Plant-Based Proteins and Their Modification and Processing for Vegan Cheese Production
Abstract
:1. Introduction
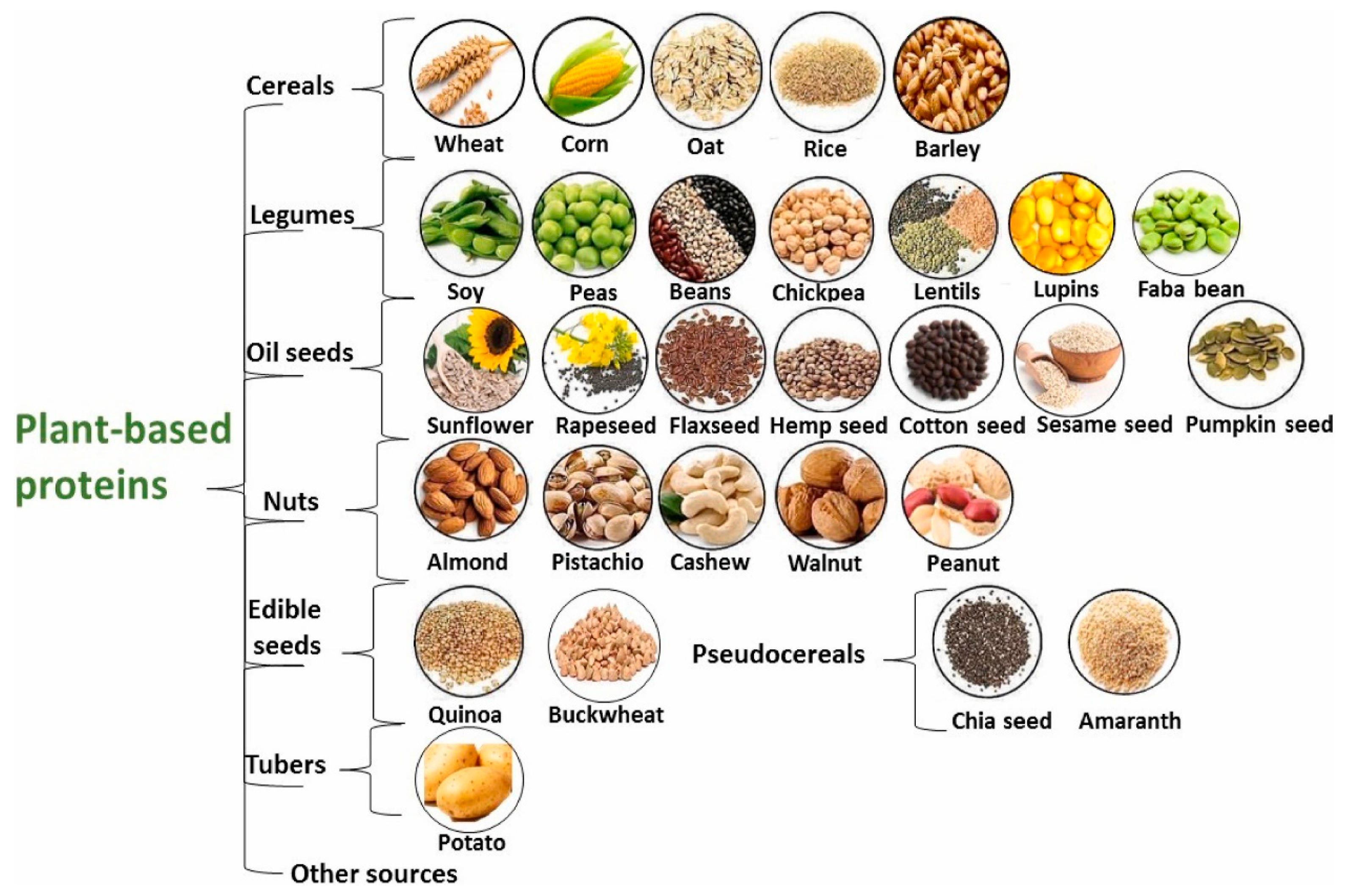
2. Natural Sources and Classification of Plant-Based Proteins
3. Plant-Based Cheese
4. Modification Approaches of Plant-Based Proteins
4.1. Physical Modification
4.1.1. High-Pressure Treatment
4.1.2. Ultrasound
4.1.3. Gamma Irradiation
4.1.4. Extrusion
4.1.5. Ultrafiltration
4.1.6. Pulsed Electric Field
4.2. Chemical Modification
4.2.1. Glycation
4.2.2. pH Shifting
4.2.3. Acylation
4.2.4. Deamidation
4.2.5. Phosphorylation
4.3. Biological Modification
4.3.1. Enzymatic Hydrolysis
4.3.2. Enzymatic Cross-Linking
4.3.3. Fermentation
5. Processing Plant-Based Cheese
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Liu, R.H. Health Benefits of Fruit and Vegetables Are from Additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Bloomberg. Plant-Based Foods Market to Hit $162 Billion in Next Decade, Projects Bloomberg Intelligence; Bloomberg: New York, NY, USA, 2021. [Google Scholar]
- Technavio. Vegan-Cheese Market by Source, Distribution Channel and Geography-Forecast and Analysis 2023–2027; Infiniti Research Limited: London, UK, 2023. [Google Scholar]
- Drozłowska, E.; Weronis, M.; Bartkowiak, A. The Influence of Thermal Hydrolysis Process on Emulsifying Properties of Potato Protein Isolate. J. Food Sci. Technol. 2020, 57, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; del Refugio Rocha-Pizaña, M.; García-Lara, S.; López-Castillo, L.M.; Serna-Saldívar, S.O. Effect of Thermal Processing and Reducing Agents on Trypsin Inhibitor Activity and Functional Properties of Soybean and Chickpea Protein Concentrates. LWT 2018, 98, 629–634. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Jung, S.L.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B. Dietary Protein Intake Is Associated with Lean Mass Change in Older, Community-Dwelling Adults: The Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef]
- Hannan, M.T.; Felson, D.T.; Dawson-Hughes, B.; Tucker, K.L.; Cupples, L.A.; Wilson, P.W.F.; Kiel, D.P. Risk Factors for Longitudinal Bone Loss in Elderly Men and Women: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2000, 15, 710–720. [Google Scholar] [CrossRef]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein Supplementation Improves Muscle Mass and Physical Performance in Undernourished Prefrail and Frail Elderly Subjects: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef]
