Heat-Resistant Polymers with Intense, Visible Photoluminescence Functionality and Fluorescence Probing Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Synthesis of 9,10-bis(4-aminophenyl)anthracene (BAPA)
2.1.2. Synthesis of Model Compounds
2.1.3. Common Monomers and Conjugated Monofunctional Compounds
2.1.4. Polycondensation of PBOs via the One-Pot Process and Film Preparation
2.1.5. Polycondensation of PAzMs and Film Preparation
2.1.6. Polyaddition of PI Precursors [Poly(amic acid) (PAA)], Imidization, and Film Preparation
2.2. Measurements
2.2.1. Ultraviolet (UV)–Visible Absorption Spectra
2.2.2. Photoluminescence (PL) Spectra and PL Quantum Yields
2.2.3. PL Image
2.2.4. Reduced Viscosities
2.2.5. Linear Coefficients of Thermal Expansion (CTE)
2.2.6. Heat Resistance
2.3. Model Reaction of Transamidation in PAA Solutions
3. Results and Discussion
3.1. PL and Thermal Properties of Heat-Resistant Polymers
3.1.1. Polybenzoxazoles
3.1.2. Terminal-Modified Polyimides with Conjugated Monoamines
3.1.3. Terminal-Modified Polyazomethines with Conjugated Monoaldehydes
3.1.4. Polyimides Derived from Common Aromatic Tetracarboxylic Dianhydrides with Cycloaliphatic Diamines
3.1.5. Polyimides Obtained Using Naphthalene-Containing Tetracarboxylic Dianhydrides
3.1.6. Polyimides Obtained Using p-Terphenylene-Containing Tetracarboxylic Dianhydride
3.1.7. Influence of Conjugation in the TCDI Units on the PL Behavior of PI Films
3.1.8. Polyimides Obtained Using Other Monomers with Further Extended Conjugation
3.2. Applications of PEDI Derivatives as Fluorescence Probes
3.2.1. Features of PEDI Fluorescence Behavior
3.2.2. Model Reaction of Transamidation Using PEDI-MBMA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imai, Y.; Taoka, I.; Uno, K.; Iwakura, Y. Polybenzoxazoles and polybenzothiazoles. Makromol. Chem. 1965, 83, 167–178. [Google Scholar] [CrossRef]
- Cassidy, P.E. Thermally Stable Polymers: Syntheses and Properties; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Mittal, K.L. (Ed.) Polyimides: Synthesis, Characterization, and Applications; Plenum Press: New York, NY, USA, 1984; Volumes 1–2. [Google Scholar]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. (Eds.) Polyimides: Thermally Stable Polymers; Plenum: New York, NY, USA, 1987. [Google Scholar]
- Feger, C.; Khojasteh, M.M.; McGrath, J.E. (Eds.) Polyimides: Materials, Chemistry and Characterization; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Sroog, C.E. Polyimide. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Abadie, M.J.M.; Sillion, B. (Eds.) Polyimides and Other High-Temperature Polymers; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Bessonov, M.I.; Zubkov, V.A. (Eds.) Polyamic Acid and Polyimides: Synthesis, Transformation and Structure; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Feger, C.; Khojasteh, M.M.; Htoo, M.S. (Eds.) Advances in Polyimide Science and Technology; Technomic Publishing: Lancaster, PA, USA, 1993. [Google Scholar]
