Impact of Exogenous Nitric Oxide Treatment on Vascularization of a Subcutaneous Device for Cell Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Fabrication
2.2. Scaffold Implantation
2.3. Blood Vessel Functionality
2.4. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Statistics
3. Results
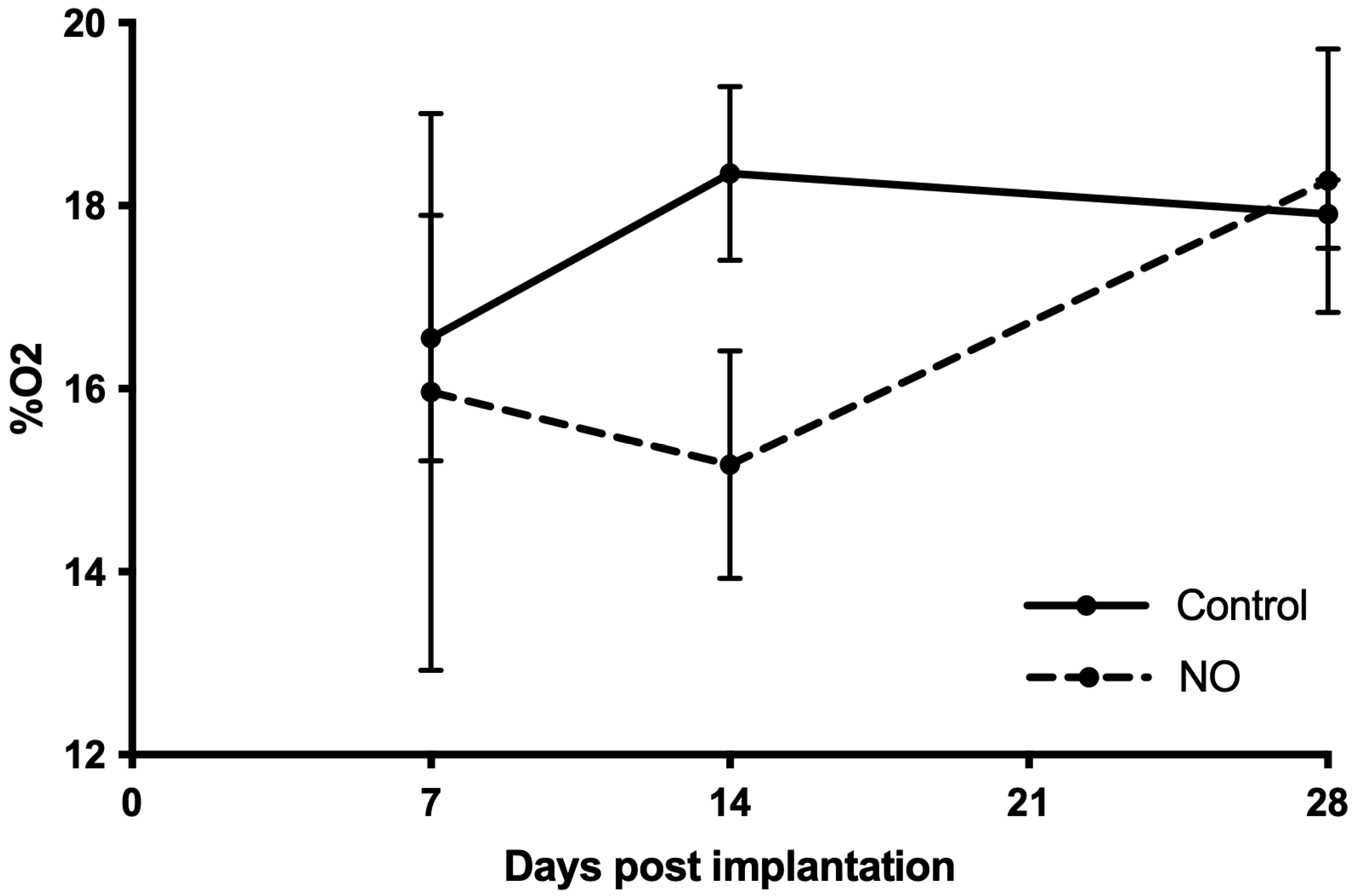
3.1. NO Impairs the Oxygenation during the Vascularization Process
3.2. Blood Vessels Are Well Perfused
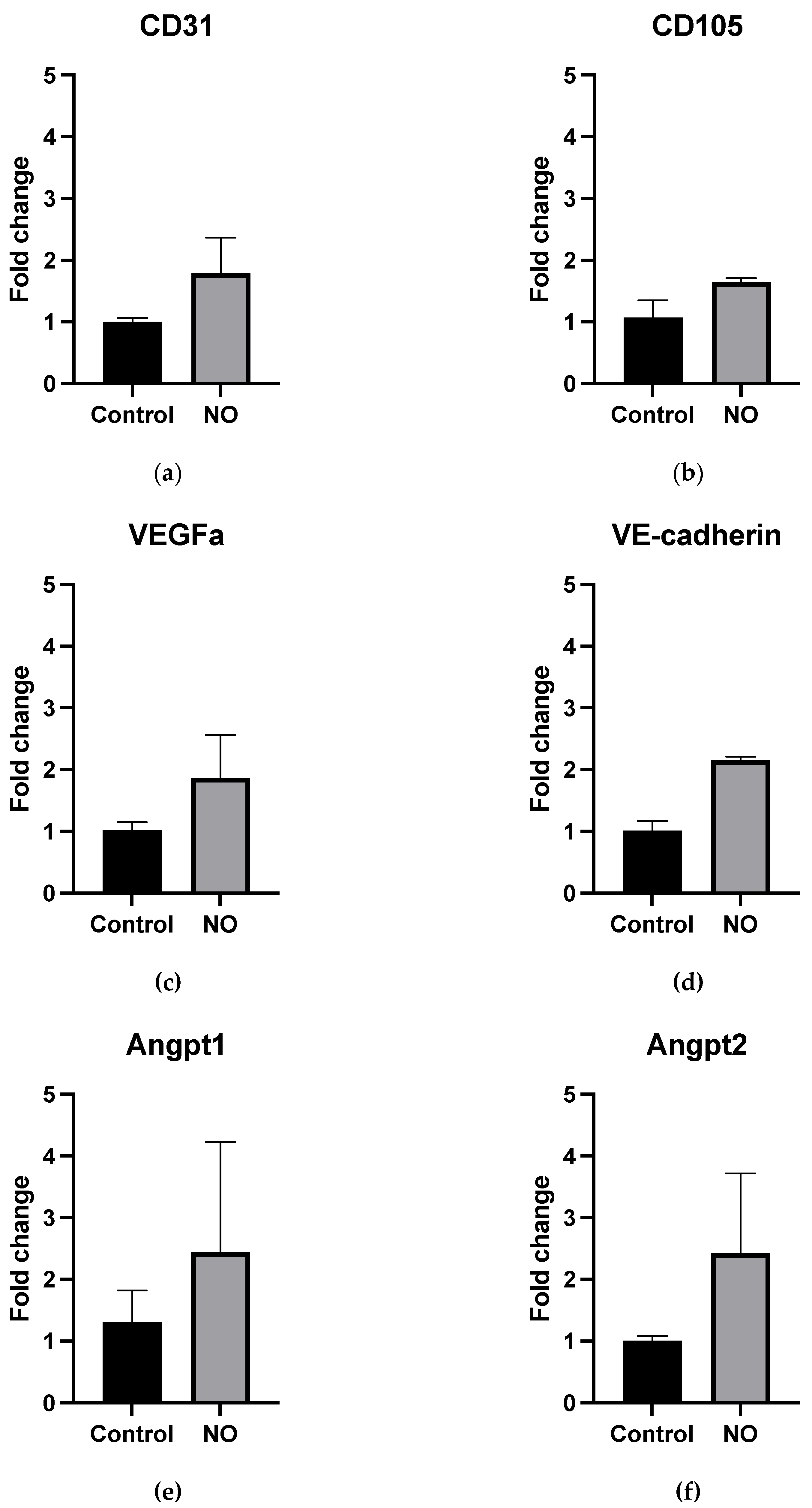
3.3. NO Treatment Does Not Increase mRNA Expression of Vascularization Genes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mobarra, N.; Soleimani, M.; Ghayour-Mobarhan, M.; Safarpour, S.; Ferns, G.A.; Pakzad, R.; Pasalar, P. Hybrid poly-l-lactic acid/poly(e-caprolactone) nanofibrous scaffold can improve biochemical and molecular markers of human induced pluripotent stem cell-derived hepatocyte-like cells. J. Cell. Physiol. 2019, 234, 11247–11255. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Pinatel, E.M.; Leszczynska, A.; Goyal, N.P.; Nishio, T.; Kim, J.; Kneiber, D.; Horcel, L.D.A.; Eguchi, A.; Ordonez, P.M.; et al. Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight 2019, 5, e125652. [Google Scholar] [CrossRef] [PubMed]
- Kamao, H.; Mandai, M.; Ohashi, W.; Hirami, Y.; Kurimoto, Y.; Kiryu, J.; Takahashi, M. Evaluation of the Surgical Device and Procedure for Extracellular Matrix-Scaffold-Supported Human iPSC-Derived Retinal Pigment Epithelium Cell Sheet Transplantation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 211–220. [Google Scholar] [CrossRef]
- Ilmarinen, T.; Thieltges, F.; Hongisto, H.; Juuti-Uusitalo, K.; Koistinen, A.; Kaarniranta, K.; Brinken, R.; Braun, N.; Holz, F.G.; Skottman, H.; et al. Survival and functionality of xeno-free human embryonic stem cell-derived retinal pigment epithelial cells on polyester substrate after transplantation in rabbits. Acta Ophthalmol. 2019, 97, e688–e699. [Google Scholar] [CrossRef]
