Abstract
The MALDI-TOF mass-spectrometry was employed to analyze the structure of the reaction products of limonene, a natural terpene, and elemental sulfur, with the objective of identifying the occurrence of side processes, such as oxidative dehydrogenation, aromatization, and the Diels–Alder reaction cascade. The MALDI-TOF mass-spectrometry was demonstrated to be effective for the analysis of high-sulfur polymers obtained by the inverse vulcanization reaction, allowing for the unambiguous separation of sulfur-containing and hydrocarbon molecular fragments and the detailed characterization of macromolecular structures. By varying the ratio of sulfur (S8) and limonene in the initial reaction system, we were able to ascertain the limiting amount of sulfur that can be covalently bonded by terpene, as well as determine the average length of polysulfide chains under the assumption of equal reactivity and complete depletion of all double bonds. The side reaction of limonene aromatization, as indicated by the MALDI-TOF spectrum of the product resulting from its interaction with elemental sulfur, was corroborated by 1H and 13C NMR spectroscopy. Consequently, the registration and interpretation of MALDI-TOF spectra of inverse vulcanization products, either independently or in conjunction with the application of 1H and 13C NMR spectroscopy methods, as well as the determination of the limiting number of sulfur atoms that can be bound to one molecule of an unsaturated compound, paves the way for new avenues of investigation into the structure and side reactions involved in the synthesis of high-sulfur polymers.
1. Introduction
Despite the fact that elemental sulfur is a readily available raw material derived from natural sources, its production consistently exceeds consumption [1,2]. In this regard, the processes of obtaining high-sulfur macromolecules through the interaction of unsaturated compounds, such as divinylbenzene [3,4,5,6], diisopropenylbenzene [7,8,9,10,11,12], styrene [13,14,15,16], limonene [17,18,19,20], squalene [21,22,23,24], myrcene [25,26,27], and unsaturated fatty acids [28,29,30,31,32,33] with S8 are of considerable interest. The polymers obtained by this reaction can have a different molar mass (102–105 g/mol) and possess an acceptable complex of physical and mechanical properties [9,16], as well as significant heat resistance [5,6,16]. The products of the reaction of sulfur with unsaturated compounds show promise as materials for the fabrication of cathodes for lithium-sulfur batteries [34,35,36,37,38,39,40,41,42], infrared optics [43,44,45,46,47,48], and sorbents for water treatment and the extraction of heavy metal cations [49,50,51,52,53,54,55,56]. Despite the extensive research conducted on the inverse vulcanization reaction, the majority of studies in this field have concentrated on the regulation of properties and the design of polymers with specific functions [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. However, there is still a lack of clarity regarding the underlying mechanism and the occurrence of numerous side reactions. The latter is attributable, at least in part, to the established methodology for the study of inverse vulcanizations products, which includes gel permeation chromatography [57,58,59,60,61], Raman spectroscopy [38,62,63,64,65,66], IR spectroscopy [63,64,65,66,67], UV-vis spectroscopy [15,64,68,69,70], and 1H, 13C NMR spectroscopy [7,14,71,72,73,74] and various elemental analysis approaches, including XPS [3,6,42,75,76], EDX [33,64,65,77,78,79,80], and ICP-OES [6]. The aforementioned methods permit the acquisition of valuable data regarding the composition and structure of high-sulfur polymers. However, in certain instances, they may prove inadequate for the detection of side reactions occurring under inverse vulcanization conditions. The utilization of a series of mass spectrometry techniques to examine the products of the interaction between unsaturated compounds and elemental sulfur is confined to a limited number of documented instances. These include the methodologies employed for the recording of spectra generated by CIS-MS [17], ToF-SIMS [73,75], and APCI-MS [21]. Concurrently, the MALDI-TOF mass-spectrometry, which has been extensively employed for structural investigations and the semi-quantitative estimation of the molecular-weight distribution of polymers [81,82,83,84,85], is seemingly underestimated for the analysis of inverse vulcanization products. As will be demonstrated subsequently, high-sulfur polymers desorb with decomposition into charged fragments under MALDI-TOF recording conditions. However, the identification of their structure can provide valuable insight into the nature of side reactions in inverse vulcanization processes. A further crucial aspect of inverse vulcanization processes is the existence of a limiting length of polysulfide fragments, at which they lose thermodynamic stability. This implies that there is a limiting capacity for elemental sulfur, which can be bound covalently, in unsaturated compounds. It is established that high-molecular-weight plastic sulfur loses its thermodynamic stability at temperatures below the lower critical temperature of polymerization [86]. This phenomenon indicates the potential for depolymerization of polysulfide bridging groups with lengths, exceeding a certain threshold, at room temperature.
The present article is devoted to the application of the MALDI-TOF mass-spectrometry for the establishment of side reactions in the inverse vulcanization process and the development of a technique for determining the ultimate capacity of unsaturated compounds by the elemental sulfur assimilated by them. The example of the interaction between the model terpene (limonene) and S8 is used to illustrate these points.
2. Materials and Methods
2.1. Materials
Elemental sulfur with a purity of more than 99% produced by “Reachim” (Moscow, Russia) and D-limonene purchased from “Merck” (Darmstadt, Germany) ( with the content of the basic substance of 98.5% were used for the inverse vulcanization reaction. Chloroform from “Merck” (Darmstadt, Germany) was used to dissolve the obtained polymer in order to determine the mass of unreacted sulfur residue.
2.2. Interaction of D-Limonene and Cyclooctasulfur (Synthesis of High-Sulfur Polymer by Inverse Vulcanization Reaction)
The elemental sulfur powder was subjected to a heating process at a temperature of 125 °C, with the use of a thermostat. D-limonene was subsequently added to the molten sulfur in order to achieve the following mass ratios of reactants: the ratios of the reactants were 1:4, 1:6, 1:8, 1:10, 1:12, 1:14, and 1:16. Subsequently, the temperature was increased to 165 °C and the resulting reaction system was incubated for 20 min. The resulting product was then cooled to room temperature at a slow rate and transferred to a vial by the addition of chloroform. It was then incubated for one hour to allow the polymer to dissolve. Subsequently, the precipitate was separated and washed once more with chloroform. Once the polymer had been fully removed, the elemental sulfur precipitate was dried and weighed. The amount of sulfur included in the polymer was determined by the difference between the weight of the initial sulfur and the weight of the sulfur remaining after the inverse vulcanization and dissolution of the synthesized polymer.
2.3. Methods for the Study of a Polymer Obtained by the Interaction of D-Limonene and Elemental Sulfur
The structure of the synthesized polymer (initial mass ratio S8/D-limonene = 4) was determined by MALDI-TOF mass spectrometry, 1H and 13C NMR spectroscopy. MALDI-TOF spectra were recorded on an Ultraflex II mass spectrometer (Bruker, Bremen, Germany) with the detection of anions and cations in reflector mode with an accelerating voltage of 25 kV. Desorption was carried out by Nd:YAG laser with a wavelength of 355 nm (3.5 eV), without the use of a matrix. In total, 0.5 μL of the polymer solution in CHCl3 with a concentration of 0.01 g/mL was applied to a ground steel MALDI target plate (MTP) from “Bruker Daltonics”. After the evaporation of the chloroform for 5 min, the MALDI-TOF mass spectrum was recorded. Although no matrix was used in the recording of mass spectra in this paper and the spectra obtained are LDI, the abbreviation MALDI-TOF will be used in further discussion of the results, as it is more frequently used in the literature even when the spectra were recorded without a matrix. Taking into account the possibility of laser desorption from MTP of non-polar and devoid of pronounced acid-base properties products of inverse vulcanization of limonene, as well as complications in interpreting spectra of signals caused by the presence of a matrix, in this work the laser desorption of macromolecules was carried out in the absence of a matrix.
The 1H and 13C NMR spectra were recorded in a CDCl3 medium (polymer concentration 0.1 g/mL) on a Bruker Avance 400 DPX spectrometer (Bruker, Germany) at 20 °C.
