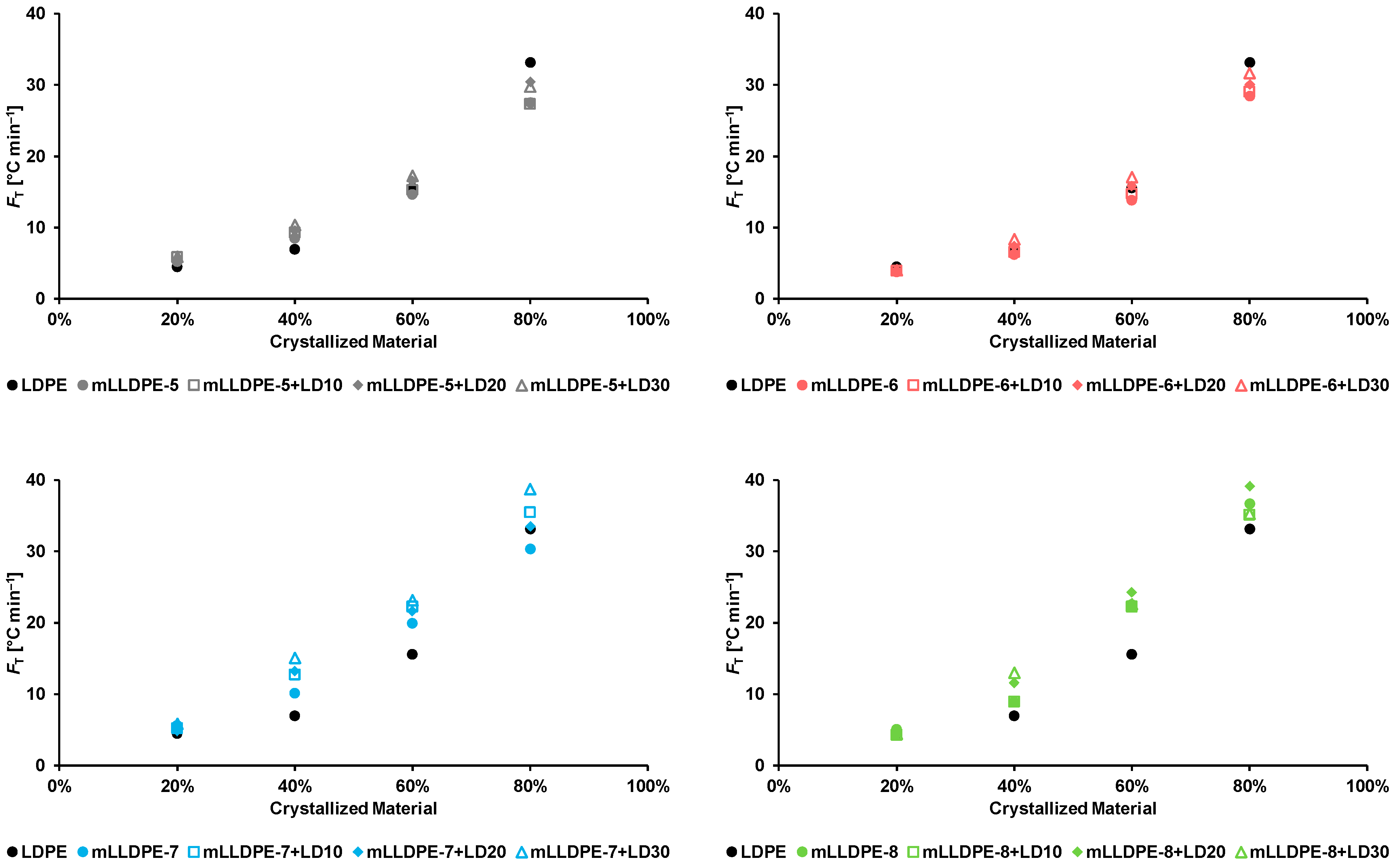
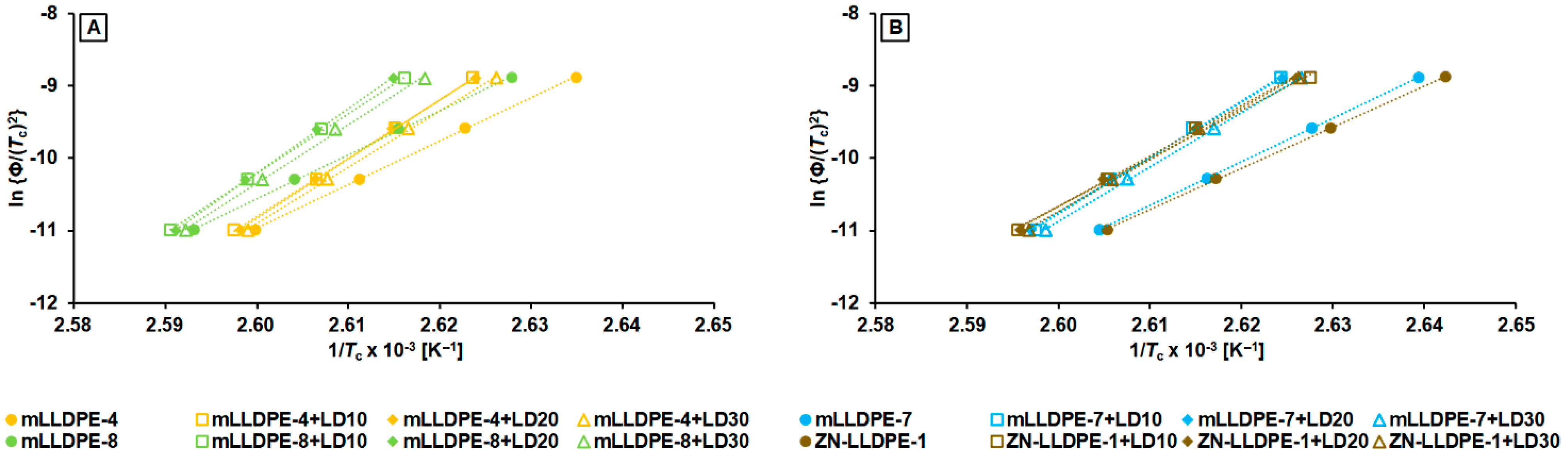
3.2.3. Non-Isothermal Crystallization Kinetics
- mLLDPE with SCB/1000C < 12, (viz., mLLDPE-2, mLLDPE-3, mLLDPE-5 and mLLDPE-6): For these mLLDPE matrices, blending with LDPE did not significantly influence Ti,c and Tc of mLLDPE. Their LPDE blends tend to crystallize faster than LDPE. In these blends, concurrent and/or separate crystallization was found to be the more preferred mode than co-crystallization of mLLDPE and LDPE.
- mLLDPE with SCB/1000C > 12, (viz., mLLDPE-1, mLLDPE-4, mLLDPE-7 and mLLDPE-8): For these mLLDPE matrices, blending with LDPE did significantly influence Ti,c and Tc of mLLDPE. Their LPDE blends tend to crystallize slower than LDPE. In these blends, co-crystallization was found to be the more preferred mode than concurrent and/or separate crystallization of mLLDPE and LDPE.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffl, T. Rheological and Film Blowing Properties of Various Low Density Polyethylenes and Their Blends. Ph.D. Thesis, Universität Erlangen-Nürnberg, Erlangen, Germany, June 2004. [Google Scholar]
- Simanke, A.G.; Galland, G.B.; Baumhardt, R.; Quijada, R.; Mauler, R.S. Influence of the type and the comonomer contents on the mechanical behavior of ethylene/α-olefin copolymers. J. Appl. Polym. Sci. 1999, 74, 1194–1200. [Google Scholar] [CrossRef]
- Usami, T.; Gotoh, Y.; Takayama, S. Generation mechanism of short-chain branching distribution in linear low-density polyethylene. Macromolecules 1986, 19, 2722–2726. [Google Scholar] [CrossRef]
- Munstedt, H.; Kurzbeck, S.; Egersdorfer, L. Influence of molecular structure on rheological properties of polyethylenes. Rheol. Acta 1998, 37, 21–29. [Google Scholar] [CrossRef]
- Wignal, G.D.; Alamo, R.G.; Ritchson, E.J.; Mandelkern, L.; Schwahn, D. SANS studies of liquid− liquid phase separation in heterogeneous and metallocene-based linear low-density polyethylenes. Macromolecules 2001, 34, 8160–8165. [Google Scholar] [CrossRef]
- Hussein, I.A.; Williams, M.C. Rheological Study of the Influence of Branch Content on the Miscibility of Octene m-LLDPE and ZNLLDPEin LDPE. Polym. Eng. Sci. 2004, 44, 660–672. [Google Scholar] [CrossRef]
