Structural, Thermal and Mechanical Assessment of Green Compounds with Natural Rubber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Devulcanization of GTR
2.3. Samples Preparation
2.4. Measurements
3. Results
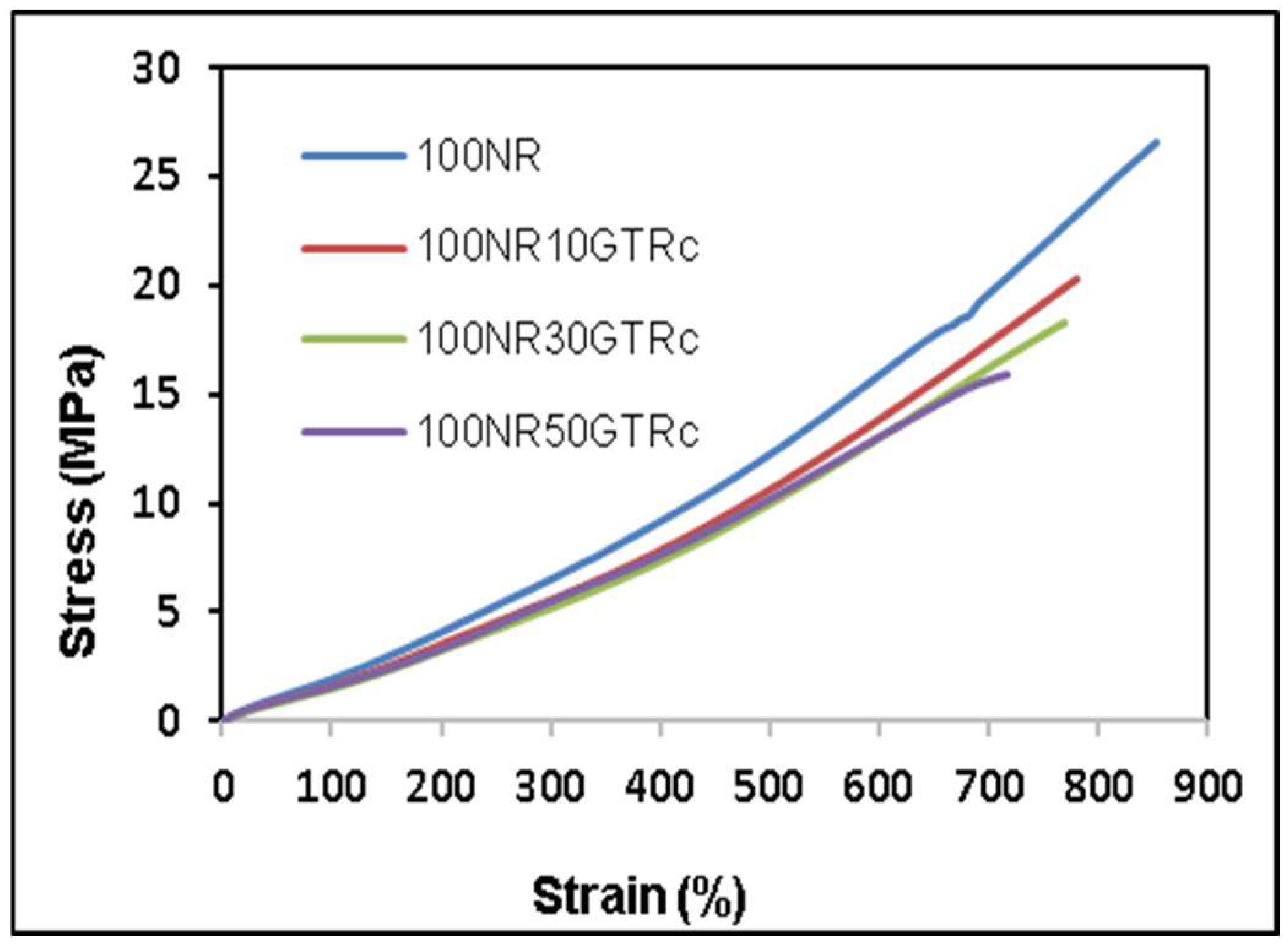
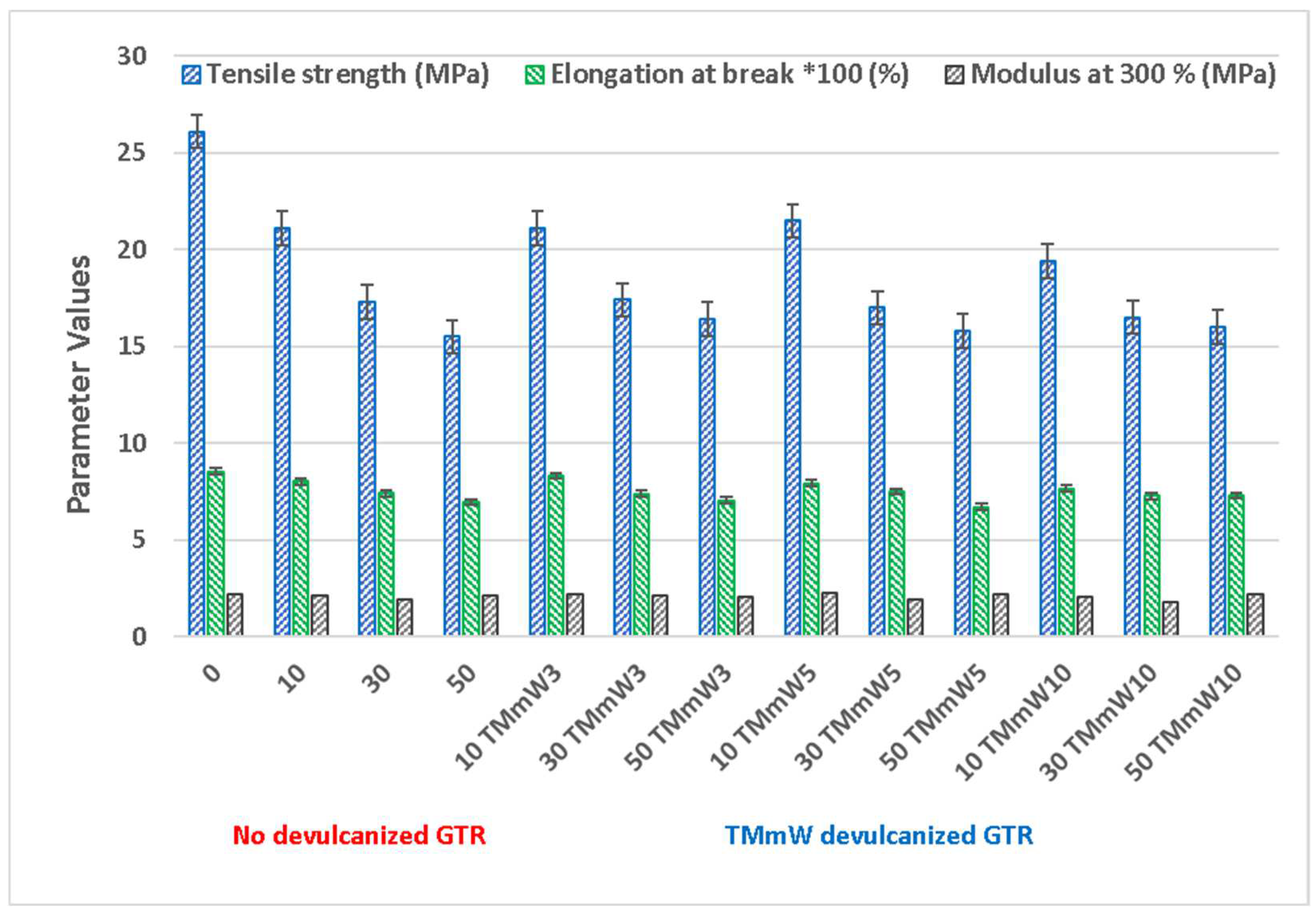
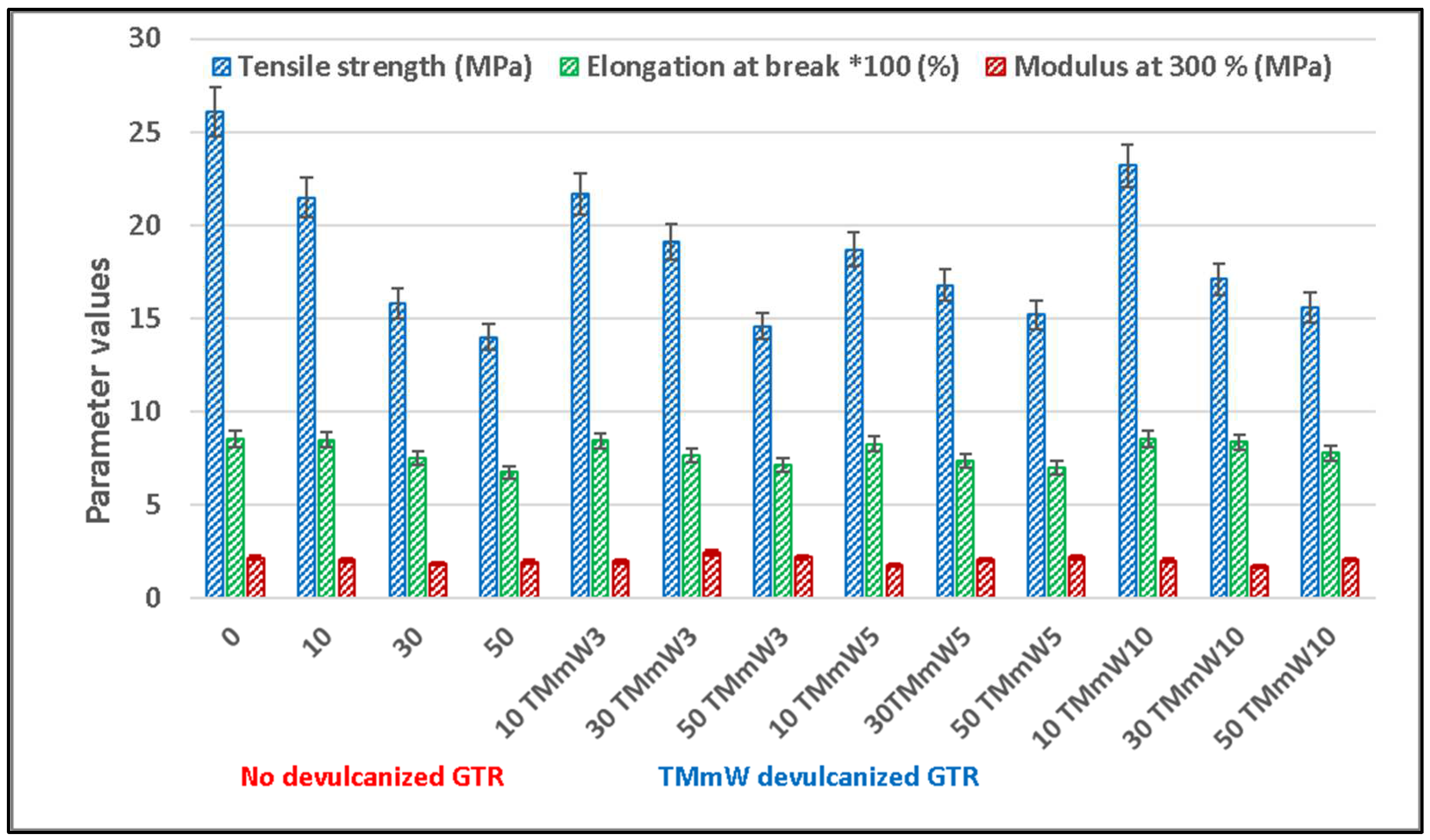
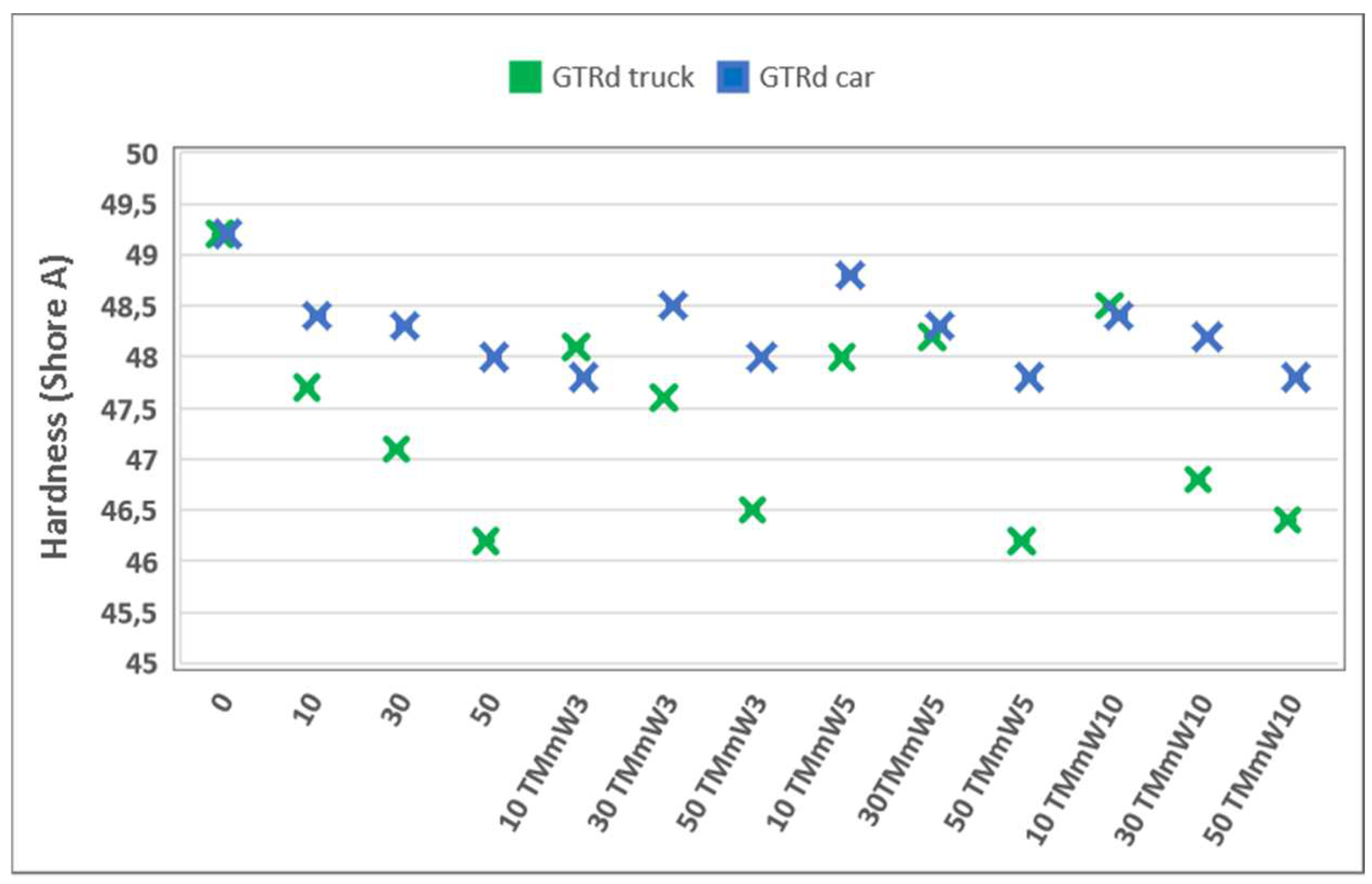
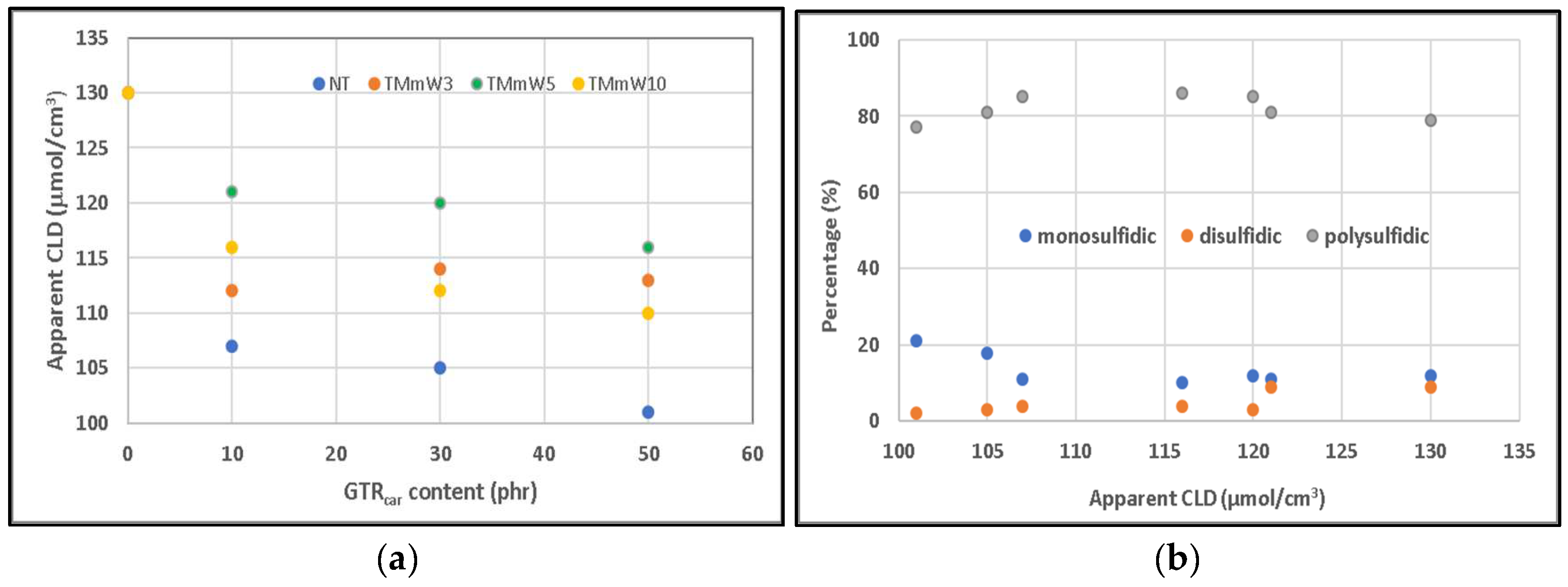
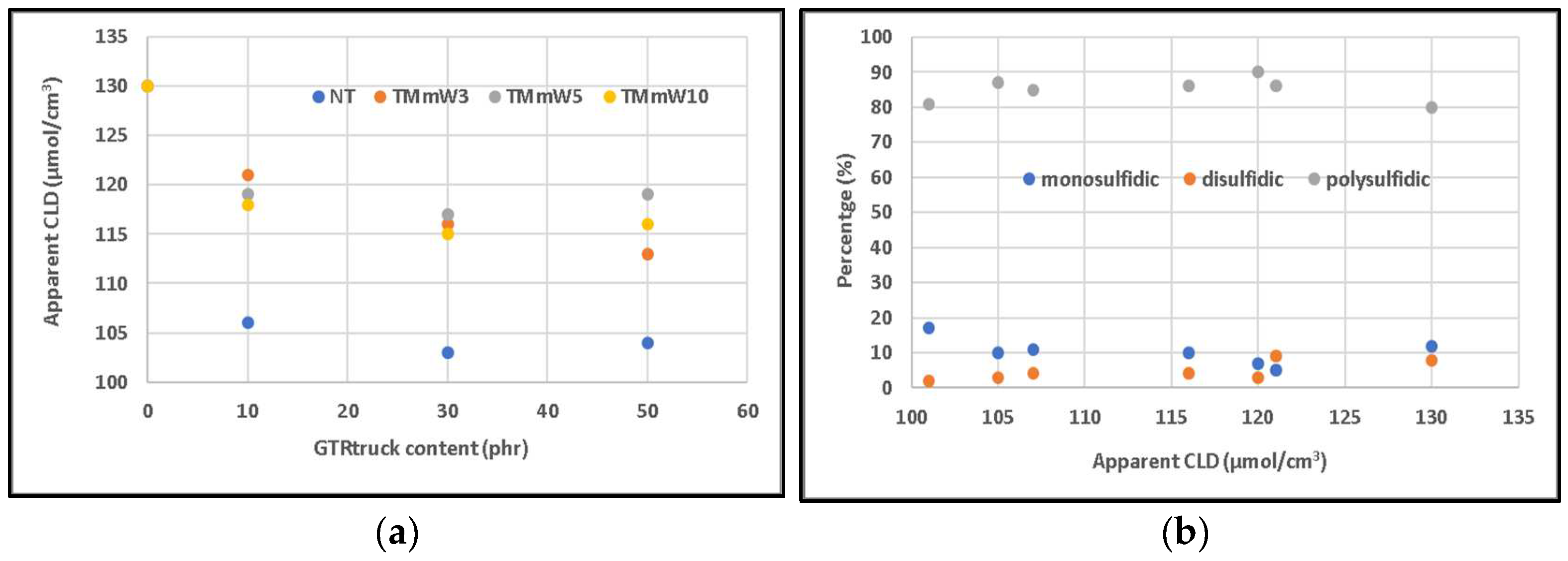
3.1. Mechanical Properties
3.1.1. Compounds including Non-Devulcanized GTR
3.1.2. Compounds including Devulcanized GTR
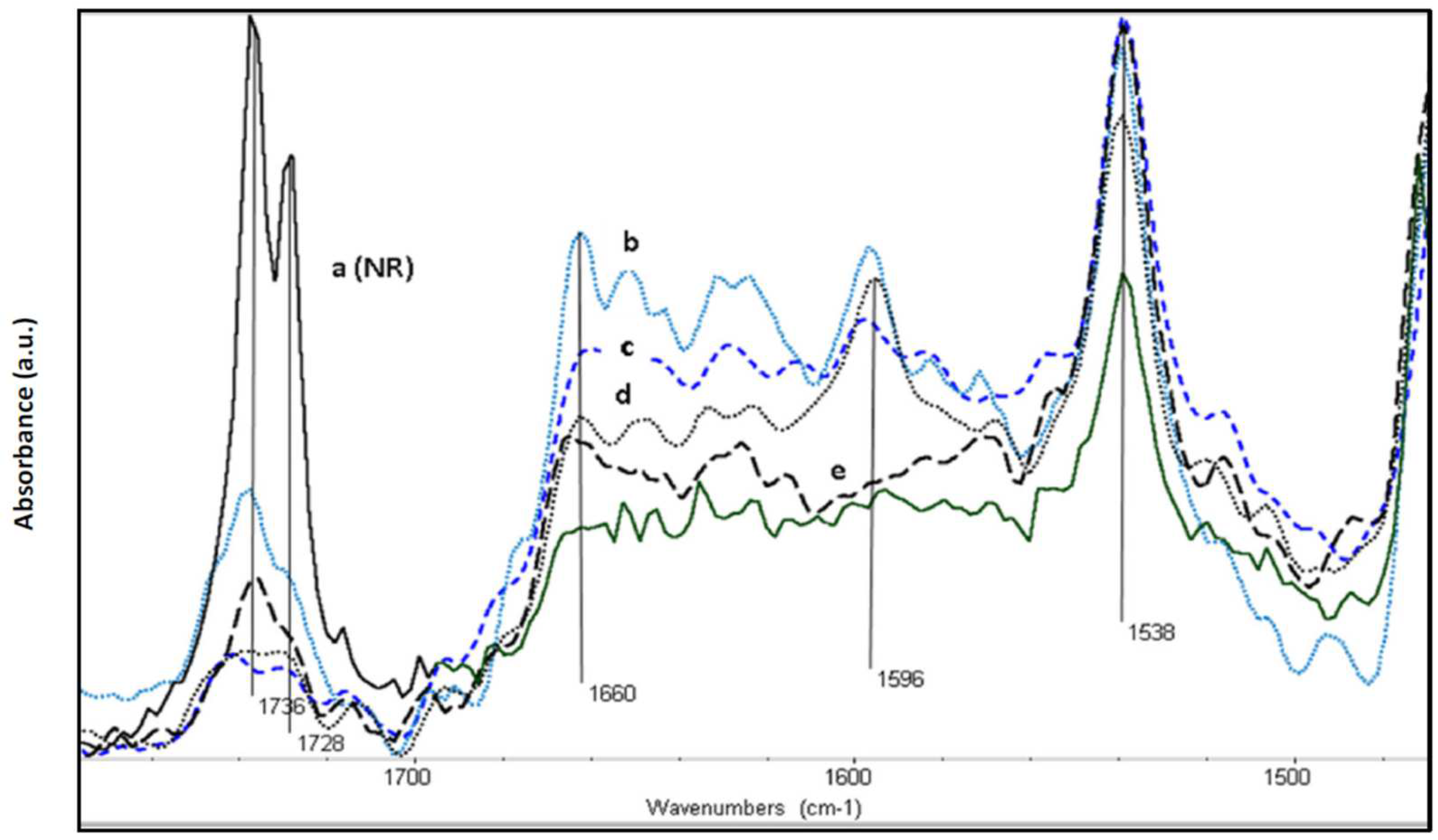
3.2. Spectroscopy Characterization
3.3. Thermogravimetric Analysis
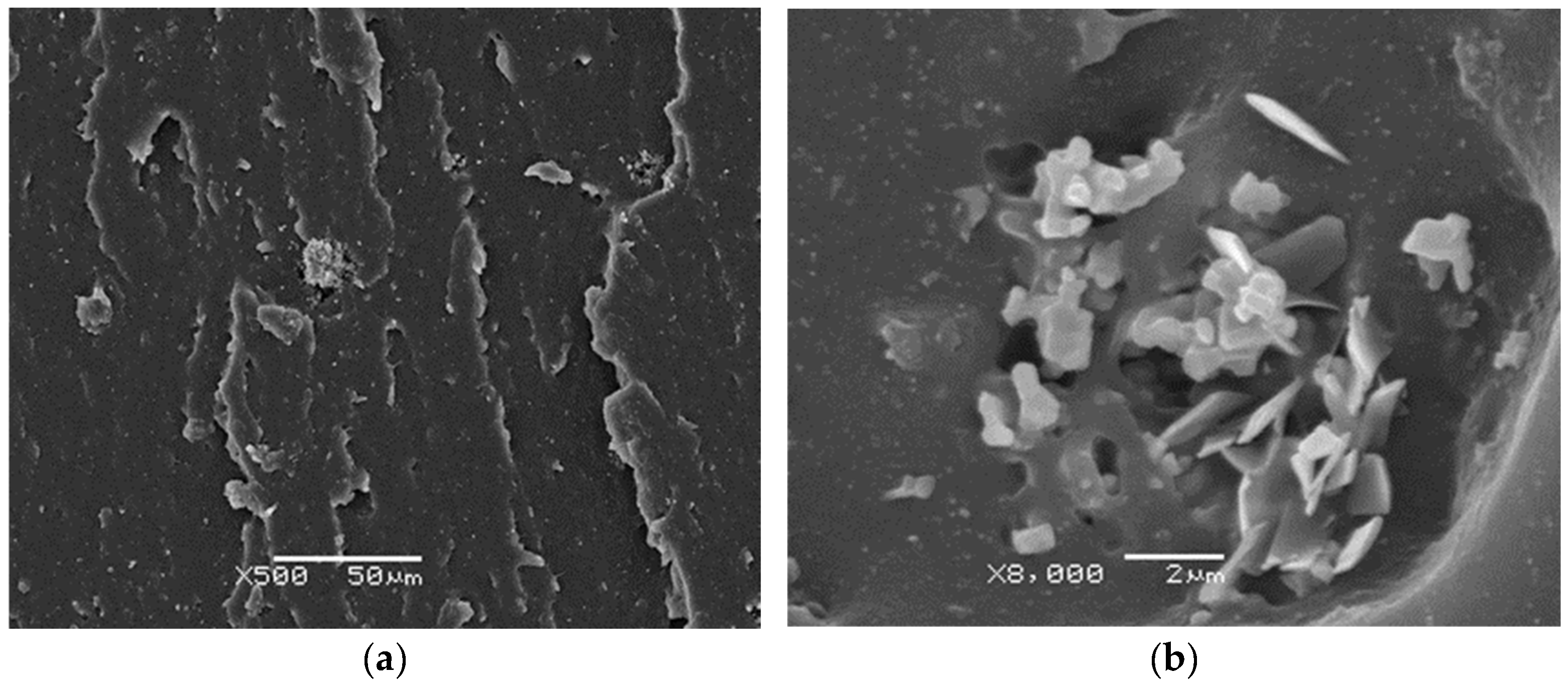
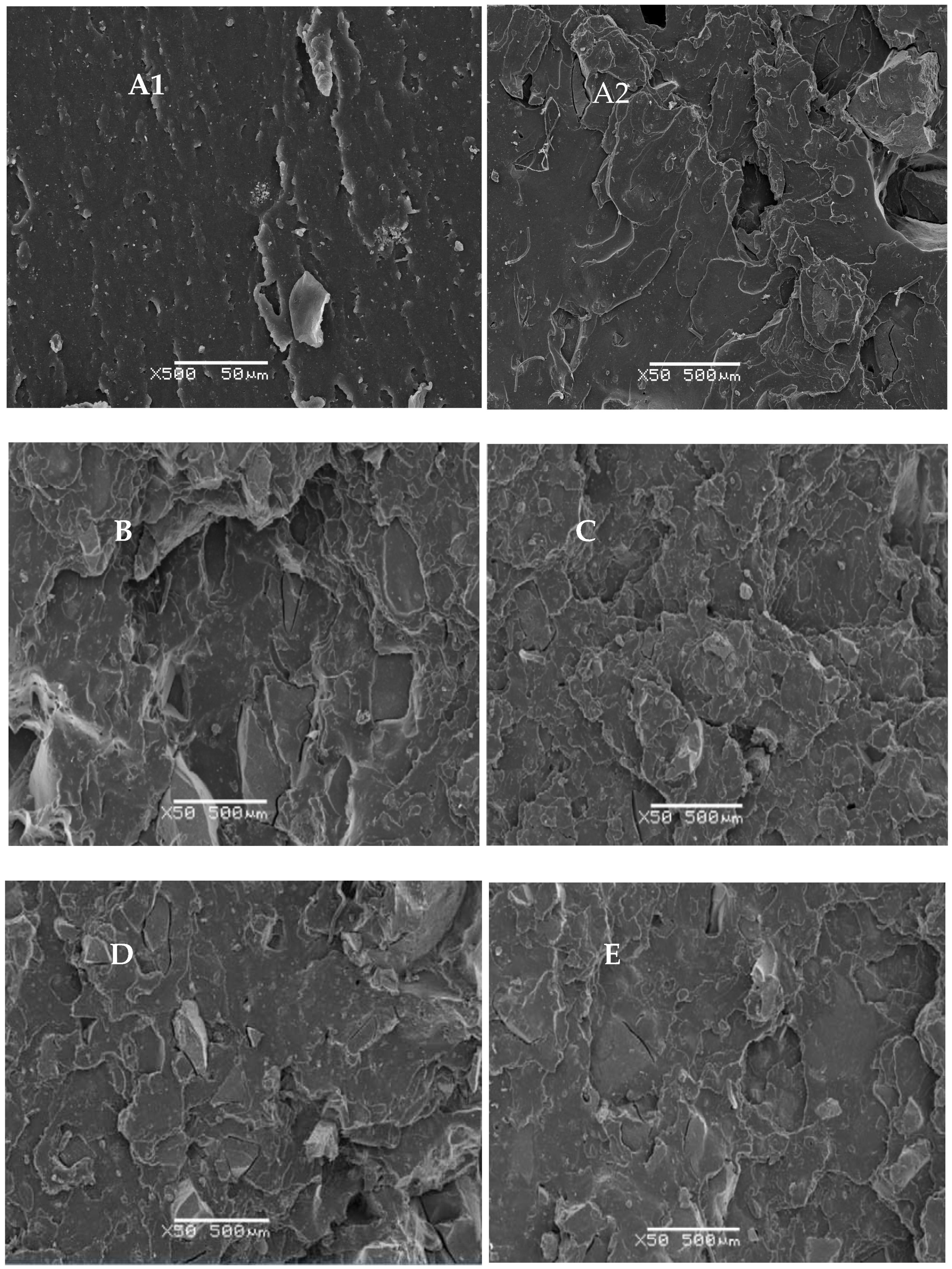
3.4. SEM Studies of NR-GTR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Tyre and Rubber Manufacturers Association. Annual Report 2020/2021 and End Life Tyre Reports; European Tyre and Rubber Manufacturers Association: Bruxelles, Belgium, 2021. [Google Scholar]