- Ghosh, M.K.; Mittal, K.L. (Eds.) Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Sachdev, H.S.; Khojasteh, M.M.; Feger, C. (Eds.) Advances in Polyimides and Low Dielectric Polymers; Society of Plastic Engineers: New York, NY, USA, 1997. [Google Scholar]
- Hergenrother, P.M. The use, design, synthesis, and properties of high performance/high temperature polymers: An overview. High Perform. Polym. 2003, 15, 3–45. [Google Scholar] [CrossRef]
- Ando, S.; Ueda, M.; Kakimoto, M.; Kochi, M.; Takeichi, T.; Hasegawa, M.; Yokota, R. (Eds.) The Latest Polyimides: Fundamentals and Applications, 2nd ed.; NTS: Tokyo, Japan, 2010. [Google Scholar]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Yang, C.Y. (Ed.) Advanced Polyimide Materials: Synthesis, Characterization, and Applications; Chemical Industry Press: Shanghai, China; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Diaham, S. (Ed.) Polyimide for Electronic and Electrical Engineering Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Fukukawa, K.; Ueda, M. Recent development of photosensitive polybenzoxazoles. Polym. J. 2006, 38, 405–418. [Google Scholar] [CrossRef]
- Fukukawa, K.; Ueda, M. Recent progress of photosensitive polyimides. Polym. J. 2008, 40, 281–296. [Google Scholar] [CrossRef]
- Freilich, S.C. Photoconductivity of donor-loaded polyimides. Macromolecules 1987, 20, 973–978. [Google Scholar] [CrossRef]
- Jeong, K.M.; Tapaswi, P.K.; Kambara, T.; Ishige, R.; Ando, S.; Ha, C.S. Photoconductive polyimides derived from a novel imid-azole-containing diamine. High Perform. Polym. 2020, 32, 620–630. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsai, C.Y.; Li, L.J.; Liaw, D.J. Colorless-to-colorful switching electrochromic polyimides with very high contrast ratio. Nat. Commun. 2019, 10, 1239. [Google Scholar] [CrossRef]
- Kim, D.Y.; Cho, H.N.; Kim, C.Y. Blue light emitting polymers. Prog. Polym. Sci. 2000, 25, 1089. [Google Scholar] [CrossRef]
- Ghassemi, H.; Zhu, J.H. Fluorescence of perylene- and naphthalene-containing polyimides: Evidence of molecular aggregation and chain coiling in chloroform solution. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1633–1639. [Google Scholar] [CrossRef]
- Li, W.; Fox, M.A. Photophysical and photochemical properties of anthryl-labeled polyimides: Fluorescence, electron transfer, and photoreaction. J. Phys. Chem. B 1997, 101, 11068–11076. [Google Scholar] [CrossRef]
- Pyo, S.M.; Kim, S.I.; Shin, T.J.; Ree, M.; Park, K.H.; Kang, J.S. Synthesis and blue light-emitting characteristic of rod-like poly(4,4′-biphenylene pyromellitimide) with fury1 side groups. Polymer 1998, 40, 125–130. [Google Scholar] [CrossRef]
- Pyo, S.M.; Kim, S.I.; Shin, T.J.; Park, H.K.; Ree, M.; Park, K.H.; Kang, J.S. Synthesis and characterization of a new blue-light-emitting polyimide. Macromolecules 1998, 31, 4777–4781. [Google Scholar] [CrossRef]
