Abstract
A series of six composites was prepared from the reaction of lignin-derived guaiacol, fatty acids, and sulfur. In this preparation, the organic comonomers undergo C–S bond-forming reactions to establish a highly crosslinked network material in which some non-covalently incorporated sulfur species are also entrapped. Both monounsaturated oleic acid and diunsaturated linoleic acid were used as fatty acid components to assess the influence of their unsaturation levels on composite properties. The ratio of organics and the proportion of sulfur (70 or 80 wt%) was also varied to assess the effect on thermal, morphological, and mechanical properties. Thermogravimetric analysis showed that composites exhibited good thermal stability up to ~220 °C. Differential scanning calorimetry revealed that the materials generally exhibit melting features for entrapped cyclo-S8, cold crystallization features for some materials, and a composition-dependent glass transition temperature. The flexural and compressive strengths of the composites revealed that some of the composites exhibit strengths significantly higher than those required of Portland cements used in residential housing fabrication and may be more sustainable structural materials. The thermal and mechanical properties could be tailored by changing the degree of unsaturation of the fatty acid comonomer or by altering the percentage of fatty acid in the monomer feed. The highest mechanical strength was achieved with greater amounts of monounsaturated oleic acid comonomer.
Keywords:
sustainable composite; sulfur; fatty acid; inverse vulcanization; guaiacol; sustainability 1. Introduction
Leveraging lower-value, waste, or bio-based materials to replace ecologically harmful mineral and plastic structural goods is vital for the success of a green economy [1,2]. Lignin, for example, is the most underutilized lignocellulosic biomass constituent, so lignin oils, comprising small molecular lignin breakdown products like guaiacol, are hotly pursued as chemical feedstocks for more sustainably sourced commodities [3,4,5,6,7,8,9,10,11,12]. Another abundant industry byproduct is elemental sulfur. Sulfur is a product of fossil fuel refining whose production outpaces all current economically viable uses by over 7 Mt per year [13,14,15,16,17,18,19]. Based on the abundance and affordability of sulfur, various high sulfur-content materials (HSMs) have been pursued for applications such as sulfur cements [20,21,22,23,24,25]. Owing to the thermal reversibility of S–S bond breakage and formation such HSMs are often thermally recyclable over many cycles without any loss in mechanical strength [26,27,28,29,30,31]. More sustainable sulfur composites can be prepared when petroleum-derived olefins are replaced with biologically produced monomers, such as fatty acids [26,32,33] or lignin derivatives [4,5,6,34]. Reaction temperatures for preparing or shaping HSMs can be lowered by using catalysts [35], mechanochemical methods [36], ternary mixtures [37], pre-formation of more reactive sulfur species [37,38,39], nucleophiles to fuse materials through S–S metathesis [40], or by compression-molding of materials [41]. Promising photochemical routes to C–S bond formation towards preparing or healing HSMs also hold potential for lowering the energy requirements of these processes [42].
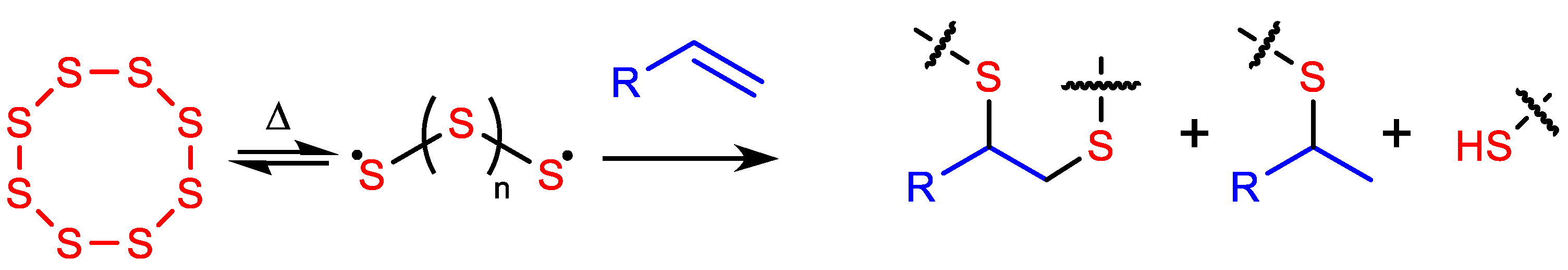
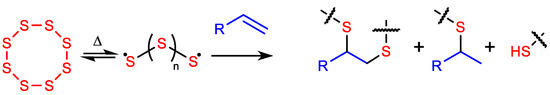
HSMs have most often been prepared by inverse vulcanization (Scheme 1) [43,44,45,46,47], a convenient process whereby thermally generated polysulfur radicals add across olefins, typically at 159–180 °C, to create highly crosslinked networks comprising oligo- and poly-sulfur chains [13,14,15,16,17,18]. Other routes to HSMs have been developed for C–S bond formation between elemental sulfur and organic thermal decomposition products, requiring higher temperatures of 220–320 °C [5,34].
Scheme 1.
Inverse vulcanization occurs when elemental sulfur reacts with olefins, resulting in high sulfur-content materials (HSMs) comprising crosslinked networks.
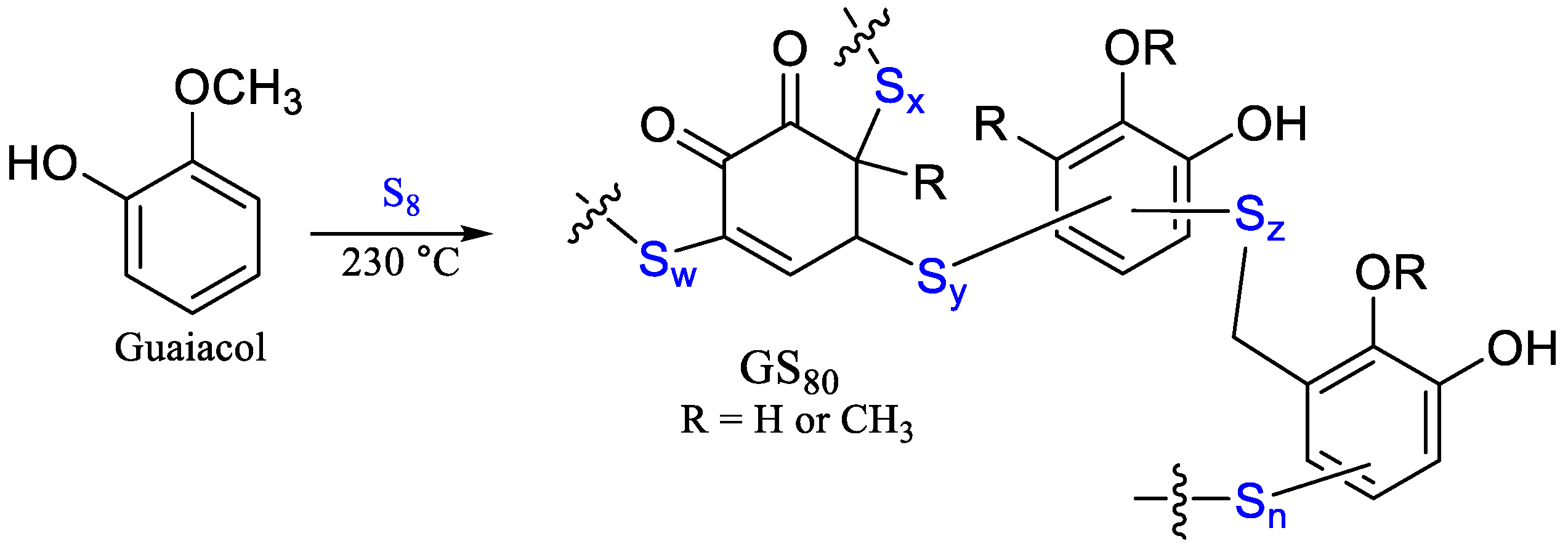
We have previously reported a guaiacol–sulfur composite (GS80, Scheme 2) formed through the reaction of guaiacol and sulfur at 230 °C to facilitate the formation of a crosslinked network via S–Caryl bond-formation [34]. GS80 is a relatively soft and flexible composite as compared to most HSMs, with relatively poor compressive strength (<10 MPa). HSMs with fatty acid crosslinkers like oleic or linoleic acid, on the other hand, are quite brittle but can exhibit compressive strengths (up to 19.4 MPa) higher than the 17 MPa required for Portland cements used in residential housing foundations [19,26,32,33].