3. Results and Discussion
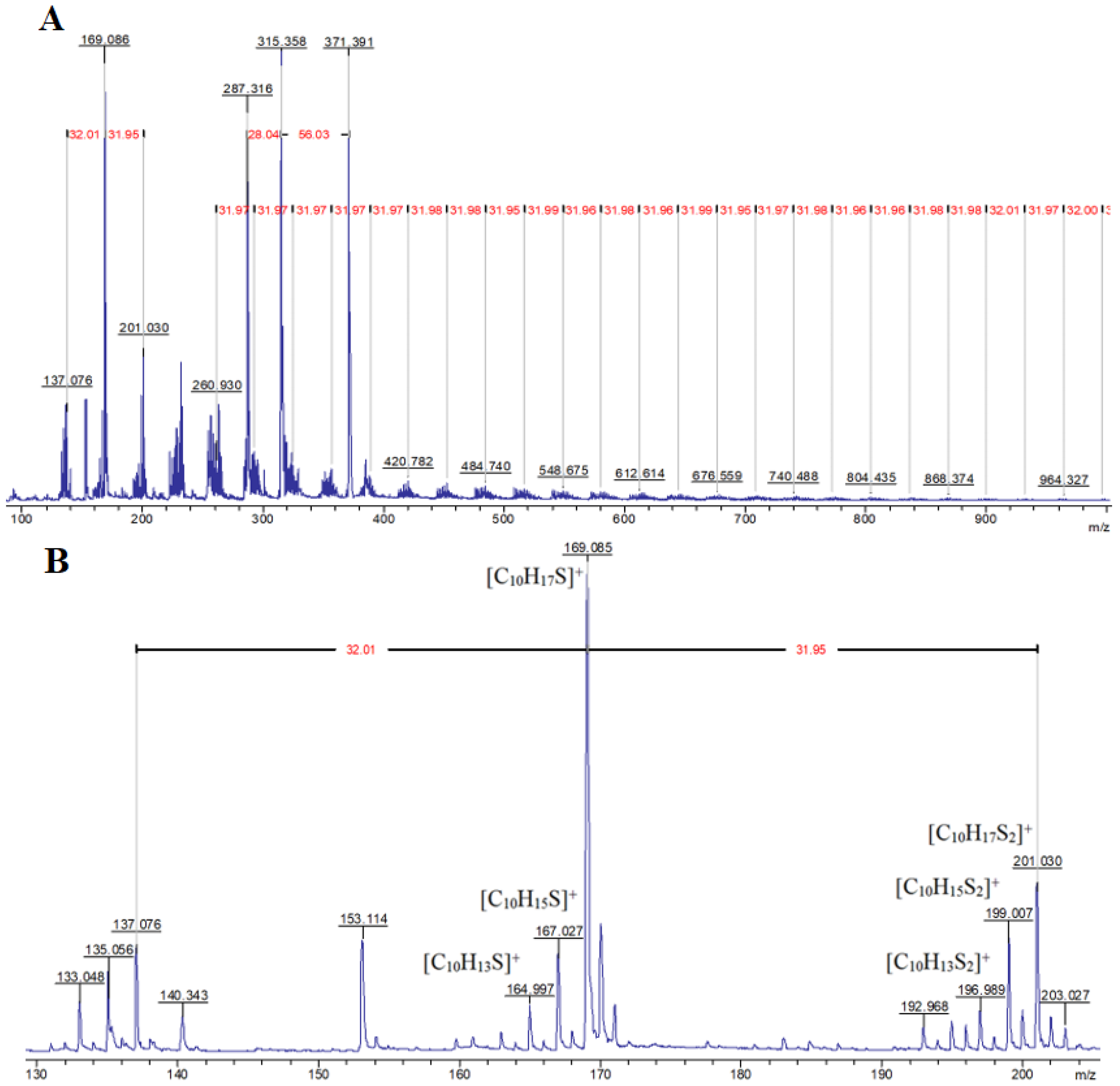
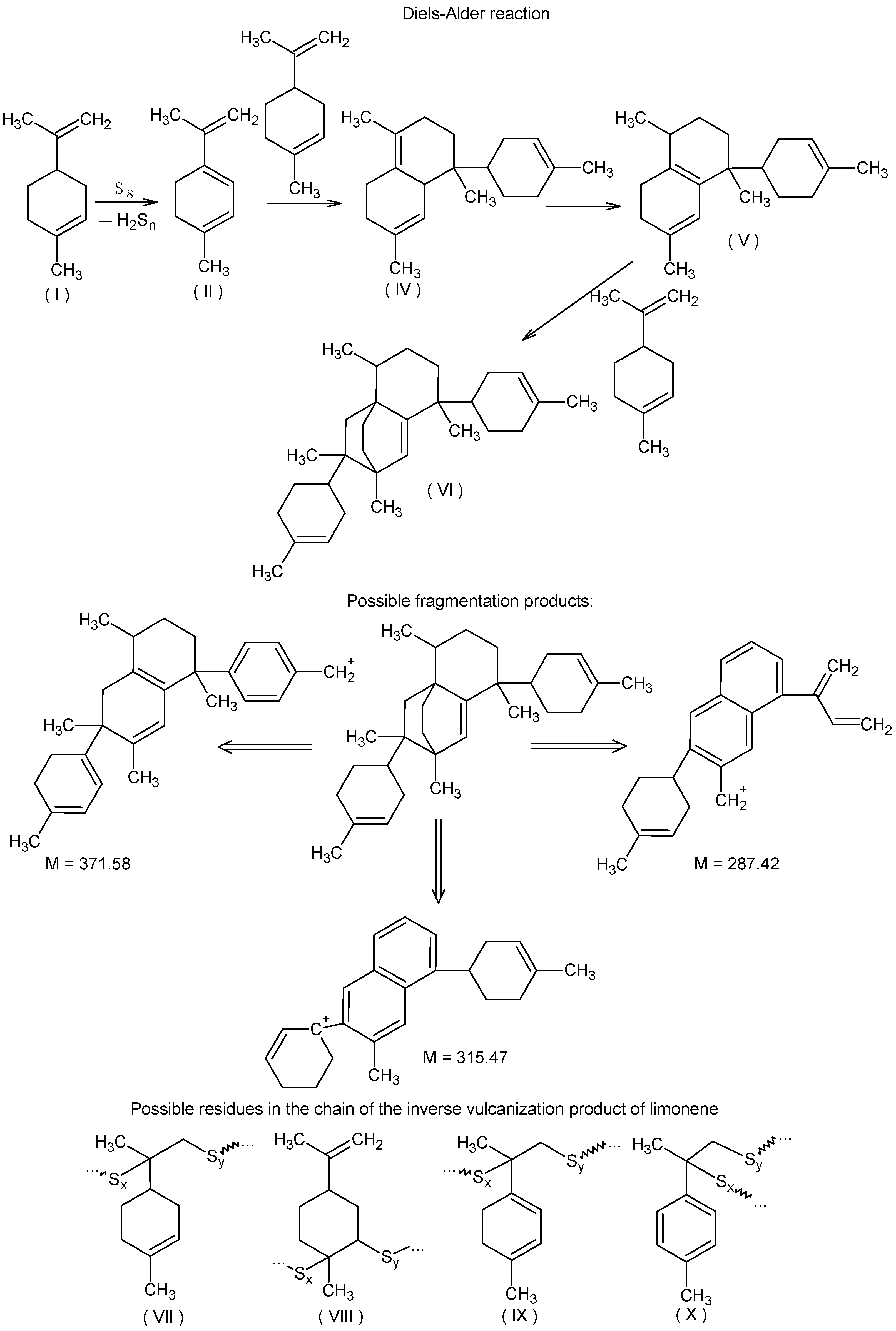
Since natural, elemental sulfur has four stable isotopes 32S, 33S, 34S, and 36S, the content of which is 95.02%, 0.75%, 4.21%, and 0.02%, respectively [87], it is possible to identify signals of fragments containing and not containing sulfur atoms in MALDI-TOF mass spectra of inverse vulcanization products. In particular, when interpreting the MALDI-TOF mass spectrum of the product of the interaction of elemental sulfur with the model terpene limonene (Figure 1A), the presence of a group of fragment signals with m/z equal to 133, 135, 137, 287.3, 315.3, and 371.4, which do not contain sulfur atoms, and fragment signals with m/z 165, 167, 169, 197, 199, and 201, which contain sulfur atoms and give accompanying isotope signals with an isotopic distribution pattern close to the theoretical one, was noted.
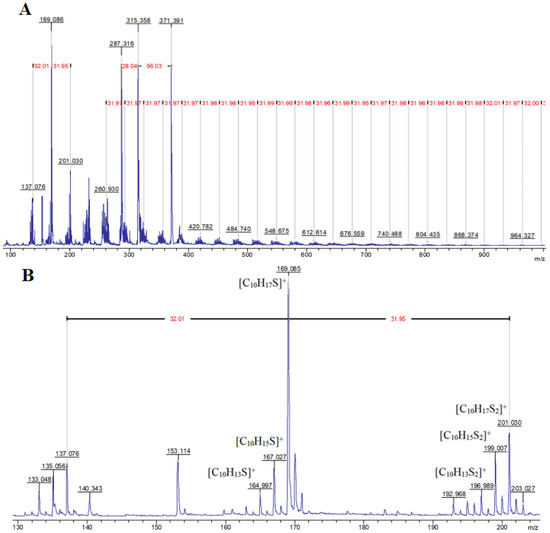
Figure 1.
MALDI-TOF mass spectrum of the reaction product of elemental sulfur with limonene in the m/z 100–1000 (A) region, as well as at m/z < 205 (B), recorded in reflector mode with cation detection.
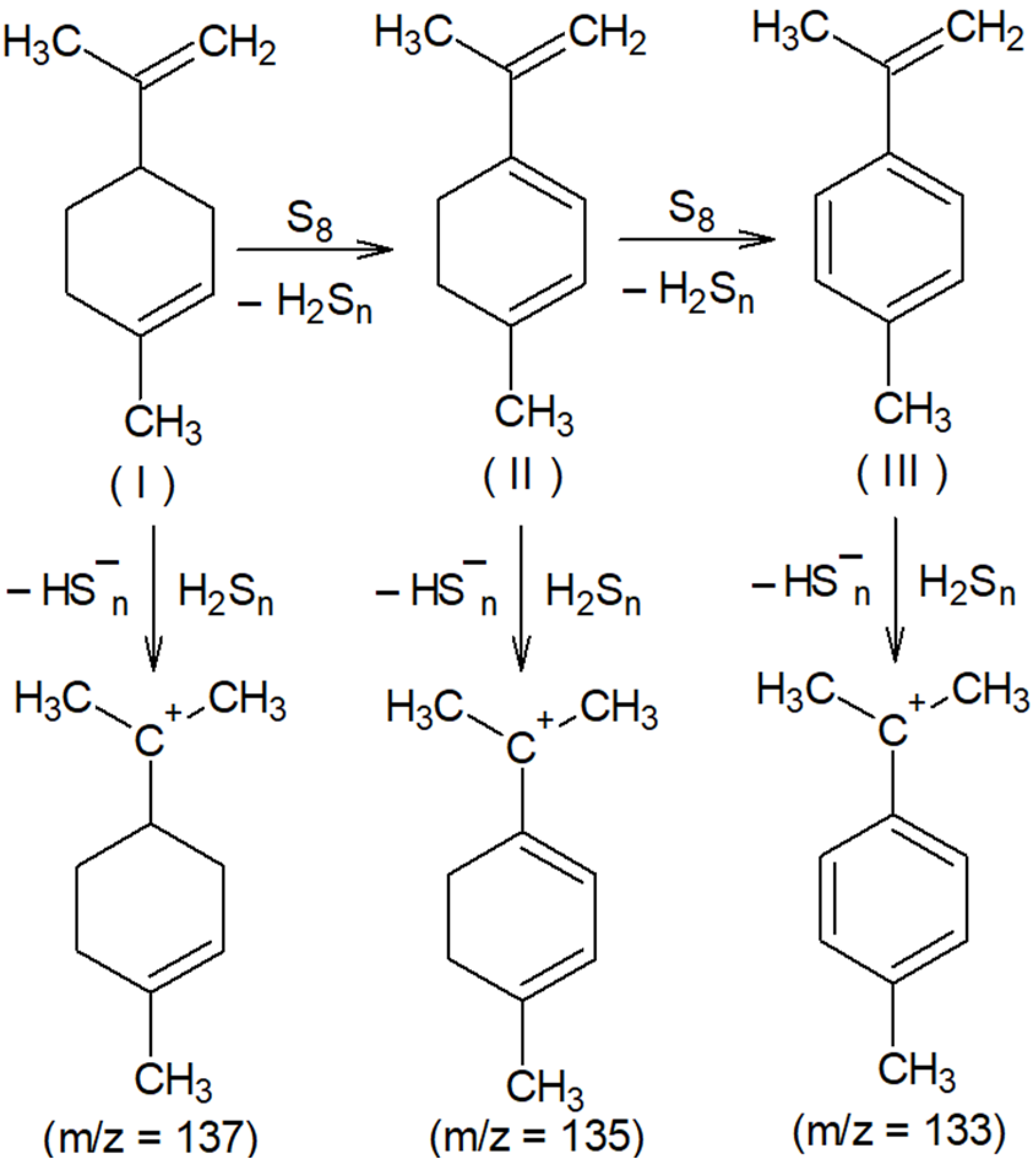
The presence of signals with m/z 133 and 135 (Figure 1B) is consistent with the assumption of partial oxidative dehydrogenation of limonene with subsequent aromatization, accompanied by the formation of 4-methylisopropenylbenzene (III). The signal with m/z 137 (Figure 1B) can be attributed to the protonation products of the initial limonene. The detection of cations corresponding to protonated molecules (I), (II), and (III) indicates the formation of compounds with pronounced acidic properties in the system, which are presumably hydrogen polysulfides (Figure 2).
