Mimic miRNA and Anti-miRNA Activated Scaffolds as a Therapeutic Strategy to Promote Bone, Cartilage, and Skin Regeneration
Abstract
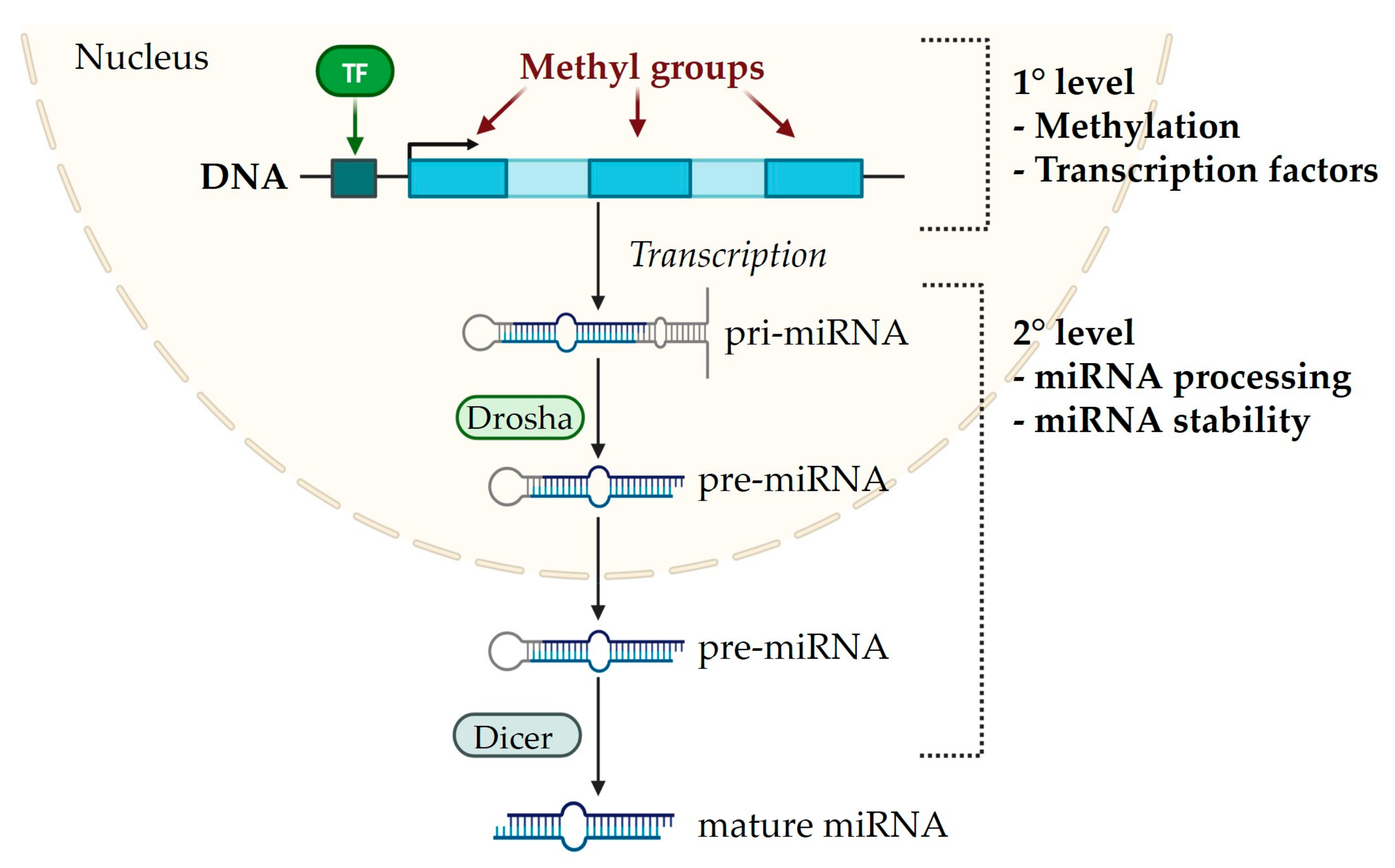
1. MiRNA Biogenesis
2. Epigenetics–miRNA Regulatory Loop
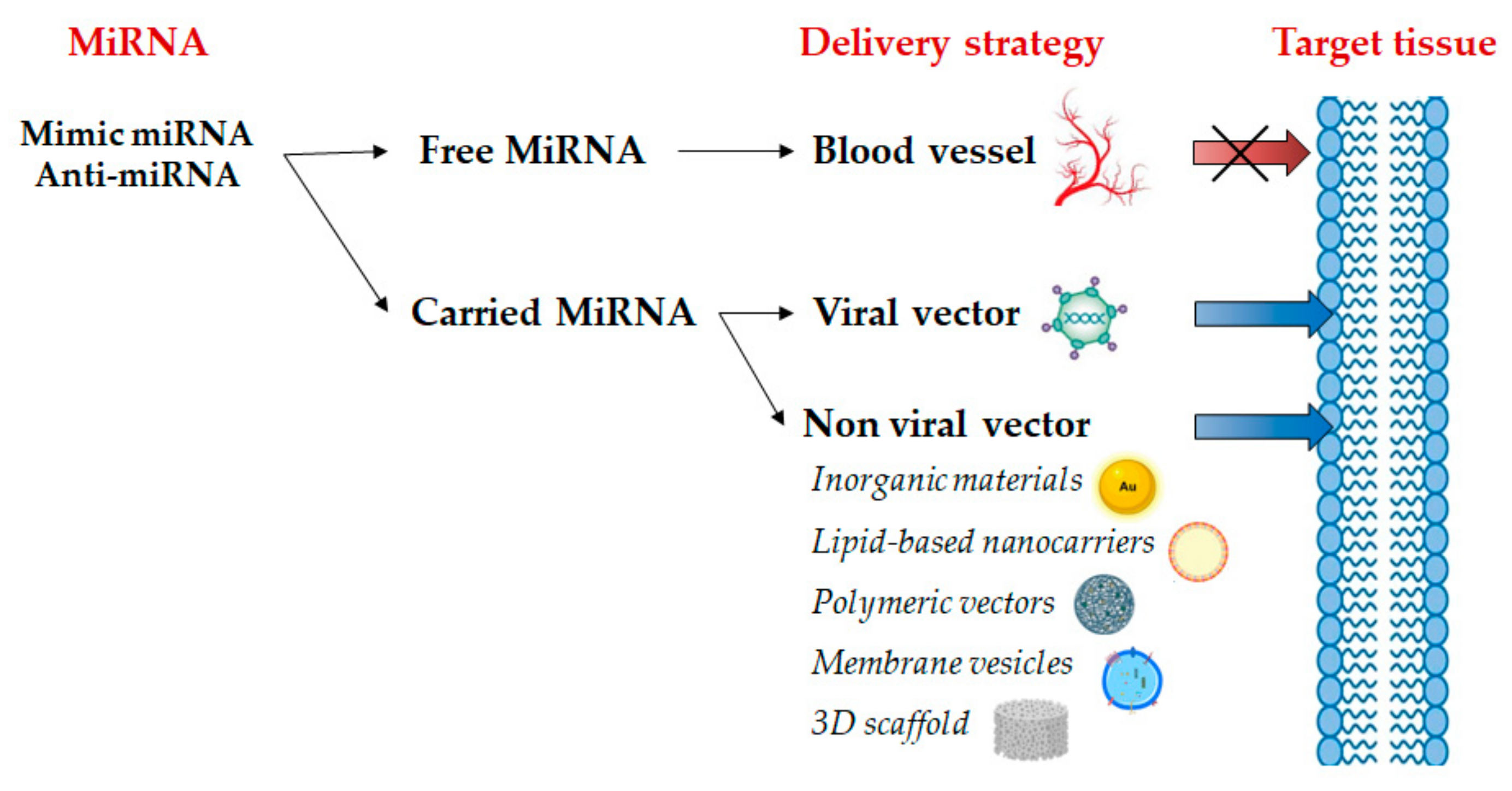
3. Emerging Clinical Application of Mimic miRNA and Anti-miRNA
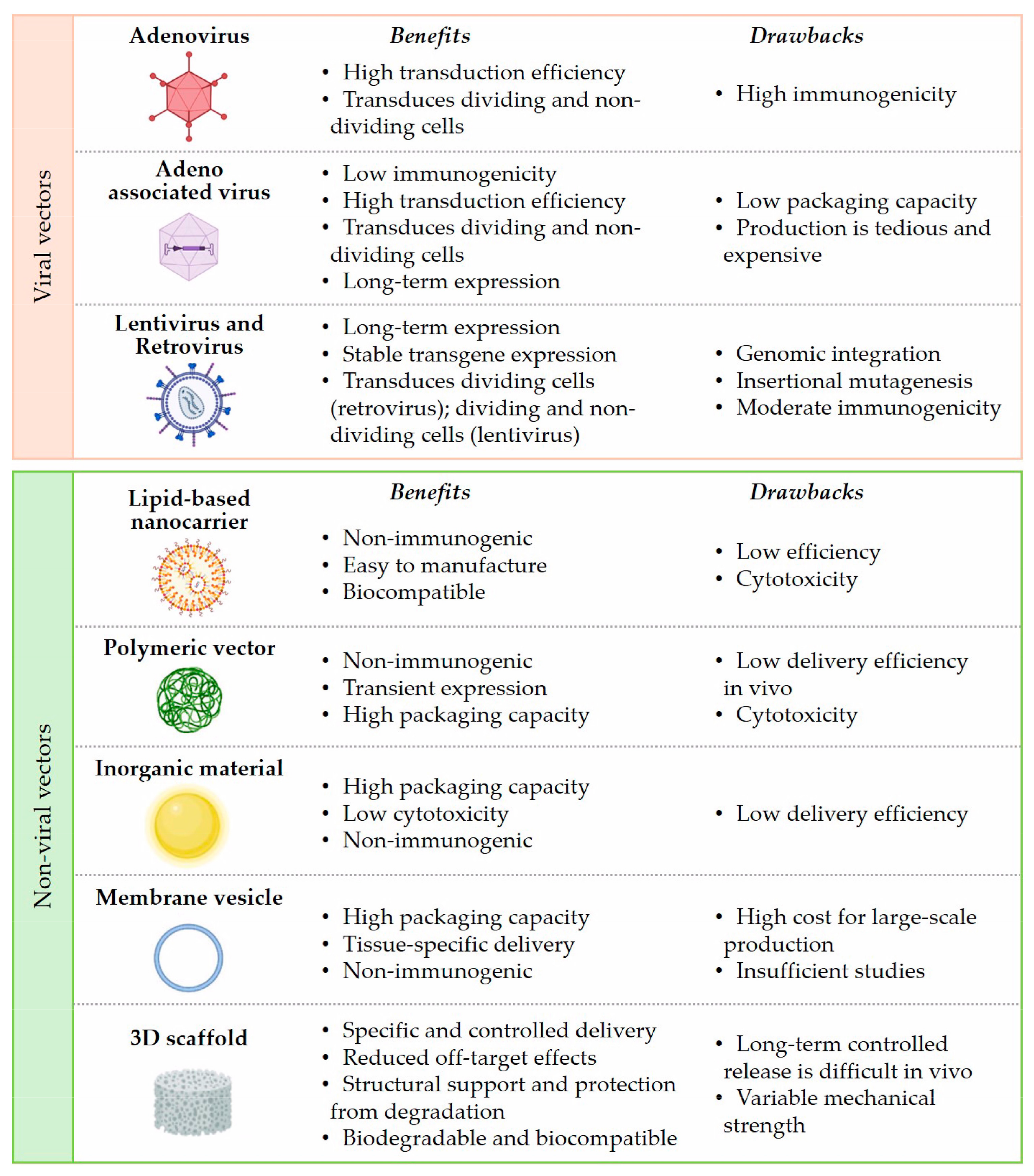
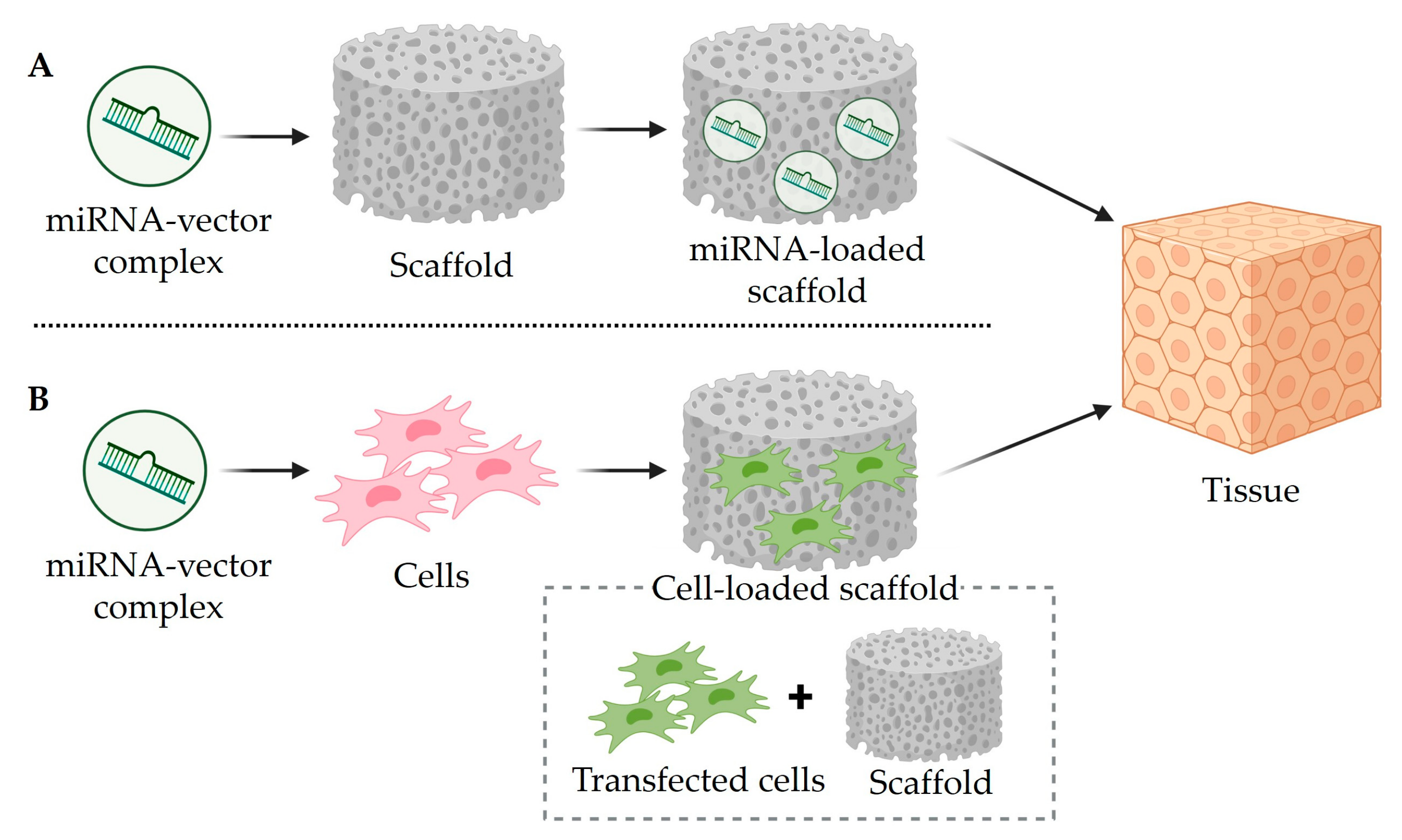
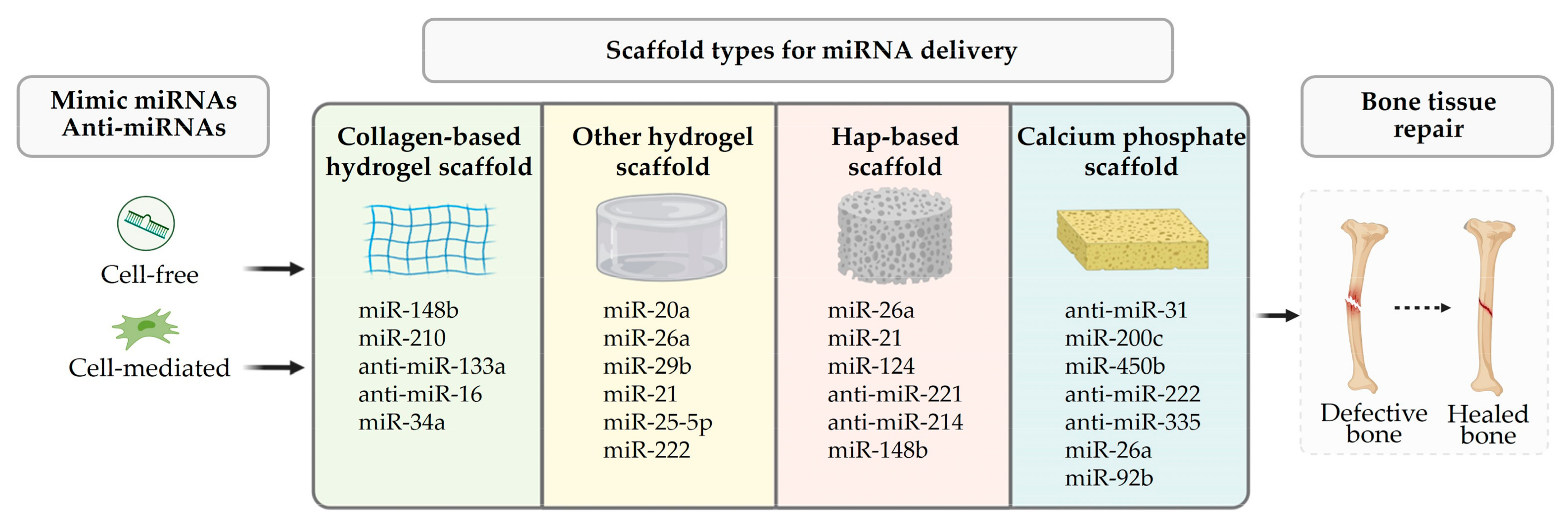
4. Mimic miRNA and Anti-miRNA Scaffold Delivery for Tissue Regeneration
| Scaffold | MiRNA | In Vitro and In Vivo | Tissue | Ref | Advantages and Disadvantages |
|---|---|---|---|---|---|
| Collagen-based hydrogel | miR-148b | BMSCs; rat calvarial defect | B | [106] | Advantages: biodegradable, highly biocompatible, easily modifiable, and versatile. Disadvantages: poor mechanical strength and stiffness; variability of isolated collagen. |
| miR-210 and anti-miR-16 | HMSCs; rat calvarial defect | B | [107] | ||
| anti-miR-133a | rat calvarial defect | B | [108,109] | ||
| miR-34a | MSCs; rat tibia | B | [110] | ||
| anti-miR-221 | hMSCs | C | [111] | ||
| Other hydrogel | miR-20a | hMSCs; rat calvarial defect | B | [112] | Advantages: high biocompatible, controlled biodegradation rate. Disadvantages: variable mechanical strength. |
| chol-miR-26a | hMSC | B | [113] | ||
| exo-miR-26a | rat calvarial defect | B | [114] | ||
| miR-29b | mouse subcutaneous implantation | B | [115] | ||
| miR-221 | mouse osteochondral defect | C | [116] | ||
| miR-99a-3p | murine models of osteoarthritis | C | [117] | ||
| miR-223 5p | macrophages J774A.1 | S | [118] | ||
| miR-17-5p | diabetic mouse | S | [119] | ||
| Hydroxyapatite | miR-21/124 | MC3T3-E1 and 4B12; mouse bilateral cranial defect | B | [120] | Advantages: bioactive, biocompatible, osteoconductive, non-toxic, and non-inflammatory. Disadvantages: brittle, slowly degradable. |
| anti-miR-221 | rat calvarial defect | B | [121] | ||
| Calcium phosphate | anti-miR-31 | rat critical-sized bone defect; canine medial orbital wall defect | B | [122,123] | Advantages: excellent biocompatibility, good, osteoconductivity, adequate mechanical strength. Disadvantages: slowly degradable, brittle, non-resorbable, poor mechanical properties. |
| miR-200c | BMSCs; rat calvarial defect | B | [124] |
4.1. MiRNA-Activated Scaffold and Bone Regeneration
- -
- Collagen-based hydrogel scaffolds
- -
- Other natural and synthetic hydrogel scaffolds
- -
- Hydroxyapatite-based scaffolds
- -
- Calcium phosphate scaffolds
4.2. MiRNA-Activated Scaffold and Cartilage Repair
4.3. MiRNA-Activated Scaffold and Wound Healing and Skin Regeneration
5. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamilton, J.P. Epigenetics: Principles and Practice. Dig. Dis. 2011, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, B. Epigenetics: The Science of Change. Environ. Health Perspect. 2006, 114, A160–A167. [Google Scholar] [CrossRef] [PubMed]
- Peschansky, V.J.; Wahlestedt, C. Non-Coding RNAs as Direct and Indirect Modulators of Epigenetic Regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Medley, J.C.; Panzade, G.; Zinovyeva, A.Y. microRNA Strand Selection: Unwinding the Rules. WIREs RNA 2021, 12, e1627. [Google Scholar] [CrossRef] [PubMed]
- Dalmay, T. Mechanism of miRNA-Mediated Repression of mRNA Translation. Essays Biochem. 2013, 54, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs Are Repressed as Efficiently by microRNA-Binding Sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Place, R.F.; Li, L.-C.; Pookot, D.; Noonan, E.J.; Dahiya, R. MicroRNA-373 Induces Expression of Genes with Complementary Promoter Sequences. Proc. Natl. Acad. Sci. USA 2008, 105, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Eiring, A.M.; Harb, J.G.; Neviani, P.; Garton, C.; Oaks, J.J.; Spizzo, R.; Liu, S.; Schwind, S.; Santhanam, R.; Hickey, C.J.; et al. miR-328 Functions as an RNA Decoy to Modulate hnRNP E2 Regulation of mRNA Translation in Leukemic Blasts. Cell 2010, 140, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sætrom, P.; Snøve, O.; Rossi, J.J. MicroRNA-Directed Transcriptional Gene Silencing in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16230–16235. [Google Scholar] [CrossRef] [PubMed]
- Mette, M.F.; Aufsatz, W.; Van Der Winden, J.; Matzke, M.A.; Matzke, A.J.M. Transcriptional Silencing and Promoter Methylation Triggered by Double-Stranded RNA. EMBO J. 2000, 19, 5194–5201. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chiu, H.; Domenger, D.; Chuang, C.-F.; Chang, C. The Lin-4 MicroRNA Targets the LIN-14 Transcription Factor to Inhibit Netrin-Mediated Axon Attraction. Sci. Signal. 2012, 5, ra43. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther.-Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Shapiro, G.I.; LoRusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M.; et al. First-in-Human Phase I Study of the Liposomal RNA Interference Therapeutic Atu027 in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Brown, D.; Stoudemire, J.; Lammers, P. Developing Therapeutic microRNAs for Cancer. Gene Ther. 2011, 18, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Gayosso-Gómez, L.V.; Ortiz-Quintero, B. Circulating MicroRNAs in Blood and Other Body Fluids as Biomarkers for Diagnosis, Prognosis, and Therapy Response in Lung Cancer. Diagnostics 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, L.; Sun, D.; Hou, J.; Ji, Z. CircRNA: A Novel Type of Biomarker for Cancer. Breast Cancer 2018, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhou, C.; Zhuang, J.; Liu, L.; Liu, C.; Li, H.; Liu, G.; Wei, J.; Sun, C. MicroRNA Expression in Cervical Cancer: Novel Diagnostic and Prognostic Biomarkers. J. Cell. Biochem. 2018, 119, 7080–7090. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Dittmar, R.L.; Sen, S. Secretory microRNAs as Biomarkers of Cancer. Semin. Cell Dev. Biol. 2018, 78, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Gromisch, C.; Ozturk, S.; Papageorgis, P.; Abdolmaleky, H.M.; Reinhard, B.M.; Thiagalingam, A.; Thiagalingam, S. MicroRNA-4417 Is a Tumor Suppressor and Prognostic Biomarker for Triple-Negative Breast Cancer. Cancer Biol. Ther. 2019, 20, 1113–1120. [Google Scholar] [CrossRef]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 Reflect Myocardial Damage in Cardiovascular Disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; De Windt, L.J.; Van Der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p As a Circulating Biomarker for Heart Failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, R.; Li, Y.; Pu, J.; Lu, Y.; Jiao, J.; Li, K.; Yu, B.; Li, Z.; Wang, R.; et al. Circulating microRNA-1 as a Potential Novel Biomarker for Acute Myocardial Infarction. Biochem. Biophys. Res. Commun. 2010, 391, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Ma, J.; Chen, Z.; Wang, F.; Li, Z.; Shang, Z.; Dong, J. Osteoarthritis Related Epigenetic Variations in miRNA Expression and DNA Methylation. BMC Med. Genom. 2023, 16, 163. [Google Scholar] [CrossRef]
- Kim, M.; Rubab, A.; Chan, W.C.W.; Chan, D. Osteoarthritis Year in Review 2022: Genetics, Genomics and Epigenetics. Osteoarthr. Cartil. 2023, 31, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, H.; Peng, X.; Chai, Y.; Wang, S.; Wen, G. Identification and Comprehensive Analysis of circRNA–miRNA–mRNA Regulatory Networks in Osteoarthritis. Front. Immunol. 2023, 13, 1050743. [Google Scholar] [CrossRef] [PubMed]
- Foessl, I.; Kotzbeck, P.; Obermayer-Pietsch, B. miRNAs as Novel Biomarkers for Bone Related Diseases. J. Lab. Precis. Med. 2019, 4, 2. [Google Scholar] [CrossRef]
- Kingsley, S.M.K.; Bhat, B.V. Role of microRNAs in Sepsis. Inflamm. Res. 2017, 66, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Q.; Cui, X.; Yang, J.; Zhang, B.; Song, G. Differential Expression of miRNA and Its Role in Sepsis. Pediatrics 2018, 142, 563. [Google Scholar] [CrossRef]
- Daka, A.; Peer, D. RNAi-Based Nanomedicines for Targeted Personalized Therapy. Adv. Drug Deliv. Rev. 2012, 64, 1508–1521. [Google Scholar] [CrossRef]
- Kadekar, S.; Nawale, G.N.; Karlsson, K.; Ålander, C.; Oommen, O.P.; Varghese, O.P. Synthetic Design of Asymmetric miRNA with an Engineered 3′ Overhang to Improve Strand Selection. Mol. Ther.-Nucleic Acids 2019, 16, 597–604. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The Multilayered Complexity of ceRNA Crosstalk and Competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Hsin, J.-P.; Lu, Y.; Loeb, G.B.; Leslie, C.S.; Rudensky, A.Y. The Effect of Cellular Context on miR-155-Mediated Gene Regulation in Four Major Immune Cell Types. Nat. Immunol. 2018, 19, 1137–1145. [Google Scholar] [CrossRef]
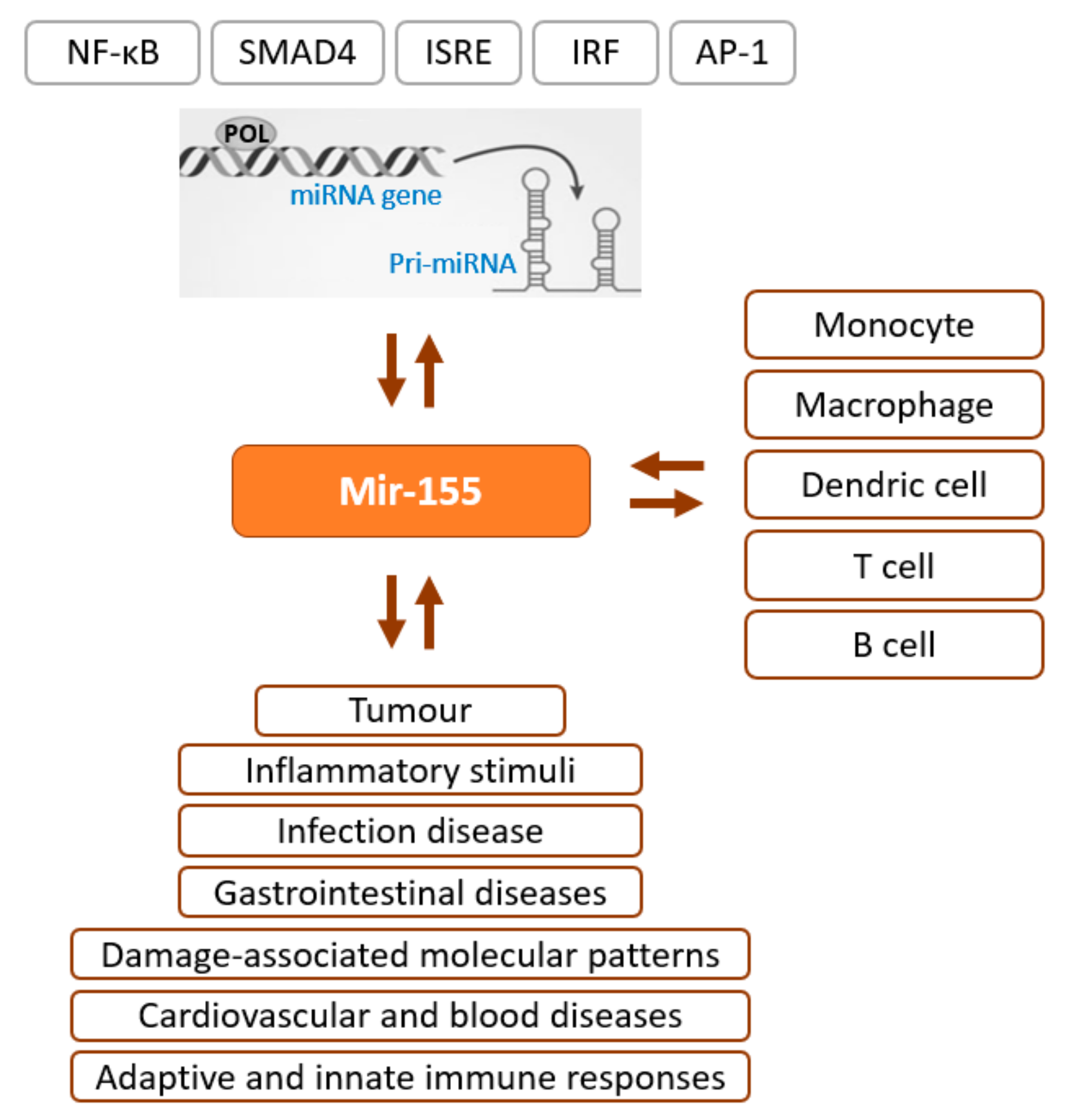
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. miR-155 Gene: A Typical Multifunctional microRNA. Biochim. Biophys. Acta 2009, 1792, 497–505. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of Bic/microRNA-155 for Normal Immune Function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 Is Induced during the Macrophage Inflammatory Response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.R.; Mangan, N.E.; Caffrey, B.E.; Gantier, M.P.; Williams, B.R.G.; Hertzog, P.J.; McCoy, C.E.; O’Neill, L.A.J. The Role of Ets2 Transcription Factor in the Induction of microRNA-155 (miR-155) by Lipopolysaccharide and Its Targeting by Interleukin-10. J. Biol. Chem. 2014, 289, 4316–4325. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of miRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Brown, D.; Winkler, M. The Promise of MicroRNA Replacement Therapy. Cancer Res. 2010, 70, 7027–7030. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Tsuchiya, S.; Meltzer, S.J.; Shimizu, K. MicroRNAs and Epigenetics. FEBS J. 2011, 278, 1598–1609. [Google Scholar] [CrossRef]
- Gulyaeva, L.F.; Kushlinskiy, N.E. Regulatory Mechanisms of microRNA Expression. J. Transl. Med. 2016, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 Host Gene in Physiological and Pathological Processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Christina, V.R.; Guo, C.; Chu, Y.; Liu, R.; Yan, Z. Involvement of MicroRNA-210 Demethylation in Steroid-Associated Osteonecrosis of the Femoral Head. Sci. Rep. 2016, 6, 20046. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in Vivo with “Antagomirs”. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef] [PubMed]