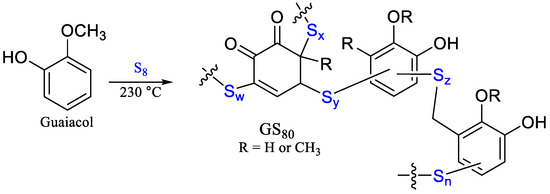
Scheme 2.
The reaction of guaiacol with elemental sulfur leads to formation of composite GS80.
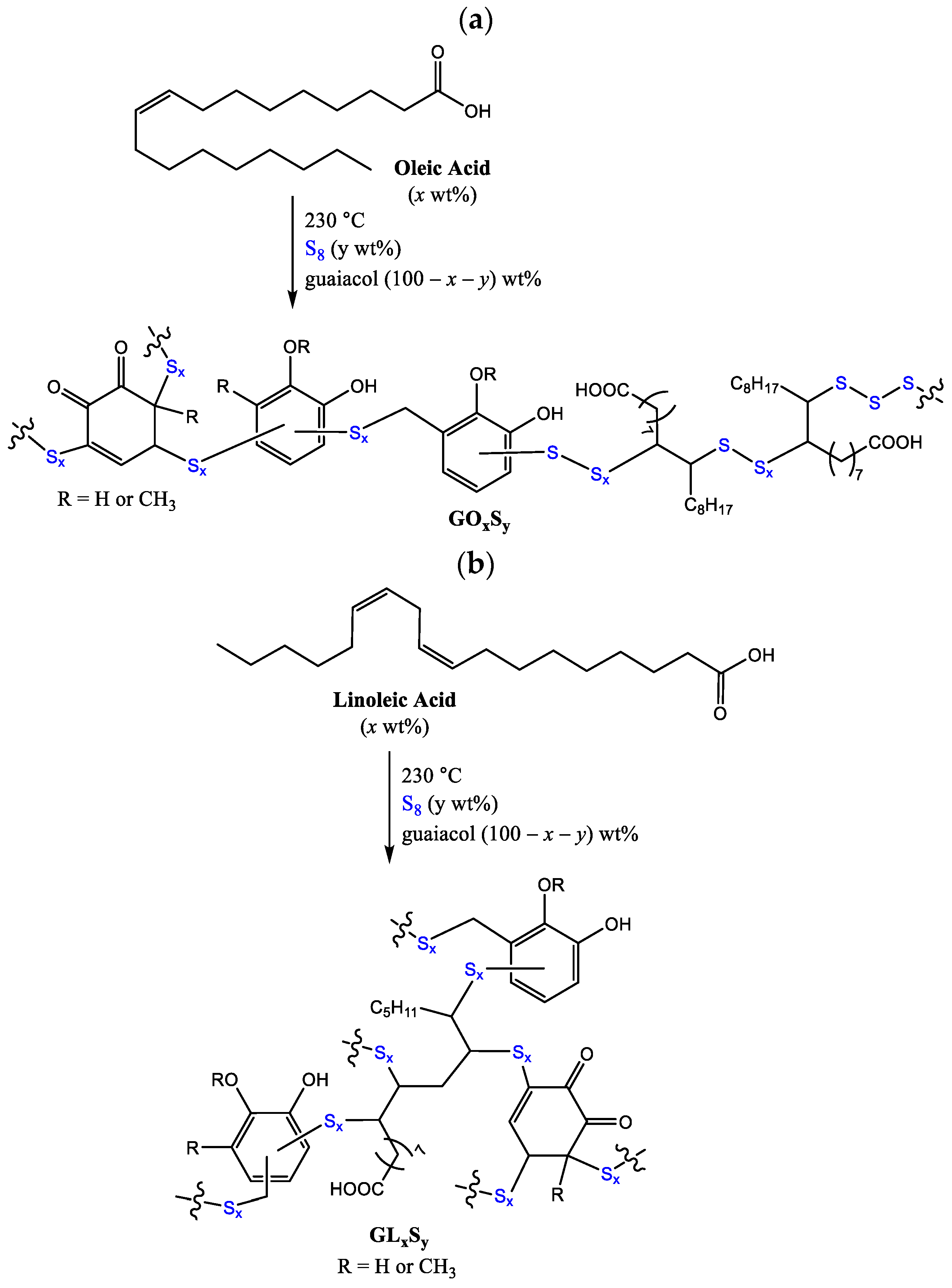
Given the contrasting properties endowed to HSMs by guaiacol versus fatty acid monomers, we hypothesized that reacting elemental sulfur with a monomer blend comprising both guaiacol and a fatty acid may lead to composites exhibiting intermediary or tunable properties suitable for a broader range of applications than accessible to either guaiacol–sulfur or fatty acid–sulfur composites. Herein we report composites of sulfur and guaiacol with either oleic acid or linoleic acid to give composites GOxSy or GLxSy, respectively (Scheme 3, x = wt% fatty acid, y = wt% sulfur, with the mass balance of guaiacol). The influences of constituent ratios on thermomorphological properties, flexural strength, and compressive strength are discussed.
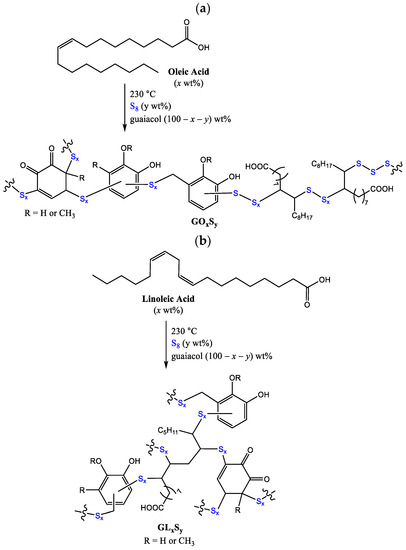
Scheme 3.
The reaction of guaiacol and fatty acids with elemental sulfur leads to formation of (a) composites GOxSy and (b) GLxSy. This is a random polymerization, so organic units attached to each end of a sulfur chain are expected to be variable.