- Roca, F.G.; Santos, L.G.; Roig, M.M.; Medina, L.M.; Martínez-Ramos, C.; Pradas, M.M. Novel Tissue-Engineered Multimodular Hyaluronic Acid-Polylactic Acid Conduits for the Regeneration of Sciatic Nerve Defect. Biomedicines 2022, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Poongodi, R.; Chen, Y.-L.; Yang, T.-H.; Huang, Y.-H.; Yang, K.D.; Lin, H.-C.; Cheng, J.-K. Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. Int. J. Mol. Sci. 2021, 22, 13347. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Pokrywczynska, M.; Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef]
- Bachul, P.J.; Golab, K.; Basto, L.; Zangan, S.; Pyda, J.S.; Perez-Gutierrez, A.; Borek, P.; Wang, L.J.; Tibudan, M.; Tran, D.K.; et al. Post-Hoc Analysis of a Randomized, Double Blind, Prospective Study at the University of Chicago: Additional Standardizations of Trial Protocol are Needed to Evaluate the Effect of a CXCR1/2 Inhibitor in Islet Allotransplantation. Cell Transplant. 2021, 30, 9636897211001774. [Google Scholar] [CrossRef]
- Smink, A.M.; Li, S.; Hertsig, D.T.; de Haan, B.J.; Schwab, L.; van Apeldoorn, A.A.; de Koning, E.; Faas, M.M.; Lakey, J.R.; de Vos, P. The Efficacy of a Prevascularized, Retrievable Poly(d,l,-lactide-co-epsilon-caprolactone) Subcutaneous Scaffold as Transplantation Site for Pancreatic Islets. Transplantation 2017, 101, e112–e119. [Google Scholar] [CrossRef]
- Kuppan, P.; Kelly, S.; Seeberger, K.; Castro, C.; Rosko, M.; Pepper, A.R.; Korbutt, G.S. Bioabsorption of Subcutaneous Nanofibrous Scaffolds Influences the Engraftment and Function of Neonatal Porcine Islets. Polymers 2022, 14, 1120. [Google Scholar] [CrossRef]
- Bourgeois, S.; Sawatani, T.; Van Mulders, A.; De Leu, N.; Heremans, Y.; Heimberg, H.; Cnop, M.; Staels, W. Towards a Functional Cure for Diabetes Using Stem Cell-Derived Beta Cells: Are We There Yet? Cells 2021, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, D.M.; Mitchell, S.A.; Sackett, S.D.; Odorico, J.S. Mimicking nature-made beta cells: Recent advances towards stem cell-derived islets. Curr. Opin. Organ. Transplant. 2019, 24, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Davalli, A.M.; Ogawa, Y.; Ricordi, C.; Scharp, D.W.; Bonner-Weir, S.; Weir, G.C. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation 1995, 59, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Anazawa, T.; Okajima, H.; Masui, T.; Uemoto, S. Current state and future evolution of pancreatic islet transplantation. Ann. Gastroenterol. Surg. 2018, 3, 34–42. [Google Scholar] [CrossRef]
- Gamble, A.; Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. The journey of islet cell transplantation and future development. Islets 2018, 10, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Rawson, J.; Barriga, A.; Gonzalez, N.; Mendez, D.; Li, J.; Omori, K.; Kandeel, F.; Mullen, Y. Posttransplant oxygen inhalation improves the outcome of subcutaneous islet transplantation: A promising clinical alternative to the conventional intrahepatic site. Am. J. Transplant. 2018, 18, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Bruni, A.; Pawlick, R.; O’Gorman, D.; Kin, T.; Thiesen, A.; Shapiro, A.M.J. Posttransplant Characterization of Long-term Functional hESC-Derived Pancreatic Endoderm Grafts. Diabetes 2019, 68, 953–962. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L. Nitric oxide and angiogenesis. J. Neurooncol. 2000, 50, 139–148. [Google Scholar] [CrossRef]
- Lee, P.C.; Kibbe, M.R.; Schuchert, M.J.; Stolz, D.B.; Watkins, S.C.; Griffith, B.P.; Billiar, T.R.; Shears, L.L., 2nd. Nitric oxide induces angiogenesis and upregulates alpha(v)beta(3) integrin expression on endothelial cells. Microvasc/Res. 2000, 60, 269–280. [Google Scholar] [CrossRef]
- Ziche, M.; Morbidelli, L.; Masini, E.; Amerini, S.; Granger, H.J.; Maggi, C.A.; Geppetti, P.; Ledda, F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J. Clin. Investig. 1994, 94, 2036–2044. [Google Scholar] [CrossRef]
- Beurton, J.; Boudier, A.; Barozzi Seabra, A.; Vrana, N.E.; Clarot, I.; Lavalle, P. Nitric Oxide Delivering Surfaces: An Overview of Functionalization Strategies and Efficiency Progress. Adv. Healthc. Mater. 2022, 11, e2102692. [Google Scholar] [CrossRef] [PubMed]