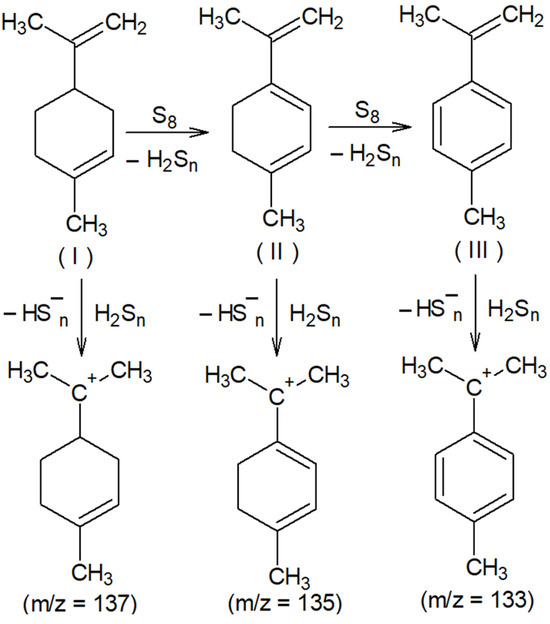
Figure 2.
Oxidative dehydrogenation of limonene with elemental sulfur and putative structures of cations detected in the MALDI-TOF mass spectrum.
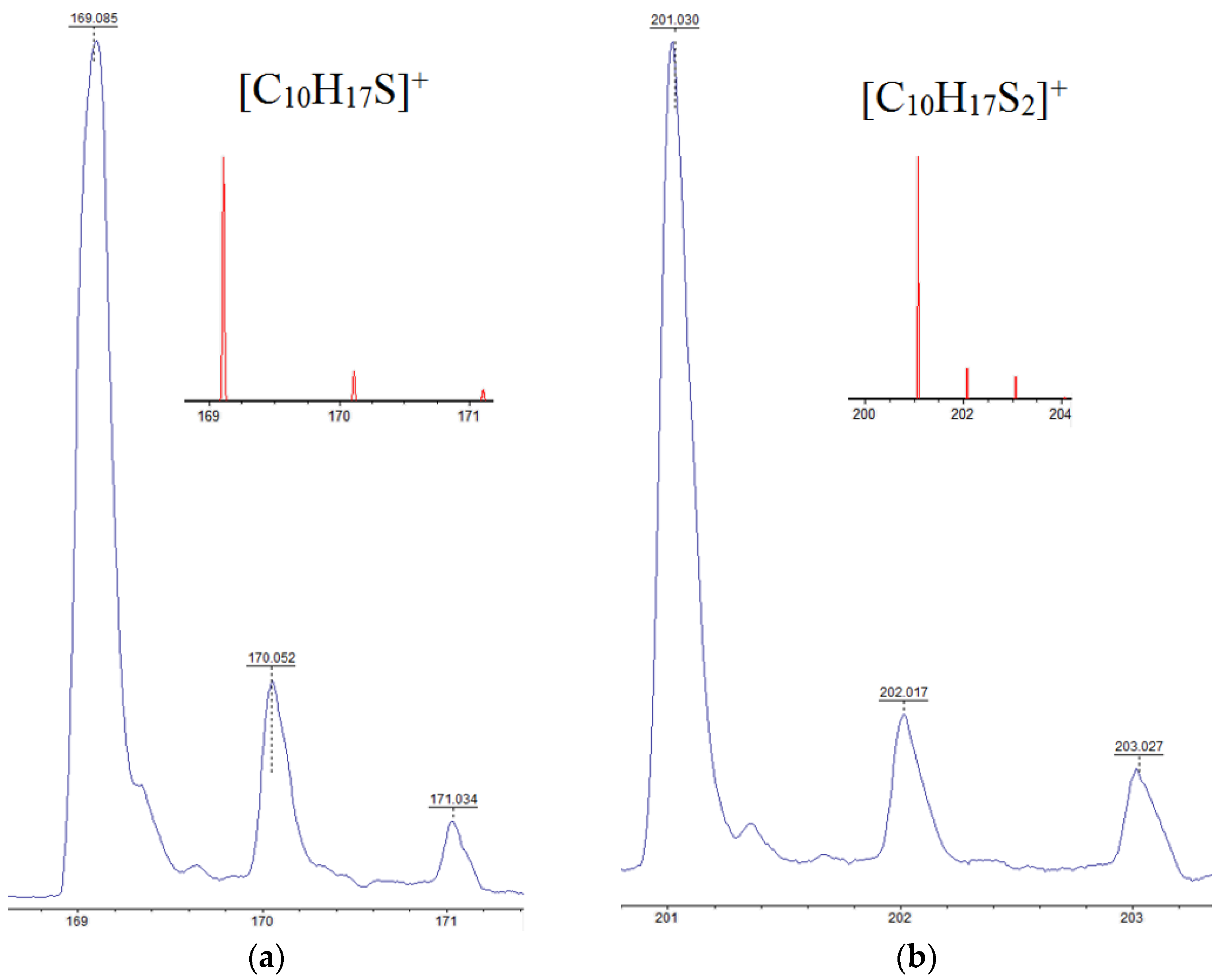
The activity of limonene (I) and the products of its oxidative dehydrogenation (II) (III) in the reaction with cyclooctasulfur has been confirmed by the presence of groups of signals with m/z 169, 167, and 165, which correspond to [C10H17S]+, and [C10H15S]+. Furthermore, the presence of the ions [C10H13S]+ and those with m/z 201, 199, and 197, corresponding to the cations [C10H17S2]+, [C10H15S2]+, and [C10H13S2]+ (Figure 2) was also confirmed. A comparison of the experimental isotopic distribution for the fragments [C10H17S]+ and [C10H17S2]+ with the theoretical one calculated using the program “Iso Pro 3.1” indicates a high degree of correspondence between the two (Figure 3).
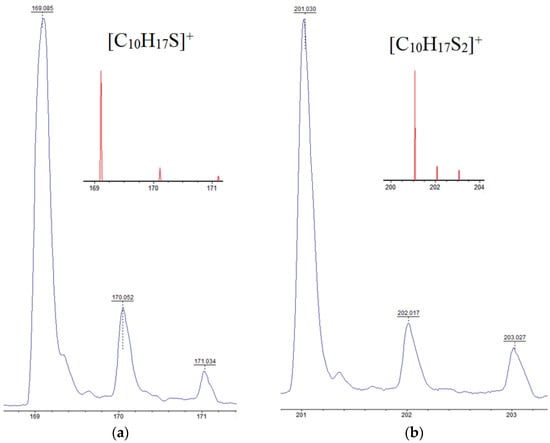
Figure 3.
The experimental and theoretical (constructed using the program “Iso Pro 3.1”) isotopic distributions of signals for cations: (a)—[C10H17S]+ and (b)—[C10H17S2]+ detected in the MALDI-TOF mass spectrum of the product of the interaction of limonene with elemental sulfur.
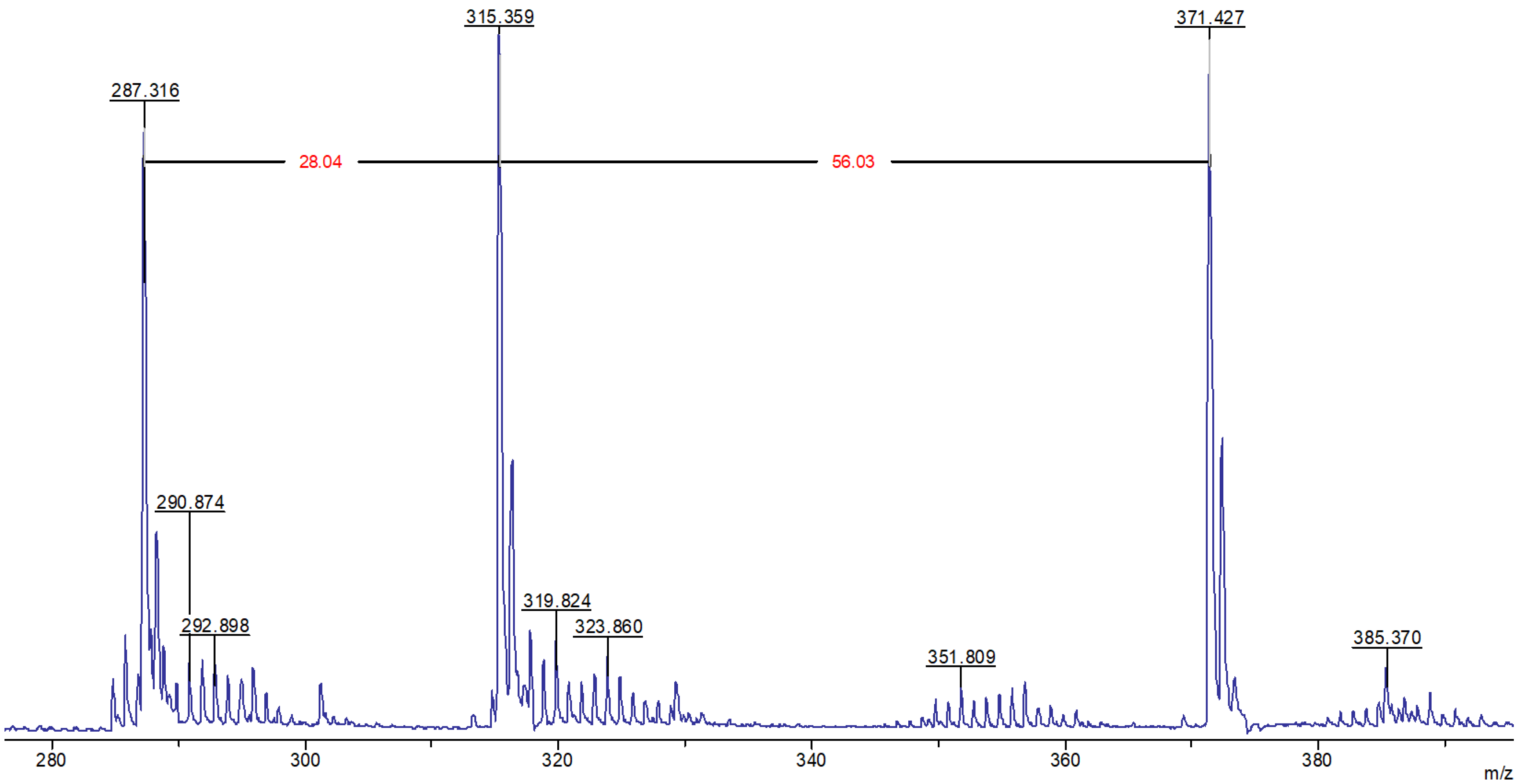
Given that the molecular mass of the limonene corresponds to m/z 136.24, the presence of signals with m/z equal to 287.3, 315.4, and 371.4 in the MALDI-TOF mass spectrum (Figure 4) belonging to fragments devoid of sulfur atoms, indicates secondary transformations of this terpene, which occur in the presence of cyclooctasulfur, which acts as the oxidizing agent.