2. Materials and Methods
2.1. Instrumentation
Thermogravimetric analysis (TGA) was recorded on a Mettler Toledo TGA 2 STARe System from 25–800 °C with a heating rate of 5 °C min−1 under a flow of N2 (20 mL·min−1).
Differential scanning calorimetry (DSC) data were acquired using a Mettler Toledo DSC 3 STARe System from −60 to 140 °C, with a heating rate of 5 °C min−1 under a flow of N2 (200 mL·min−1). Each DSC measurement was carried out over three heat–cool cycles to screen out thermal history. The data reported were taken from the third cycle of the experiment to minimize thermal history effects and allow for their comparison to the reported data for other HSMs discussed in this report.
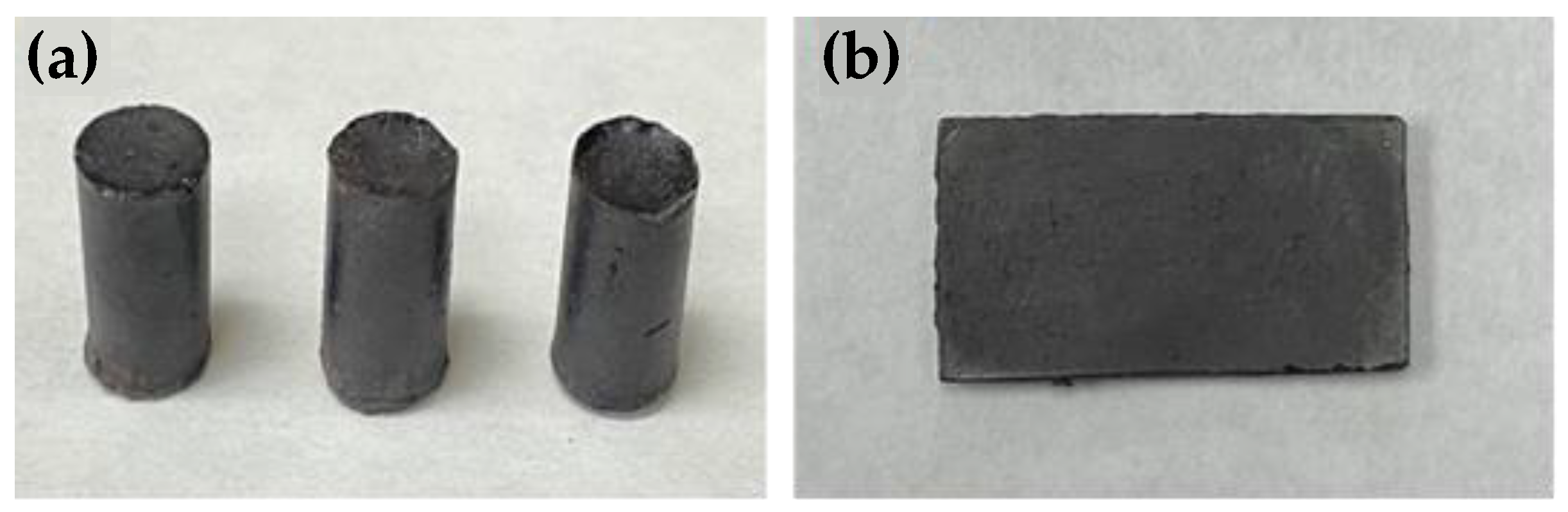
Compressive measurements were acquired on cylinders (Figure 1) using a Mark-10 ES30 Manual Test Stand equipped with a Mark 10 M3-200 Force Gauge by a modified ASTM C39 standard. Terpenoid–sulfur composite materials were aged for 4 d prior to compressive strength testing. The 4 d aging period was selected after assessing material properties over shorter and longer times for one set of samples and the properties were leveled off after 4 d (Figure S2, Supplementary Materials).
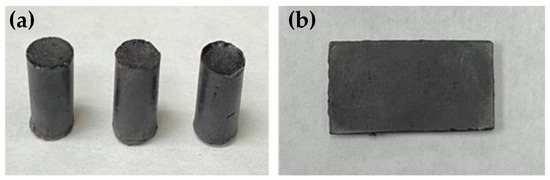
Figure 1.
(a) Representative photos of compressive cylinders and (b) rectangular prisms for flexural strength testing of the guaiacol–fatty acid–sulfur composites. All of the composites have the same visual appearance. Samples of GO10S80 are shown as an example.
Flexural strength was determined via dynamic mechanical analysis (DMA) using a Mettler Toledo DMA 1 STARe System in single cantilever mode. DMA samples were cast from silicone resin molds using a commercial Smooth-On Oomoo® 30 tin-cure kit. Samples were manually sanded to ensure uniform dimensions of approximately 15 × 8 × 1.5 mm. Sample dimensions were measured using a digital caliper with 0.01 mm resolution. The force was varied from 0 to 10 N with a ramp rate of 0.2 N·min−1 measured isothermally at 25 °C.
SEM was acquired on a Schottky Field Emission Scanning Electron Microscope SU5000 operating in variable pressure mode with an accelerating voltage of 15 keV.
2.2. Materials and Precautions
Guaiacol (98%, TCI America, Portland, OR, USA), elemental sulfur (99.5%, Alfa Aesar, Ward Hill, MA, USA), linoleic acid (98%, Acros Organics, Verona, Italy), and oleic acid (99%, Alfa Aesar, Ward Hill, MA, USA) were used without further purification.
CAUTION: Heating elemental sulfur with organics can result in the formation of H2S gas. H2S is toxic, foul-smelling, and corrosive. Heating sealed tubes presents significant danger of the vessel bursting and such reactions must be carried out behind ballistic glass or other shielding that can withstand vessel failure [48,49,50].
2.3. Synthesis
To a heavy-walled glass pressure tube (Chemglass CG-1880-01, maximum pressure rating 150 PSI) sealed with a Viton O-ring-equipped Teflon stopper were added a Teflon-coated magnetic stir bar, elemental sulfur, and the requisite organics according to the ratios provided in Table 1. The flask was sealed under an atmosphere of N2 in a glove box and then transferred to an oil bath at 230 ± 5 °C. The reaction mixture was stirred for 24 h, after which the reaction mixture was a black, visually homogenous solution. Cooling to room temperature gave the requisite materials in quantitative yield. Amounts used to prepare each composite are indicated below.

Table 1.
Monomer feed mass ratios used to prepare guaiacol–fatty acid–sulfur composites.
2.3.1. GO1S70
This material was made according to the general procedure described above using 7 g of sulfur, 0.1 g of oleic acid, and 2.9 g of guaiacol, and was isolated in quantitative yield as a black solid. IR spectra and TGA traces are provided in the Supporting Information. DSC data are provided in Table 2 and Figure 2.

Table 2.
Thermal properties of sulfur and composites.