- Chen, S.-J.; Su, H.-B.; Chang, J.-E.; Lee, W.-J.; Huang, K.-L.; Hsieh, L.-T.; Huang, Y.-C.; Lin, W.-Y.; Lin, C.-C. Emissions of polycyclic aromatic hydrocarbons (PAHs) from the pyrolysis of scrap tires. Atmos. Environ. 2007, 41, 1209. [Google Scholar] [CrossRef]
- Korger-Kocsis, J. Editorial corner-a personal view Waste tyre rubber—What to do next? Express Polym. Lett. 2013, 7, 406. [Google Scholar] [CrossRef]
- Kojima, M.; Tosaka, M.; Ikeda, Y.; Kohjiya, S. Devulcanization of Carbon Black filled Natural Rubber using Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2005, 95, 137. [Google Scholar] [CrossRef]
- Sun, X.; Isayev, A.I. Ultrasound devulcanization: Comparison of synthetic isoprene and natural rubbers. J. Mater. Sci. 2007, 42, 7520. [Google Scholar] [CrossRef]
- Formela, K.; Klein, M.; Colom, X.; Saeb, M.R. Investigating the combined impact of plasticizer and shear force on the efficiency of low temperature reclaiming of ground tire rubber. Polym. Degrad. Stab. 2016, 125, 1. [Google Scholar] [CrossRef]
- De, D.; Panda, P.K.; Roy, M.; Bhunia, S. Reinforcing effect of reclaim rubber on natural rubber/polybutadiene rubber blends. Mater. Des. 2013, 46, 142. [Google Scholar] [CrossRef]