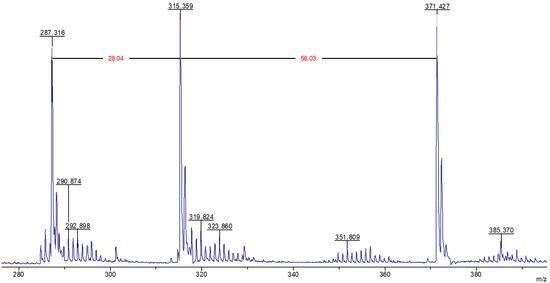
Figure 4.
MALDI-TOF mass spectrum of the product of the interaction of elemental sulfur with limonene in the region 280–400 m/z, recorded in reflector mode with cation detection.
It seems reasonable to posit that following the formation of compound (II) as the result of the oxidative dehydrogenation of limonene (Figure 2), a cascade of Diels–Alder reactions ensues, culminating in the generation of limonene trimers with a structural resemblance to compound (VI) (Figure 5). As can be observed, compound (VI) lacks diene fragments and all its double bonds are sterically hindered, preventing further participation in the Diels–Alder reaction. This may explain the absence of signals for hydrocarbon fragments containing no sulfur atoms with m/z exceeding 371.4. Figure 5 illustrates the potential structures of fragmentation products (VI) (or their isomers) with m/z 287.3, 315.3, and 371.4, which were identified during MALDI-TOF mass spectral registration. The primary pathways of fragmentation for compound (VI) are likely to be the Diels–Alder retro-reaction, dehydrogenation, and methane loss. In addition, the MALDI-TOF mass spectrum contains extended sequences of signals with an m/z difference of one unit with peaks dominating in intensity with m/z equal to 290.9, 319.8, 351.8, and 385.4, corresponding to cations formed from sulfur-containing fragments (VII)–(X) included in the macromolecules (Figure 5).
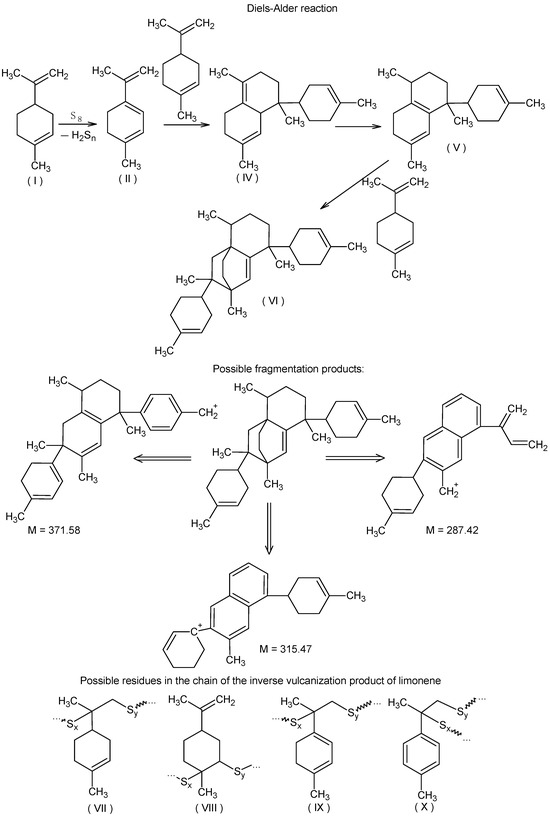
Figure 5.
Oxidative dehydrogenation of limonene with elemental sulfur followed by a Diels–Alder reaction cascade, possible fragmentation products of compound (VI) under MALDI-TOF mass spectral recording conditions, and some putative structures in the macromolecular chain.
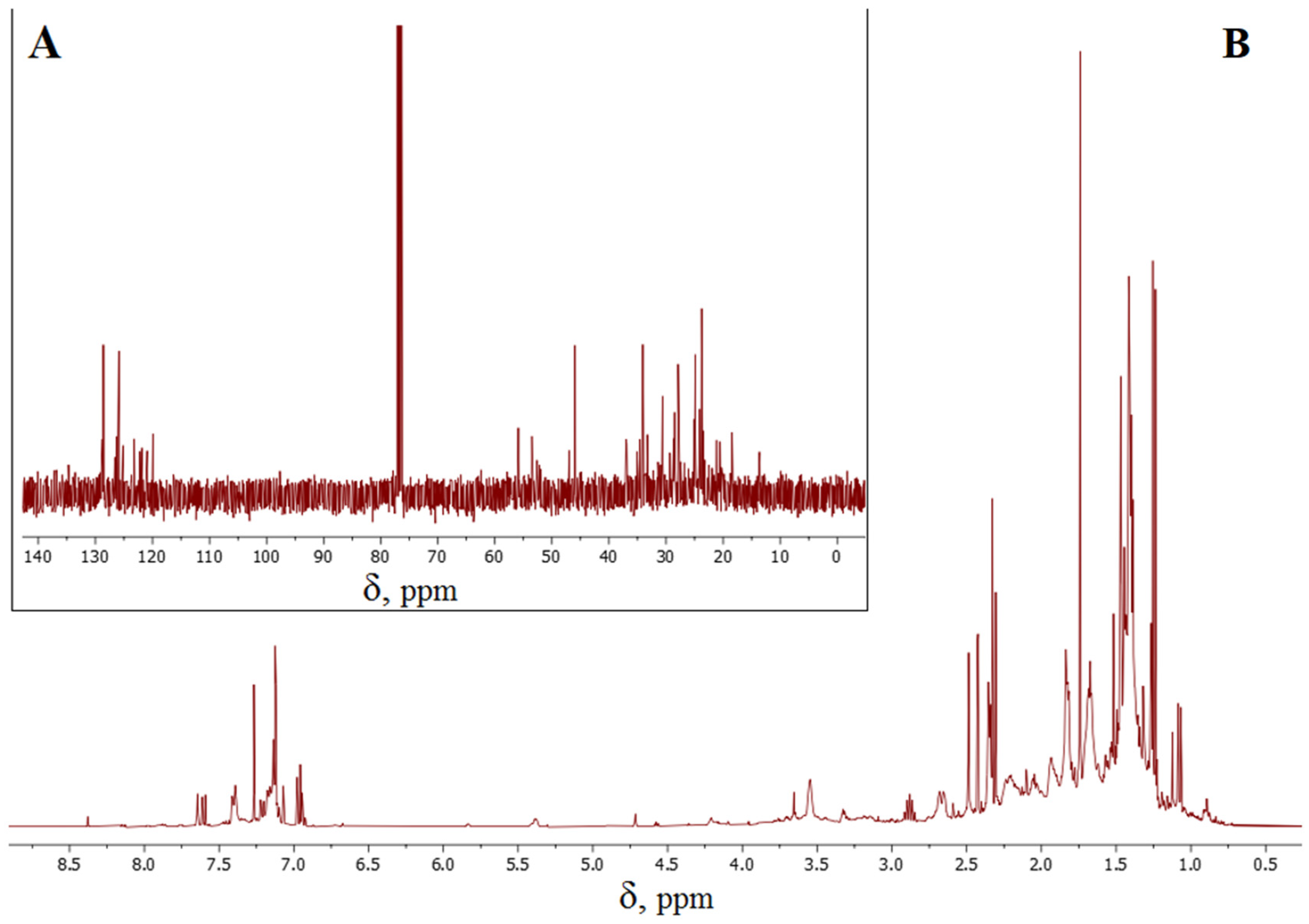
The putative compounds (II)–(VI) (Figure 5), as well as the initial limonene, contain double carbon–carbon bonds and are thus capable of reacting with S8. It would appear that the CH2=CR2 double bonds of the acyclic fragments are the most active in the interaction with cyclooctasulfur, as evidenced by the lack of signals with chemical shifts around 110 ppm and 150 ppm in the 13C NMR spectrum (Figure 6A), which are characteristic of this fragment. Concurrently, multiple bonds within the cyclic fragments are partially preserved, as evidenced by the presence of signals with chemical shifts of 120–130 ppm in the 13C NMR spectrum of inverse vulcanization products (Figure 6A). The 1H NMR spectrum (Figure 6B) displays proton signals with chemical shifts of 7.0–7.6 ppm, which corroborates the oxidative aromatization of limonene under the conditions of its reaction with S8. This phenomenon has previously been documented in the literature [19,88,89]. It can thus be surmised that the presence of signals with a chemical shift of 120–130 ppm in the 13C NMR spectrum (Figure 6A) may be attributed to aromatization, given that the double bonds of the benzoic ring are inactive in inverse vulcanization. The 13C NMR and 1H NMR spectroscopy data indicate the formation of a complex mixture of hydrocarbons and the corresponding polysulfides, which is consistent with the MALDI-TOF mass spectrometry data on positively charged ions discussed above. Although the identification of the structure of individual links of the polymer chain using NMR spectroscopy data is difficult due to the formation of their complex mixture, the presented results allow us to conclude that the dehydrogenation of limonene is not a consequence of destructive processes under conditions of laser ionization/desorption of macromolecules, since the formation of the corresponding fragments is independently shown by 13C NMR and 1H NMR spectroscopy methods using low-energy radio wave radiation.
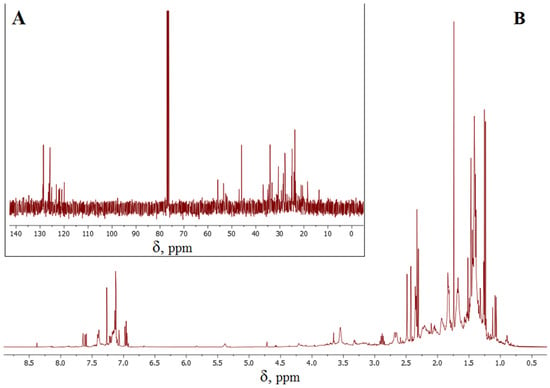
Figure 6.
13C NMR (A) and 1H NMR (B) spectra of the product of the reaction of limonene with elemental sulfur.