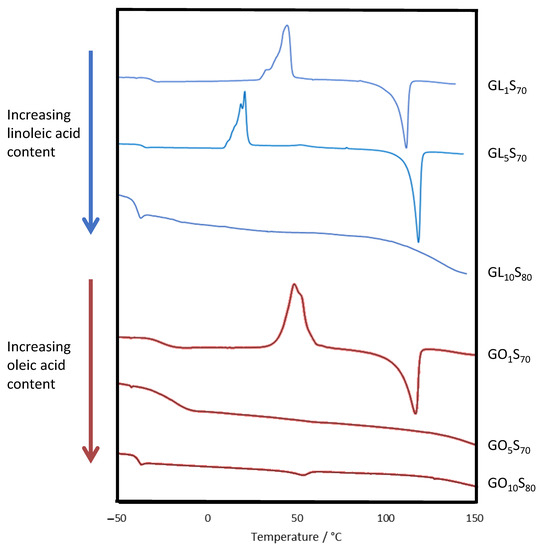
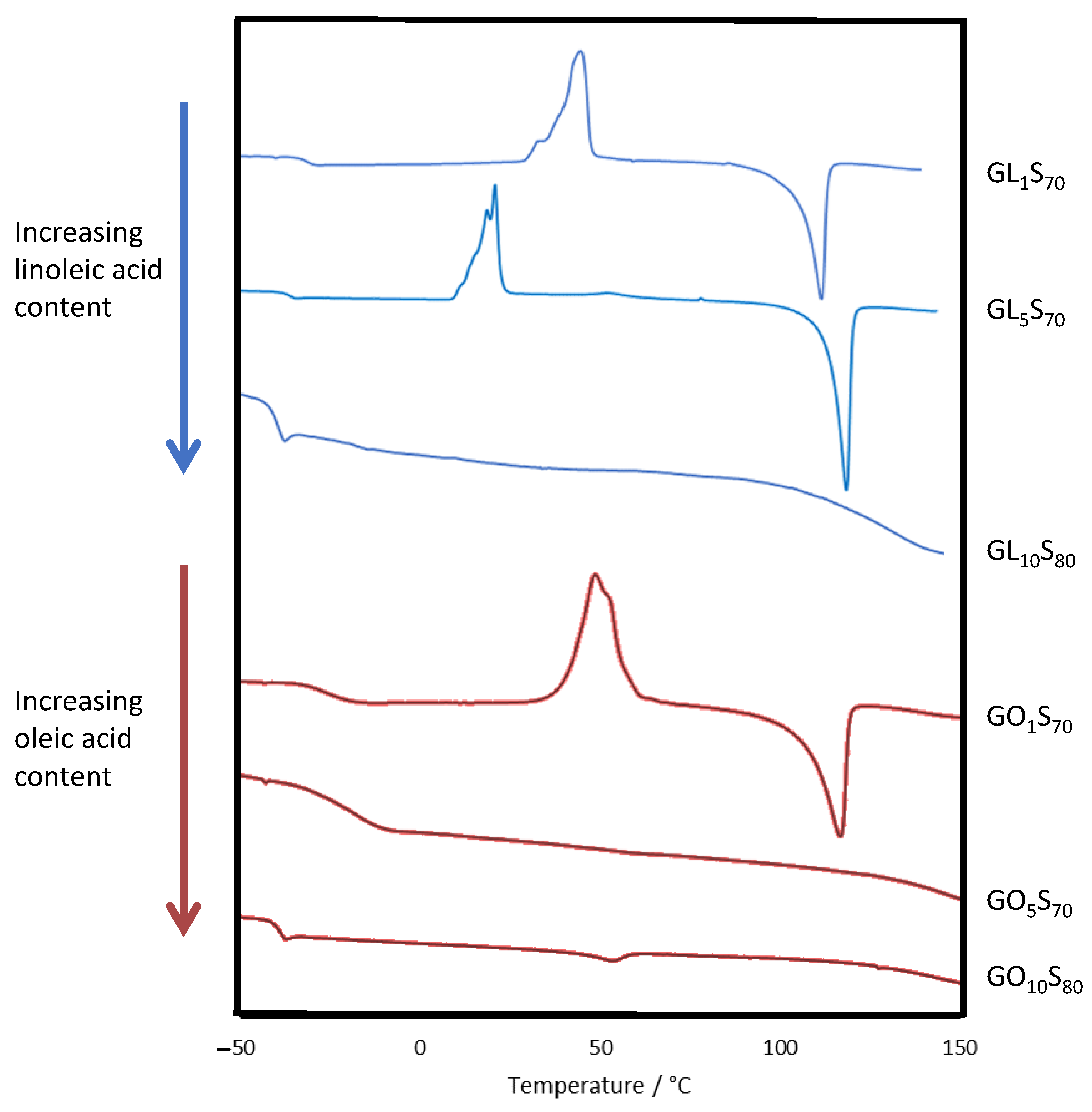
Figure 2.
DSC traces for composites.
2.3.2. GO5S70
This material was made according to the general procedure described above using 7 g of sulfur, 0.5 g of oleic acid, and 2.5 g of guaiacol, and was isolated in quantitative yield as a black solid. IR spectra and TGA traces are provided in the Supporting Information. DSC data are provided in Table 2 and Figure 2.
2.3.3. GO10S80
This material was made according to the general procedure described above using 8 g of sulfur, 1 g of oleic acid, and 1 g of guaiacol, and was isolated in quantitative yield as a black solid. IR spectra and TGA traces are provided in the Supporting Information. DSC data are provided in Table 2 and Figure 2.
2.3.4. GL1S70
This material was made according to the general procedure described above using 7 g of sulfur, 0.1 g of linoleic acid, and 2.9 g of guaiacol, and was isolated in quantitative yield as a black solid. IR spectra and TGA traces are provided in the Supporting Information. DSC data are provided in Table 2 and Figure 2.
2.3.5. GL5S70
This material was made according to the general procedure described above using 7 g of sulfur, 0.5 g of linoleic acid, and 2.5 g of guaiacol, and was isolated in quantitative yield as a black solid. IR spectra and TGA traces are provided in the Supporting Information. DSC data are provided in Table 2 and Figure 2.
2.3.6. GL10S80
This material was made according to the general procedure described above using 8 g of sulfur, 1 g of linoleic acid, and 1 g of guaiacol, and was isolated in quantitative yield as a black solid. IR spectra and TGA traces are provided in the Supporting Information. DSC data are provided in Table 2 and Figure 2.
3. Results and Discussion
3.1. Synthesis and Surface Properties
Preparation of guaiacol–fatty acid–sulfur composites was accomplished by the reaction of guaiacol, the requisite fatty acid, and elemental sulfur in the ratios shown in Table 1 under conditions we previously reported for the preparation of composite GS80 (Scheme 2). These reactions all produced quantitative yields of dark solids that could be readily remelted when heated above 150 °C, facilitating their fabrication by simply pouring the molten materials into silicone molds and allowing them to cool to room temperature (25 °C). In this way, variously shaped samples needed for mechanical tests, such as cylinders for compressional strength testing (Figure 1a), and rectangular prisms for flexural strength testing (Figure 1b) were prepared.