- Mal’tsev, E.I.; Brusentseva, M.A.; Lypenko, D.A.; Berendyaev, V.I.; Kolesnikov, V.A.; Kotov, B.V.; Vannikov, A.V. Electrolumi-nescent properties of anthracene-containing polyimides. Polym. Adv. Technol. 2000, 11, 325–329. [Google Scholar] [CrossRef]
- Huang, W.; Yan, D.; Lu, Q.; Huang, Y. Synthesis and characterization of highly soluble fluorescent main chain copolyimides containing perylene units. Eur. Polym. J. 2003, 39, 1099–1104. [Google Scholar] [CrossRef]
- Kudo, K.; Imai, T.; Hamada, T.; Sakamoto, S. Synthesis of blue emitting alicyclic polyimides using one-pot alternating copoly-merization method. High Perform. Polym. 2006, 18, 749–759. [Google Scholar] [CrossRef]
- Ishizaka, T.; Kasai, H.; Oikawa, H.; Nakanishi, H. Unique luminescence properties of Eu3+-doped polyimide. J. Photochem. Pho-tobiol. A Chem. 2006, 183, 280–284. [Google Scholar] [CrossRef]
- Wu, J.H.; Liou, G.S. High-efficiency fluorescent polyimides based on locally excited triarylamine-containing dianhydride moieties. Polym. Chem. 2015, 6, 5225–5232. [Google Scholar] [CrossRef]
- Wakita, J.; Sekino, H.; Sakai, K.; Urano, Y.; Ando, S. Molecular design, synthesis, and properties of highly fluorescent polyimides. J. Phys. Chem. B 2009, 113, 15212–15224. [Google Scholar] [CrossRef] [PubMed]
- Kanosue, K.; Hirata, S.; Vacha, M.; Augulis, R.; Gulbinas, V.; Ishige, R.; Ando, S. A colorless semi-aromatic polyimide derived from a sterically hindered bromine-substituted dianhydride exhibiting dual fluorescence and phosphorescence emission. Mater. Chem. Front. 2019, 3, 39–49. [Google Scholar] [CrossRef]
- Liang, N.; Fujiwara, E.; Nara, M.; Ishige, R.; Ando, S. Colorless copolyimide films exhibiting large stokes-shifted photolumines-cence applicable for spectral conversion. ACS Appl. Polym. Mater. 2021, 3, 3911–3921. [Google Scholar] [CrossRef]
- Tabuchi, A.; Hayakawa, T.; Kuwata, S.; Ishige, R.; Ando, S. Synthesis and characterization of white-light luminescent end-capped polyimides based on FRET and excited state intramolecular proton transfer. Polymers 2021, 13, 4050. [Google Scholar] [CrossRef]
- Ando, S. Characteristic changes in the structures and properties of polyimides induced by very high pressures up to 8 GPa. Polym. J. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ichikawa, K.; Takahashi, S.; Ishii, J. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride: Strategies to reduce the coefficients of thermal expansion by maximizing the spontaneous chain orientation behavior during solution casting. Polymers 2022, 14, 1131. [Google Scholar] [CrossRef]
- Horii, S.; Hasegawa, M. Photoluminescent high-temperature polymers (6). Polyimides, polybenzoxazoles, and polyazomethines. Polym. Prepr. Jpn. 2006, 55, 4089. [Google Scholar]
- Nitta, H.; Hasegawa, M. Photoluminescent high-temperature polymers (9). Highly blue-fluorescent soluble polyimides. Polym. Prepr. Jpn. 2008, 57, 4183. [Google Scholar]
- Etienne, A.; Arcos, J.C. ms-Bis(aminophenyl)anthradiols. I. ms-Bis(p-dialkylaminophenyl)anthradiols. Bull. Soc. Chim. Fr. 1951, 236, 727–732. [Google Scholar]