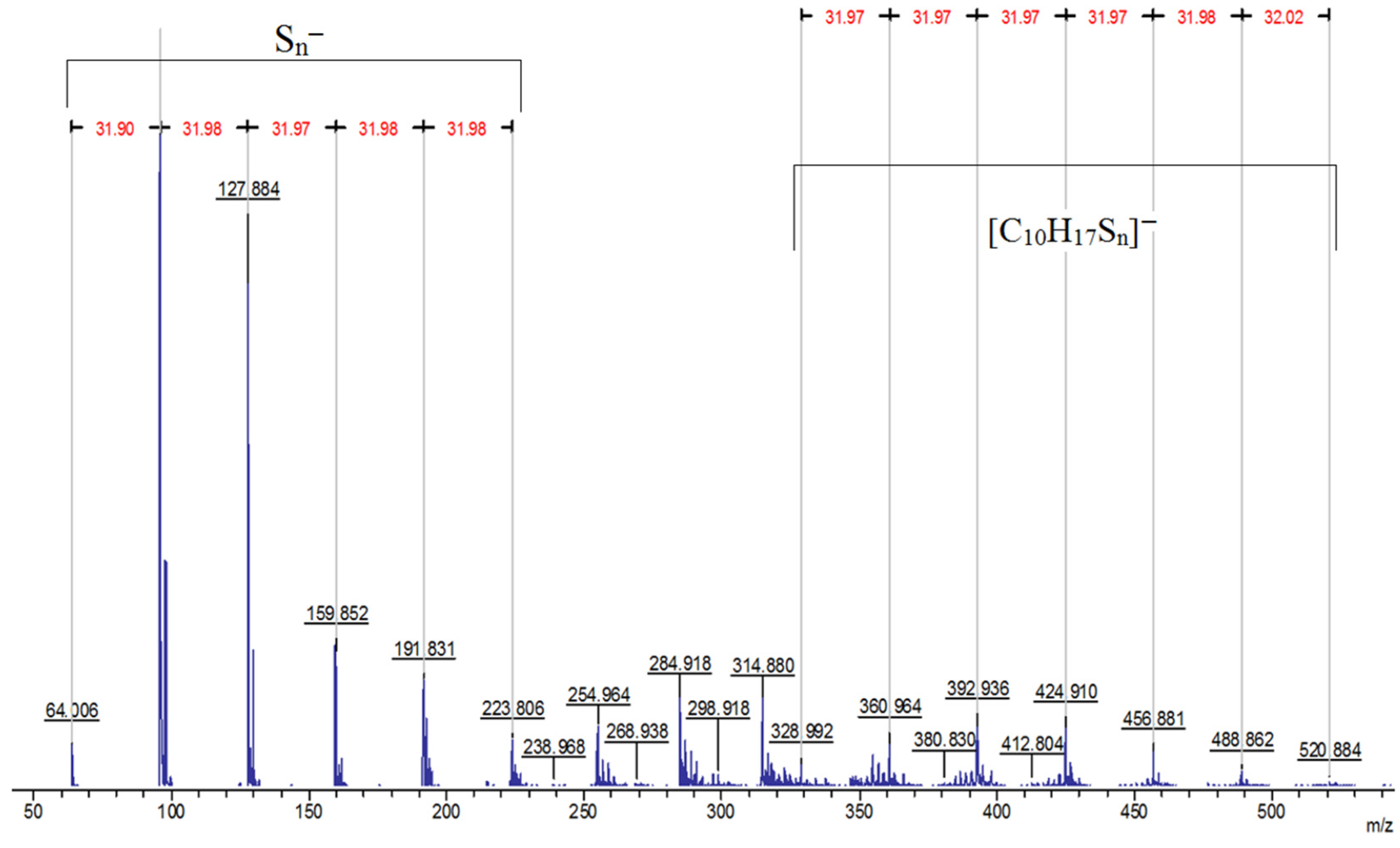
The MALDI-TOF spectrum reveals the presence of anion signals from to in the low molar mass region, concomitant with the observation of signals of [C10H17Sn]− fragments in the high molar mass region (Figure 7).
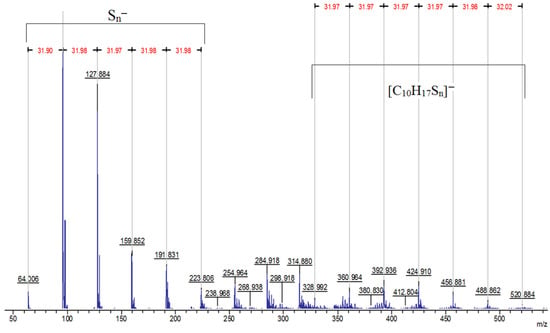
Figure 7.
MALDI-TOF mass spectrum of the product of the reaction of elemental sulfur with limonene recorded in reflector mode with anion detection.
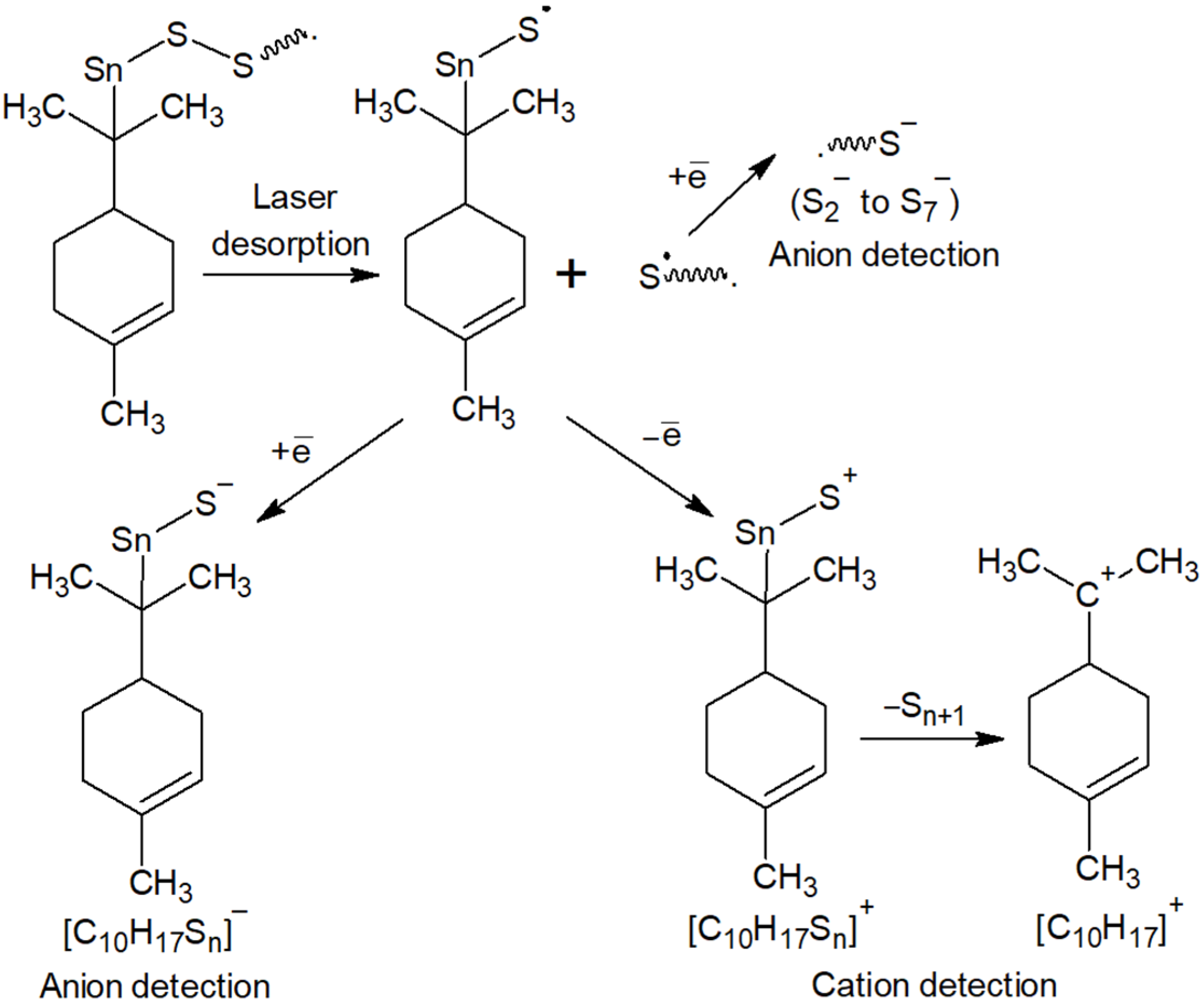
The series of signals from to are caused by the destruction of polysulfide bridges between hydrocarbon residues in the macromolecular chain. The signals [C10H17Sn]− observed during the detection of anions are related in origin to the signals [C10H17Sn]+ found in the cation detection mode (Figure 3) and are a consequence of the formation of fragment ions containing limonene residues and sulfur atoms (Figure 8). The driving force for the formation of fragment ions is the relative lability of S–S bonds in long polysulfide sequences [90].
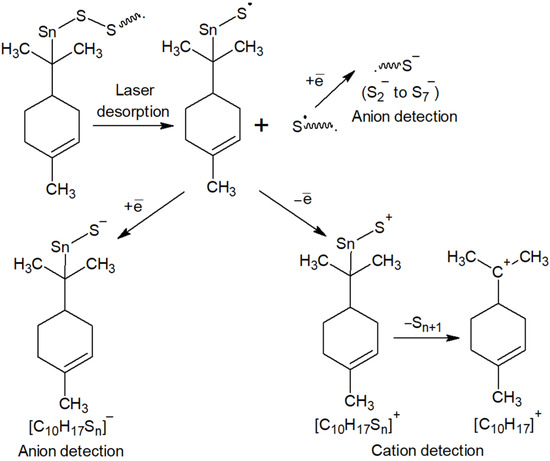
Figure 8.
Scheme of fragmentation of products of inverse vulcanization of limonene under laser desorption/ionization conditions.
The study of the reaction products of cyclooctasulfur with unsaturated compounds by MALDI-TOF spectrometry offers a promising avenue for elucidating the structural details of synthesized macromolecules. This is achieved by considering the potential mechanisms of their destruction at laser ionization (Figure 8). Additionally, this approach serves as a robust method for identifying side reactions in inverse vulcanization processes. On the other hand, the possibility of using MALDI-TOF mass spectrometry to determine the structural details of the products of inverse vulcanization of the natural terpene limonene is an expansion of the scope of this method, which has proven itself to be a reliable tool for the analysis of many biopolymers [91,92], their derivatives and polymers for pharmaceutical purposes [93].