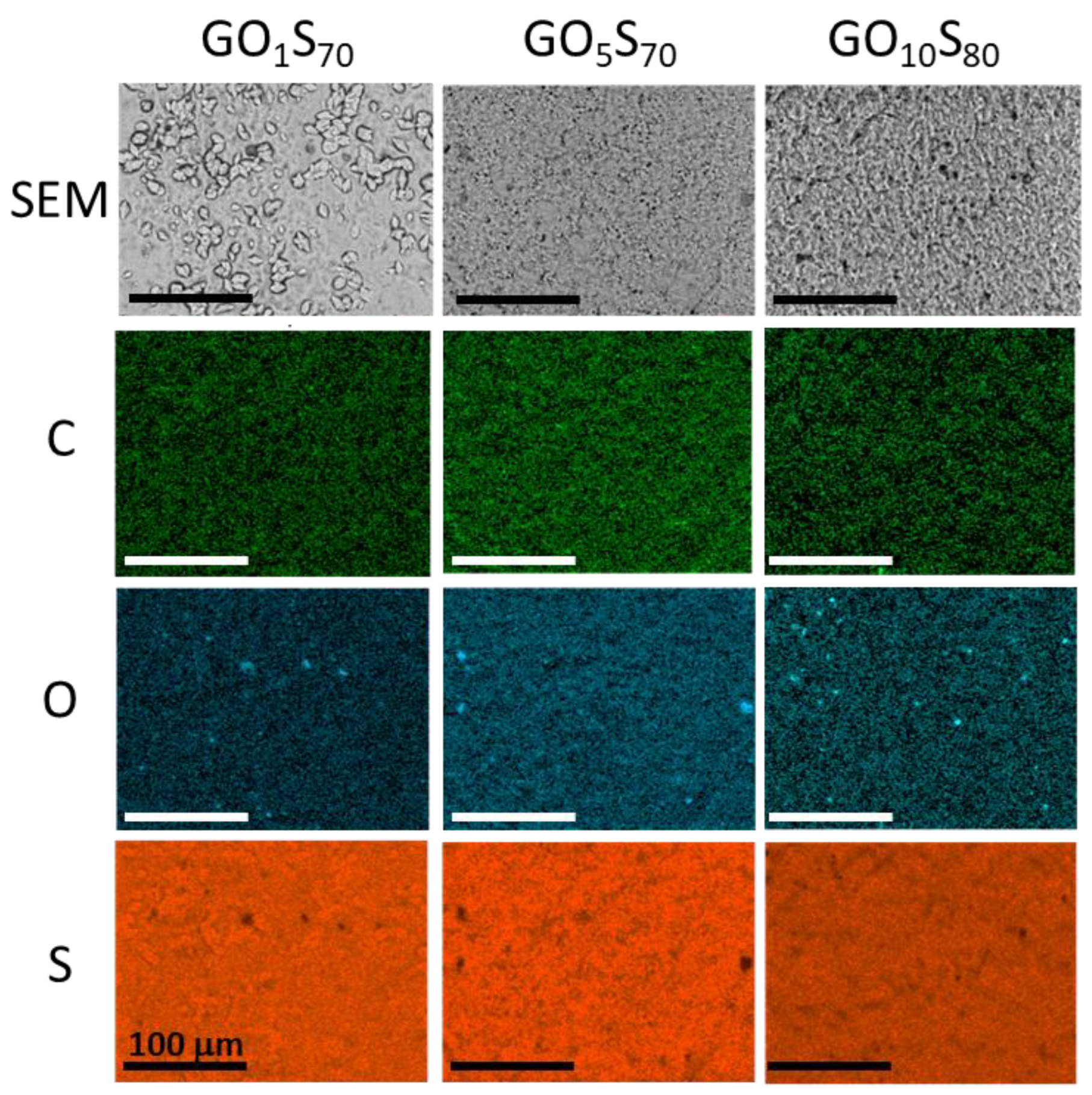
Because the oleic acid-containing composites proved most promising from the standpoint of mechanical strength (vide infra), the surface properties of those composites were further investigated. Scanning electron microscopy with elemental mapping by energy dispersive X-ray analysis (SEM-EDX) was used to analyze composites GO1S70, GO5S70, and GO10S80 (Figure 3). The elemental mapping from EDX shows the expected abundance of elements C, O and S, and good distribution of carbon and oxygen throughout the majority of the sulfur composites without significant observable bulk macroscale phase separation other than the aforementioned sulfur crystallites in GO1S70.
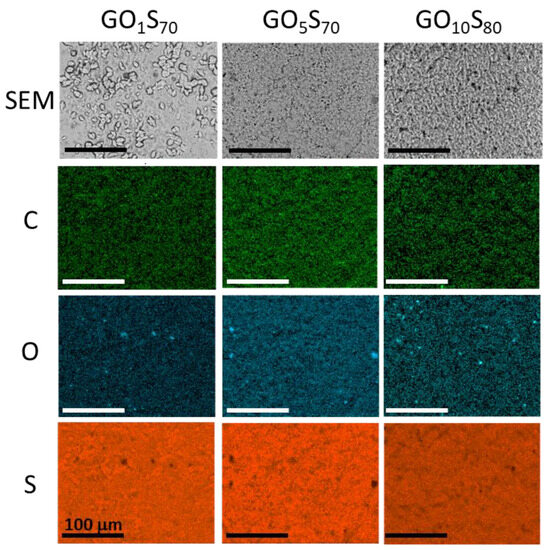
Figure 3.
Surface analysis of the composites by SEM (top row) with elemental mapping by EDX (rows 2–4). The scale bar in each image is 100 microns.
The composites were also characterized by Fourier-transform infrared spectroscopy (FTIR, Figure S1, Supplementary Materials). Most notably, IR spectra revealed consumption of olefin units as evidenced by the disappearance of out-of-plane alkene C–H bends, which appear at ~933 cm−1 in both oleic and linoleic acid, but which are absent or very weak in the composites.
3.2. Thermal Properties
The thermally induced mass-loss properties of the composites were assessed by thermogravimetric analysis (TGA, Table 2, original traces for all materials are provided in Figure S2, Supplementary Materials). HSMs comprising fatty acids and majority constituent sulfur generally exhibit a Td (5% mass loss under N2) at around the same temperature (229 °C), for example compounds ZOS90, ZPLS90, ZPLS95 and ZPLS99 in Table 2 (Td values of 220–231 °C) [24,30]. This trend holds for materials comprising ≥80 wt% S in the current series as well. Lower Td values were observed, however, for several of the materials having the highest percent composition of the organic components, with two examples having Td below 200 °C, specifically for GO1S70 (Td = 159 °C) and GO5S70 (Td = 184 °C), reflecting the lower thermal stability of the organic component. The fatty acid has a notable impact on the Td, with higher values (by 16–60 °C) uniformly observed in each GLxSy composite when compared to its GOxSy analogue. The improved thermal stability may be attributable to the greater crosslinking afforded by linoleic versus oleic acid. Such a trend in Td values of HSMs has been noted in some previous cases [30,51,52], but is not universally observed [41].
The thermomorphological properties of the composites were assessed by differential scanning calorimetry (DSC, Figure 2 and Table 2). Polymeric sulfur domains within the crosslinked network produced a diagnostic glass transition temperature (Tg) near −37 °C, while the melting temperature (Tm) around 118 °C reflects the presence of entrapped cyclo-S8 [53,54,55]. In the oleic acid-containing composites, the cyclo-S8 melting feature was only observed in GO1S70, consistent with the observation of cyclo-S8 crystals in the SEM-EDX image for this composite (Figure 3). Cold crystallization features were also generally observed in HSMs due to morphological changes in partially organized oligomeric/polymeric sulfur catenate domains [41,56,57,58,59,60]. The cold crystallization temperatures (Tcc) for the current guaiacol–fatty acid–sulfur composites ranged from 21 to 44 °C, somewhat lower than observed in the composites of 80–90 wt% sulfur with guaiacol (GS80, Tcc = 57 °C) or linoleic acid (ZPLS90, Tcc = 87 °C). Several of these notable morphological changes underwent progressive changes as the monomer composition was varied (Figure 2). For composites containing linoleic acid, when the organic component was 30 wt%, composites showed significant cold crystallization features. In contrast, for composites comprising 20 wt% organics, the cold crystallization and melting features were absent as longer sulfur chains and lower crosslink density dictated material properties. Results from oleic acid-containing composites exhibited this same general pattern. Oleic acid provides fewer crosslinking sites (one unsaturation) than linoleic acid (two degrees of unsaturation), so oleic acid-containing composites have longer average sulfur crosslink lengths [2]. As a result, cold crystallization features were not observed in the GO5S70 but were prominent in its homolog GL5S70.