- Kearney, J. Food Consumption Trends and Drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary. Recommended Dietary Allowances; National Academies Press: Washington, DC, USA, 1989; ISBN 978-0-309-04633-6. [Google Scholar]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. History of Soymilk and Other Non-Dairy Milks (1226 to 2013); Soyinfo Center: Lafayette, CA, USA, 2013; ISBN 9781928914587. [Google Scholar]
- Saraco, M. Functionality of the Ingredients Used in Commercial Dairy-Free Imitation Cheese and Analysis of Cost-Related, Food Safety and Legal Implications; Department of Healthcare and Food, Cardiff School of Sport & Health Sciences: Cardiff, UK, 2019; Volume 2019, pp. 1–15. [Google Scholar]
- Pickard, R.S.; McKevith, B.J. The Role of Cereals in the Diet. In Using Cereal Science and Technology for the Benefit of Consumers; Cauvain, S.P., Salmon, S.S., Young, L.S., Eds.; Woodhead Publishing: Sawston, UK, 2005; ISBN 9781845690632. [Google Scholar]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B. Pseudocereals as Super Foods of 21st Century: Recent Technological Interventions. J. Agric. Food Res. 2020, 2, 100052. [Google Scholar] [CrossRef]
- USDA US Department of Agriculture, Agricultural Research Service. ARS Annual Report on Science 2020. Available online: https://www.ars.usda.gov/research/ars-annual-report-on-science/2020-ars-annual-report-on-science/ (accessed on 15 October 2023).
- Kelly, A.; Becker, W.; Helsing, E. Food Balance Sheets; WHO: Geneva, Switzerland, 1991; pp. 39–48. [Google Scholar]
- Augustin, J.; Klein, B. Legumes: Chemistry, Technology, and Human Nutrition Food Science and Technology; Mattwes, R.M., Ed.; Marcel Dekker: New York, NU, USA, 1989; ISBN 978-0824780425. [Google Scholar]
- Waseem, S.; Imadi, S.R.; Gul, A.; Ahmad, P. Oilseed Crops: Present Scenario and Future Prospects. In Oilseed Crops: Yield and Adaptations under Environmental Stress; Wiley: Hoboken, NJ, USA, 2017; pp. 1–306. [Google Scholar]
- Ibrahim, O.S. Chemical Composition and Nutritional Characterization of Cotton Seed as Potential Feed Supplement. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 977–982. [Google Scholar]
- USDA. USDA Department of Agriculture, National Nutrient Database for Standard Reference; 2018. Available online: https://www.ars.usda.gov/research/publications/publication/?seqno115=349687 (accessed on 15 October 2023).