A further crucial element of the synthesis of sulfur-containing polymers via the interaction of unsaturated compounds and S8 is the inability to achieve covalent bonding of more than a specific number of sulfur atoms. This is due to the fact that polysulfide sequences become thermodynamically unstable as their length increases [94]. It is established that polymeric sulfur is unstable at temperatures below the lower critical temperature of polymerization, resulting in depolymerization and the formation of the initial cyclooctasulfur [95]. It is therefore essential to establish the ultimate sulfur capacity of unsaturated compounds, that is to say, the maximum amount of sulfur that can be covalently assimilated. The inverse vulcanization process was conducted at varying initial ratios of sulfur/limonene, followed by the dissolution of the resulting polymer in chloroform and the determination of the residual amount of unreacted sulfur. This approach enabled the establishment of two key observations: firstly, that the mass of unreacted sulfur increased in proportion to the amount present in the reaction system, and secondly, that the amount of sulfur incorporated into the polymer remained constant at a significant excess of S8 (Table 1).

Table 1.
Amounts of initial cyclooctasulfur, limonene, remaining unreacted sulfur, and covalently bound sulfur under inverse vulcanization conditions.
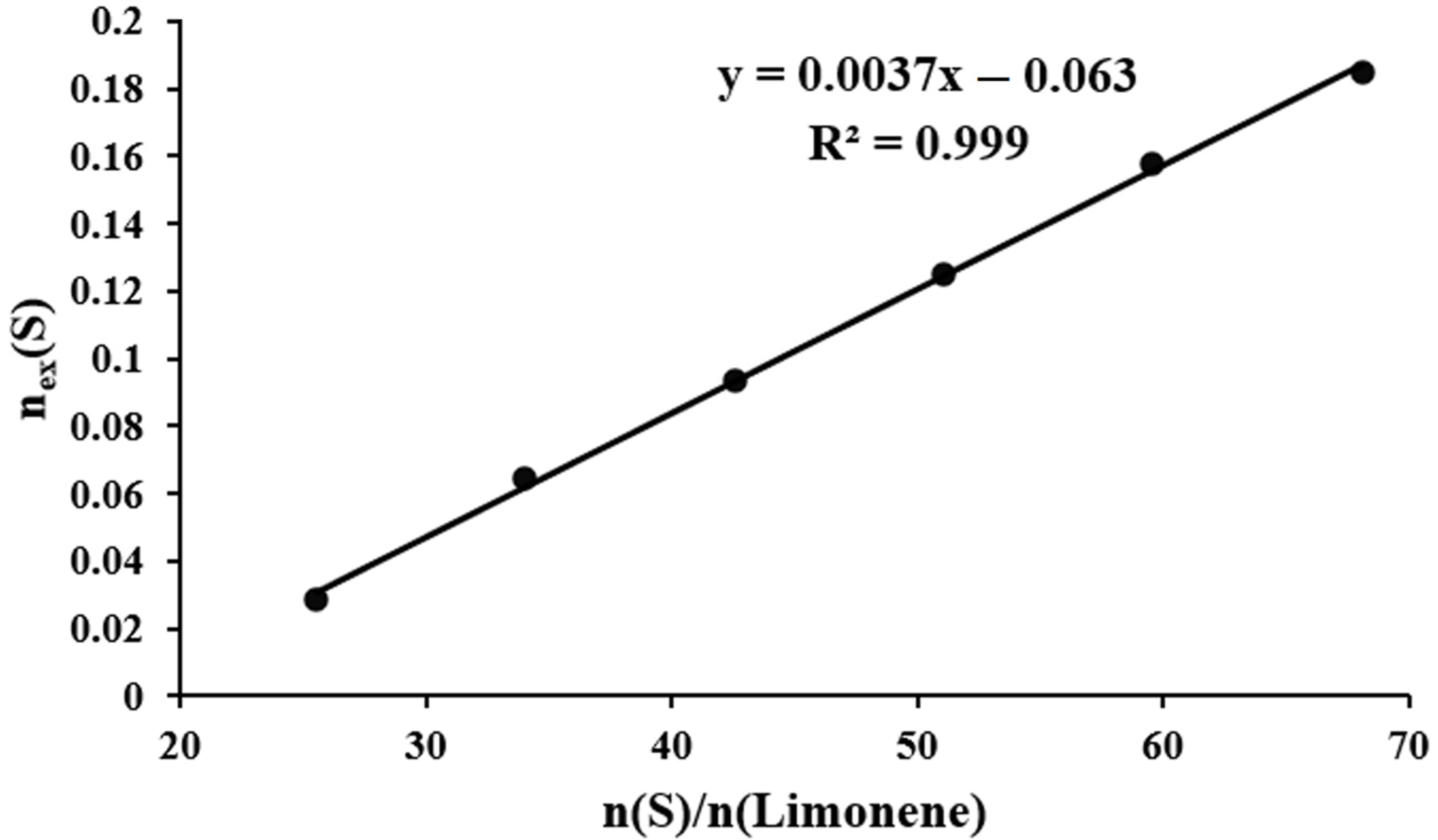
The mass of unreacted elemental sulfur present in the final mixture is found to be dependent upon the molar ratio of sulfur and limonene present in the initial mixture. This dependence is linear (Table 1, Figure 9) and can be described by the following Equation (1):
where —number of moles of unreacted sulfur, and —number of moles of sulfur and limonene in the initial mixture.
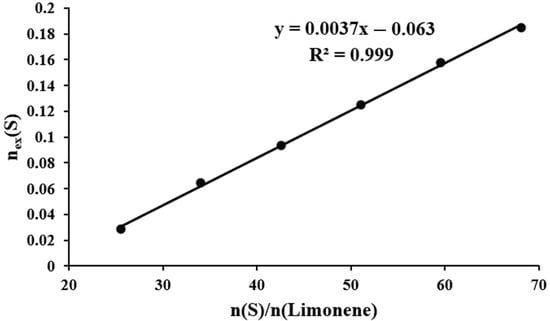
Figure 9.
Dependence of the number of moles of unreacted sulfur () on the molar ratio of elemental sulfur and limonene ().
It can be demonstrated that the limiting amount of sulfur that can be covalently bonded by one mole of limonene is equal to the value of the amount of unreacted sulfur, = 0. This implies that, on average, approximately 17 sulfur atoms can be bonded by one limonene molecule. This figure corresponds to the inclusion of an average of 4.25 sulfur atoms per carbon atom of the double bonds of the original limonene (seventeen sulfur atoms per four carbon atoms of the two double bonds of limonene). The mean length of polysulfide bridges is 8.5 sulfur atoms when the conditions of equal reactivity and complete conversion of all double bonds of limonene are met. As evidenced by NMR spectroscopy data (Figure 6), a portion of the double bonds present within the cyclohexene fragments of limonene are retained in the products of inverse vulcanization, suggesting the presence of polysulfide bridges with an average length exceeding 8.5 sulfur atoms.
4. Conclusions
The application of the MALDI-TOF mass-spectrometry to analyze the structure of limonene inverse vulcanization products has been demonstrated to facilitate the registration of an informative spectrum of high-sulfur polymer degradation. The advantage of MALDI-TOF mass spectrometry is that it allows for the separation of signals from molecular fragments containing and not containing sulfur atoms based on the character of the isotopic distribution. Additionally, it enables the identification of side transformations of unsaturated compounds under conditions of interaction with cyclooctasulfur. In the inverse vulcanization of limonene, additional processes were observed, including oxidative dehydrogenation and aromatization of the initial terpene, as well as the activity of the monodehydrogenation product of limonene in successive Diels–Alder reactions, which led to the formation of polycyclic molecules. By varying the ratio of elemental sulfur and limonene in the initial reaction mixture, it was determined that limonene is capable of binding no more than seventeen sulfur atoms, with the average length of polysulfide bridges slightly exceeding 8.5 sulfur atoms. Therefore, the utilization of a combination of MALDI-TOF mass spectrometry techniques and the determination of the maximum capacity of unsaturated compounds for sulfur atoms enables the characterization of the details of the structure of inverse vulcanization products, the identification of which by other methods is challenging. Furthermore, this approach facilitates the detection of side reactions in the synthesis of high-sulfur polymers. Thus, the presented results not only provide new opportunities for analyzing the structure and mechanism of the formation of reverse vulcanization products of natural and synthetic unsaturated compounds, but also expands the scope of the application of the MALDI-TOF method, traditionally used to study biopolymers.