- McLeod, B.W.; Hayman, M.L.; Purcell, A.L.; Marcus, J.S.; Veitenheimer, E. The “real World” Utility of miRNA Patents: Lessons Learned from Expressed Sequence Tags. Nat. Biotechnol. 2011, 29, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Chang, S.; Sang, Y.; Ding, P.; Wang, L.; Nan, X.; Xu, R.; Liu, F.; Gu, L.; Zheng, Y.; et al. Circular RNA circCCDC85A Inhibits Breast Cancer Progression via Acting as a miR-550a-5p Sponge to Enhance MOB1A Expression. Breast Cancer Res. BCR 2022, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic Advances of miRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Grixti, J.M.; Ayers, D.; Day, P.J.R. An Analysis of Mechanisms for Cellular Uptake of miRNAs to Enhance Drug Delivery and Efficacy in Cancer Chemoresistance. Non-Coding RNA 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Alhakamy, N.A.; Karim, S.; Gabr, G.A.; Iqubal, M.K.; Murshid, S.S.A. Signaling Pathway Inhibitors, miRNA, and Nanocarrier-Based Pharmacotherapeutics for the Treatment of Lung Cancer: A Review. Pharmaceutics 2021, 13, 2120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Gu, Y.; Bian, C.; Xie, X.; Bai, Y.; Zhang, N. Applications of Nonviral Biomaterials for microRNA Transfection in Bone Tissue Engineering. Front. Mater. 2022, 9, 932157. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.-Y.; Huang, L. In Vivo Delivery of miRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Chow, M.Y.T.; Qiu, Y.; Lam, J.K.W. Inhaled RNA Therapy: From Promise to Reality. Trends Pharmacol. Sci. 2020, 41, 715–729. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and Characteristics Governing Cellular Internalization and Trans-Barrier Trafficking of Nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar] [CrossRef]
- Midoux, P.; Pichon, C.; Yaouanc, J.-J.; Jaffrès, P.-A. Chemical Vectors for Gene Delivery: A Current Review on Polymers, Peptides and Lipids Containing Histidine or Imidazole as Nucleic Acids Carriers. Br. J. Pharmacol. 2009, 157, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and Nonviral Delivery Systems for Gene Delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, N. An Overview of Viral and Nonviral Delivery Systems for microRNA. Int. J. Pharm. Investig. 2015, 5, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.M.; Castaño, I.M.; O’Brien, F.J. Scaffold-Based microRNA Therapies in Regenerative Medicine and Cancer. Adv. Healthc. Mater. 2018, 7, 1700695. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Huang, Z. Recent Progress in microRNA-Based Delivery Systems for the Treatment of Human Disease. ExRNA 2019, 1, 24. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non Viral Vectors in Gene Therapy—An Overview. J. Clin. Diagn. Res. JCDR 2015, 9, GE01–GE06. [Google Scholar] [CrossRef] [PubMed]
- Yang, N. Nonviral Gene Delivery System. Int. J. Pharm. Investig. 2012, 2, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, H.; Jiang, X.; Zhou, W.; Zhu, B.; Zeng, Y.; Yao, K.; Ren, C. Lipofectamine RNAiMAX: An Efficient siRNA Transfection Reagent in Human Embryonic Stem Cells. Mol. Biotechnol. 2008, 40, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, D.; Hu, Z.; Cheng, J.; Zhuo, C.; Fang, X.; Xing, Y. MicroRNA-26a-Modified Adipose-Derived Stem Cells Incorporated with a Porous Hydroxyapatite Scaffold Improve the Repair of Bone Defects. Mol. Med. Rep. 2015, 12, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, Y.; Leong, K.W. MicroRNA Delivery for Regenerative Medicine. Adv. Drug Deliv. Rev. 2015, 88, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther.-Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, H.; Butowska, K.; Swingle, K.L.; Alameh, M.-G.; Weissman, D.; Mitchell, M.J. An Ionizable Lipid Toolbox for RNA Delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef] [PubMed]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of Lipid-Based and Polymer-Based Non-Viral Vectors in Nucleic Acid Delivery for Next-Generation Gene Therapy. Molecules 2020, 25, 2866. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, H.; Li, G.; Su, J.; Wei, Y.; Xu, C. Lipid Nanovehicles Overcome Barriers to Systemic RNA Delivery: Lipid Components, Fabrication Methods, and Rational Design. Acta Pharm. Sin. B 2023, 14, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Schade, A.; Delyagina, E.; Scharfenberg, D.; Skorska, A.; Lux, C.; David, R.; Steinhoff, G. Innovative Strategy for MicroRNA Delivery in Human Mesenchymal Stem Cells via Magnetic Nanoparticles. Int. J. Mol. Sci. 2013, 14, 10710–10726. [Google Scholar] [CrossRef] [PubMed]
- Bitar, A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Silica-Based Nanoparticles for Biomedical Applications. Drug Discov. Today 2012, 17, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Agarwal, V.; Agarwal, M.; Singh, M. Exosomes: Structure, Biogenesis, Types and Application in Diagnosis and Gene and Drug Delivery. Curr. Gene Ther. 2020, 20, 195–206. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Eberhardt, M.; Schmitz, U.; Vera, J. Systems Biology-Based Investigation of Cooperating microRNAs as Monotherapy or Adjuvant Therapy in Cancer. Nucleic Acids Res. 2019, 47, 7753–7766. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Cho, W.C. The Role of microRNAs in Toxicology. Arch. Toxicol. 2015, 89, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Steer, C.J. Special Issue: MicroRNA Regulation in Health and Disease. Genes 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol. Biol. 2017, 1617, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 Study of MRX34, a Liposomal miR-34a Mimic, in Patients with Advanced Solid Tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef] [PubMed]
