Surface Microfabrication of Lactic Acid–Glycolic Acid Copolymers Using a Gas-Permeable Porous Mold
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2-SiO2 Gas-Permeable Porous Mold Material
2.2. Mixing of Lactic Acid–Glycolic Acid Copolymer (LG-80)
2.3. Mixing of Materials
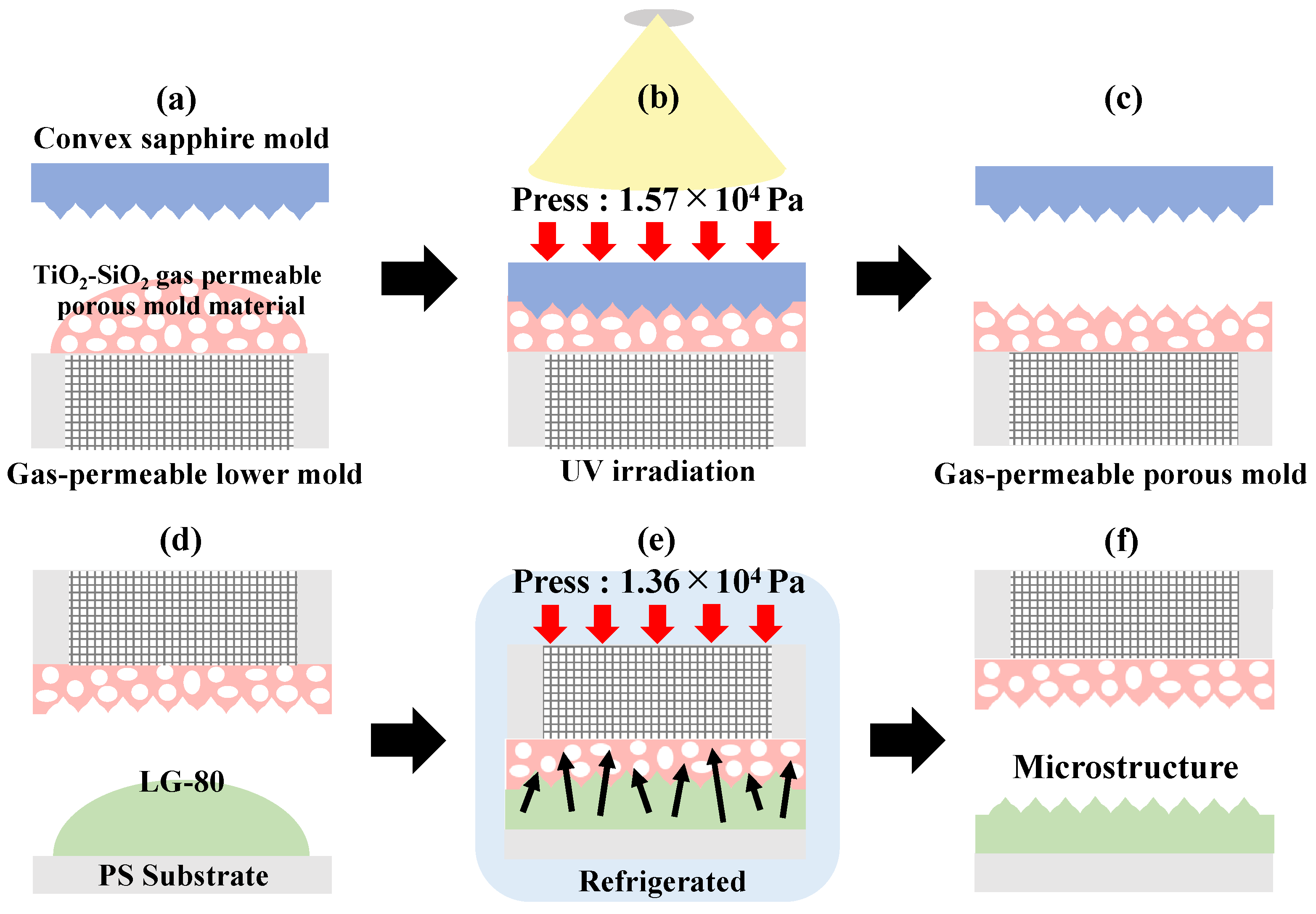
2.4. Surface Micromachining Process
2.5. Surface Micromachining SEM Observation
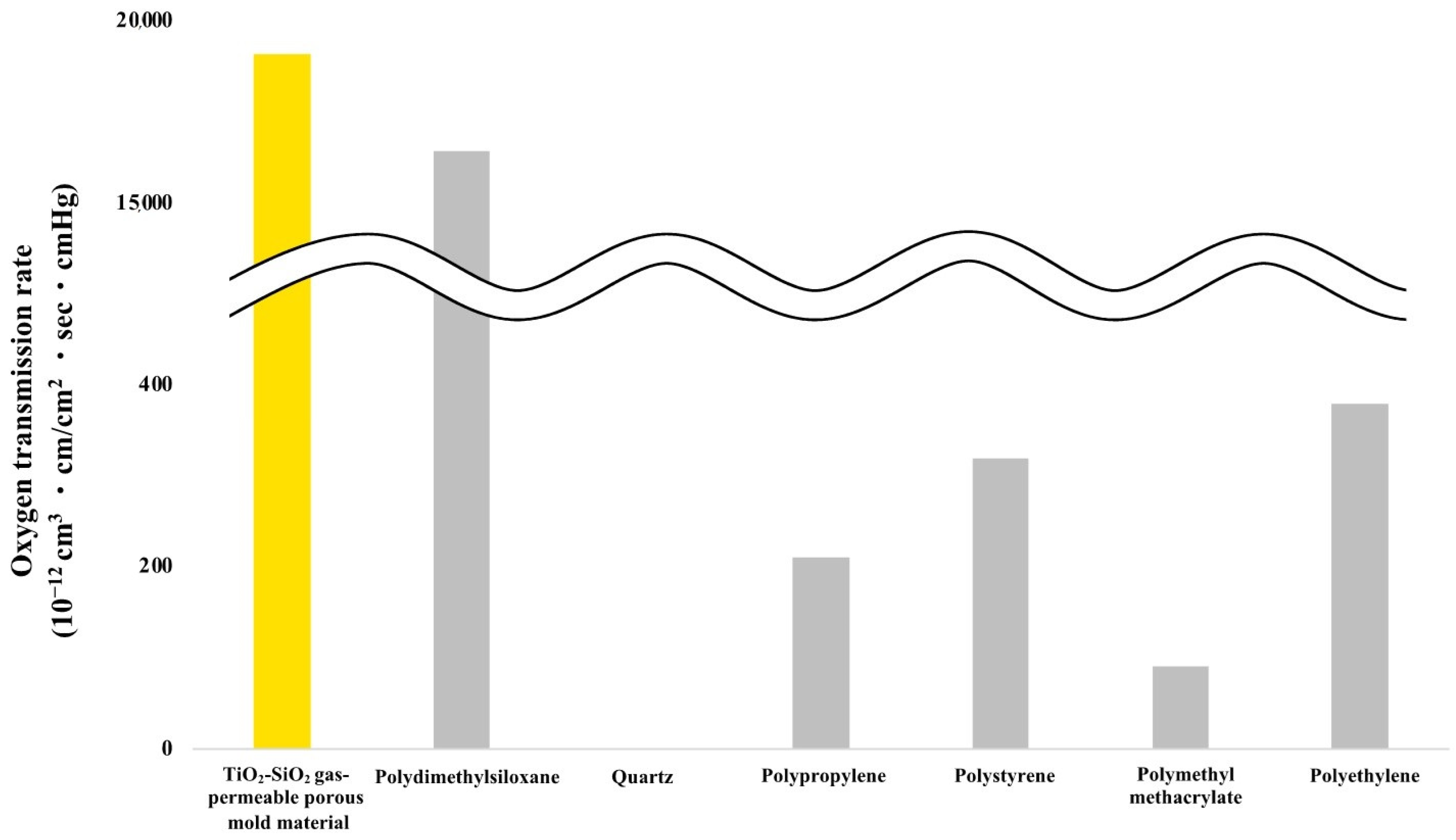
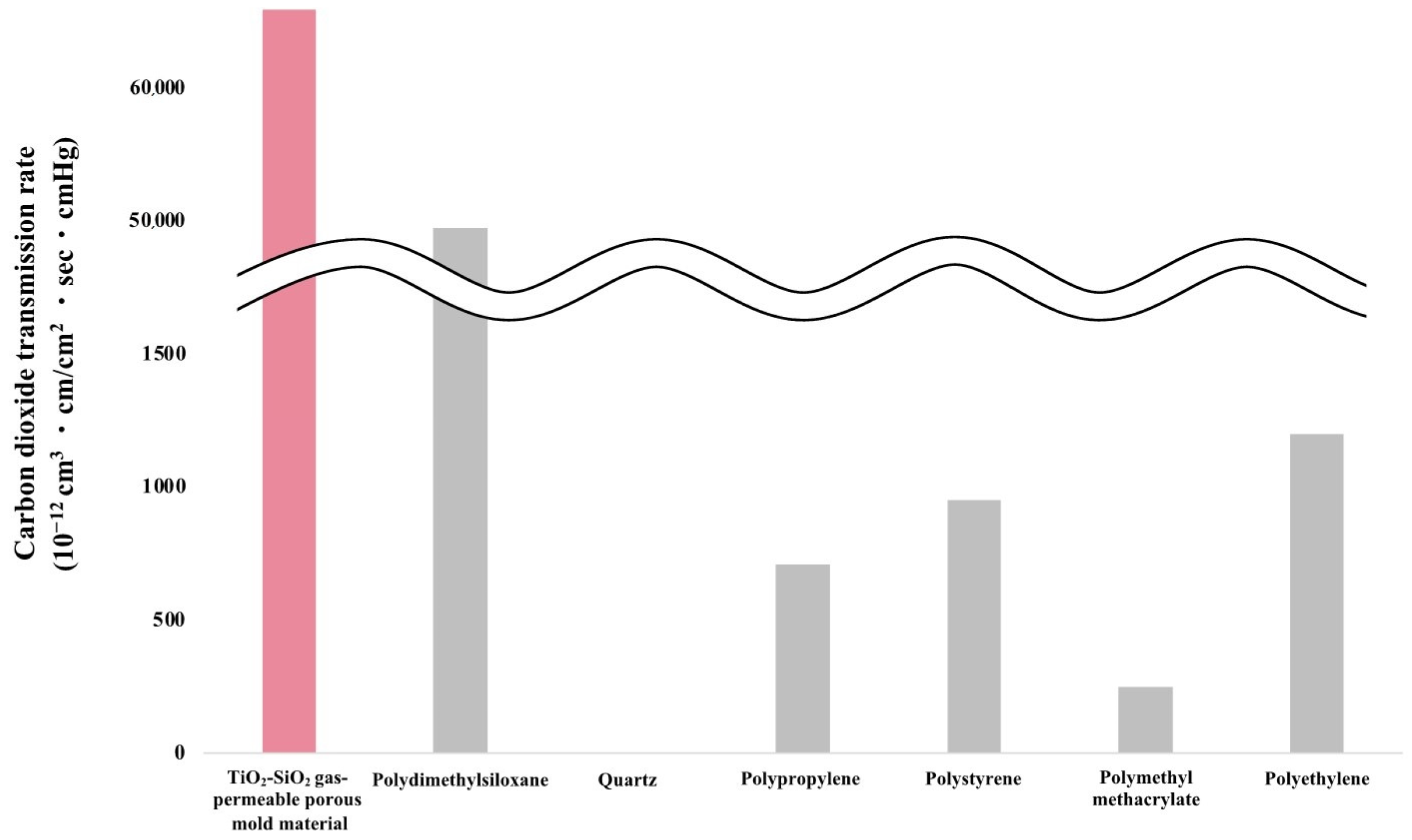
2.6. Oxygen and Carbon Dioxide Gas Permeability Measurement
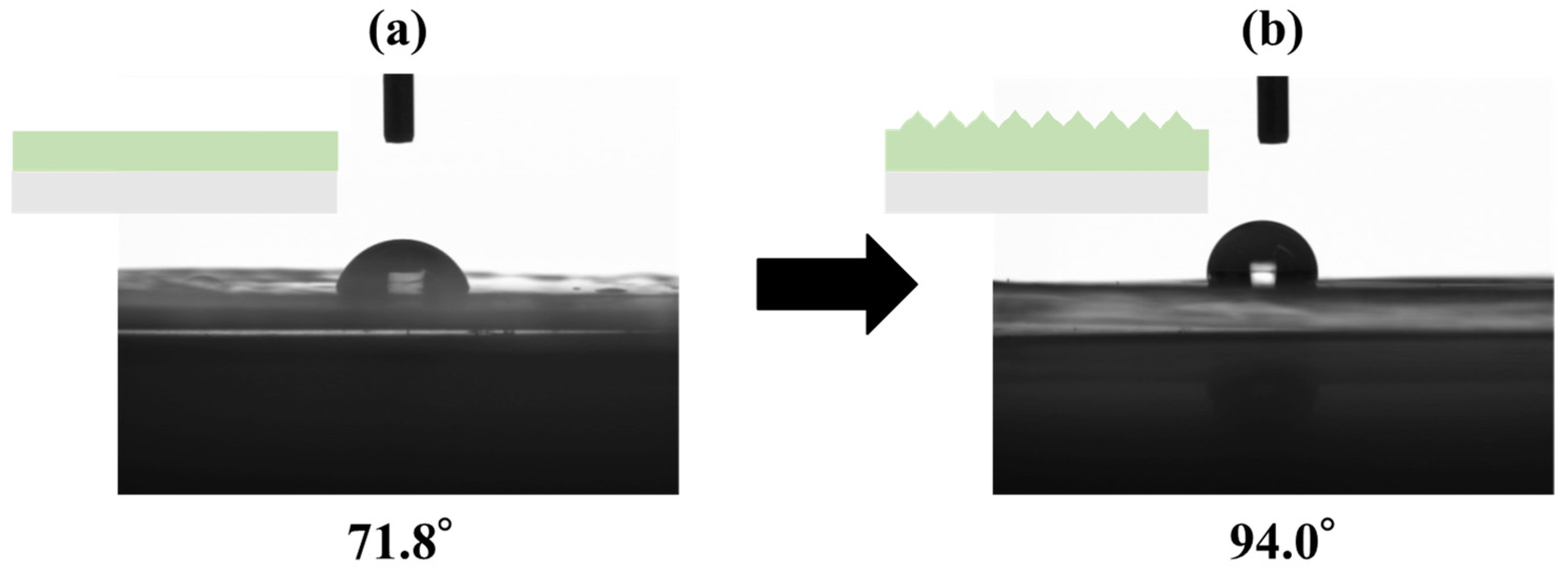
2.7. Contact Angle Measurement
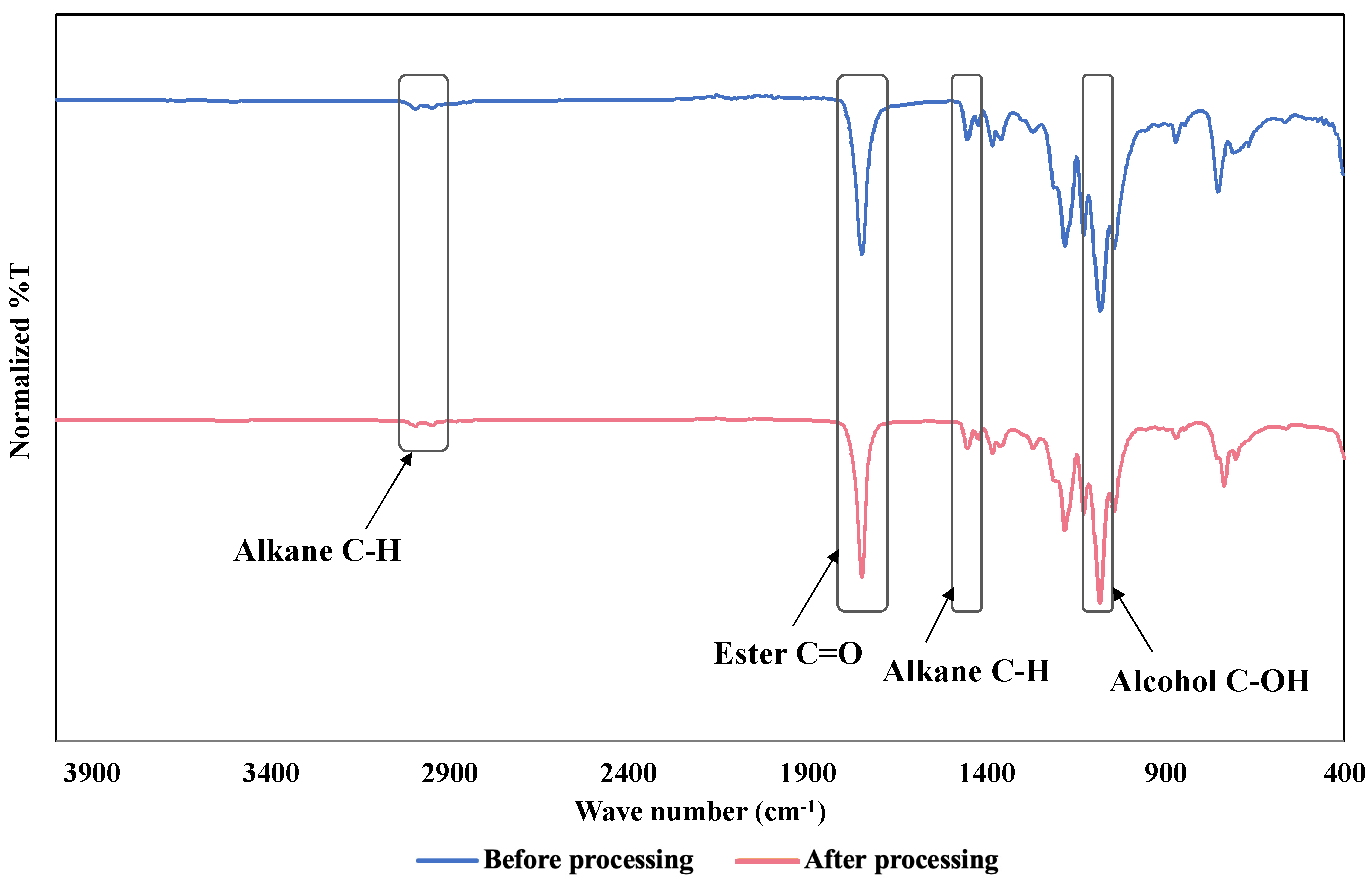
2.8. FT-IR Measurement
3. Results
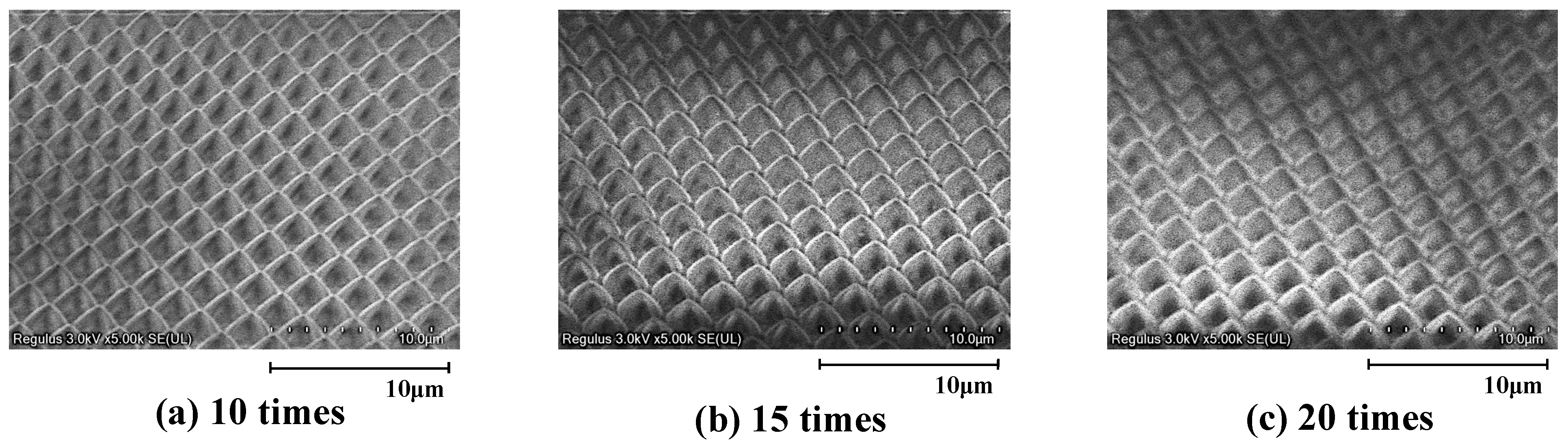
3.1. Surface Micromachining Results for LG-80 Using Gas-Permeable Porous Mold
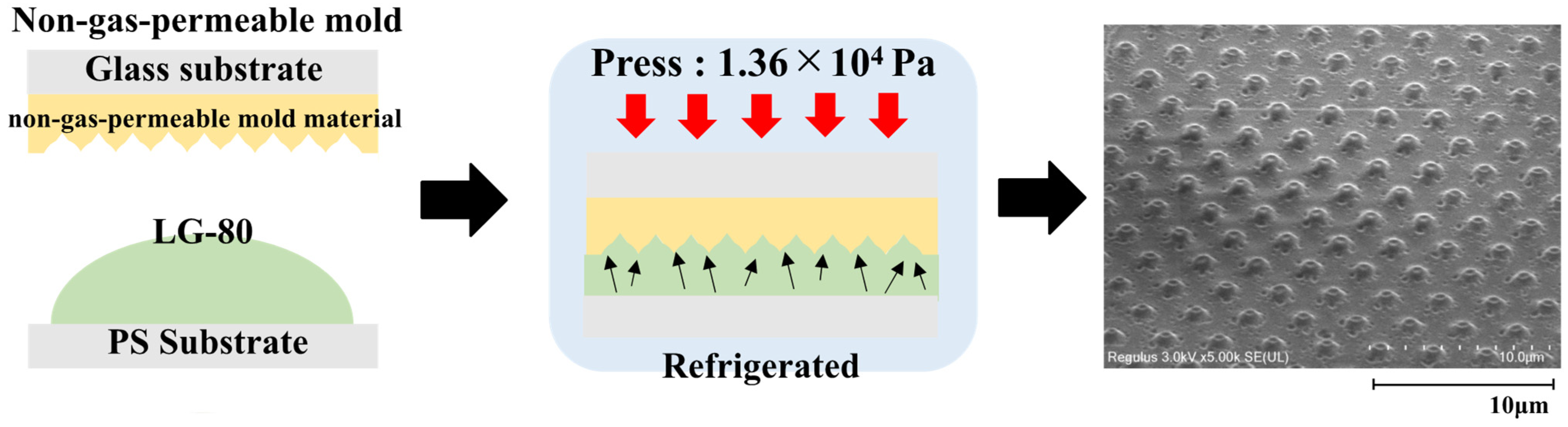
3.2. Results of Surface Micromachining of LG-80 Using Non-Gas-Permeable Mold
3.3. Oxygen Gas Permeability Measurement Results
3.4. Contact Angle Measurement Results
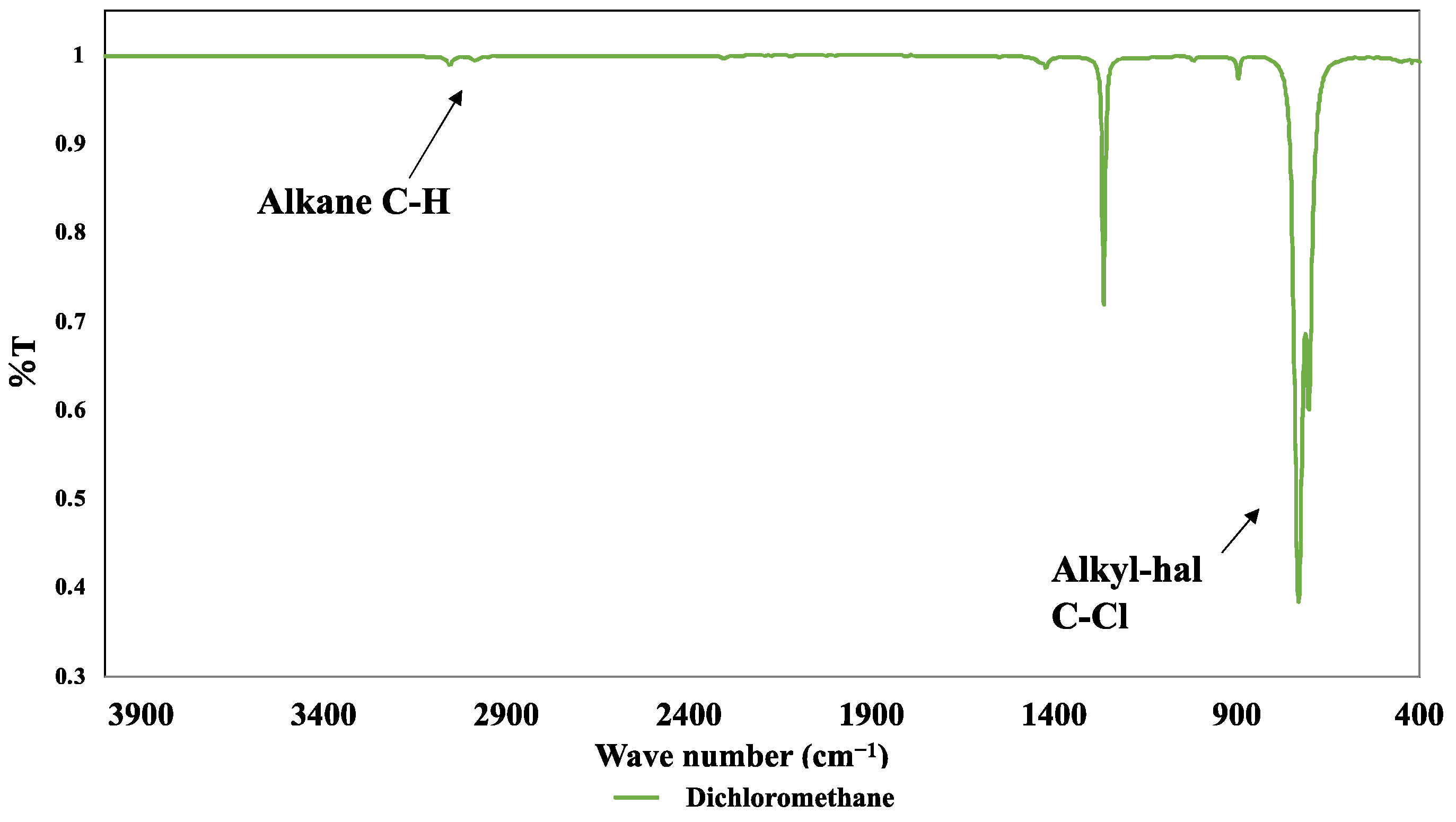
3.5. FT-IR Measurement Results
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisecky, L.; Luger, M.; Klasan, A.; Gotterbarm, T.; Klotz, M.C.; Hochgatterer, R. Bioabsorbable implants in forefoot surgery: A review of materials, possibilities and disadvantages. EFORT Open Rev. 2021, 6, 1132–1139. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: A review on polymers and magnesium-based materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.; Fonseca, R.; Pinto, L.F.; Ferreira, F.C.; Almeida, A.; Rodrigues, A. Strategy to improve the mechanical properties of bioabsorbable materials based on chitosan for orthopedic fixation applications. J. Mech. Behav. Biomed. Mater. 2020, 103, 103572. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.L.; Shine, C.J.; Kelly, N.; Sweeney, C.A.; McHugh, P.E. A review of material degradation modelling for the analysis and design of bioabsorbable stents. Ann. Biomed. Eng. 2016, 44, 341–356. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Woolf, S.K.; Friedman, R.J. Pre-clinical in vivo evaluation of orthopaedic bioabsorbable devices. Biomaterials 2000, 21, 2635–2652. [Google Scholar] [CrossRef] [PubMed]
- Morsada, Z.; Hossain, M.M.; Islam, M.T.; Mobin, M.A.; Saha, S. Recent progress in biodegradable and bioresorbable materials: From passive implants to active electronics. Appl. Mater. Today 2021, 25, 101257. [Google Scholar] [CrossRef]
- Adekomaya, O.; Majozi, T. Bioresorbable polymers and their composites for biomedical applications. In Bioresorbable Polymers and Their Composites; Woodhead Publishing: Cambridge, UK, 2024; pp. 23–40. [Google Scholar]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What affects the biocompatibility of polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Delivery Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, L.; Zhang, H.; Chang, F.; Li, S.; Ma, S.; Zhang, Y.; Ren, L. Biomechanical comparison between bioabsorbable and medical titanium screws in distal chevron osteotomy of first metatarsal in hallux valgus treatment. J. Mech. Behav. Biomed. Mater. 2022, 131, 105260. [Google Scholar] [CrossRef]