Author Contributions
Conceptualization, N.T., E.K. and Y.M.; methodology, E.K. and I.T.; software, A.Z. and E.K.; validation, N.T. and Y.M.; formal analysis, I.T., E.K., N.T., E.K. and Y.M.; investigation, E.K., A.Z., I.T. and D.K.; resources, N.T.; data curation, N.T. and E.K.; writing—original draft preparation, E.K., D.K. and Y.M.; writing—review and editing, N.T. and Y.M.; visualization, D.K. and A.Z.; supervision, N.T. and E.K.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Russian Science Foundation, project no. 23-23-00543.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors express their gratitude for the assistance in the preparation of the manuscript to the Network center for advanced research “Green Chemistry for Sustainable Development: from fundamental principles to new materials” and the D.I. Mendeleev Center for Collective Use.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seong-Ju, L.; Gi-Yeon, H.; Mo-Beom, Y.; Jong-Ho, B.; Hyun-Joong, K. Inverse vulcanization of sulfur and solvent-based depolymerization for preparation of pressure-sensitive adhesives. J. Mater. Res. Technol. 2024, 29, 1798–1804. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Smirnov, Y.D.; Lisay, V.V.; Borowski, G. Issues of the Impact of Granulated Sulfur Transportation on the Environmental Components. J. Ecol. Eng. 2023, 24, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.; Mecerreyes, D.; Blazquez, J.A.; Leonet, O.; Ben Youcef, H.; Li, C.; Gómez-Cámer, J.L.; Bondarchuk, O.; Rodriguez-Martinez, L. Inverse vulcanization of sulfur with divinylbenzene: Stable and easy processable cathode material for lithium-sulfur batteries. J. Power Sources 2016, 329, 72–78. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Cengiz, E.C.; Demir-Cakan, R.; Yagci, Y. Inverse vulcanization of bismaleimide and divinylbenzene by elemental sulfur for lithium sulfur batteries. Eur. Polym. J. 2016, 80, 70–77. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.; Cho, H.; Lim, J.; Char, K. Inverse Vulcanization Polymers with Enhanced Thermal Properties via Divinylbenzene Homopolymerization-Assisted Cross-Linking. ACS Macro Lett. 2019, 8, 1670–1675. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; McBride, F.; Cai, D.; Dale, J.; Hanna, V.; Hasell, T. Mechanochemical synthesis of inverse vulcanized polymers. Nat. Commun. 2022, 13, 4824. [Google Scholar] [CrossRef]
- Bao, J.; Martin, K.P.; Cho, E.; Kang, K.-S.; Glass, R.S.; Coropceanu, V.; Bredas, J.-L.; Parker, W.O., Jr.; Njardarson, J.T.; Pyun, J. On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. Soc. 2023, 145, 12386–12397. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Astashkin, A.V.; Glass, R.S.; Mackay, M.E.; Char, K.; Pyun, J. Preparation of Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur. ACS Macro Lett. 2014, 3, 1258–1261. [Google Scholar] [CrossRef]
- Ghumman, A.S.M.; Nasef, M.M.; Shamsuddin, M.R.; Abbasi, A. Evaluation of properties of sulfur-based polymers obtained by inverse vulcanization: Techniques and challenges. Polym. Polym. Compos. 2020, 29, 1333–1352. [Google Scholar] [CrossRef]
- Hanna, V.; Graysmark, M.; Willcock, H.; Hasell, T. Liquid polybutadiene reinforced inverse vulcanised polymers. J. Mater. Chem. A 2024, 12, 1211–1217. [Google Scholar] [CrossRef]
- Dirlam, P.T.; Simmonds, A.G.; Shallcross, R.C.; Arrington, K.J.; Chung, W.J.; Griebel, J.J.; Hill, L.; Glass, R.S.; Char, K.; Pyun, J. Improving the Charge Conductance of Elemental Sulfur via Tandem Inverse Vulcanization and Electropolymerization. ACS Macro Lett. 2015, 4, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, A.G.; Griebel, J.J.; Park, J.; Kim, K.R.; Chung, W.J.; Oleshko, V.P.; Kim, J.; Kim, E.T.; Glass, R.S.; Soles, C.L.; et al. Inverse Vulcanization of Elemental Sulfur to Prepare Polymeric Electrode Materials for Li–S Batteries. ACS Macro Lett. 2014, 3, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Griebel, J.J.; Dirlam, P.T.; Nguyen, N.A.; Glass, R.S.; Mackay, M.E.; Char, K.; Pyun, J. Inverse vulcanization of elemental sulfur and styrene for polymeric cathodes in Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2016, 55, 107–116. [Google Scholar] [CrossRef]
- Pyun, J.; Carrozza, C.F.; Silvano, S.; Boggioni, L.; Losio, S.; Alberto de Angelis, R.; Parker, W.O., Jr. Nuclear magnetic resonance structural characterization of sulfur-derived copolymers from inverse vulcanization. Part 1: Styrene. J. Polym. Sci. 2022, 60, 3471–3477. [Google Scholar] [CrossRef]
- Onose, Y.; Ito, Y.; Kuwabara, J.; Kanbara, T. Tracking side reactions of the inverse vulcanization process and developing monomer selection guidelines. Polym. Chem. 2022, 13, 5486–5493. [Google Scholar] [CrossRef]
- Bischoff, D.J.; Lee, T.; Kang, K.-S.; Molineux, J.; Parker, W.O., Jr.; Pyun, J.; Mackay, M.E. Unraveling the rheology of inverse vulcanized polymers. Nat. Commun. 2023, 14, 7553. [Google Scholar] [CrossRef]
- Tedjini, R.; Viveiros, R.; Casimiro, T.; Bonifácio, V.D.B. Iron-free mechanochemical limonene inverse vulcanization. RSC Mechanochem. 2024, 1, 176–180. [Google Scholar] [CrossRef]
- Orhan, R.; Aydoğmuş, E. Synthesis and Characterization of Limonene-Based Sulfur Polymer. Eur. J. Sci. Technol. 2021, 28, 1517–1520. [Google Scholar] [CrossRef]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.H.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.L.; Chalker, J.M. Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil. Ang. Chem. Int. Ed. 2016, 55, 1714–1718. [Google Scholar] [CrossRef]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.H.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T.; et al. Crosslinker Copolymerization for Property Control in Inverse Vulcanization. Chem.—Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef]
- Giansanti, L.; Aleandri, S.; Altieri, B.; Caretti, F.; Mancini, G.; Morosetti, S.; Ventura, S.; Pérez-Fernández, V.; Gentil, A. Liquid chromatography/mass spectrometry identification of intermediates and vulcanization products by using squalene as vulcanization model compound. Rapid Commun. Mass Spectrom. 2016, 30, 1339–1348. [Google Scholar] [CrossRef]
- Wang, D.; Tang, Z.; Fang, S.; Wu, S.; Zeng, H.; Wang, A.; Guo, B. The use of inverse vulcanised polysulfide as an intelligent interfacial modifier in rubber/carbon black composites. Carbon 2021, 184, 409–417. [Google Scholar] [CrossRef]
- Kuwabara, J.; Oi, K.; Watanabe, M.M.; Fukuda, T.; Kanbara, T. Algae-Inspired, Sulfur-Based Polymer with Infrared Transmission and Elastic Function. ACS Appl. Polym. Mater. 2020, 2, 5173–5178. [Google Scholar] [CrossRef]
- Sahu, T.S.; Choi, S.; Jaumaux, P.; Zhang, J.; Wang, C.; Zhou, D.; Wang, G. Squalene-derived sulfur-rich copolymer@ 3D graphene-carbon nanotube network cathode for high-performance lithium-sulfur batteries. Polyhedron 2019, 162, 147–154. [Google Scholar] [CrossRef]
- Gomez, I.; Leonet, O.; Blazquez, J.A.; Mecerreyes, D. Inverse Vulcanization of Sulfur using Natural Dienes as Sustainable Materials for Lithium–Sulfur Batteries. ChemSusChem 2016, 9, 3419–3425. [Google Scholar] [CrossRef]
- Dale, J.J.; Hanna, V.; Hasell, T. Manipulating Inverse Vulcanization Comonomers to Generate High-Tensile-Strain Polymers. ACS Appl. Polym. Mater. 2023, 5, 6761–6765. [Google Scholar] [CrossRef]
- Ghumman, A.S.M.; Shamsuddin, R.; Sabir, R.; Waheed, A.; Sami, A.; Almohamadid, H. Synthesis and performance evaluation of slow-release fertilizers produced from inverse vulcanized copolymers obtained from industrial waste. RSC Adv. 2023, 13, 7867–7876. [Google Scholar] [CrossRef]
- Smith, A.D.; Tennyson, A.G.; Smith, R.C. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustain. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Smith, A.D.; McMillen, C.D.; Smith, R.C.; Tennyson, A.G. Copolymers by Inverse Vulcanization of Sulfur with Pure or Technical-Grade Unsaturated Fatty Acids. J. Polym. Sci. 2020, 58, 438–445. [Google Scholar] [CrossRef]
- Shen, H.; Zheng, B.; Zhang, H. A Decade Development of Inverse Vulcanization Towards Green and Sustainable Practices. Polym. Rev. 2024, 64, 1211–1266. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Klaic, R.; Guimarães, G.G.F.; Ribeiro, C. Sulfur fertilizer based on inverse vulcanization process with soybean oil. Polym. Degrad. Stab. 2019, 162, 102–105. [Google Scholar] [CrossRef]
- Tikoalu, A.D.; Lundquist, N.A.; Chalker, J.M. Mercury Sorbents Made By Inverse Vulcanization of Sustainable Triglycerides: The Plant Oil Structure Influences the Rate of Mercury Removal from Water. Adv. Sustain. Syst. 2020, 4, 1900111. [Google Scholar] [CrossRef]
- Nayeem, A.; Ali, M.F.; Shariffuddin, J.H. Polysulfide Synthesis Using Waste Cooking Palm Oil via Inverse Vulcanization. Chem. Eng. Technol. 2022, 45, 971–978. [Google Scholar] [CrossRef]
- Ye, H.; Sun, J.; Zhang, S.; Lin, H.; Zhang, T.; Yao, Q.; Lee, J.Y. Stepwise Electrocatalysis as a Strategy against Polysulfide Shuttling in Li–S Batteries. ACS Nano 2019, 13, 14208–14216. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Feng, W. Recent Advances in Applying Vulcanization/Inverse Vulcanization Methods to Achieve High-Performance Sulfur-Containing Polymer Cathode Materials for Li–S Batteries. Small Methods 2018, 2, 1800156. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Z.; Zhang, W.; Deng, N.; Liu, J.; Zhao, F. Inverse Vulcanization of a Natural Monoene with Sulfur as Sustainable Electrochemically Active Materials for Lithium-Sulfur Batteries. Molecules 2021, 26, 7039. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-Sulfur Batteries: Advances and Trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, F.; Deng, X.; Huang, Y.; Li, Y.; Zhao, C.; Ren, W.; Zou, C.; Li, X.; Wang, M.; et al. Novel Sulfur-Containing Polymeric Cathode Material Prepared via an Inverse Vulcanization Method for Advanced Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2022, 5, 7617–7626. [Google Scholar] [CrossRef]
- Choudhury, S.; Srimuk, P.; Raju, K.; Tolosa, A.; Fleischmann, S.; Zeiger, M.; Ozoemena, K.I.; Borchardt, L.; Presser, V. Carbon onion/sulfur hybrid cathodes via inverse vulcanization for lithium–sulfur batteries. Sustain. Energy Fuels 2018, 2, 133–146. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 173–177. [Google Scholar] [CrossRef]