- Jardim, T.; Domingues, M.R.M.; Alves, E. An Overview on Lipids in Nuts and Oily Fruits: Oil Content, Lipid Composition, Health Effects, Lipidomic Fingerprinting and New Biotechnological Applications of Their by-Products. Crit. Rev. Food Sci. Nutr. 2023, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Vinson, J.A.; Cai, Y. Nuts, Especially Walnuts, Have Both Antioxidant Quantity and Efficacy and Exhibit Significant Potential Health Benefits. Food Funct. 2012, 3, 134–140. [Google Scholar] [CrossRef] [PubMed]
- USDA. Food Composition Database; USDA: Washington, DC, USA, 2018. [Google Scholar]
- Lieberman, J.A.; Gupta, R.S.; Knibb, R.C.; Haselkorn, T.; Tilles, S.; Mack, D.P.; Pouessel, G. The Global Burden of Illness of Peanut Allergy: A Comprehensive Literature Review. Allergy 2021, 76, 1367–1384. [Google Scholar] [CrossRef]
- Pua, A.; Tang, V.C.Y.; Goh, R.M.V.; Sun, J.; Lassabliere, B.; Liu, S.Q. Ingredients, Processing, and Fermentation: Addressing the Organoleptic Boundaries of Plant-Based Dairy Analogues. Foods 2022, 11, 875. [Google Scholar] [CrossRef]
- Walther, B.; Guggisberg, D.; Badertscher, R.; Egger, L.; Portmann, R.; Dubois, S.; Haldimann, M.; Kopf-Bolanz, K.; Rhyn, P.; Zoller, O.; et al. Comparison of Nutritional Composition between Plant-Based Drinks and Cow’s Milk. Front. Nutr. 2022, 9, 2645. [Google Scholar] [CrossRef]
- Pam Ismail, B.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein Demand: Review of Plant and Animal Proteins Used in Alternative Protein Product Development and Production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. The Science of Plant-Based Foods: Approaches to Create Nutritious and Sustainable Plant-Based Cheese Analogs. Trends Food Sci. Technol. 2021, 118, 207–229. [Google Scholar] [CrossRef]
- Tang, Q.; Roos, Y.H.; Miao, S. Plant Protein versus Dairy Proteins: A PH-Dependency Investigation on Their Structure and Functional Properties. Foods 2023, 12, 368. [Google Scholar] [CrossRef]
- Foh, M.B.K.; Wenshui, X.; Amadou, I.; Jiang, Q. Influence of PH Shift on Functional Properties of Protein Isolated of Tilapia (Oreochromis niloticus) Muscles and of Soy Protein Isolate. Food Bioprocess Technol. 2012, 5, 2192–2200. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Macnaughtan, W.; Harding, S.; Wilde, P.; Wolf, B. Stabilisation of Oil-in-Water Emulsions with Non-Chemical Modified Gelatinised Starch. Food Hydrocoll. 2018, 81, 409–418. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Scolaro, B.; Milne, G.L.; Castro, I.A. Oxidation Products from Omega-3 and Omega-6 Fatty Acids during a Simulated Shelf Life of Edible Oils. LWT 2019, 101, 113–122. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey Proteins Solubility as Function of Temperature and PH. LWT Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Salunkhe, D.K.; Deshpande, U.S. Food Additive Toxicology; Maga, J.A., Tu, A.Y., Eds.; Marcel Dekker: New York, NY, USA, 1994; pp. 11–88. ISBN 0-8247-9245-9. [Google Scholar]
- Short, E.C.; Kinchla, A.J.; Nolden, A.A. Plant-Based Cheeses: A Systematic Review of Sensory Consumer Acceptance. Foods 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Santagiuliana, M.; Broers, L.; Marigómez, I.S.; Stieger, M.; Piqueras-Fiszman, B.; Scholten, E. Strategies to Compensate for Undesired Gritty Sensations in Foods. Food Qual. Prefer. 2020, 81, 103842. [Google Scholar] [CrossRef]