- Sheik-Ali, S.; Guets, W. Absorbable vs non absorbable sutures for wound closure. Systematic review of systematic reviews. Wound Med. 2018, 23, 35–37. [Google Scholar] [CrossRef]
- Gillanders, S.L.; Anderson, S.; Mellon, L.; Heskin, L. A systematic review and meta-analysis: Do absorbable or non-absorbable suture materials differ in cosmetic outcomes in patients requiring primary closure of facial wounds? JPRAS 2018, 71, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.M.; Durisin, M.; Goldman, J.; Drelich, J.W. Recent advances in biodegradable metals for medical sutures: A critical review. Adv. Healthc. Mater. 2015, 4, 1915–1936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Jiang, Z.; Wang, J.; Xu, Z.; Meng, K.; Zhao, H. Poly (glyceryl sebacate)/silk fibroin small-diameter artificial blood vessels with good elasticity and compliance. Smart Mater. Med. 2021, 2, 74–86. [Google Scholar] [CrossRef]
- Cheng, S.; Jin, Y.; Wang, N.; Cao, F.; Zhang, W.; Bai, W.; Zheng, W.; Jiang, X. Self-adjusting, polymeric multilayered roll that can keep the shapes of the blood vessel scaffolds during biodegradation. Adv. Mater. 2017, 29, 1700171. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Kapadia, J.R. Current knowledge on biodegradable microspheres in drug delivery. Expert Opin. Drug Delivery 2015, 12, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A.A.; Mishra, N. Polymers in drug delivery. J. Biosci. Med. 2015, 4, 69–84. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Pina, S.; Joaquim, M.O.; Rui, L.R. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef]