- Knuuttila, H.; Lehtinen, A.; Nummila-Pakarinen, A. Advanced polyethylene technologies—Controlled material properties. In Long Term Properties of Polyolefins; Springer: Berlin/Heidelberg, Germany, 2004; pp. 13–28. [Google Scholar]
- Malpass, D.B. An overview of industrial polyethylene processes. In Introduction to Industrial Polyethylene: Properties, Catalysts, Processes; Scrivener Publishing LLC: Beverly, MA, USA, 2010; pp. 71–82. [Google Scholar]
- Hussein, I.A. Influence of composition distribution and branch content on the miscibility of m-LLDPE and HDPE blends: Rheological investigation. Macromolecules 2003, 36, 2024–2031. [Google Scholar] [CrossRef]
- Hussein, I.A.; Hameed, T.; Abu Sharkh, B.F.; Mezghani, K. Miscibility of hexene-LLDPE and LDPE blends: Influence of branch content and composition distribution. Polymer 2003, 44, 4665–4672. [Google Scholar] [CrossRef][Green Version]
- Pérez, R.; Rojo, E.; Fernández, M.; Leal, V.; Lafuente, P.; Antamaría, A.S. Basic and applied rheology of m-LLDPE/LDPE blends: Miscibility and processing features. Polymer 2005, 46, 8045–8053. [Google Scholar] [CrossRef]
- Delgadillo-Velázquez, O.; Hatzikiriakos, S.G.; Sentmanat, M. Thermorheological properties of LLDPE/LDPE blends. Rheol. Acta 2008, 47, 19–31. [Google Scholar] [CrossRef]
- Mamun, A.; Chen, X.; Alamo, R.G. Interplay between a strong memory effect of crystallization and liquid–liquid phase separation in melts of broadly distributed ethylene–1-alkene copolymers. Macromolecules 2014, 47, 7958–7970. [Google Scholar] [CrossRef]
- Groeninckx, G.; Vanneste, M.; Everaert, V. Crystallization, morphological structure and melting of polymer blends. In Polymer Blends Handbook; Utracki, L.A., Ed.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2003; pp. 215–240. [Google Scholar]
- Galgali, G.; Kaliappan, S.K. Microstructure–sealing performance linkage of metallocene linear low density polyethylene and low density polyethylene blends. Polym. Cryst. 2021, 4, e10206. [Google Scholar] [CrossRef]
- ISO 6721-10:2015; Plastics—Determination of Dynamic Mechanical Properties—Part 10: Complex Shear Viscosity Using a Parallel-Plate Oscillatory Rheometer. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 11357-3:2018; Plastics—Differential Scanning Calorimetry (DSC)—Part 3: Determination of Temperature and Enthalpy of melting and Crystallization. International Organization for Standardization: Geneva, Switzerland, 2018.