- Rooj, S.; Basak, G.C.; Maji, P.K.; Bhowmnick, A.K. New Route for devulcanization of Natural Rubber and the properties of devulcanized rubber. J. Polym. Environ. 2011, 19, 382. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Improvement of the properties of natural rubber/ground tire rubber composites through biological desulfuration of GTR. J. Polym. Res. 2012, 19, 9864. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo-Navarrete, F.; Saeb, M.R.; Marin, M.; Formela, K.; Cañavate, J. Evaluation and rationale of the performance of several elastomeric composites incorporating devulcanized EPDM. Polym. Test. 2023, 121, 107976. [Google Scholar] [CrossRef]
- Colom, X.; Saeb, M.R.; Cañavate, J. Microstructural phenomena in ground tire rubber (GTR) devulcanized via combined Thermochem mechanical and microwave processes monitored by FTIR and DTGA assisted by other techniques. Express Polym. Lett. 2024, 18, 950. [Google Scholar] [CrossRef]
- Kleps, T.; Piaskiewicz, M.; Parasiewicz, W. The use of thermogravimetry in the study of rubber devulcanization. J. Therm. Anal. Calorim. 2000, 60, 271. [Google Scholar] [CrossRef]
- Scuracchio, C.H.; Waki, D.A.; da Silva, M.L.P. Thermal analysis of ground tire rubber devulcanized by Microwave. J. Therm. Anal. Calorim. 2007, 87, 893. [Google Scholar] [CrossRef]