- Takei, S.; Hanabata, M. High-resolution nanopatterning of biodegradable polylactide by thermal nanoimprint lithography using gas permeable mold. AIP Adv. 2017, 7, 035110. [Google Scholar] [CrossRef]
- Budak, K.; Oguz, S.; Umran, A.S. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020, 27, 208. [Google Scholar] [CrossRef]
- Wang, R.; Sun, X.; Chen, L.; Liang, W. Morphological and mechanical properties of biodegradable poly (glycolic acid)/poly (butylene adipate-co-terephthalate) blends with in situ compatibilization. RSC Adv. 2021, 11, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Niu, D.Y.; Liu, B.; Xu, P.W.; Yang, W.J.; Lemstra, P.J.; Ma, P.M. Improvement on the mechanical performance and resistance towards hydrolysis of poly (glycolic acid) via solid-state drawing. Chin. J. Polym. Sci. 2023, 41, 14–23. [Google Scholar] [CrossRef]
- Deng, H.; Yu, J.; Liu, C.; Zhao, Y.; Pan, H.; Ni, H.; Wang, Z.; Bian, J.; Han, L.; Zhang, H. Crystallization and. heat resistance properties of poly (glycolic acid) reinforced poly (lactic acid)/poly (butylene adipate-co-terephthalate) blends. Thermochim. Acta 2024, 731, 179628. [Google Scholar] [CrossRef]