3.3. Mechanical Properties
A wide range of organic monomer mixtures has been employed in preparing HSMs for which mechanical strength properties have been reported, but knowledge of how comonomer composition and additives may be used to tune mechanical properties is in its early stages, bolstered by insightful recent studies by the Chalker and Hasell groups [16,61,62,63]. The compressive and flexural strengths of the composites were thus assessed for comparison to those of other HSMs and to delineate any predictable trends in these metrics with composition (Table 3: representative stress-strain plots are provided in Figure S3 for compressional and Figure S4 for flexural analyses). All measurements were performed in triplicate and the errors reported were the standard deviations of the set of measurements for each material.

Table 3.
Mechanical properties of composites and sulfur.
Portland cement used for building foundations is required by the American Concrete Institute (code 332.1R-06) to have a minimum compressive strength of 17 MPa and flexural strength ranges from 2 to 7 MPa [43]. The current composites generally have low flexural strength and are brittle materials. However, several of the materials exhibit promising compressive strengths for load-bearing applications, notably GO10S80, which has a compressive strength of 30.4 ± 0.5 MPa.
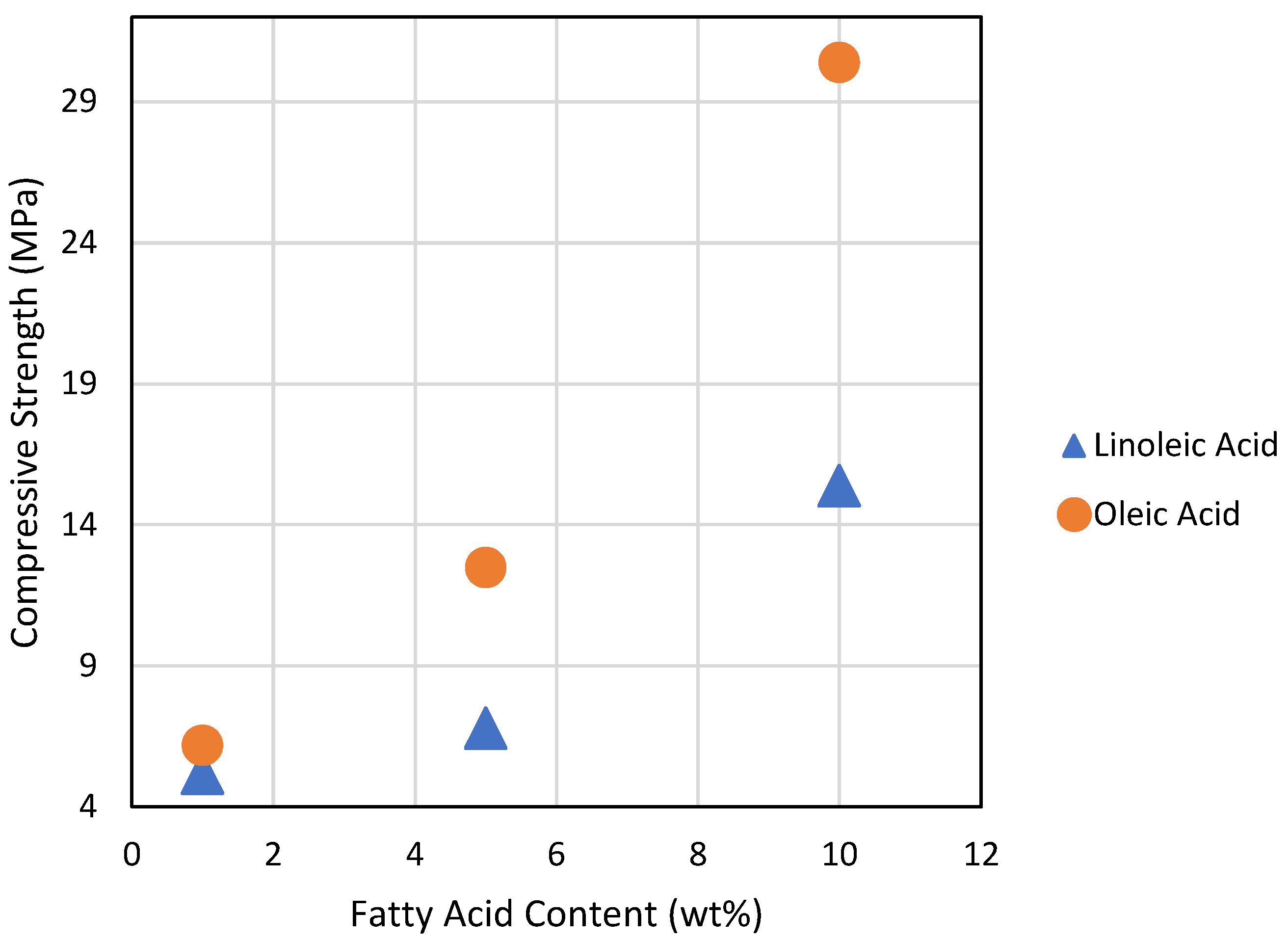
An interesting trend in compressive strength with respect to the amount and identity of the added fatty acid was observed (Figure 4). First, oleic acid-containing composites outperform linoleic acid-containing composites for a given percent of fatty acid composition. Second, a higher amount of fatty acid relative to guaiacol significantly improves compressive strength performance. When compared to the DSC data, these trends suggest that fewer sites for crosslinking, which generally correlates with longer sulfur crosslinking chains [2,32], leads to materials with greater compressive strength. Flexural strength does not follow this same trend. In fact, composites having the highest fatty acid content have by far the lowest flexural strengths of all the composites. Prior studies show that aryl-crosslinked HSMs generally have the highest flexural and tensile properties among HSMs [42,54,57]. Although it is clear that replacing aryl guaiacol with fatty acid crosslinkers diminishes the flexural strength in the current case as well, a molecular basis for this phenomenon is unclear.
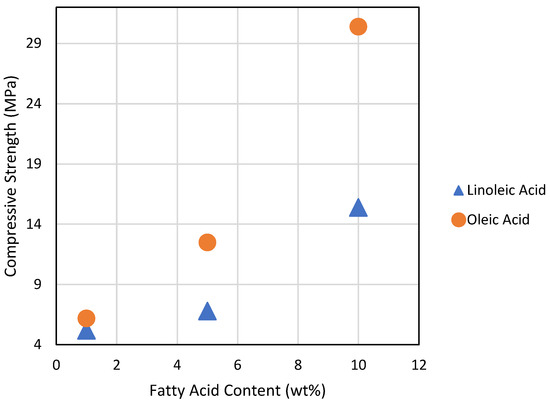
Figure 4.