- Popp, F.D.; McEwen, W.E. Polyphosphoric acid as a reagent in organic chemistry. Chem. Rev. 1958, 58, 321–401. [Google Scholar] [CrossRef]
- Birks, J.B. Fluorescence quantum yield measurements. J. Res. Natl. Bur. Stand. 1976, 80A, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.E.; Kamat, P.V. Photochemistry of 3,4,9,10-perylenetetracarboxylic dianhydride dyes. 3. Singlet and triplet excited-state properties of the bis(2,5-di-tert-butylphenyl)imide derivative. J. Phys. Chem. 1987, 91, 6373–6380. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hasegawa, M. Highly Tough and Highly Transparent Soluble Polybenzoxazoles. High Perform. Polym. 2007, 19, 243–269. [Google Scholar] [CrossRef]
- Chou, P.T.; Cooper, W.C.; Clements, J.H.; Studer, S.L.; Chang, C.P. A comparative study. The photophysics of 2-phenylbenzoxazoles and 2-phenylbenzothiazoles. Chem. Phys. Lett. 1993, 216, 300–304. [Google Scholar] [CrossRef]
- Bains, G.; Patel, A.B.; Narayanaswami, V. Pyrene: A probe to study protein conformation and conformational changes. Mole-cules 2011, 16, 7909–7935. [Google Scholar] [CrossRef]
- Barik, S.; Skene, W.G. Turning-on the quenched fluorescence of azomethines through structural modifications. Eur. J. Org. Chem. 2013, 2013, 2563–2572. [Google Scholar] [CrossRef]
- Kaya, I.; Yıldırım, M.; Avcı, A. Synthesis and characterization of fluorescent polyphenol species derived from methyl substitut-ed aminopyridine based Schiff bases: The effect of substituent position on optical, electrical, electrochemical, and fluorescence properties. Synth. Met. 2010, 160, 911–920. [Google Scholar] [CrossRef]
- Yoshino, J.; Kano, N.; Kawashima, T. Fluorescence properties of simple N-substituted aldimines with a B-N interaction and their fluorescence quenching by a cyanide ion. J. Org. Chem. 2009, 74, 7496–7503. [Google Scholar] [CrossRef]
- Ishii, J.; Oshima, N.; Tanaka, Y.; Hasegawa, M. Film properties of polyazomethines (1). Effect of incorporation of iintramolecular cyclodehydrating uunits. High Perform. Polym. 2010, 22, 259–273. [Google Scholar] [CrossRef]
- Morgan, P.W.; Kwolek, S.L.; Pletcher, T.C. Aromatic azomethine polymers and fibers. Macromolecules 1987, 20, 729–739. [Google Scholar] [CrossRef]
- Ishida, H.; Wellinghoff, S.T.; Baer, E.; Koenig, J.L. Spectroscopic studies of poly[N,N′-bis(phenoxyphenyl)pyromellitimide]. 1. Structures of the polyimide and three model compounds. Macromolecules 1980, 13, 826–834. [Google Scholar] [CrossRef]
- Rao, C.N.R. Ultra-violet and visible spectroscopy. In Chemical Applications, 2nd ed.; Butterworth & Co.: London, UK, 1967. [Google Scholar]
- Hasegawa, M.; Koyanaka, M. Polyimides containing trans-1,4-cyclohexane unit. Polymerizability of their precursors and low-CTE, low-k and high-Tg properties. High Perform. Polym. 2003, 15, 47–64. [Google Scholar] [CrossRef]