- Jem, K.J.; Bowen, T. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Piyasin, P.; Rattakarn, Y.; Supree, P. Size-controllable melt-electrospun polycaprolactone (PCL) fibers with a sodium chloride additive. Polymers 2019, 11, 1768. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.; Bazuin, C.G.; Prud’homme, R.E. Morphologies of various polycaprolactone/polymer blends in ultrathin films. Macromolecules 2015, 48, 1412–1417. [Google Scholar] [CrossRef]
- Sugioka, K. Progress in ultrafast laser processing and future prospects. Nanophotonics 2017, 6, 393–413. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Luo, W.; Cheng, X. Nanoindentation behavior of UV-curable resist and its correlation with patterning defect in nanoimprint lithography. J. Micromech. Microeng. 2020, 30, 065010. [Google Scholar] [CrossRef]
- Park, W.I.; Park, T.W.; Choi, Y.J.; Lee, S.; Ryu, S.; Liang, X.; Jung, Y.S. Extreme-Pressure Imprint Lithography for Heat and Ultraviolet-Free Direct Patterning of Rigid Nanoscale Features. ACS Nano 2021, 15, 10464–10471. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.; Güniat, L.; Morgan, N.; Ramanandan, S.P.; Rudra, A.; Piazza, V.; Morral, A.I.; Dede, D. The implementation of thermal and UV nanoimprint lithography for selective area epitaxy. Nanotechnology 2023, 34, 445301. [Google Scholar] [CrossRef] [PubMed]
- Takei, S. Nanoimprinting of TiO2–SiO2 photocurable materials with high titanium concentration for CF4/O2 etch selectivity. Micro Nano Lett. 2023, 8, 1–4. [Google Scholar] [CrossRef]
- Miura, S.; Yamagishi, R.; Sugino, N.; Yokoyama, Y.; Miyazaki, R.; Yasuda, K.; Ando, M.; Hachikubo, Y.; Murashita, T.; Kameda, T.; et al. Nanoimprint lithography and microinjection molding using gas-permeable hybrid mold for antibacterial nanostructures. J. Photopolym. Sci. Technol. 2023, 36, 183–190. [Google Scholar] [CrossRef]