- Fan, Z.J.; Williams, M.C.; Choi, P.A. molecular dynamics study of the effects of branching characteristics of LDPE on its miscibility with HDPE. Polymer 2002, 43, 1497–1502. [Google Scholar] [CrossRef]
- Abu-Sharkh, B.F.; Giri, A.M.; Hussein, I.A. Influence of branch content on the microstructure of blends of linear and octene-branched polyethylene: A MD simulation study. Eur. Polym. J. 2004, 40, 1177–1182. [Google Scholar] [CrossRef]
- Van Gurp, M.; Palmen, J. Time-temperature superposition for polymeric blends. J. Rheol. Bull. 1998, 67, 5–8. [Google Scholar]
- Hatzikiriakos, S.G. Long chain branching and polydispersity effects on the rheological properties of polyethylenes. Polym. Eng. Sci. 2000, 40, 2279–2287. [Google Scholar] [CrossRef]
- Kanso, M.A.; Giacomin, A. Van Gurp–Palmen relations for long-chain branching from general rigid bead-rod theory. J. Phys. Fluids 2020, 32, 033101-15. [Google Scholar] [CrossRef]
- Hassager, O. Kinetic theory and rheology of bead—Rod models for macromolecular solutions. II. Linear unsteady flow properties. J. Chem. Phys. 1974, 60, 4001–4008. [Google Scholar] [CrossRef]
- Olabishi, O.; Robeson, L.M.; Shaw, M.Y. Polymer-Polymer Miscibility; Academic Press: New York, NY, USA, 1979; pp. 105–133. [Google Scholar]
- Mandelkern, L. Crystallisation of Polymers: Volume 2. Kinetics and Mechanisms; Cambridge University Press: Cambridge, UK, 2004; pp. 67–96. [Google Scholar]
- Young, R.J.; Lovell, P.A. Introduction to Polymers; CRC Press: New York, NY, USA, 2011; pp. 168–186. [Google Scholar]
- Yamaguchi, M.; Abe, S. LLDPE/LDPE blends. I. Rheological, thermal, and mechanical properties. J. Appl. Polym. Sci. 1999, 74, 3153–3159. [Google Scholar] [CrossRef]
- Sweed, M. Co-Crystallization in Polyolefin Blends Studied by Various Crystallization Analysis Techniques. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, April 2006. [Google Scholar]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly (aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger Method in Kinetics of Materials: Things to Beware and Be Aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.J.; Kim, J.; Kim, Y.C. Effect of starch content on the non-isothermal crystallization behavior of HDPE/silicate nanocomposites. J. Ind. Eng. Chem. 2010, 16, 406–410. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.J.; Zhang, J. Non-isothermal crystallization kinetics of partially miscible ethylene-vinyl acetate copolymer/low density polyethylene blends. Express Polym. Lett. 2010, 4, 141–152. [Google Scholar] [CrossRef]