A plot of compressive strength versus fatty acid content for composites comprising 70 wt% sulfur.
4. Conclusions
The current study employs lignocellulosic biomass-derived guaiacol, low nutritional value fatty acids, and fossil fuel processing by-product sulfur to prepare durable composites simply by heating the components together in a single reaction stage. During this process, the fatty acid-derived olefins undergo inverse vulcanization with sulfur to form new C–S bonds and create a crosslinked network that appears primarily responsible for dictating the mechanical strength of the composites. Guaiacol undergoes thermal decomposition during heating to form additional C–S bonds within the network. The resultant materials are best described as composites in which some non-covalently incorporated sulfur species is entrapped within the network. The materials show uniform distribution of elements on the micron level, ruling out any macroscopic phase separation. The extent to which cold crystallization features are observed tracks with the degree of unsaturation of the organic crosslinking components, but the observed Tg values reflect the presence of oligomeric and polymeric sulfur chains as major contributors to the thermomorphological transitions in all cases. Both the degree of unsaturation and amount of the fatty acid monomer added to the composite formulation influence on the overall composite compressive strengths, with the highest observed compressive strengths achieved when greater amounts of monounsaturated oleic acid comonomer were employed. The flexural and compressive strengths of the composites revealed that some of the composites exhibit strengths significantly higher than that of Portland cements used in residential housing fabrication. The utility of more sustainable feedstocks and waste materials/by-products in the current formulation may thus provide insight into ways to achieve more sustainable structural materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/macromol3040038/s1. Figure S1: (A) Region from 650–1850 cm−1 of the FTIR spectra of composites; Figure S2: TGA traces for GOxSy (A) and GLxSy (B); Figure S3: Representative stress-strain plots for compressive strength analysis of GLxSy and GOxSy; Figure S4: Representative stress-strain plots for flexural strength analysis of GLxSy and GOxSy.
Author Contributions
Conceptualization, R.C.S.; methodology, R.C.S.; formal analysis, C.P.M.; investigation, C.P.M. (lead) and N.L.K.D. (supporting); resources, R.C.S.; data curation, C.P.M.; writing—original draft preparation, R.C.S.; writing—review and editing, all authors; supervision, R.C.S.; funding acquisition, R.C.S. and A.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Science Foundation grant number CHE-2203669 and a seed grant from the Animal Coproducts Research and Education Center.
Data Availability Statement
Data supporting the manuscript are provided in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merino-Saum, A.; Clement, J.; Wyss, R.; Baldi, M.G. Unpacking the Green Economy concept: A quantitative analysis of 140 definitions. J. Clean. Prod. 2020, 242, 118339. [Google Scholar] [CrossRef]
- Loiseau, E.; Saikku, L.; Antikainen, R.; Droste, N.; Hansjürgens, B.; Pitkänen, K.; Leskinen, P.; Kuikman, P.; Thomsen, M. Green economy and related concepts: An overview. J. Clean. Prod. 2016, 139, 361–371. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Maladeniya, C.P.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Durable Composites by Vulcanization of Oleyl-Esterified Lignin. RSC Adv. 2023, 13, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Facile new approach to high sulfur-content materials and preparation of sulfur-lignin copolymers. J. Mater. Chem. A 2020, 8, 548–553. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Smith, R.C. Valorization of Lignin as a Sustainable Component of Structural Materials and Composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Thiounn, T.; Smith, R.C.; Tennyson, A.G. Valorization of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A 2019, 7, 15683–15690. [Google Scholar] [CrossRef]
- Thi, H.D.; Van Aelst, K.; Van den Bosch, S.; Katahira, R.; Beckham, G.T.; Sels, B.F.; Van Geem, K.M. Identification and quantification of lignin monomers and oligomers from reductive catalytic fractionation of pine wood with GC × GC–FID/MS. Green. Chem. 2022, 24, 191–206. [Google Scholar]
- Van Aelst, K.; Van Sinay, E.; Vangeel, T.; Cooreman, E.; Van den Bossche, G.; Renders, T.; Van Aelst, J.; Van den Bosch, S.; Sels, B. Reductive catalytic fractionation of pine wood: Elucidating and quantifying the molecular structures in the lignin oil. Chem. Sci. 2020, 11, 11498–11508. [Google Scholar] [CrossRef]
- Ebikade, E.O.; Samulewicz, N.; Xuan, S.; Sheehan, J.D.; Wu, C.; Vlachos, D.G. Reductive catalytic fractionation of agricultural residue and energy crop lignin and application of lignin oil in antimicrobials. Green. Chem. 2020, 22, 7435–7447. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, A.T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-first biorefinery: A reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive catalytic fractionation of corn stover lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Glass, R.S.; Char, K.; Pyun, J. Recent advances in the polymerization of elemental sulphur, inverse vulcanization and methods to obtain functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs). Polym. Chem. 2019, 10, 4078–4105. [Google Scholar] [CrossRef]
- Kleine, T.S.; Glass, R.S.; Lichtenberger, D.L.; MacKay, M.E.; Char, K.; Norwood, R.A.; Pyun, J. 100th Anniversary of Macromolecular Science Viewpoint: High Refractive Index Polymers from Elemental Sulfur for Infrared Thermal Imaging and Optics. ACS Macro Lett. 2020, 9, 245–259. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Zhang, B.; Jiang, L.; Petcher, S.; Smith, J.A.; Parker, D.J.; Cooper, A.I.; Lei, J.; Hasell, T. Inverse vulcanized polymers with shape memory, enhanced mechanical properties, and vitrimer behavior. Angew. Chem. Int. Ed. 2020, 59, 13371–13378. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green. Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Chalker, J.M.; Worthington, M.J.H.; Lundquist, N.A.; Esdaile, L.J. Synthesis and Applications of Polymers Made by Inverse Vulcanization. Top. Curr. Chem. 2019, 377, 16. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Carbon-Negative Polymer Cements by Copolymerization of Waste Sulfur, Oleic Acid, and Pozzolan Cements. Sust. Chem. Pharm. 2020, 16, 100249. [Google Scholar] [CrossRef]
- Gutarowska, B.; Piotrowska, M.; Kozirog, A.; Berlowska, J.; Dziugan, P.; Kotynia, R.; Bielinski, D.; Anyszka, R.; Wreczycki, J. New Sulfur Organic Polymer-Concrete Composites Containing Waste Materials: Mechanical Characteristics and Resistance to Biocorrosion. Materials 2019, 12, 2602. [Google Scholar] [CrossRef]
- Mohammed, S.; Poornima, V. Strength and durability study of sulphur concrete with replaced fine aggregate. Mater. Today Proc. 2018, 5, 23888–23897. [Google Scholar] [CrossRef]