- Lopez, F.L.; Mistry, P.; Batchelor, H.K.; Bennett, J.; Coupe, A.; Ernest, T.B.; Orlu, M.; Tuleu, C. Acceptability of Placebo Multiparticulate Formulations in Children and Adults. Sci. Rep. 2018, 8, 9210. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Novák, P.; Havlíček, V. Protein Extraction and Precipitation. In Proteomic Profiling and Analytical Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 52–62. [Google Scholar] [CrossRef]
- Dahal, Y.R.; Schmit, J.D. Ion Specificity and Nonmonotonic Protein Solubility from Salt Entropy. Biophys. J. 2018, 114, 76–87. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gomes, M.T.d.M.S. Whey Proteins Solubility Curves at Several Temperatures Values. Cienc. Nat. 2008, 30, 17–25. [Google Scholar]
- Pandurangan, S.; Sandercock, M.; Beyaert, R.; Conn, K.L.; Hou, A.; Marsolais, F. Differential Response to Sulfur Nutrition of Two Common Bean Genotypes Differing in Storage Protein Composition. Front. Plant Sci. 2015, 6, 92. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of Plant Proteins for Improved Functionality: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. An Update on High Hydrostatic Pressure, from the Laboratory to Industrial Applications. Food Eng. Rev. 2011, 3, 44–61. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, Y.; Acosta-Muñiz, C.; Olivas, G.I.; Guerrero-Beltrán, J.; Rodrigo-Aliaga, D.; Sepúlveda, D.R. High Hydrostatic Pressure Processing of Cheese. Compr. Rev. Food Sci. Food Saf. 2012, 11, 399–416. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Puppo, M.C.; Speroni, F.; Chapleau, N.; De Lamballerie, M.; Añón, M.C.; Anton, M. Effect of High-Pressure Treatment on Emulsifying Properties of Soybean Proteins. Food Hydrocoll. 2005, 19, 289–296. [Google Scholar] [CrossRef]
- Tang, C.H.; Ma, C.Y. Effect of High Pressure Treatment on Aggregation and Structural Properties of Soy Protein Isolate. LWT 2009, 42, 606–611. [Google Scholar] [CrossRef]
- Wang, X.S.; Tang, C.H.; Li, B.S.; Yang, X.Q.; Li, L.; Ma, C.Y. Effects of High-Pressure Treatment on Some Physicochemical and Functional Properties of Soy Protein Isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Tatsumi, E.; Isobe, S. High-Pressure Treatment Effects on Proteins in Soy Milk. LWT 2005, 38, 7–14. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of Ultrasound on the Technological Properties and Bioactivity of Food: A Review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for Future Foods: A Roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Su, J.; Cavaco-Paulo, A. Effect of Ultrasound on Protein Functionality. Ultrason. Sonochem. 2021, 76, 105653. [Google Scholar] [CrossRef] [PubMed]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of Ultrasound Treatment on Solubility and Foaming Properties of Whey Protein Suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of Ultrasound on the Structure and Physical Properties of Black Bean Protein Isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Davies, K.J.; Delsignore, M.E. Protein Damage and Degradation by Oxygen Radicals. III. Modification of Secondary and Tertiary Structure. J. Biol. Chem. 1987, 262, 9908–9913. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Song, K. Bin Effect of Gamma-Irradiation on the Physicochemical Properties of Porcine and Bovine Blood Plasma Proteins. Food Chem. 2003, 82, 521–526. [Google Scholar] [CrossRef]
- Cho, Y.; Song, K. Bin Effect of γ-Irradiation on the Molecular Properties of Bovine Serum Albumin and β-Lcatoglobulin. J. Biochem. Mol. Biol. 2000, 33, 133–137. [Google Scholar]