- Hasegawa, M.; Shindo, Y.; Sugimura, T.; Ohshima, S.; Horie, K.; Kochi, M.; Yokota, R.; Mita, I. Photophysical processes in ar-omatic polyimides. Studies with model compounds. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 1617–1625. [Google Scholar] [CrossRef]
- Ishii, J.; Horii, S.; Sensui, N.; Hasegawa, M.; Vladimirov, L.; Kochi, M.; Yokota, R. Polyimides containing trans-1,4-cyclohexane unit (III). Ordered structure and intermolecular interaction in s-BPDA/CHDA polyimide. High Perform. Polym. 2009, 21, 282–303. [Google Scholar] [CrossRef]
- Hemker, D.J.; Frank, C.W.; Thomas, J.W. Photophysical studies of amorphous orientation in poly(ethylene terephthalate) films. Polymer 1988, 29, 437–447. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sensui, N.; Shindo, Y.; Yokota, R. Structure and properties of novel asymmetric biphenyl-type polyimides. Ho-mo- and copolymers and blends. Macromolecules 1999, 32, 387–396. [Google Scholar] [CrossRef]
- Tong, Y.; Huang, W.; Luo, J.; Ding, M. Synthesis and properties of aromatic polyimides derived from 2,2′,3,3′-biphenyltetracarboxylic dianhydride. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1425–1433. [Google Scholar] [CrossRef]
- Rozhanskii, I.; Okuyama, K.; Goto, K. Synthesis and properties of polyimides derived from isomeric biphenyltetracarboxylic dianhydrides. Polymer 2000, 41, 7057–7065. [Google Scholar] [CrossRef]
- Hasegawa, M.; Koseki, K. Poly(ester imide)s possessing low CTE and low water absorption. High Perform. Polym. 2006, 18, 697–717. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirai, T.; Ishigami, T.; Takahashi, S.; Ishii, J. Optically transparent aromatic poly(ester imide)s with low coeffi-cients of thermal expansion (2) Effect of introduction of alkyl-substituted p-biphenylene units. Polym. Int. 2018, 67, 431–444. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horii, S. Low-CTE polyimides derived from 2,3,6,7-naphthalenetetracarboxylic dianhydride. Polym. J. 2007, 39, 610–621. [Google Scholar] [CrossRef]
- Doi, M.; Muto, K.; Nara, M.; Liang, N.; Sano, K.; Mori, H.; Ishige, R.; Ando, S. Photoluminescence properties of copolyimides containing naphthalene core and analysis of excitation energy transfer between the dianhydride moieties. J. Photopolym. Sci. Technol. 2021, 34, 423–430. [Google Scholar] [CrossRef]
- Aveline, B.M.; Matsugo, S.; Redmond, R.W. Photochemical mechanisms responsible for the versatile application of naph-thalimides and naphthaldiimides in biological systems. J. Am. Chem. Soc. 1997, 119, 11785–11795. [Google Scholar] [CrossRef]
- Ueshima, K.; Kikuchi, T. Some properties of polyimides derived from p- or m-terphenyltetracarboxylic dianhydride. Polym. Prepr. Jpn. 1990, 39, 796–798. [Google Scholar]
- Inoue, T.; Kakimoto, M.; Imai, Y.; Watanabe, J. First observation of a thermotropic liquid crystal in a simple polyimide derived from 1,11-diaminoundecane and 4,4′-terphenyltetracarboxylic acid. Macromolecules 1996, 28, 6368–6370. [Google Scholar] [CrossRef]