- Mohamed, A.-M.O.; Gamal, M.E. Sulfur Concrete for the Construction Industry; Ross, J., Ed.; J. Ross Publishing: Fort Lauderdale, FL, USA, 2010; p. 424. [Google Scholar]
- Okumura, H.A. Early sulfur concrete installations. Concr. Int. 1998, 20, 72–75. [Google Scholar]
- Weber, H.H.; McBee, W.C.; Krabbe, E.A. Sulfur concrete composite materials for construction and maintenance. Mater. Perform. 1990, 29, 73–77. [Google Scholar]
- Pickard, S.S. Sulfur concrete for acid resistance. Chem. Eng. 1985, 92, 77–78+80. [Google Scholar]
- Smith, A.D.; Thiounn, T.; Lyles, E.W.; Kibler, E.K.; Smith, R.C.; Tennyson, A.G. Combining agriculture and energy industry waste products to yield recyclable, thermally healable copolymers of elemental sulfur and oleic acid. J. Poly. Sci. A 2019, 57, 1704–1710. [Google Scholar] [CrossRef]
- Michal, B.T.; Jaye, C.A.; Spencer, E.J.; Rowan, S.J. Inherently Photohealable and Thermal Shape-Memory Polydisulfide Networks. ACS Macro Lett. 2013, 2, 694–699. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; MacKay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Pasparakis, G. Stimuli responsive self-healing polymers: Gels, elastomers and membranes. Polym. Chem. 2017, 8, 6464–6484. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Recycling and Self-Healing of Polybenzoxazines with Dynamic Sulfide Linkages. Sci. Rep. 2017, 7, 5207. [Google Scholar] [CrossRef]
- Takahashi, A.; Goseki, R.; Ito, K.; Otsuka, H. Thermally Healable and Reprocessable Bis(hindered amino)disulfide-Cross-Linked Polymethacrylate Networks. ACS Macro Lett. 2017, 6, 1280–1284. [Google Scholar] [CrossRef]
- Smith, A.D.; McMillin, C.D.; Smith, R.C.; Tennyson, A.G. Copolymers by Inverse Vulcanization of Sulfur with Pure or Technical Grade Unsaturated Fatty Acids. J. Poly. Sci. 2020, 58, 438–445. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sus. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 10035–10044. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; McBride, F.; Cai, D.; Dale, J.; Hanna, V.; Hasell, T. Mechanochemical synthesis of inverse vulcanized polymers. Nat. Commun. 2022, 13, 4824. [Google Scholar] [CrossRef]
- Zhang, B.; Petcher, S.; Hasell, T. A ternary system for delayed curing inverse vulcanisation. Chem. Commun. 2019, 55, 10681–10684. [Google Scholar] [CrossRef]
- Westerman Clayton, R.; Walker Princess, M.; Jenkins Courtney, L. Synthesis of Terpolymers at Mild Temperatures Using Dynamic Sulfur Bonds in Poly(S-Divinylbenzene). J. Vis. Exp. 2019. [Google Scholar] [CrossRef]
- Sahu, S.; Lochab, B. Facile Strategy for Room Temperature Knitting of Sulfur in Polybenzoxazine: A New Class of Solution Processable Copolymers. ACS Sustain. Chem. Eng. 2022, 10, 12355–12364. [Google Scholar] [CrossRef]
- Mann, M.; Pauling, P.J.; Tonkin, S.J.; Campbell, J.A.; Chalker, J.M. Chemically Activated S-S Metathesis for Adhesive-Free Bonding of Polysulfide Surfaces. Macromol. Chem. Phys. 2022, 223, 2100333. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Tikoalu, A.D.; Worthington, M.J.H.; Shapter, R.; Tonkin, S.J.; Stojcevski, F.; Mann, M.; Gibson, C.T.; Gascooke, J.R.; Karton, A.; et al. Reactive Compression Molding Post-Inverse Vulcanization: A Method to Assemble, Recycle, and Repurpose Sulfur Polymers and Composites. Chem. A Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef]
- Jia, J.; Liu, J.; Wang, Z.-Q.; Liu, T.; Yan, P.; Gong, X.-Q.; Zhao, C.; Chen, L.; Miao, C.; Zhao, W.; et al. Photoinduced inverse vulcanization. Nat. Chem. 2022, 14, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.-J.; van der Laan, J.; Nguyen, N.A.; et al. New Infrared Transmitting Material via Inverse Vulcanization of Elemental Sulfur to Prepare High Refractive Index Polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse Vulcanization of Elemental Sulfur to Prepare Polymeric Electrode Materials for Li-S Batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Dirlam, P.T.; Simmonds, A.G.; Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Klever, A.O.; Florian, A.; Costanzo, P.J.; Theato, P.; Mackay, M.E.; et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li-S batteries. RSC Adv. 2015, 5, 24718–24722. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Poly. Sci. A 2015, 53, 173–177. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Combining Elemental Sulfur with Polybenzoxazines via Inverse Vulcanization. Macromolecules 2016, 49, 767–773. [Google Scholar] [CrossRef]
- Ng, P.C.; Hendry-Hofer, T.B.; Witeof, A.E.; Brenner, M.; Mahon, S.B.; Boss, G.R.; Haouzi, P.; Bebarta, V.S. Hydrogen sulfide toxicity: Mechanism of action, clinical presentation, and countermeasure development. J. Med. Toxicol. 2019, 15, 287–294. [Google Scholar] [CrossRef]
- Beauchamp, R.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A.; Leber, P. A critical review of the literature on hydrogen sulfide toxicity. CRC Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- Truong, D.H.; Eghbal, M.A.; Hindmarsh, W.; Roth, S.H.; O’Brien, P.J. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab. Rev. 2006, 38, 733–744. [Google Scholar] [CrossRef]
- Yoon, K.-B.; Ryu, H.M.; Lee, G.H.; Gopalan, A.I.; Sai-anand, G.; Lee, D.-E. Enhanced compressive strength of rammed earth walls stabilized with eco-friendly multi-functional polymeric system. Renew. Sustain. Energy Rev. 2021, 152, 111681. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green. Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Meyer, B. Solid allotropes of sulfur. Chem. Rev. 1964, 64, 429–451. [Google Scholar] [CrossRef]
- Meyer, B.; Oommen, T.V.; Jensen, D. Color of liquid sulfur. J. Phys. Chem. 1971, 75, 912–917. [Google Scholar] [CrossRef]
- Meyer, C.B.; Stroyer-Hansen, T.; Jensen, D.; Oommen, T.V. Color of liquid sulfur. J. Amer. Chem. Soc. 1971, 93, 1034–1035. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable Polysulfides for Oil Spill Remediation: Repurposing Industrial Waste for Environmental Benefit. Adv. Sust. Syst. 2018, 2, 1800024. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying Waste to Mercury: Inexpensive Sorbents Made from Sulfur and Recycled Cooking Oils. Chem. A Eur. J. 2017, 23, 16219–16230. [Google Scholar] [CrossRef]
- Orme, K.; Fistrovich, A.H.; Jenkins, C.L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization. Macromolecules 2020, 53, 9353–9361. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic Sulfur Bonds Initiate Polymerization of Vinyl and Allyl Ethers at Mild Temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.H.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T.; et al. Crosslinker Copolymerization for Property Control in Inverse Vulcanization. Chem. A Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef]
- Hanna, V.; Yan, P.; Petcher, S.; Hasell, T. Incorporation of fillers to modify the mechanical performance of inverse vulcanised polymers. Polym. Chem. 2022, 13, 3930–3937. [Google Scholar] [CrossRef]
- Yan, P.; Wang, H.; Dodd, L.J.; Hasell, T. Processable crosslinked terpolymers made from elemental sulfur with wide range of thermal and mechanical properties. ChemRxiv 2023, 1–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).