- Štajner, D.; Milošević, M.; Popović, B.M. Irradiation Effects on Phenolic Content, Lipid and Protein Oxidation and Scavenger Ability of Soybean Seeds. Int. J. Mol. Sci. 2007, 8, 618–627. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.Y.; Ryu, G.H. Effects of Extrusion Types, Screw Speed and Addition of Wheat Gluten on Physicochemical Characteristics and Cooking Stability of Meat Analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef] [PubMed]
- Nosworthy, M.G.; Medina, G.; Franczyk, A.J.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; Tar’an, B.; House, J.D. Thermal Processing Methods Differentially Affect the Protein Quality of Chickpea (Cicer arietinum). Food Sci. Nutr. 2020, 8, 2950–2958. [Google Scholar] [CrossRef]
- Omosebi, M.O.; Osundahunsi, O.F.; Fagbemi, T.N. Effect of Extrusion on Protein Quality, Antinutritional Factors, and Digestibility of Complementary Diet from Quality Protein Maize and Soybean Protein Concentrate. J. Food Biochem. 2018, 42, e12508. [Google Scholar] [CrossRef]
- Manoi, K.; Rizvi, S.S.H. Emulsification Mechanisms and Characterizations of Cold, Gel-like Emulsions Produced from Texturized Whey Protein Concentrate. Food Hydrocoll. 2009, 23, 1837–1847. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of Processing on the Chemistry and Functionality of Food Proteins, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081007228. [Google Scholar]
- Boye, J.I.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S.H. Comparison of the Functional Properties of Pea, Chickpea and Lentil Protein Concentrates Processed Using Ultrafiltration and Isoelectric Precipitation Techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Doost, A.S.; Nasrabadi, M.N.; Van Der Meeren, P.; Chemistry, S. Production of Food Nanomaterials by Specialized Equipment 5. In Handbook of Food Nanotechnology: Applications and Approaches; Academic Press: London, UK, 2020; ISBN 9780128158661. [Google Scholar]
- Li, Y.; Chen, Z.; Mo, H. Effects of Pulsed Electric Fields on Physicochemical Properties of Soybean Protein Isolates. LWT 2007, 40, 1167–1175. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Ngadi, M.O.; Ochoa-Martinez, L.A.; Simpson, M.V. Pulsed Electric Field-Induced Structural Modification of Whey Protein Isolate. Food Bioprocess Technol. 2011, 4, 1341–1348. [Google Scholar] [CrossRef]
- Van Boekel, M.A.J.S. Kinetic Aspects of the Maillard Reaction: A Critical Review. Nahrung Food 2001, 45, 150–159. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.C.; Coimbra, J.S.d.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food Protein-Polysaccharide Conjugates Obtained via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, P.; Sozer, N. Impact of Ultrasound Treatment and Ph-Shifting on Physicochemical Properties of Protein-Enriched Barley Fraction and Barley Protein Isolate. Foods 2020, 9, 1055. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.; Chen, J. PH Shifting Alters Solubility Characteristics and Thermal Stability of Soy Protein Isolate and Its Globulin Fractions in Different PH, Salt Concentration, and Temperature Conditions. J. Agric. Food Chem. 2010, 58, 8035–8042. [Google Scholar] [CrossRef]
- Li, Q.; Clarke, I.J.; Smith, A.I. Acetylation. In Handbook of Biologically Active Peptides; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1711–1714. [Google Scholar]
- Ponnampalam, R.; Goulet, G.; Amiot, J.; Chamberland, B.; Brisson, G.J. Some Functional Properties of Acetylated and Succinylated Oat Protein Concentrates and a Blend of Succinylated Oat Protein and Whey Protein Concentrates. Food Chem. 1988, 29, 109–118. [Google Scholar] [CrossRef]