- Garcia, P.S.; de Sousa, F.D.B.; de Lima, J.A.; Cruz, S.A.; Scuracchio, C.H. Devulcanization of ground tire rubber: Physical and Chemical changes after different microwave exposure times. Express Polym. Lett. 2015, 9, 1015. [Google Scholar] [CrossRef]
- Colom, X.; Faliq, A.; Formela, K.; Cañavate, J. FTIR spectroscopic and thermogravimetric characterization of ground tyre rubber devulcanized by microwave treatment. Polym. Test. 2016, 52, 200. [Google Scholar] [CrossRef]
- Aprem, A.S.; Joseph, K.; Thomas, S. Recent developments in crosslinking of elastomers. Rubber Chem. Technol. 2005, 78, 458–488. [Google Scholar] [CrossRef]
- Ismail, H.; Nodin, R.; Noor, A.M. Cure characteristics, tensile properties and swelling bahaviour of recycled rubber powder-filled natural rubber compounds. Polym. Test. 2002, 21, 565. [Google Scholar] [CrossRef]
- Mangili, I.; Lasagni, M.; Anzano, M.; Collina, E.; Tatangelo, V.; Franzetti, A.; Caracino, P.; Isayev, A.I. Mechanical and rheological properties of natural rubber compounds containing devulcanization ground tire rubber from several methods. Polym. Degrad. Stab. 2015, 121, 369. [Google Scholar] [CrossRef]