- Abareshi, M.; Zebarjad, S.M.; Goharshadi, E.K. Non-isothermal crystallization kinetics of polyethylene–clay nanocomposites prepared by high-energy ball milling. Bull. Mater. Sci. 2004, 37, 1113–1121. [Google Scholar] [CrossRef]

| Sample | MFR2 [g 10 min−1] | Density [kg m−3] | Mn [kg mol−1] | Mw [kg mol−1] | MWD = Mw/Mn [-] | SHI(1/100) [-] |
|---|---|---|---|---|---|---|
| LDPE | 2.0 | 923 | 17 | 108 | 6.3 | 27.4 |
| mLLDPE-1 | 1.5 | 918 | 25 | 104 | 4.2 | 2.0 |
| mLLDPE-2 | 1.0 | 918 | 32 | 113 | 3.5 | 1.5 |
| mLLDPE-3 | 1.0 | 920 | 24 | 114 | 4.7 | 7.4 |
| mLLDPE-4 | 1.0 | 918 | 26 | 118 | 4.6 | 2.7 |
| mLLDPE-5 | 1.3 | 927 | 28 | 107 | 3.9 | 1.5 |
| mLLDPE-6 | 1.3 | 927 | 24 | 106 | 4.4 | 1.8 |
| mLLDPE-7 | 1.0 | 918 | 25 | 105 | 4.2 | 4.5 |
| mLLDPE-8 | 1.5 | 918 | 27 | 111 | 4.1 | 1.7 |
| ZN-LLDPE-1 | 0.9 | 919 | 23 | 119 | 5.1 | 3.7 |
| ZN-LLDPE-2 | 0.2 | 931 | 11 | 192 | 18.2 | 10.2 |
| Sample | Comonomer Type [-] | Total Comonomer Content [mol.%] | SCB/1000C [-] |
|---|---|---|---|
| mLLDPE-1 | C4 + C6 | 2.8 | 14.4 |
| mLLDPE-2 | C6 | 2.1 | 10.8 |
| mLLDPE-3 | C6 | 1.1 | 6.1 |
| mLLDPE-4 | C4 | 3.5 | 18.1 |
| mLLDPE-5 | C6 | 1.1 | 6.2 |
| mLLDPE-6 | C4 + C6 | 1.4 | 7.8 |
| mLLDPE-7 | C8 | 2.5 | 14.1 |
| mLLDPE-8 | C4 | 4.2 | 21.7 |
| ZN-LLDPE-1 | C8 | 2.1 | 10.8 |
| ZN-LLDPE-2 | C4 + C6 | 2.0 | 11.9 |
| Sample | Ea [kJ mol−1] |
|---|---|
| mLLDPE-1 | 36.4 |
| mLLDPE-1 + LD30 | 40.2 |
| mLLDPE-2 | 35.0 |
| mLLDPE-2 + LD30 | 37.3 |
| mLLDPE-3 | 39.8 |
| mLLDPE-3 + LD30 | 43.2 |
| Sample | 20 °C min−1 | 2.5 °C min−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ti,c [°C] | Tc [°C] | Tf,c [°C] | ΔHc [J g−1] | Ti,c [°C] | Tc [°C] | Tf,c [°C] | ΔHc [J g−1] | |
| LDPE | 105.6 | 100.9 | 27.3 | 130.6 | 108.3 | 104.0 | 10.9 | 159.5 |
| mLLDPE-1 | 112.5 | 106.6 | 19.4 | 129.8 | 116.7 | 111.9 | 29.0 | 155.4 |
| mLLDPE-1 + LD20 | 113.0 | 108.9 | 21.8 | 130.6 | 116.5 | 112.6 | 38.1 | 156.2 |
| mLLDPE-2 | 115.2 | 107.1 | 39.5 | 118.6 | 118.1 | 112.3 | 29.9 | 138.1 |
| mLLDPE-2 + LD20 | 114.9 | 107.1 | 37.7 | 121.3 | 118.7 | 112.2 | 35.9 | 151.8 |