- Miura, S.; Yamagishi, R.; Miyazaki, R.; Yasuda, K.; Kawano, Y.; Yokoyama, Y.; Sugino, N.; Kameda, T.; Takei, S. Fabrication of high-resolution fine microneedles derived from hydrolyzed hyaluronic acid gels in vacuum environment imprinting using water permeable mold. Gels 2022, 8, 785. [Google Scholar] [CrossRef]
- Takei, S. Direct nanoimprint lithography of polyethersulfone using cellulose-based mold. Macromol. Mater. Eng. 2020, 305, 1900853. [Google Scholar] [CrossRef]
- Takei, S. Fabrication of moth-eye gold nanostructures by nanoimprint lithography using solvent-permeable porous cross-link molds derived from hydroxypropyl-cyclodextrin. Appl. Phys. Express 2019, 12, 046501. [Google Scholar] [CrossRef]
- Yamagishi, R.; Miura, S.; Ando, M.; Hachikubo, Y.; Murashita, T.; Sugino, N.; Kameda, T.; Yokoyama, Y.; Kawano, Y.; Yasuda, K.; et al. Ultraviolet-curable material with high fluorine content for biomimetic functional structures achieved by nanoimprint lithography with gas-permeable template for life science and electronic applications. J. Photopolym. Sci. Technol. 2023, 36, 83–90. [Google Scholar] [CrossRef]
- Yamagishi, R.; Miura, S.; Yasuda, K.; Sugino, N.; Kameda, T.; Kawano, Y.; Yokoyama, Y.; Takei, S. Thermal nanoimprint lithography of sodium hyaluronate solutions with gas permeable inorganic hybrid mold for cosmetic and pharmaceutical applications. Appl. Phys. Express 2022, 15, 046502. [Google Scholar] [CrossRef]