- Lindow, M.; Kauppinen, S. Discovering the First microRNA-Targeted Drug. J. Cell Biol. 2012, 199, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.-S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther.-Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Soler-Bistué, A.; Zorreguieta, A.; Tolmasky, M.E. Bridged Nucleic Acids Reloaded. Molecules 2019, 24, 2297. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.J. RNA Stability: Remember Your Driver. Nat. Rev. Genet. 2012, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Nöth, U.; Rackwitz, L.; Steinert, A.F.; Tuan, R.S. Cell Delivery Therapeutics for Musculoskeletal Regeneration. Adv. Drug Deliv. Rev. 2010, 62, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent Advances in Nanomaterials for Gene Delivery—A Review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, M.A.; Ding, M.-H.; Gutierrez, A.S.; Chew, S.A. The Application of microRNAs in Biomaterial Scaffold-Based Therapies for Bone Tissue Engineering. Biotechnol. J. 2019, 14, 1900084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, Y.; Zhu, W.; Zhang, G.; Yang, Y.P.; Zhao, C. The Role of MicroRNAs in Tendon Injury, Repair, and Related Tissue Engineering. Biomaterials 2021, 277, 121083. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhu, Y.Y.; Smiley, E.; Bonadio, J.; Rouleau, J.P.; Goldstein, S.A.; McCauley, L.K.; Davidson, B.L.; Roessler, B.J. Stimulation of New Bone Formation by Direct Transfer of Osteogenic Plasmid Genes. Proc. Natl. Acad. Sci. USA 1996, 93, 5753–5758. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, J.; Smiley, E.; Patil, P.; Goldstein, S. Localized, Direct Plasmid Gene Delivery in Vivo: Prolonged Therapy Results in Reproducible Tissue Regeneration. Nat. Med. 1999, 5, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Bidram, E.; Bigham, A.; Atari, M.; Nasr Azadani, R.; Tavakoli, M.; Salehi, S.; Mirhaj, M.; Basiri, A.; Mirzavandi, Z.; et al. Exploring the Evolution of Tissue Engineering Strategies over the Past Decade: From Cell-Based Strategies to Gene-Activated Matrix. Alex. Eng. J. 2023, 81, 137–169. [Google Scholar] [CrossRef]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Kulus, M.; Sibiak, R.; Stefańska, K.; Zdun, M.; Wieczorkiewicz, M.; Piotrowska-Kempisty, H.; Jaśkowski, J.M.; Bukowska, D.; Ratajczak, K.; Zabel, M.; et al. Mesenchymal Stem/Stromal Cells Derived from Human and Animal Perinatal Tissues—Origins, Characteristics, Signaling Pathways, and Clinical Trials. Cells 2021, 10, 3278. [Google Scholar] [CrossRef]
- Eguchi, T.; Kuboki, T. Cellular Reprogramming Using Defined Factors and MicroRNAs. Stem Cells Int. 2016, 2016, 7530942. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, M.; Chen, Y.; You, M.; Chen, Q. MicroRNAs as Important Regulators Mediate the Multiple Differentiation of Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2021, 9, 619842. [Google Scholar] [CrossRef] [PubMed]
- Thomaidou, A.C.; Goulielmaki, M.; Tsintarakis, A.; Zoumpourlis, P.; Toya, M.; Christodoulou, I.; Zoumpourlis, V. miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy. Int. J. Mol. Sci. 2023, 24, 9189. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Aydin, R.S.T.; Abu-Laban, M.; Heo, D.N.; Rizk, E.; Tucker, S.M.; Lewis, G.S.; Hayes, D.; Ozbolat, I.T. Collagen-Infilled 3D Printed Scaffolds Loaded with miR-148b-Transfected Bone Marrow Stem Cells Improve Calvarial Bone Regeneration in Rats. Mater. Sci. Eng. C 2019, 105, 110128. [Google Scholar] [CrossRef] [PubMed]
- Castaño, I.M.; Raftery, R.M.; Chen, G.; Cavanagh, B.; Quinn, B.; Duffy, G.P.; Curtin, C.M.; O’Brien, F.J. Dual Scaffold Delivery of miR-210 Mimic and miR-16 Inhibitor Enhances Angiogenesis and Osteogenesis to Accelerate Bone Healing. Acta Biomater. 2023, 172, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Castaño, I.M.; Raftery, R.M.; Chen, G.; Cavanagh, B.; Quinn, B.; Duffy, G.P.; O’Brien, F.J.; Curtin, C.M. Rapid Bone Repair with the Recruitment of CD206+M2-like Macrophages Using Non-Viral Scaffold-Mediated miR-133a Inhibition of Host Cells. Acta Biomater. 2020, 109, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Mencía Castaño, I.; Curtin, C.M.; Duffy, G.P.; O’Brien, F.J. Next Generation Bone Tissue Engineering: Non-Viral miR-133a Inhibition Using Collagen-Nanohydroxyapatite Scaffolds Rapidly Enhances Osteogenesis. Sci. Rep. 2016, 6, 27941. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, Y.; Feng, X.; Li, L.; Jiao, Y.; Bai, S.; Feng, Z.; Yu, H.; Li, X.; Zhao, Y. miR-34a Promotes Bone Regeneration in Irradiated Bone Defects by Enhancing Osteoblastic Differentiation of Mesenchymal Stromal Cells in Rats. Stem Cell Res. Ther. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Intini, C.; Ferreras, L.B.; Casey, S.; Dixon, J.E.; Gleeson, J.P.; O’Brien, F.J. An Innovative miR-Activated Scaffold for the Delivery of a miR-221 Inhibitor to Enhance Cartilage Defect Repair. Adv. Ther. 2023, 6, 2200329. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Jeon, O.; Krebs, M.D.; Schapira, D.; Alsberg, E. Sustained Localized Presentation of RNA Interfering Molecules from in Situ Forming Hydrogels to Guide Stem Cell Osteogenic Differentiation. Biomaterials 2014, 35, 6278–6286. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; Zhou, Q.; Ge, J.; Zhao, J.; Wang, Y.; Yan, Q.; Wu, C.; Yu, H.; Xiao, Q.; Wang, W.; et al. Precise In-Situ Release of microRNA from an Injectable Hydrogel Induces Bone Regeneration. Acta Biomater. 2021, 135, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Ma, J.; Chi, X.; Fu, Q.; Zhu, Q.; Cao, W.; Zhang, P.; Xie, X. Integrated Osteoinductive Factors─Exosome@MicroRNA-26a Hydrogel Enhances Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 22805–22816. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Song, W.; Xin, H.; Yu, H.; Wang, H.; Ma, D.; Cao, X.; Wang, Y. MicroRNA-Activated Hydrogel Scaffold Generated by 3D Printing Accelerates Bone Regeneration. Bioact. Mater. 2021, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lolli, A.; Narcisi, R.; Lambertini, E.; Penolazzi, L.; Angelozzi, M.; Kops, N.; Gasparini, S.; Van Osch, G.J.V.M.; Piva, R. Silencing of Antichondrogenic MicroRNA-221 in Human Mesenchymal Stem Cells Promotes Cartilage Repair In Vivo. Stem Cells 2016, 34, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Qin, C.; Pan, S.; Shi, C.; Wu, G.; Feng, Y.; Zhang, J.; Yu, Z.; Liang, B.; Gui, J. Injectable Hyperbranched PEG Crosslinked Hyaluronan Hydrogel Microparticles Containing Mir-99a-3p Modified Subcutaneous ADSCs-Derived Exosomes Was Beneficial for Long-Term Treatment of Osteoarthritis. Mater. Today Bio 2023, 23, 100813. [Google Scholar] [CrossRef] [PubMed]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small Weinh. Bergstr. Ger. 2019, 15, e1902232. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Su, J.; Meng, S.; Wang, Y.; Ma, K.; Li, B.; Chu, Z.; Huang, Q.; Hu, W.; Wang, Z.; et al. MiR-17-5p-engineered sEVs Encapsulated in GelMA Hydrogel Facilitated Diabetic Wound Healing by Targeting PTEN and P21. Adv. Sci. 2024, 2307761. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Śmieszek, A.; Kornicka-Garbowska, K.; Pielok, A.; Janeczek, M.; Lipińska, A.; Nikodem, A.; Filipiak, J.; Sobierajska, P.; Nedelec, J.-M.; et al. Novel Nanohydroxyapatite (nHAp)-Based Scaffold Doped with Iron Oxide Nanoparticles (IO), Functionalized with Small Non-Coding RNA (miR-21/124) Modulates Expression of Runt-Related Transcriptional Factor 2 and Osteopontin, Promoting Regeneration of Osteoporotic Bone in Bilateral Cranial Defects in a Senescence-Accelerated Mouse Model (SAM/P6). PART 2. Int. J. Nanomed. 2021, 16, 6049–6065. [Google Scholar] [CrossRef]
- Sadeghi, M.; Bakhshandeh, B.; Dehghan, M.M.; Mehrnia, M.R.; Khojasteh, A. Functional Synergy of Anti-Mir221 and Nanohydroxyapatite Scaffold in Bone Tissue Engineering of Rat Skull. J. Mater. Sci. Mater. Med. 2016, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, H.; Gu, P.; Fan, X. Repair of Canine Medial Orbital Bone Defects With miR-31–Modified Bone Marrow Mesenchymal Stem Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6016–6023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deng, Y.; Zhou, H.; Zou, D.; Xie, Q.; Bi, X.; Gu, P.; Fan, X. The Role of miR-31-Modified Adipose Tissue-Derived Stem Cells in Repairing Rat Critical-Sized Calvarial Defects. Biomaterials 2013, 34, 6717–6728. [Google Scholar] [CrossRef] [PubMed]
- Remy, M.T.; Akkouch, A.; He, L.; Eliason, S.; Sweat, M.E.; Krongbaramee, T.; Fei, F.; Qian, F.; Amendt, B.A.; Song, X.; et al. Rat Calvarial Bone Regeneration by 3D-Printed β-Tricalcium Phosphate Incorporating MicroRNA-200c. ACS Biomater. Sci. Eng. 2021, 7, 4521–4534. [Google Scholar] [CrossRef] [PubMed]
- Janko, M.; Dietz, K.; Rachor, J.; Sahm, J.; Schroder, K.; Schaible, A.; Nau, C.; Seebach, C.; Marzi, I.; Henrich, D. Improvement of Bone Healing by Neutralization of microRNA-335-5p, but Not by Neutralization of microRNA-92A in Bone Marrow Mononuclear Cells Transplanted into a Large Femur Defect of the Rat. Tissue Eng. Part A 2019, 25, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.T.; Doyle, A.; Chen, C.; Coulon, D.; Dasa, V.; Piero, F.D.; Levi, B.; Monroe, W.T.; Gimble, J.M.; Hayes, D.J. Photoactivated miR-148b–Nanoparticle Conjugates Improve Closure of Critical Size Mouse Calvarial Defects. Acta Biomater. 2015, 12, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chang, H.; Hou, Y.; Wang, Y.; Zhou, Z.; Wang, M.; Huang, Z.; Yu, B. Lentivirus-mediated microRNA-26a Overexpression in Bone Mesenchymal Stem Cells Facilitates Bone Regeneration in Bone Defects of Calvaria in Mice. Mol. Med. Rep. 2018, 18, 5317–5326. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wei, W.; Ruan, J.; Ding, Y.; Zhuang, A.; Bi, X.; Sun, H.; Gu, P.; Wang, Z.; Fan, X. Effects of miR-146a on the Osteogenesis of Adipose-Derived Mesenchymal Stem Cells and Bone Regeneration. Sci. Rep. 2017, 7, 42840. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, T.; Taipaleenmäki, H.; Stenvang, J.; Abdallah, B.M.; Ditzel, N.; Nossent, A.Y.; Bak, M.; Kauppinen, S.; Kassem, M. MicroRNA-138 Regulates Osteogenic Differentiation of Human Stromal (Mesenchymal) Stem Cells in Vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 6139–6144. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Holmstrøm, K.; Qiu, W.; Ditzel, N.; Shi, K.; Hokland, L.; Kassem, M. MicroRNA-34a Inhibits Osteoblast Differentiation and in Vivo Bone Formation of Human Stromal Stem Cells. Stem Cells 2014, 32, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hassan, M.Q.; Volinia, S.; van Wijnen, A.J.; Stein, J.L.; Croce, C.M.; Lian, J.B.; Stein, G.S. A microRNA Signature for a BMP2-Induced Osteoblast Lineage Commitment Program. Proc. Natl. Acad. Sci. USA 2008, 105, 13906–13911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, W.; He, M.; Xie, W.; Lv, Q.; Wan, G.; Li, G.; Wang, H.; Lu, G.; Hu, X.; et al. MiRNA-20a Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells by Co-Regulating BMP Signaling. RNA Biol. 2011, 8, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fan, J.; Liu, Y.; Li, T.; Xu, H.; Yang, Y.; Deng, L.; Li, H.; Zhao, R.C. miR-450b Promotes Osteogenic Differentiation In Vitro and Enhances Bone Formation In Vivo by Targeting BMP3. Stem Cells Dev. 2018, 27, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, L.; Liu, S.; Liu, W.; Zhang, H.; Zhou, T.; Wu, D.; Yang, P.; Shen, L.; Chen, J.; et al. The Promotion of Bone Regeneration through Positive Regulation of Angiogenic–Osteogenic Coupling Using microRNA-26a. Biomaterials 2013, 34, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, L.; Hu, J.; Zhang, L.; Liao, L.; Liu, S.; Wu, D.; Yang, P.; Shen, L.; Chen, J.; et al. MiR-26a Rescues Bone Regeneration Deficiency of Mesenchymal Stem Cells Derived From Osteoporotic Mice. Mol. Ther. 2015, 23, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Z.; Hou, Y.; Fang, W. Potential Mechanisms Underlying the Runx2 Induced Osteogenesis of Bone Marrow Mesenchymal Stem Cells. Am. J. Transl. Res. 2015, 7, 2527–2535. [Google Scholar] [PubMed]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Vijayalekha, A.; Anandasadagopan, S.K.; Pandurangan, A.K. An Overview of Collagen-Based Composite Scaffold for Bone Tissue Engineering. Appl. Biochem. Biotechnol. 2023, 195, 4617–4636. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ruan, C.; Niu, X. Collagen-Based Bioinks for Regenerative Medicine: Fabrication, Application and Prospective. Med. Nov. Technol. Devices 2023, 17, 100211. [Google Scholar] [CrossRef]
- Mörö, A.; Samanta, S.; Honkamäki, L.; Rangasami, V.K.; Puistola, P.; Kauppila, M.; Narkilahti, S.; Miettinen, S.; Oommen, O.; Skottman, H. Hyaluronic Acid Based next Generation Bioink for 3D Bioprinting of Human Stem Cell Derived Corneal Stromal Model with Innervation. Biofabrication 2023, 15, 015020. [Google Scholar] [CrossRef] [PubMed]
- Shams, R.; Behmanesh, A.; Mazhar, F.N.; Vaghari, A.A.; Hossein-Khannazer, N.; Agarwal, T.; Vosough, M.; Padrón, J.M. Developed Bone Biomaterials Incorporated with MicroRNAs to Promote Bone Regeneration: A Systematic Review, Bioinformatics, and Meta-Analysis Study. ACS Biomater. Sci. Eng. 2023, 9, 5186–5204. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Chen, L.; Lv, Y. RNA-Based Scaffolds for Bone Regeneration: Application and Mechanisms of mRNA, miRNA and siRNA. Theranostics 2020, 10, 3190–3205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel Scaffolds in Bone Regeneration: Their Promising Roles in Angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Jeon, O.; Dang, P.N.; Huynh, C.T.; Varghai, D.; Riazi, H.; McMillan, A.; Herberg, S.; Alsberg, E. RNA Interfering Molecule Delivery from in Situ Forming Biodegradable Hydrogels for Enhancement of Bone Formation in Rat Calvarial Bone Defects. Acta Biomater. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- AlMuraikhi, N.; Binhamdan, S.; Alaskar, H.; Alotaibi, A.; Tareen, S.; Muthurangan, M.; Alfayez, M. Inhibition of GSK-3β Enhances Osteoblast Differentiation of Human Mesenchymal Stem Cells through Wnt Signalling Overexpressing Runx2. Int. J. Mol. Sci. 2023, 24, 7164. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J.; et al. miRNA-21 Promotes Osteogenesis via the PTEN/PI3K/Akt/HIF-1α Pathway and Enhances Bone Regeneration in Critical Size Defects. Stem Cell Res. Ther. 2019, 10, 65. [Google Scholar] [CrossRef]