- Sato, M.; Nakamoto, Y.; Yonetake, K.; Kido, J. Preparation, thermotropic liquid-crystalline and fluorescent properties of semi-rigid homo- and copoly(ester-imide)s composed of 3,3″,4,4″-p-terphenyltetracarboxdiimide and 3,3′,4,4′-biphenyltetracarboxdiimide. Polym. J. 2002, 34, 601–607. [Google Scholar] [CrossRef]
- Ishii, J.; Takata, A.; Oami, Y.; Yokota, R.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides in-duced by thermal imidization (6). Mechanism of negative in-plane CTE generation in non-stretched polyimide films. Eur. Polym. J. 2010, 46, 681–693. [Google Scholar] [CrossRef]
- Ebisawa, S.; Ishii, J.; Sato, M.; Vladimirov, L.; Hasegawa, M. Spontaneous molecular orientation of polyimides induced by thermal imidization (5). Effect of ordered structure formation in polyimide precursors on CTE. Eur. Polym. J. 2010, 46, 283–297. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. The measurement of photoluminescence quantum yields. A review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar]
- Suzuki, H.; Abe, T.; Takaishi, K.; Narita, M.; Hamada, F. The synthesis and X-ray structure of 1,2,3,4-cyclobutane tetracarbox-ylic dianhydride and the preparation of a new type of polyimide showing excellent transparency and heat resistance. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 108–116. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kasamatsu, K.; Koseki, K. Colorless poly(ester imide)s derived from hydrogenated trimellitic anhydride. Eur. Polym. J. 2012, 48, 483–498. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishii, J.; Shindo, Y. Unique fluorescence behavior of perylenetetracarboxydiimides in polyimide systems. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 827–840. [Google Scholar] [CrossRef]
- Ishii, J.; Shimizu, N.; Ishihara, N.; Ikeda, Y.; Sensui, N.; Matano, T.; Hasegawa, M. Spontaneous molecular orientation of polyi-mides induced by thermal imidization (4). Casting- and melt-induced in-plane orientation. Eur. Polym. J. 2010, 46, 69–80. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishii, J.; Shindo, Y. Polyimide/Polyimide blend miscibility probed by perylenetetracarboxydiimide fluorescence. Macromolecules 1999, 32, 6111–6119. [Google Scholar] [CrossRef]
- Kreuz, J.A.; Coff, D.L. Materials Science of High Temperature Polymers for Microelectronics (Materials Research Society Symposium Proceedings); Grubb, D.T., Mita, I., Yoon, D.Y., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1991; Volume 227, p. 11. [Google Scholar]












| No. | Bis(o-aminophenol) (mol%) | Dicarboxylic Acid (mol%) | ηred (dL/g) | Tg (°C) | Td5 (N2) (°C) | Td5 (Air) (°C) | CTE (ppm/K) | Abs at λEX | λEX (nm) | λEM (Peak) (nm) | ΦPL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DAR | 1,3-CHDCA (a) | 2.17 | 325 | 48.0 | 0.36 | 350 | 391 | 0.026 | ||
| 2 | p-HAB | Bis-B-AF | 1.51 | 340 | 529 | 524 | 40.0 | >3 | 350 | 427 | 0.011 |