- Zhao, C.B.; Zhang, H.; Xu, X.Y.; Cao, Y.; Zheng, M.Z.; Liu, J.S.; Wu, F. Effect of Acetylation and Succinylation on Physicochemical Properties and Structural Characteristics of Oat Protein Isolate. Process Biochem. 2017, 57, 117–123. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, N.; Li, Y.; Cheng, S.; Jiang, C.; Lin, S. Effects of Electron Beam Irradiation (EBI) on Structure Characteristics and Thermal Properties of Walnut Protein Flour. Food Res. Int. 2017, 100, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Sebii, H.; Karra, S.; Bchir, B.; Nhouchi, Z.; Ghribi, A.M.; Karoui, R.; Blecker, C.; Besbes, S. Effect of Succinylation on the Secondary Structures, Surface, and Thermal Properties of Date Palm Pollen Protein Concentrate. J. Food Sci. Technol. 2021, 58, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.S.; Adebowale, K.O. Effect of Acetylation and Succinylation on Solubility Profile, Water Absorption Capacity, Oil Absorption Capacity and Emulsifying Properties of Mucuna Bean (Mucuna pruriens) Protein Concentrate. Nahrung Food 2004, 48, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Takai, R.; Toraya, K.; Ogawa, T.; Muramoto, K.; Mohri, S.; Ishikawa, D.; Fujii, T.; Chi, H.; Cho, S.J. Effects of Alkaline Deamidation on the Chemical Properties of Rice Bran Protein. Food Sci. Technol. Res. 2017, 23, 697–704. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pouramin, S. Optimization of Extraction and Deamidation of Edible Protein from Evening Primrose (Oenothera biennis L.) Oil Processing by-Products and Its Effect on Structural and Techno-Functional Properties. Food Chem. 2021, 334, 127613. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-q.; Sontag-Strohm, T.; Salovaara, H.; Sibakov, J.; Kanerva, P.; Loponen, J. Oat Protein Solubility and Emulsion Properties Improved by Enzymatic Deamidation. J. Cereal Sci. 2015, 64, 126–132. [Google Scholar] [CrossRef]
- Matheis, G.; Whitaker, J.R. Chemical Phosphorylation of Food Proteins: An Overview and a Prospectus. J. Agric. Food Chem. 1984, 32, 699–705. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, L.; Sun, Y.; Xiong, H.; Ogra, Y. Improvement of the Solubility and Emulsifying Properties of Rice Bran Protein by Phosphorylation with Sodium Trimetaphosphate. Food Hydrocoll. 2019, 96, 288–299. [Google Scholar] [CrossRef]
- Chemisto, F. Phosphorylation of Food Proteins with Phosphorus Oxychloridc Improvement of Functional and Nutritional Properties: A Review. Food Chem. 1991, 39, 13–26. [Google Scholar]
- Aguilar, J.G.d.S.; Granato Cason, V.; de Castro, R.J.S. Improving Antioxidant Activity of Black Bean Protein by Hydrolysis with Protease Combinations. Int. J. Food Sci. Technol. 2019, 54, 34–41. [Google Scholar] [CrossRef]
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The Enzymatic Hydrolysis of Soy Protein Isolate by Corolase PP under High Hydrostatic Pressure and Its Effect on Bioactivity and Characteristics of Hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.K.; Demain, A.L. Protein Hydrolysates in Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–229. [Google Scholar] [CrossRef]
- Valencia, P.; Espinoza, K.; Ceballos, A.; Pinto, M.; Almonacid, S. Novel Modeling Methodology for the Characterization of Enzymatic Hydrolysis of Proteins. Process Biochem. 2015, 50, 589–597. [Google Scholar] [CrossRef]
- Barros, R.M.; Xavier Malcata, F. A Kinetic Model for Hydrolysis of Whey Proteins by Cardosin A Extracted from Cynara Cardunculus. Food Chem. 2004, 88, 351–359. [Google Scholar] [CrossRef]
- O’Meara, G.M.; Munro, P.A. Kinetics of the Hydrolysis of Lean Meat Protein by Alcalase: Derivation of Two Alternative Rate Equations and Their Fit to Experimental Data. Biotechnol. Bioeng. 1985, 27, 861–869. [Google Scholar] [CrossRef]