- Lan, Y.; Xie, H.; Jin, Q.; Zhao, X.; Shi, Y.; Zhou, Y.; Hu, Z.; Ye, Y.; Huang, X.; Sun, Y.; et al. Extracellular Vesicles Derived from Neural EGFL-Like 1-Modified Mesenchymal Stem Cells Improve Acellular Bone Regeneration via the miR-25-5p-SMAD2 Signaling Axis. Bioact. Mater. 2022, 17, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable Colloidal Hydrogel with Mesoporous Silica Nanoparticles for Sustained Co-Release of microRNA-222 and Aspirin to Achieve Innervated Bone Regeneration in Rat Mandibular Defects. J. Mater. Chem. B 2019, 7, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite Scaffolds Exhibit in Vitro Immunological Inertness and Promote Robust Osteogenic Differentiation of Human Mesenchymal Stem Cells without Osteogenic Stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Ou, L.; Lan, Y.; Feng, Z.; Feng, L.; Yang, J.; Liu, Y.; Bian, L.; Tan, J.; Lai, R.; Guo, R. Functionalization of SF/HAP Scaffold with GO-PEI-miRNA Inhibitor Complexes to Enhance Bone Regeneration through Activating Transcription Factor 4. Theranostics 2019, 9, 4525–4541. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.G.; Awartani, F.A.; Niazy, A.A.; Jansen, J.A.; Alghamdi, H.S. A Combination of Biphasic Calcium Phosphate (Maxresorb®) and Hyaluronic Acid Gel (Hyadent®) for Repairing Osseous Defects in a Rat Model. Appl. Sci. 2020, 10, 1651. [Google Scholar] [CrossRef]
- Denry, I.; Kuhn, L.T. Design and Characterization of Calcium Phosphate Ceramic Scaffolds for Bone Tissue Engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Miri, Z.; Haugen, H.J.; Loca, D.; Rossi, F.; Perale, G.; Moghanian, A.; Ma, Q. Review on the Strategies to Improve the Mechanical Strength of Highly Porous Bone Bioceramic Scaffolds. J. Eur. Ceram. Soc. 2024, 44, 23–42. [Google Scholar] [CrossRef]
- Yuan, J.; Cui, L.; Zhang, W.J.; Liu, W.; Cao, Y. Repair of Canine Mandibular Bone Defects with Bone Marrow Stromal Cells and Porous Beta-Tricalcium Phosphate. Biomaterials 2007, 28, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, Z.; Bi, X.; Zhou, H.; Wang, Y.; Gu, P.; Fan, X. Effects of miR-31 on the Osteogenesis of Human Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2014, 446, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Venø, M.T.; Chen, L.; Ditzel, N.; Le, D.Q.S.; Dillschneider, P.; Kassem, M.; Kjems, J. Global MicroRNA Profiling in Human Bone Marrow Skeletal—Stromal or Mesenchymal–Stem Cells Identified Candidates for Bone Regeneration. Mol. Ther. 2018, 26, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lin, W.; Li, Y.; Sun, Y.; Liu, Y.; Chen, C.; Jiang, X.; Li, G.; Xu, L. De-Osteogenic-Differentiated Mesenchymal Stem Cells Accelerate Fracture Healing by Mir-92b. J. Orthop. Transl. 2021, 27, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours That Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Van Osch, G.J.V.M.; Brittberg, M.; Dennis, J.E.; Bastiaansen-Jenniskens, Y.M.; Erben, R.G.; Konttinen, Y.T.; Luyten, F.P. Cartilage Repair: Past and Future—Lessons for Regenerative Medicine. J. Cell. Mol. Med. 2009, 13, 792–810. [Google Scholar] [CrossRef] [PubMed]
- Gordeladze, J.O.; Reseland, J.E.; Karlsen, T.A.; Jakobsen, R.B.; Engebretsen, L.; Lyngstadaas, P.; Duroux-Richard, I.; Jorgensen, C.; Brinchmann, J.E. Bone and Cartilage from Stem Cells: Growth Optimalization and Stabilization of Cell Phenotypes. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; InTech: Melbourne, FL, USA, 2011; ISBN 978-953-307-663-8. [Google Scholar]
- Jeon, S.Y.; Park, J.S.; Yang, H.N.; Lim, H.J.; Yi, S.W.; Park, H.; Park, K.-H. Co-Delivery of Cbfa-1-Targeting siRNA and SOX9 Protein Using PLGA Nanoparticles to Induce Chondrogenesis of Human Mesenchymal Stem Cells. Biomaterials 2014, 35, 8236–8248. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Thakore, P.I.; O’Connor, S.K.; Willard, V.P.; Brunger, J.M.; Christoforou, N.; Leong, K.W.; Gersbach, C.A.; Guilak, F. Knockdown of the Cell Cycle Inhibitor P21 Enhances Cartilage Formation by Induced Pluripotent Stem Cells. Tissue Eng. Part A 2015, 21, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kumar, H.; Cha, B.-H.; Park, S.; Arai, Y.; Han, I.; Park, S.G.; Lee, S.-H. AIMP1 Downregulation Restores Chondrogenic Characteristics of Dedifferentiated/Degenerated Chondrocytes by Enhancing TGF-β Signal. Cell Death Dis. 2016, 7, e2099. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Terao, F.; Miyazaki, K.; Yoshizaki, K.; Takahashi, I. MicroRNA-200a Regulates the Development of Mandibular Condylar Cartilage. J. Dent. Res. 2015, 94, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Lolli, A.; Penolazzi, L.; Narcisi, R.; van Osch, G.J.V.M.; Piva, R. Emerging Potential of Gene Silencing Approaches Targeting Anti-Chondrogenic Factors for Cell-Based Cartilage Repair. Cell. Mol. Life Sci. 2017, 74, 3451–3465. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-Based Delivery of Nucleic Acid Therapeutics for Enhanced Bone and Cartilage Repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Lolli, A.; Sivasubramaniyan, K.; Vainieri, M.L.; Oieni, J.; Kops, N.; Yayon, A.; Van Osch, G.J.V.M. Hydrogel-Based Delivery of antimiR-221 Enhances Cartilage Regeneration by Endogenous Cells. J. Control. Release 2019, 309, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.A.; Jakobsen, R.B.; Mikkelsen, T.S.; Brinchmann, J.E. microRNA-140 Targets RALA and Regulates Chondrogenic Differentiation of Human Mesenchymal Stem Cells by Translational Enhancement of SOX9 and ACAN. Stem Cells Dev. 2014, 23, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Chubinskaya, S.; Di Matteo, B.; Lovato, L.; Iacono, F.; Robinson, D.; Kon, E. Agili-C Implant Promotes the Regenerative Capacity of Articular Cartilage Defects in an Ex Vivo Model. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1953–1964. [Google Scholar] [CrossRef]