| 3 | m-HAB | 1,3-CHDCA (a) | 2.33 | 241 | 51.1 | 0.30 | 350 | 424 | 0.017 | ||
| 4 | ADPE | Bis-B-AF | 1.46 | 298 | 528 | 517 | 57.0 | 1.78 | 370 | 420 | 0.013 |
| 5 | 1,3-CHDCA (a) | 1.30 | 195 | 470 | 443 | 55.3 | >3 | 310 | 363/477 | 0.015 | |
| 6 | 1,4-CHDCA (a) | 6.08 | 217 | 486 | 418 | 68.1 | >3 | 310 | 367/558 | 0.0032 | |
| 7 | 6FAP | Bis-B-AF | 0.56 | 320 | 513 | 510 | 55.0 | 0.66 | 350 | 404/428 | 0.22 |
| 8 | OBA | 0.89 | 302 | 533 | 522 | 51.0 | 0.32 | 350 | 387/409 | 0.065 | |
| 9 | 1,4-CHDCA (a) | 2.16 | 269 | 483 | 377 | 64.5 | 0.25 | 325 | 365/380 | 0.14 | |
| 10 | OBA (99) 3,9-PEDCA (1) | 0.39 | 291 | 530 | 520 | 55.7 | 1.08 | 469 | 515 | 0.26 |
| No. | Diamine (mol%) Monoamine (mol%) | ηred (PAA) (dL/g) | Tg (°C) | Td5 (N2) (°C) | Td5 (Air) (°C) | CTE (ppm/K) | λEX (nm) | λEM (Peak) (nm) | ΦPL |
|---|---|---|---|---|---|---|---|---|---|
| 11 | MBCHA (99.5) 1-APY (1.0) | 1.16 | 334 | 429 | 372 | 66.0 | 330 | 375, 394 | 0.26 |
| 12 | MBCHA (99.5) 1-AAN (1.0) | 0.820 | 334 | 429 | 373 | 63.9 | 365 | 392, 413, 438 | 0.12 |
| 13 | MBCHA (99.5) 2-AAN (1.0) | 0.704 | 330 | 424 | 367 | 62.4 | 343 | 387, 408, 441, 470 | 0.12 |
| No. | Tetracarboxylic Dianhydride | Diamine | ηred (PAA) (dL/g) | Tg (°C) | Td5 (N2) (°C) | Td5 (Air) (°C) | CTE (ppm/K) | λEX (nm) | λEM (Peak) (nm) | ΦPL |
|---|---|---|---|---|---|---|---|---|---|---|
| 14 | PMDA | MBCHA | 1.44 | 342 | 464 | 390 | 55.0 | 300 | 430 | 0.0019 |
| 15 | M-MBCHA | 1.17 | 307 | 436 | 360 | 59.0 | 300 | 435 | 0.0016 | |
| 16 | s-BPDA | t-CHDA | 1.55 | 360 | 481 | 444 | 9.5 | 300 | 405 | 0.080 |
| 17 | MBCHA | 1.21 | 232 | 470 | 442 | 62.3 | 300 | 414 | 0.050 | |
| 18 | M-MBCHA | 1.00 | 253 | 438 | 365 | 53.0 | 300 | 406 | 0.024 | |
| 19 | TFMB | 1.65 | 310 | >550 | >550 | 34.8 | 300 | 479 (CT) | 0.013 | |
| 20 | p-PDA | 1.78 | 370 | 587 | 571 | 10.3 | 300 | 537 (CT) | 0.0021 | |
| 21 | a-BPDA | MBCHA | 0.623 | 289 | 465 | 426 | 56.1 | 300 | 396 | 0.0012 |
| 22 | i-BPDA | MBCHA | 0.281 | - (a) | - (a) | - (a) | - (a) | 300 | 394 | 0.0078 |
| 23 | HQDA | MBCHA | 1.50 | 217 | - | - | 75.3 | 350 | 434 | 0.061 (0.11) (b) |
| 24 | CBDA (90) HQDA (10) | MBCHA | 0.78 | 346 | 435 | 386 | 63.8 | 350 | 424 | 0.064 (0.067) (c) |
| 25 | TA-HQ | MBCHA (d) | 2.41 | ND (e) | 437 | 376 | 51.5 | 350 | 465 | 0.0016 |
| 26 | TA-44BP | MBCHA | 0.49 | 212 | 447 | 396 | 62.4 | 350 | 530 | 0.0019 |
| No. | Tetracarboxylic Dianhydride | Diamine | ηred (PAA) (dL/g) | Tg (°C) | Td5 (N2) (°C) | Td5 (Air) (°C) | CTE (ppm/K) | λEX (nm) | λEM (Peak) (nm) | ΦPL |
|---|---|---|---|---|---|---|---|---|---|---|
| 27 | 2,3,6,7-NTDA | MBCHA | 1.94 | 376 | 465 | 403 | 45.2 | 350 | 406 | 0.014 |
| 28 | M-MBCHA | 2.00 | 347 | 448 | 381 | 51.0 | 350 | 404 | 0.021 | |
| 29 | TFMB | 2.15 | ND | 582 | 559 | –3.2 | 350 | 510 (CT) | 0.0051 | |
| 30 | p-PDA | 3.21 | ND | >590 | 580 | 3.1 | 350 | 580 (CT) | 0.0007 | |
| 31 | CBDA (50) 2,3,6,7-NTDA (50) | MBCHA | 0.76 | 340 | 432 | 382 | 55.6 | 350 | 404 | 0.034 |