- Trusek-Holownia, A. Production of Protein Hydrolysates in an Enzymatic Membrane Reactor. Biochem. Eng. J. 2008, 39, 221–229. [Google Scholar] [CrossRef]
- Keillor, J.W.; Clouthier, C.M.; Apperley, K.Y.P.; Akbar, A.; Mulani, A. Acyl Transfer Mechanisms of Tissue Transglutaminase. Bioorg. Chem. 2014, 57, 186–197. [Google Scholar] [CrossRef]
- Isaschar-Ovdat, S.; Fishman, A. Crosslinking of Food Proteins Mediated by Oxidative Enzymes—A Review. Trends Food Sci. Technol. 2018, 72, 134–143. [Google Scholar] [CrossRef]
- Nivala, O.; Mäkinen, O.E.; Kruus, K.; Nordlund, E.; Ercili-Cura, D. Structuring Colloidal Oat and Faba Bean Protein Particles via Enzymatic Modification. Food Chem. 2017, 231, 87–95. [Google Scholar] [CrossRef]
- Glusac, J.; Isaschar-Ovdat, S.; Fishman, A. Transglutaminase Modifies the Physical Stability and Digestibility of Chickpea Protein-Stabilized Oil-in-Water Emulsions. Food Chem. 2020, 315, 126301. [Google Scholar] [CrossRef] [PubMed]
- Djoullah, A.; Husson, F.; Saurel, R. Gelation Behaviors of Denaturated Pea Albumin and Globulin Fractions during Transglutaminase Treatment. Food Hydrocoll. 2018, 77, 636–645. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation Properties of Salt-Extracted Pea Protein Isolate Catalyzed by Microbial Transglutaminase Cross-Linking. Food Hydrocoll. 2011, 25, 25–31. [Google Scholar] [CrossRef]
- Ma, H.; Forssell, P.; Partanen, R.; Buchert, J.; Boer, H. Improving Laccase Catalyzed Cross-Linking of Whey Protein Isolate and Their Application as Emulsifiers. J. Agric. Food Chem. 2011, 59, 1406–1414. [Google Scholar] [CrossRef]
- Heijnis, W.H.; Wierenga, P.A.; Van Berkel, W.J.H.; Gruppen, H. Directing the Oligomer Size Distribution of Peroxidase-Mediated Cross-Linked Bovine α-Lactalbumin. J. Agric. Food Chem. 2010, 58, 5692–5697. [Google Scholar] [CrossRef]
- Saricay, Y.; Wierenga, P.; De Vries, R. Nanostructure Development during Peroxidase Catalysed Cross-Linking of α-Lactalbumin. Food Hydrocoll. 2013, 33, 280–288. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-Enabled Wellness Foods: A Fresh Perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F. Fermentation for Future Food Systems. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef]
- Barus, T.; Giovania, G.; Lay, B.W. Lactic Acid Bacteria from Tempeh and Their Ability to Acidify Soybeans in Tempeh Fermentation. Microbiol. Indones. 2020, 14, 149–155. [Google Scholar] [CrossRef]
- Garofalo, C.; Norici, A.; Mollo, L.; Osimani, A.; Aquilanti, L. Fermentation of Microalgal Biomass for Innovative Food Production. Microorganisms 2022, 10, 2069. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.; Tyndall, S. Innovation in Precision Fermentation for Food Ingredients. Crit. Rev. Food Sci. Nutr. 2023, 2023, 2166014. [Google Scholar] [CrossRef]
- Tarapata, J.; Smoczyński, M.; Maciejczyk, M.; Zulewska, J. Effect of Calcium Chloride Addition on Properties of Acid-Rennet Gels. Int. Dairy J. 2020, 106, 104707. [Google Scholar] [CrossRef]
- Grasso, N.; Roos, Y.H.; Crowley, S.V.; Arendt, E.K.; O’Mahony, J.A. Composition and Physicochemical Properties of Commercial Plant-Based Block-Style Products as Alternatives to Cheese. Future Foods 2021, 4, 100048. [Google Scholar] [CrossRef]
- Bergsma, J. Vegan Cheese Analogue. Patent WO2017150973A1, 1 March 2017. pp. 1–28. [Google Scholar]