- Wo, Y.; Brisbois, E.J.; Wu, J.; Li, Z.; Major, T.C.; Mohammed, A.; Wang, X.; Colletta, A.; Bull, J.L.; Matzger, A.J.; et al. Reduction of Thrombosis and Bacterial Infection via Controlled Nitric Oxide (NO) Release from S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated CarboSil Intravascular Catheters. ACS Biomater. Sci. Eng. 2017, 3, 349–359. [Google Scholar] [CrossRef]
- Brisbois, E.J.; Major, T.C.; Goudie, M.J.; Bartlett, R.H.; Meyerhoff, M.E.; Handa, H. Improved hemocompatibility of silicone rubber extracorporeal tubing via solvent swelling-impregnation of S-nitroso-N-acetylpenicillamine (SNAP) and evaluation in rabbit thrombogenicity model. Acta Biomater. 2016, 37, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.D.; Headen, D.M.; Hunckler, M.D.; Coronel, M.M.; Stabler, C.L.; Garcia, A.J. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials 2018, 172, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L.; Donnini, S.; Ziche, M. Role of nitric oxide in the modulation of angiogenesis. Curr. Pharm. Des. 2003, 9, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kabirian, F.; Brouki Milan, P.; Zamanian, A.; Heying, R.; Mozafari, M. Additively manufactured small-diameter vascular grafts with improved tissue healing using a novel SNAP impregnation method. J. Biomed. Mater Res. B Appl. Biomater. 2019, 108, 1322–1331. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; Xiong, K.; Li, X.; Qi, P.; Tu, Q.; Jing, F.; Weng, Y.; Wang, J.; Huang, N. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials 2015, 63, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Chen, S.; Tang, D.; Jiang, L.; Kong, D.; Wang, S. Electrospun poly-e-caprolactone scaffold modified with catalytic nitric oxide generation and heparin for small-diameter vascular graft. RSC Adv. 2017, 7, 18775–18784. [Google Scholar] [CrossRef]
- Joseph, C.A.; McCarthy, C.W.; Tyo, A.G.; Hubbard, K.R.; Fisher, H.C.; Altscheffel, J.A.; He, W.; Pinnaratip, R.; Liu, Y.; Lee, B.P.; et al. Development of an Injectable Nitric Oxide Releasing Poly(ethylene) Glycol-Fibrin Adhesive Hydrogel. ACS Biomater. Sci. Eng. 2019, 5, 959–969. [Google Scholar] [CrossRef]