| 32 | CBDA (90) 2,3,6,7-NTDA (10) | MBCHA | 0.64 | 337 | 420 | 381 | 62.6 | 350 | 402 | 0.088 |
| 33 | CBDA (98) 2,3,6,7-NTDA (2) | MBCHA | 0.69 | 336 | 427 | 372 | 63.6 | 350 | 401 | 0.15 |
| 34 | CBDA (98) 2,3-NA (4) | MBCHA | 1.15 | 329 | 427 | 366 | 62.3 | 358 | 385 | 0.13 |
| 35 | CBDA (90) 1,4,5,8-NTDA (10) | MBCHA | 0.65 | 334 | 426 | 379 | 60.7 | 360 | ~500 | 0.0008 |
| 36 | CBDA (98) 1,8-NA (4) | MBCHA | 0.93 | 350 | 432 | 377 | 65.8 | 358 | 422 | 0.049 |
| No. | Tetracarboxylic Dianhydride (mol%) | Diamine (mol%) | ηred (PAA) (dL/g) | Tg (°C) | Td5 (N2) (°C) | Td5 (Air) (°C) | CTE (ppm/K) | λEX (nm) | λEM (Peak) (nm) | ΦPL |
|---|---|---|---|---|---|---|---|---|---|---|
| 37 | TPDA | t-CHDA | 1.17 | 316 | - | - | 8.0 | 350 | 434 | 0.15 |
| 38 | MBCHA | 1.16 | 222 | 477 | 444 | 51.9 | 350 | 434 | 0.26 | |
| 39 | TFMB | 1.02 | 254 | 591 | - | 37.2 | 350 | 444 | 0.16 | |
| 40 | p-PDA | 2.11 | ND | - | - | 3.0 | 350 | 523 (CT) | 0.0028 | |
| 41 | CBDA (90) TPDA (10) | MBCHA | 1.25 | 328 | 432 | 377 | 70.8 | 350 | 424 | 0.41 |
| 42 | CBDA | MBCHA (99.8) PEDI-MBMA (0.2) | 1.52 | 318 | 428 | 373 | 65.3 | 490 | 542, 581, 625 | 0.67 |
| 43 | CBDA | MBCHA (98) BAPA (2) | 0.60 | 339 | 438 | 391 | 63.1 | 399 | 441 | 0.17 |
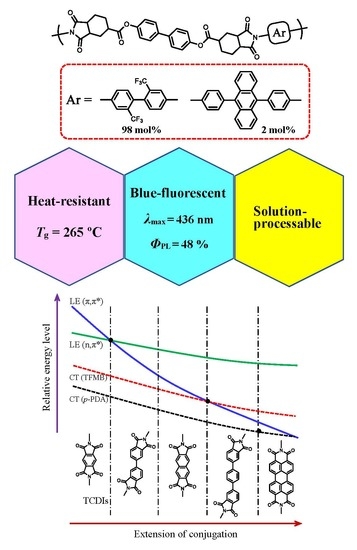
| 44 | HTA-44BP | TFMB (98) BAPA (2) | 1.32 (PAA) 0.84 (PI) | 265 | 423 | 396 | 69.6 | 378 | 436 | 0.48 (0.804) (a) |
| TCDA System | S1 State of CHA-Based Model Compounds in Solution | Effect of Weakened Chain Stacking on PL Yield | Fluorophore Dilution Effect on PL Yield | PL Character of TFMB-Based PIs | PL Character of p-PDA-Based PIs |
|---|---|---|---|---|---|
| PMDA | (n, π*) | Slight decrease or little change | CT | CT | |
| s-BPDA | (π, π*) | Decrease | CT | CT | |
| 2,3,6,7-NTDA | (π, π*) | Some increase | Remarkable enhancement | CT | CT |
| TPDA | (π, π*) | Some increase | Some increase | LE | CT |
 | |||||
| Structures of PEDI Analogs | ΦPL |
|---|---|
(a) | 0.90 |
(b) | 7.4 × 10−4 |
(c) | 8.4 × 10−3 |
(d) | 3.7 × 10−3 |
(e) | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, M.; Horii, S. Heat-Resistant Polymers with Intense, Visible Photoluminescence Functionality and Fluorescence Probing Application. Macromol 2023, 3, 245-274. https://doi.org/10.3390/macromol3020016
Hasegawa M, Horii S. Heat-Resistant Polymers with Intense, Visible Photoluminescence Functionality and Fluorescence Probing Application. Macromol. 2023; 3(2):245-274. https://doi.org/10.3390/macromol3020016
Chicago/Turabian StyleHasegawa, Masatoshi, and Shunichi Horii. 2023. "Heat-Resistant Polymers with Intense, Visible Photoluminescence Functionality and Fluorescence Probing Application" Macromol 3, no. 2: 245-274. https://doi.org/10.3390/macromol3020016
APA StyleHasegawa, M., & Horii, S. (2023). Heat-Resistant Polymers with Intense, Visible Photoluminescence Functionality and Fluorescence Probing Application. Macromol, 3(2), 245-274. https://doi.org/10.3390/macromol3020016