| mLLDPE-3 | 107.7 | 103.2 | 42.9 | 115.9 | 110.5 | 107.0 | 19.7 | 137.0 |
| mLLDPE-3 + LD20 | 108.3 | 103.2 | 28.1 | 133.5 | 110.6 | 107.3 | 19.5 | 148.4 |
| mLLDPE-4 | 111.9 | 106.4 | 28.6 | 120.9 | 115.8 | 111.5 | 27.6 | 135.6 |
| mLLDPE-4 + LD20 | 112.2 | 108.0 | 24.4 | 121.0 | 115.9 | 111.7 | 11.0 | 158.2 |
| mLLDPE-5 | 116.3 | 110.9 | 39.9 | 121.7 | 119.0 | 115.1 | 40.3 | 135.9 |
| mLLDPE-5 + LD20 | 116.4 | 111.1 | 36.5 | 139.5 | 119.6 | 114.9 | 40.7 | 157.4 |
| mLLDPE-6 | 113.7 | 109.2 | 31.5 | 142.6 | 116.4 | 113.3 | 27.7 | 167.6 |
| mLLDPE-6 + LD20 | 113.4 | 109.6 | 33.6 | 143.9 | 116.5 | 113.2 | 35.3 | 163.4 |
| mLLDPE-7 | 111.4 | 105.7 | 39.9 | 110.6 | 115.4 | 110.8 | 15.4 | 129.2 |
| mLLDPE-7 + LD20 | 112.4 | 107.9 | 30.5 | 117.0 | 115.2 | 111.9 | 34.7 | 149.0 |
| mLLDPE-8 | 114.2 | 107.4 | 23.1 | 125.5 | 116.5 | 112.5 | 21.2 | 139.6 |
| mLLDPE-8 + LD20 | 113.5 | 109.3 | 24.9 | 128.8 | 116.3 | 112.8 | 24.4 | 149.6 |
| ZN-LLDPE-1 | 111.5 | 105.3 | 28.3 | 126.2 | 117.2 | 110.7 | 25.7 | 144.8 |
| ZN-LLDPE-1 + LD30 | 112.7 | 107.7 | 22.6 | 131.1 | 115.7 | 112.1 | 40.4 | 144.3 |
| ZN-LLDPE-2 | 119.1 | 114.7 | 33.3 | 158.4 | 121.7 | 117.7 | 25.4 | 184.3 |
| ZN-LLDPE-2 + LD30 | 118.4 | 114.6 | 39.7 | 142.6 | 120.9 | 117.6 | 34.1 | 173.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgali, G.; Kaliappan, S.K.; Pandit, T. Influence of Ethylene-1-Alkene Copolymers Microstructure on Thermo-Rheological Behavior of Model Blends for Enhanced Recycling. Macromol 2022, 2, 168-183. https://doi.org/10.3390/macromol2020011
Galgali G, Kaliappan SK, Pandit T. Influence of Ethylene-1-Alkene Copolymers Microstructure on Thermo-Rheological Behavior of Model Blends for Enhanced Recycling. Macromol. 2022; 2(2):168-183. https://doi.org/10.3390/macromol2020011
Chicago/Turabian StyleGalgali, Girish, Senthil Kumar Kaliappan, and Tej Pandit. 2022. "Influence of Ethylene-1-Alkene Copolymers Microstructure on Thermo-Rheological Behavior of Model Blends for Enhanced Recycling" Macromol 2, no. 2: 168-183. https://doi.org/10.3390/macromol2020011
APA StyleGalgali, G., Kaliappan, S. K., & Pandit, T. (2022). Influence of Ethylene-1-Alkene Copolymers Microstructure on Thermo-Rheological Behavior of Model Blends for Enhanced Recycling. Macromol, 2(2), 168-183. https://doi.org/10.3390/macromol2020011