- Ando, M.; Yamagishi, R.; Miura, S.; Hachikubo, Y.; Murashita, T.; Sugino, N.; Kameda, T.; Yokoyama, Y.; Kawano, Y.; Yasuda, K.; et al. Surface nanopatterning of bioabsorbable materials using thermal imprinting technology. J. Photopolym. Sci. Technol. 2023, 36, 277–282. [Google Scholar] [CrossRef]
- Oyama, T.G.; Kimura, A.; Nagasawa, N.; Oyama, K.; Taguchi, M. Development of advanced biodevices using quantum beam microfabrication technology. Quantum Beam Sci. 2020, 4, 14. [Google Scholar] [CrossRef]
- Malinauskas, M.; Lukosevicius, L.; Butkus, S.; Paipulas, D. Femtosecond pulse light. filament-assisted microfabrication of biodegradable polylactic acid (PLA) material. J. Laser Micro/Nanoeng. 2015, 10, 222–228. [Google Scholar] [CrossRef]
- Aguilar, C.A.; Lu, Y.; Mao, S.; Chen, S. Direct micro-patterning of biodegradable polymers using ultraviolet and femtosecond lasers. Biomaterials 2005, 26, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Demko, M.T.; Cheng, J.C.; Pisano, A.P. Rigid, vapor-permeable poly (4-methyl-2-pentyne) templates for high resolution patterning of nanoparticles and polymers. ACS Nano 2012, 6, 6890–6896. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Subiyanto, I.; Choi, W.; Park, Y.C.; Cho, C.H.; Kim, H. CO2/N2 and O2/N2 separation using mixed-matrix membranes with MOF-74 nanocrystals synthesized via microwave reactions. Bull. Korean Chem. Soc. 2021, 42, 459–462. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Fujikawa, S. Molecular hybridization of polydimethylsiloxane with zirconia for highly gas permeable membranes. ACS Appl. Polym. Mater. 2019, 1, 1165–1174. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Crawford, R.J.; Ivanova, E.P. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review. Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
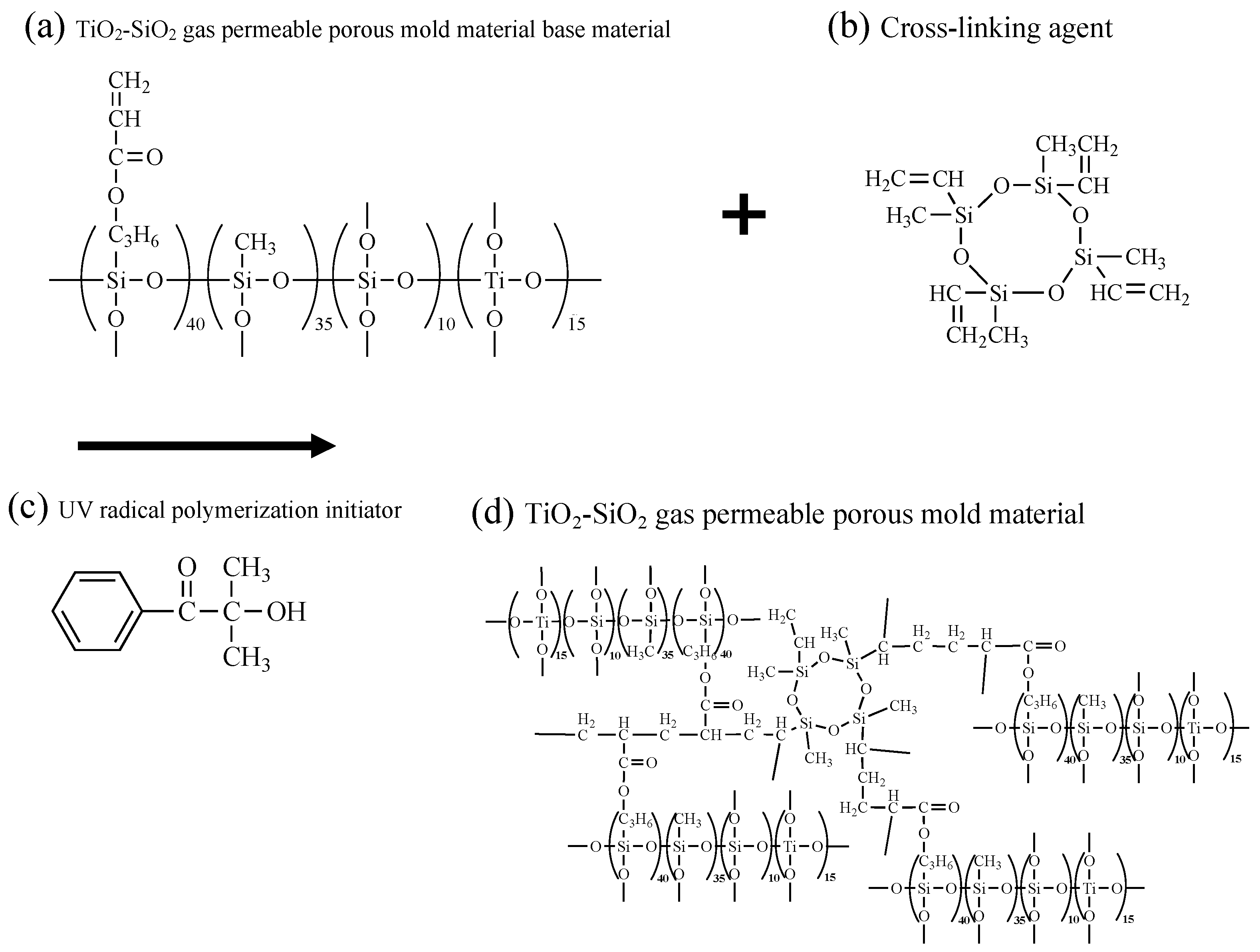
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, M.; Hachikubo, Y.; Miura, S.; Yamagishi, R.; Sugino, N.; Kameda, T.; Yokoyama, Y.; Takei, S. Surface Microfabrication of Lactic Acid–Glycolic Acid Copolymers Using a Gas-Permeable Porous Mold. Macromol 2024, 4, 544-555. https://doi.org/10.3390/macromol4030032
Ando M, Hachikubo Y, Miura S, Yamagishi R, Sugino N, Kameda T, Yokoyama Y, Takei S. Surface Microfabrication of Lactic Acid–Glycolic Acid Copolymers Using a Gas-Permeable Porous Mold. Macromol. 2024; 4(3):544-555. https://doi.org/10.3390/macromol4030032
Chicago/Turabian StyleAndo, Mano, Yuna Hachikubo, Sayaka Miura, Rio Yamagishi, Naoto Sugino, Takao Kameda, Yoshiyuki Yokoyama, and Satoshi Takei. 2024. "Surface Microfabrication of Lactic Acid–Glycolic Acid Copolymers Using a Gas-Permeable Porous Mold" Macromol 4, no. 3: 544-555. https://doi.org/10.3390/macromol4030032
APA StyleAndo, M., Hachikubo, Y., Miura, S., Yamagishi, R., Sugino, N., Kameda, T., Yokoyama, Y., & Takei, S. (2024). Surface Microfabrication of Lactic Acid–Glycolic Acid Copolymers Using a Gas-Permeable Porous Mold. Macromol, 4(3), 544-555. https://doi.org/10.3390/macromol4030032