- ISO 37; Rubber, Vulcanised or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2013.
- ISO 7619-1; Rubber, Vulcanized or Thermoplastic—Determination of Indentation Hardness—Part 1: Durometer Method (Shore Hardness). ISO: Geneva, Switzerland, 2011.
- ASTM D6814-02; Standard Test Method for Determination of Percent Devulcanization of Crumb Rubber Based on Crosslink Density. ASTM International: West Conshohocken, PA, USA, 2018.
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the crosslink characteristics and mechanical properties of natural rubber compound via accelerators and reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Kraus, G.J. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861. [Google Scholar] [CrossRef]
- Saiwari, S.; Dierkes, W.K.; Noordermeer, J.W.M. Recycling of individual waste rubbers. In Rubber Recycling; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 186–232. [Google Scholar]
- ISO 2781; Rubber, Vulcanized or Thermoplastic—Determination of Density. ISO: Geneva, Switzerland, 2015.
- Bilgili, E.; Arastoopour, H.; Bernstein, B. Pulverization of rubber granulates using the solid-state shear extrusion process: Part II. Powder characterization. Powder Technol. 2001, 115, 277–289. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Carrillo, F.; Suñol, J.J. Effect of the Particle Size and Acid pretreatments on Compatibility and Properties of Recycled HDPE Plastic Bottles Filled with Ground Tyre Powder. J. Appl. Polym. Sci. 2009, 112, 1882. [Google Scholar] [CrossRef]
- Pehlken, A.; Essadiqi, E. Scrap Tire Recycling in Canada; Technical Report; CANMET Materials Technology Laboratory: Hamilton, ON, Canada, 2005. [Google Scholar] [CrossRef]
- Formela, K.; Haponiuk, J.T. Curing characteristics, mechanical properties and morphology of butyl rubber filled with ground tire rubber (GTR). Iran Polym. J. 2014, 23, 185. [Google Scholar] [CrossRef]
- Sambastsompop, N.; Kumnuantip, C. Rheology, Cure characteristics, Physical and Mechanical properties of Tire Tread Reclaimed Rubber/Natural Rubber Compounds. J. Appl. Polym. Sci. 2003, 87, 1723. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, C.; Liang, M. Properties of natural rubber vulcanizates containing mechanochemically devulcanized ground tire rubber. J. Polym. Res. 2009, 16, 411. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; Van Baarle, B. Influence of Zinc Oxide during Different Stages of Sulfur Vulcanization. Elucidated by Model Compound Studies. J. Appl. Polym. Sci. 2005, 95, 1388. [Google Scholar] [CrossRef]
- Cañavate, J.; Carrillo, F.; Casas, P.; Colom, X.; Suñol, J.J. The Use of Waxes and Wetting Additives to Improve Compatibility Between HDPE and Ground Tyre Rubber. J. Compos. Mater. 2010, 44, 1233–1245. [Google Scholar] [CrossRef]
- Gonen, M.; Balkose, D.; Inal, F.; Ulku, S. Zinc Stearate Production by Precipitation and Fusion Processes. Ind. Eng. Chem. Res. 2005, 44, 1627. [Google Scholar] [CrossRef]



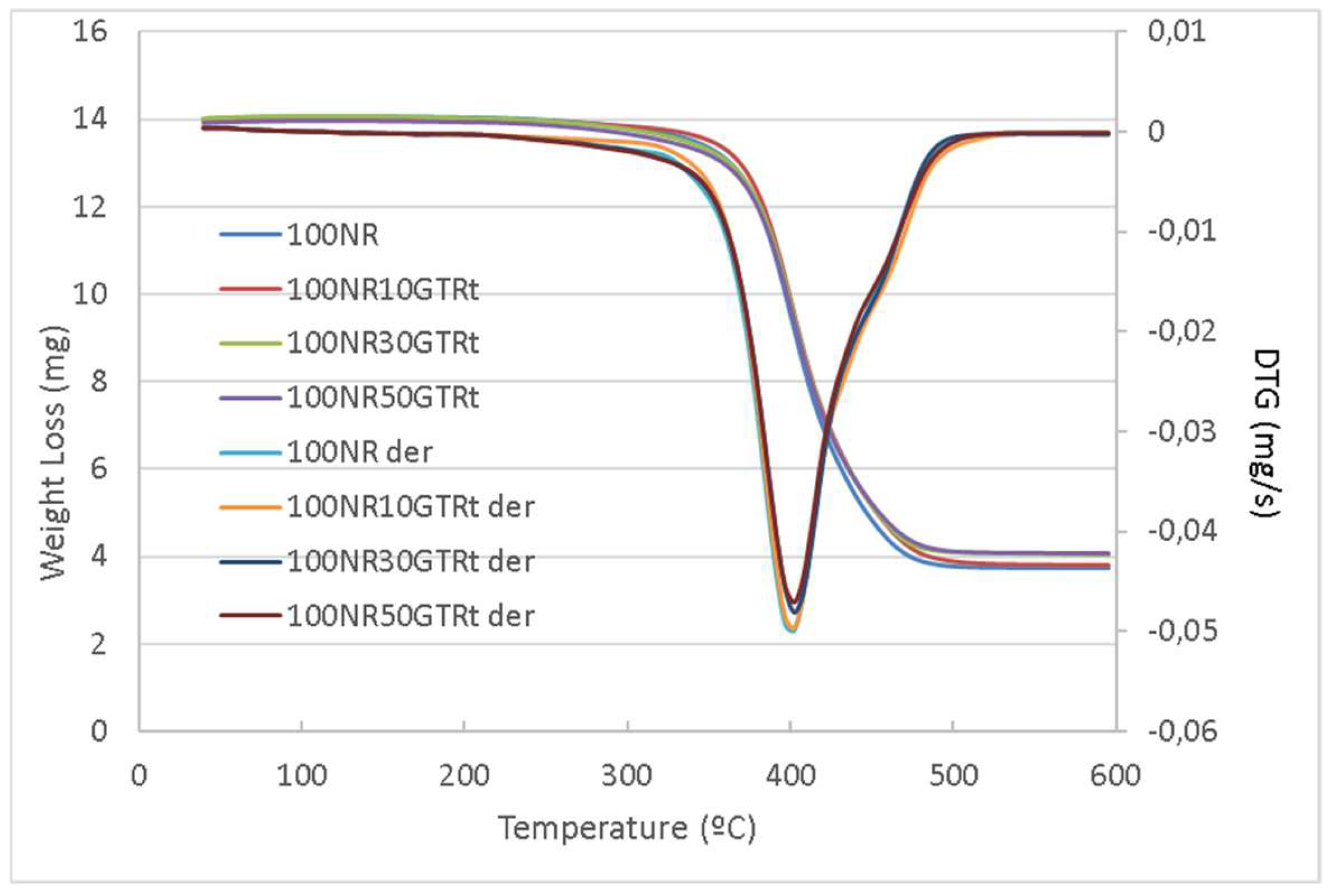
| Sample | Tm (°C) NR | Tm (°C) SynR | Char Residue |
|---|---|---|---|
| 100NR | 398.5 | - | 516.0 |
| 100NR10GTRc | 401.3 | 458.0 | 517.5 |
| 100NR30GTRc | 402.1 | 459.0 | 517.1 |
| 100NR50GTRc | 402.9 | 459.3 | 518.3 |
| 100NR10GTRc5 | 407.1 | 460.1 | 519.3 |
| 100NR30GTRc5 | 408.3 | 459.6 | 520.8 |
| 100NR50GTRc5 | 408.5 | 460.3 | 520.6 |
| 100NR10GTRc10 | 406.3 | 458.9 | 519.9 |
| 100NR30GTRc10 | 406.7 | 459.1 | 520.0 |