- Celik, N.; Kim, M.H.; Hayes, D.J.; Ozbolat, I.T. miRNA Induced Co-Differentiation and Cross-Talk of Adipose Tissue-Derived Progenitor Cells for 3D Heterotypic Pre-Vascularized Bone Formation. Biofabrication 2021, 13, 044107. [Google Scholar] [CrossRef]
- Deka Dey, A.; Yousefiasl, S.; Kumar, A.; Dabbagh Moghaddam, F.; Rahimmanesh, I.; Samandari, M.; Jamwal, S.; Maleki, A.; Mohammadi, A.; Rabiee, N.; et al. miRNA-Encapsulated Abiotic Materials and Biovectors for Cutaneous and Oral Wound Healing: Biogenesis, Mechanisms, and Delivery Nanocarriers. Bioeng. Transl. Med. 2023, 8, e10343. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Pedroza-González, S.C.; Sánchez-Salazar, M.G.; Álvarez, M.M.; Trujillo De Santiago, G. Structural and Biological Engineering of 3D Hydrogels for Wound Healing. Bioact. Mater. 2023, 24, 197–235. [Google Scholar] [CrossRef]
- Clark, R.A.F.; Ghosh, K.; Tonnesen, M.G. Tissue Engineering for Cutaneous Wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and Innovative Approaches for Wound Healing and Skin Regeneration: Current Status and Advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Sen, C.K. microRNA and Wound Healing. In microRNA: Medical Evidence; Santulli, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2015; Volume 888, pp. 291–305. ISBN 978-3-319-22670-5. [Google Scholar]
- Xie, J.; Wu, W.; Zheng, L.; Lin, X.; Tai, Y.; Wang, Y.; Wang, L. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front. Pharmacol. 2022, 13, 828627. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Wang, X.Y.; Zhou, L.Y.; Lao, G.J.; Liu, D.; Wang, C.; Hu, M.D.; Zeng, T.T.; Yan, L.; et al. MicroRNA-129 and -335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes 2018, 67, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- de Kerckhove, M.; Tanaka, K.; Umehara, T.; Okamoto, M.; Kanematsu, S.; Hayashi, H.; Yano, H.; Nishiura, S.; Tooyama, S.; Matsubayashi, Y.; et al. Targeting miR-223 in Neutrophils Enhances the Clearance of Staphylococcus Aureus in Infected Wounds. EMBO Mol. Med. 2018, 10, e9024. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.P.; Leelahavanichkul, A. Over-Expression of miR-223 Induces M2 Macrophage through Glycolysis Alteration and Attenuates LPS-Induced Sepsis Mouse Model, the Cell-Based Therapy in Sepsis. PLoS ONE 2020, 15, e0236038. [Google Scholar] [CrossRef]
- Monaghan, M.; Browne, S.; Schenke-Layland, K.; Pandit, A. A Collagen-Based Scaffold Delivering Exogenous Microrna-29B to Modulate Extracellular Matrix Remodeling. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Lakra, R.; Kiran, M.S.; Sai Korrapati, P. Collagen Scaffold Reinforced with Furfural for Wound Healing Application. Mater. Lett. 2022, 315, 131956. [Google Scholar] [CrossRef]
| Drug | Disease | MiRNA | Pharma Company |
|---|---|---|---|
| Miravirsen | Hepatitis C virus | miR-122 | Santaris Pharma |
| MRX34 | Different types of cancers | miR-34a | miRNA Therapeutics |
| RG-101 | Viral diseases | miR-122 | Regulus Therapeutics |
| RGLS4326 | Polycystic kidney disease | miR-17 | Regulus Therapeutics |
| MGN-1374 | Post-myocardial infarction | miRNA-15/195 | miRagen therapeutics |
| MGN-2677 | Vascular disease | miR-143/145 | miRagen therapeutics |
| MGN-4220 | Cardiac fibrosis | miR-29 | miRagen therapeutics |
| MGN-4893 | Abnormal red blood | miR-451 | miRagen therapeutics |
| MGN-5804 | Cardiometabolic disease | miR-378 | miRagen therapeutics |
| MGN-6114 | Peripheral arterial disease | miR-92 | miRagen therapeutics |
| MGN-9103 | Chronic heart failure | miR-208 | miRagen therapeutics |
| Cobomarsen | Cutaneous T-cell lymphoma | miR-155 | miRagen therapeutics |
| MRG-107 | Amyotrophic lateral sclerosis | miR-155 | miRagen therapeutics |
| MRG-110 | Ischemia | miR-92a | miRagen therapeutics |
| Remlarsen | Fibrosis | miR-29 | miRagen therapeutics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guelfi, G.; Capaccia, C.; Anipchenko, P.; Ciancabilla, F.; Oommen, O.P.; Bufalari, A.; Zerani, M.; Maranesi, M. Mimic miRNA and Anti-miRNA Activated Scaffolds as a Therapeutic Strategy to Promote Bone, Cartilage, and Skin Regeneration. Macromol 2024, 4, 165-189. https://doi.org/10.3390/macromol4020009
Guelfi G, Capaccia C, Anipchenko P, Ciancabilla F, Oommen OP, Bufalari A, Zerani M, Maranesi M. Mimic miRNA and Anti-miRNA Activated Scaffolds as a Therapeutic Strategy to Promote Bone, Cartilage, and Skin Regeneration. Macromol. 2024; 4(2):165-189. https://doi.org/10.3390/macromol4020009
Chicago/Turabian StyleGuelfi, Gabriella, Camilla Capaccia, Polina Anipchenko, Francesco Ciancabilla, Oommen Podiyan Oommen, Antonello Bufalari, Massimo Zerani, and Margherita Maranesi. 2024. "Mimic miRNA and Anti-miRNA Activated Scaffolds as a Therapeutic Strategy to Promote Bone, Cartilage, and Skin Regeneration" Macromol 4, no. 2: 165-189. https://doi.org/10.3390/macromol4020009
APA StyleGuelfi, G., Capaccia, C., Anipchenko, P., Ciancabilla, F., Oommen, O. P., Bufalari, A., Zerani, M., & Maranesi, M. (2024). Mimic miRNA and Anti-miRNA Activated Scaffolds as a Therapeutic Strategy to Promote Bone, Cartilage, and Skin Regeneration. Macromol, 4(2), 165-189. https://doi.org/10.3390/macromol4020009