- Nichols, S.P.; Koh, A.; Brown, N.L.; Rose, M.B.; Sun, B.; Slomberg, D.L.; Riccio, D.A.; Klitzman, B.; Schoenfisch, M.H. The effect of nitric oxide surface flux on the foreign body response to subcutaneous implants. Biomaterials 2012, 33, 6305–6312. [Google Scholar] [CrossRef] [Green Version]
- Smink, A.M.; Hertsig, D.T.; Schwab, L.; van Apeldoorn, A.A.; de Koning, E.; Faas, M.M.; de Haan, B.J.; de Vos, P. A Retrievable, Efficacious Polymeric Scaffold for Subcutaneous Transplantation of Rat Pancreatic Islets. Ann. Surg. 2017, 266, 149–157. [Google Scholar] [CrossRef] [PubMed]

| Gene | Assay ID |
|---|---|
| GAPDH | Mm99999915_g1 |
| CD31 (PECAM1) | Mm01242576_m1 |
| VEGFa | Mm00437306_m1 |
| VE-Cadherin (CDH5) | Mm00486938_m1 |
| CD105 (Endoglin) | Mm00468252_m1 |
| Angiopoietin 1 | Mm00456503_m1 |
| Angiopoietin 2 | Mm00545822_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smink, A.M.; Ceballos, B.; Koster, T.; Rodriquez, S.; Alexander, M.; Lakey, J.R.T.; de Vos, P. Impact of Exogenous Nitric Oxide Treatment on Vascularization of a Subcutaneous Device for Cell Transplantation. Macromol 2022, 2, 476-484. https://doi.org/10.3390/macromol2030029
Smink AM, Ceballos B, Koster T, Rodriquez S, Alexander M, Lakey JRT, de Vos P. Impact of Exogenous Nitric Oxide Treatment on Vascularization of a Subcutaneous Device for Cell Transplantation. Macromol. 2022; 2(3):476-484. https://doi.org/10.3390/macromol2030029
Chicago/Turabian StyleSmink, Alexandra M., Bryan Ceballos, Taco Koster, Samuel Rodriquez, Michael Alexander, Jonathan R. T. Lakey, and Paul de Vos. 2022. "Impact of Exogenous Nitric Oxide Treatment on Vascularization of a Subcutaneous Device for Cell Transplantation" Macromol 2, no. 3: 476-484. https://doi.org/10.3390/macromol2030029
APA StyleSmink, A. M., Ceballos, B., Koster, T., Rodriquez, S., Alexander, M., Lakey, J. R. T., & de Vos, P. (2022). Impact of Exogenous Nitric Oxide Treatment on Vascularization of a Subcutaneous Device for Cell Transplantation. Macromol, 2(3), 476-484. https://doi.org/10.3390/macromol2030029