| 100NR50GTRc10 | 406.0 | 459.8 | 520.3 |
| 100NR10GTRt | 401.1 | 457.1 | 517.1 |
| 100NR30GTRt | 401.8 | 458.4 | 517.1 |
| 100NR50GTRt | 402.1 | 459.0 | 518.3 |
| 100NR10GTRt5 | 407.2 | 459.3 | 519.4 |
| 100NR30GTRt5 | 408.0 | 459.0 | 519.8 |
| 100NR50GTRt5 | 408.1 | 459.1 | 520.1 |
| 100NR10GTRt10 | 406.2 | 458.9 | 520.3 |
| 100NR30GTRt10 | 406.1 | 459.3 | 520.1 |
| 100NR50GTRt10 | 405.6 | 458.8 | 520.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colom, X.; Sans, J.; de Bruijn, F.; Carrillo, F.; Cañavate, J. Structural, Thermal and Mechanical Assessment of Green Compounds with Natural Rubber. Macromol 2024, 4, 566-581. https://doi.org/10.3390/macromol4030034
Colom X, Sans J, de Bruijn F, Carrillo F, Cañavate J. Structural, Thermal and Mechanical Assessment of Green Compounds with Natural Rubber. Macromol. 2024; 4(3):566-581. https://doi.org/10.3390/macromol4030034
Chicago/Turabian StyleColom, Xavier, Jordi Sans, Frederic de Bruijn, Fernando Carrillo, and Javier Cañavate. 2024. "Structural, Thermal and Mechanical Assessment of Green Compounds with Natural Rubber" Macromol 4, no. 3: 566-581. https://doi.org/10.3390/macromol4030034