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the Size and Content of Protein Aggregates on the Rheological and Structural Properties of Soy Protein Isolate Emulsion Gels Induced by CaSO4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lu, J.; Liu, F.; Nsor-Atindana, J.; Xu, F.; Goff, H.D.; Ma, J.; Zhong, F. Study on the Emulsifying Stability and Interfacial Adsorption of Pea Proteins. Food Hydrocoll. 2019, 88, 247–255. [Google Scholar] [CrossRef]
- Gonzalez Rodriguez, A. Vegetable-Based Cheese and Method of Making the Same. U.S. Patent 15/166,127, 26 May 2016. pp. 1–39. [Google Scholar]
- Janahar, J.J.; Balasubramaniam, V.M.; Jiménez-Flores, R.; Campanella, O.H.; Patel, B.; Ortega-Anaya, J. Impact of Ultra-Shear Technology on Quality Attributes of Model Dairy-Pea Protein Dispersions with Different Fat Levels. Curr. Res. Food Sci. 2023, 6, 100439. [Google Scholar] [CrossRef]






| Energy (Kcal) | Protein (%) | Carbohydrate (%) | Dietary Fibre (%) | Lipid (%) | |
|---|---|---|---|---|---|
| CEREALS | |||||
| Wheat | 340 | 13.2 | 71.9 | 10.7 | 2.5 |
| Barley | 352 | 9.9 | 77.7 | 15.6 | 1.2 |
| Oat | 379 | 13.2 | 67.7 | 10.1 | 6.5 |
| Rice | 367 | 7.5 | 76.3 | 3.6 | 1.4 |
| PSEUDO-CEREALS | |||||
| Quinoa | 368 | 14.2 | 64.2 | 7.0 | 6.1 |
| Chia seeds | 486 | 16.5 | 42.1 | 34.4 | 30.7 |
| Amaranth | 371 | 13.6 | 65.3 | 6.7 | 7.0 |
| Buckwheat | 343 | 13.3 | 71.5 | 10.0 | 3.4 |
| Energy (Kcal) | Protein (%) | Carbohydrate (%) | Dietary Fibre (%) | Lipid (%) | |
|---|---|---|---|---|---|
| Cow peas | 339 | 22.0 | 59.1 | 4.5 | 1.4 |
| Pigeon peas | 336 | 22.4 | 51.2 | 5.5 | 1.7 |
| Red kidney | 336 | 23.1 | 62.7 | - | 1.7 |
| Mung bean | 345 | 22.2 | 62.9 | 4.40 | 1.8 |
| Jack bean | 389 | 30.3 | 54.0 | - | 2.9 |
| Soybean | 335 | 38.0 | 31.3 | 3.80 | 18.0 |
| Energy (Kcal) | Protein (%) | Carbohydrate (%) | Dietary Fibre (%) | Lipid (%) | |
|---|---|---|---|---|---|
| Cottonseeds | 253 | 24.7 | 19.4 | 6.0 | 25.2 |
| Sunflower | 627 | 27.5 | 19.4 | 9.0 | 50.8 |
| Pumpkin seeds | 591 | 21.4 | 18.9 | 18.4 | 48.0 |
| Sesame seeds | 573 | 20–28 | 14–26 | 11.8 | 48–55 |
| Flaxseeds | 530 | 20.3 | 27.3 | 4.8 | 37.1 |
| Palm kernel | 514 | 14.8 | 50.3 | 16.7 | 7.9 |
| Energy (Kcal) | Protein (%) | Carbohydrate (%) | Dietary Fibre (%) | Lipid (%) | |
|---|---|---|---|---|---|
| Peanuts | 587 | 24.4 | 21.3 | 8.4 | 49.7 |
| Almonds | 607 | 21.4 | 17.9 | 10.7 | 53.6 |
| Cashews | 579 | 18.4 | 28.9 | 2.6 | 47.4 |
| Walnuts | 654 | 15.2 | 13.7 | 6.7 | 65.2 |
| Pistachios | 571 | 21.4 | 28.6 | 10.7 | 46.4 |
| Pecans | 679 | 7.1 | 21.4 | 7.0 | 67.9 |
| Ingredient | Sources |
|---|---|
| Carbohydrate | Tapioca, potato, and corn starches |
| Plant protein | Legume, nut, and seed proteins |
| Vegetable oil | Coconut, cocoa, and palm oils |
| Salt | Sodium citrate and sodium phosphate |
| Texturizer | Xanthan gum, agar, and alginic acid |
| Acidulent | Acetic acid, citric acid, and lactic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, J.; Bechtold, T.; Pham, T. Plant-Based Proteins and Their Modification and Processing for Vegan Cheese Production. Macromol 2024, 4, 23-41. https://doi.org/10.3390/macromol4010002
Kovačević J, Bechtold T, Pham T. Plant-Based Proteins and Their Modification and Processing for Vegan Cheese Production. Macromol. 2024; 4(1):23-41. https://doi.org/10.3390/macromol4010002
Chicago/Turabian StyleKovačević, Jelica, Thomas Bechtold, and Tung Pham. 2024. "Plant-Based Proteins and Their Modification and Processing for Vegan Cheese Production" Macromol 4, no. 1: 23-41. https://doi.org/10.3390/macromol4010002
APA StyleKovačević, J., Bechtold, T., & Pham, T. (2024). Plant-Based Proteins and Their Modification and Processing for Vegan Cheese Production. Macromol, 4(1), 23-41. https://doi.org/10.3390/macromol4010002