- Yeşilot, S.; Küçükköylü, S.; Demirb, E.; Demir-Cakan, R. Phosphazene based star-branched polymeric cathode materials via inverse vulcanization of sulfur for lithium–sulfur batteries. Polym. Chem. 2020, 11, 4124–4132. [Google Scholar] [CrossRef]
- Yeşilot, S.; Küçükköylü, S.; Mutlu, T.; Demir, E.; Demir-Cakan, R. Highly sulfur-rich polymeric cathode materials via inverse vulcanization of sulfur for lithium–sulfur batteries. Mater. Chem. Phys. 2022, 285, 126168. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.-J.; van der Laan, J.; Nguyen, N.A.; et al. New Infrared Transmitting Material via Inverse Vulcanization of Elemental Sulfur to Prepare High Refractive Index Polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Norwood, R.A.; Pyun, J. Infrared plastic optics and photonic devices using chalcogenide hybrid inorganic/organic polymers via inverse vulcanization of elemental sulfur. Prog. Polym. Sci. 2024, 156, 101865. [Google Scholar] [CrossRef]
- Tavella, C.; Lova, P.; Marsotto, M.; Luciano, G.; Patrini, M.; Stagnaro, P.; Comoretto, D. High Refractive Index Inverse Vulcanized Polymers for Organic Photonic Crystals. Crystals 2020, 10, 154. [Google Scholar] [CrossRef]
- Kleine, T.S.; Nguyen, N.A.; Anderson, L.E.; Namnabat, S.; LaVilla, E.A.; Showghi, S.A.; Dirlam, P.T.; Arrington, C.A.; Manchester, M.S.; Schwiegerling, J.; et al. High Refractive Index Copolymers with Improved Thermomechanical Properties via the Inverse Vulcanization of Sulfur and 1,3,5-Triisopropenylbenzene. ACS Macro Lett. 2016, 5, 1152–1156. [Google Scholar] [CrossRef]
- Boyd, D.A.; Nguyen, V.Q.; McClain, C.C.; Kung, F.H.; Baker, C.C.; Myers, J.D.; Hunt, M.P.; Kim, W.; Sanghera, J.S. Optical Properties of a Sulfur-Rich Organically Modified Chalcogenide Polymer Synthesized via Inverse Vulcanization and Containing an Organometallic Comonomer. ACS Macro Lett. 2019, 8, 113–116. [Google Scholar] [CrossRef]
- Müller, F.G.; Lisboa, L.S.; Chalker, J.M. Inverse Vulcanized Polymers for Sustainable Metal Remediation. Adv. Sustain. Syst. 2023, 7, 2300010. [Google Scholar] [CrossRef]
- Nayeem, A.; Ali, M.F.; Shariffuddin, J.H. The recent development of inverse vulcanized polysulfide as an alternative adsorbent for heavy metal removal in wastewater. Environ. Res. 2023, 216, 114306. [Google Scholar] [CrossRef]
- Pan, Q.; Hong, Q.; Fan, Y.; Sun, X.; Huang, W.; Yan, N.; Qu, Z.; Xu, H. Efficient selective uptake of mercury ions using inverse vulcanization-synthesized sulfur-rich adsorbents. Sep. Purif. Technol. 2024, 333, 125917. [Google Scholar] [CrossRef]
- Lyu, S.; Abidin, Z.Z.; Yaw, T.C.S.; Resul, M.F.M.G. Inverse vulcanization induced oxygen modified porous polysulfides for efficient sorption of heavy metals. Environ. Sci. Pollut. Res. 2024, 31, 16940–16957. [Google Scholar] [CrossRef]
- Parker, D.J.; Jones, H.A.; Petcher, S.; Cervini, L.; Griffin, J.M.; Akhtar, R.; Hasell, T. Low cost and renewable sulfur-polymers by inverse vulcanisation, and their potential for mercury capture. J. Mater. Chem. A 2017, 5, 11682–11692. [Google Scholar] [CrossRef]
- Cai, D.; Dale, J.J.; Petcher, S.; Wu, X.; Hasell, T. Investigating the Effect of UV Irradiation and TiO2 Addition on Heavy Metal Adsorption by Inverse Vulcanized Sulfur Polymers. Chem. Eur. J. 2024, e202402194. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Chalker, J.M. Confining a spent lead sorbent in a polymer made by inverse vulcanization prevents leaching. Sustain. Mater. Technol. 2020, 26, e00222. [Google Scholar] [CrossRef]
- Lyu, S.; Abidin, Z.Z.; Yaw, T.C.S.; Resul, M.F.M.G. Synthesis of surface-modified porous polysulfides from soybean oil by inverse vulcanization and its sorption behavior for Pb(II), Cu(II), and Cr(III). Environ. Sci. Pollut. Res. 2024, 31, 29264–29279. [Google Scholar] [CrossRef]
- Berk, H.; Balci, B.; Ertan, S.; Kaya, M.; Cihaner, A. Functionalized polysulfide copolymers with 4-vinylpyridine via inverse vulcanization. Mater. Today Commum. 2019, 19, 336–341. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Yan, P.; Petcher, S.; Hasell, T. Inverse vulcanization below the melting point of sulfur. Mater. Chem. Front. 2020, 4, 669–675. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Tonkin, S.J.; Chalker, J.M.; Schiller, T.L.; Hasell, T. Stretchable and Durable Inverse Vulcanized Polymers with Chemical and Thermal Recycling. Chem. Mater. 2022, 34, 1167–1178. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Combining Elemental Sulfur with Polybenzoxazines via Inverse Vulcanization. Macromolecules 2016, 49, 767–773. [Google Scholar] [CrossRef]
- Carothers, K.; Lee, K.M.; McConney, M.E.; Stevenson, P.R.; Godman, N.P. Inverse Vulcanization of Vinyl-polycyclic Aromatic Hydrocarbon Monomers and Dynamic Covalent Polymerization with Liquid Crystalline Monomers. ACS Appl. Polym. Mater. 2024, 6, 11118–11126. [Google Scholar] [CrossRef]
- Dodd, L.J.; Lima, C.; Costa-Milan, D.; Neale, A.R.; Saunders, B.; Zhang, B.; Sarua, A.; Goodacre, R.; Hardwick, L.J.; Kuballe, M.; et al. Raman analysis of inverse vulcanised polymers. Polym. Chem. 2023, 14, 1369–1386. [Google Scholar] [CrossRef]
- Anyszka, R.; Kozanecki, M.; Czaderna, A.; Olejniczak, M.; Sielski, J.; Imiela, M.; Wręczycki, J.; Pietrzak, D.; Gozdek, T.; Okraska, M.; et al. Inverse vulcanization of sulfur with vinylic POSS. J. Sulfur Chem. 2019, 40, 587–597. [Google Scholar] [CrossRef]
- Wadi, V.S.; Jena, K.K.; Halique, K.; Rožič, B.; Cmok, L.; Tzitzios, V.; Alhassan, S.M. Scalable High Refractive Index polystyrene-sulfur nanocomposites via in situ inverse vulcanization. Sci. Rep. 2020, 10, 14924. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Zhang, Y.; Chen, Y.; Wang, L.; Zan, X.; Zhang, L. Density-Adjustable Bio-Based Polysulfide Composite Prepared by Inverse Vulcanization and Bio-Based Fillers. Polymers 2020, 12, 2127. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Si, G.; Tan, C. Self-Healing and Recyclable Vulcanized Polyisoprene Based on a Sulfur-Rich Copolymer Cross-Linking Agent Derived from Inverse Vulcanization. ACS Sustain. Chem. Eng. 2024, 12, 2212–2224. [Google Scholar] [CrossRef]
- Scheiger, J.M.; Hoffmann, M.; Falkenstein, P.; Wang, Z.; Rutschmann, M.; Scheiger, V.W.; Grimm, A.; Urbschat, K.; Sengpiel, T.; Matysik, J.; et al. Inverse Vulcanization of Norbornenylsilanes: Soluble Polymers with Controllable Molecular Properties via Siloxane Bonds. Angew. Chem. Int. Ed. 2022, 61, e202114896. [Google Scholar] [CrossRef] [PubMed]
- Diniz, V.; Bear, J.C.; Rath, S.; Crick, C.R. UV-stable photoactive superhydrophobic coatings utilizing “inverse vulcanization” sulfur polymers. Surf. Interfaces 2024, 51, 104691. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Xu, Z.; Sun, H.; Zheng, Y.; Peng, L.; Liu, Z.; Gao, C.; Gao, M. Solution processible hyperbranched inverse-vulcanized polymers as new cathode materials in Li–S batteries. Polym. Chem. 2015, 6, 973–982. [Google Scholar] [CrossRef]
- Ghumman, A.S.M.; Shamsuddin, R.; Nasef, M.M.; Yahya, W.Z.N.; Abbasi, A.; Almohamadi, H. Sulfur enriched slow-release coated urea produced from inverse vulcanized copolymer. Sci. Total Environ. 2022, 846, 157417. [Google Scholar] [CrossRef]
- Wadi, V.K.S.; Jena, K.K.; Khawaja, S.Z.; Yannakopoulou, K.; Fardis, M.; Mitrikas, G.; Karagianni, M.; Papavassiliou, G.; Alhassan, S.M. NMR and EPR Structural Analysis and Stability Study of Inverse Vulcanized Sulfur Copolymers. ACS Omega 2018, 3, 3330–3339. [Google Scholar] [CrossRef]
- Park, S.; Chung, M.; Lamprou, A.; Seidel, K.; Song, S.; Schade, C.; Lim, J.; Char, K. High strength, epoxy cross-linked high sulfur content polymers from one-step reactive compatibilization inverse vulcanization. Chem. Sci. 2022, 13, 566–572. [Google Scholar] [CrossRef]
- Grimm, A.P.; Plank, M.; Stihl, A.; Schmitt, C.W.; Voll, D.; Schacher, F.H.; Lahann, J.; Theato, P. Inverse Vulcanization of Activated Norbornenyl Esters—A Versatile Platform for Functional Sulfur Polymers. Angew. Chem. 2024, 136, e202411010. [Google Scholar] [CrossRef]
- Smith, J.A.; Wu, X.; Berry, N.G.; Hasell, T. High sulfur content polymers: The effect of crosslinker structure on inverse vulcanization. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1777–1781. [Google Scholar] [CrossRef]
- Scheiger, J.M.; Direksilp, C.; Falkenstein, P.; Welle, A.; Koenig, M.; Heissler, S.; Matysik, J.; Levkin, P.A.; Theato, P. Inverse Vulcanization of Styrylethyltrimethoxysilane–Coated Surfaces, Particles, and Crosslinked Materials. Angew. Chem. 2020, 132, 18798–18804. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, L.; Wang, X.; Lin, P.; Yang, Z.; Bai, T.; Shen, H.; Zhang, H. Structural evolution during inverse vulcanization. Nat. Commun. 2024, 15, 5507. [Google Scholar] [CrossRef]
- Grimm, A.; Scheiger, J.M.; Roesky, P.W.; Theato, P. Inverse vulcanization of trimethoxyvinylsilane particles. Polym. Chem. 2022, 13, 5852–5860. [Google Scholar] [CrossRef]
- Khawaja, S.Z.; Kumar, S.V.; Jena, K.K.; Alhassan, S.M. Flexible sulfur film from inverse vulcanization technique. Mater. Lett. 2017, 203, 58–61. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Tikoalu, A.D.; Worthington, M.J.H.; Shapter, R.; Tonkin, S.J.; Stojcevski, F.; Mann, M.; Gibson, C.T.; Gascooke, J.R.; Karton, A.; et al. Reactive Compression Molding Post-Inverse Vulcanization: A Method to Assemble, Recycle, and Repurpose Sulfur Polymers and Composites. Chem. Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef]
- Tonkin, S.J.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Karton, A.; Hasell, T.; Chalker, J.M. Chemically induced repair, adhesion, and recycling of polymers made by inverse vulcanization. Chem. Sci. 2020, 11, 5537–5546. [Google Scholar] [CrossRef]
- Mandal, I.; Kilbinger, A.F.M. Mechanistic Insights into the cis-Selective Catalytic Ring-Opening Metathesis Polymerization. J. Am. Chem. Soc. 2023, 146, 32072–32079. [Google Scholar] [CrossRef]
- Payne, M.E.; Grayson, S.M. Characterization of Synthetic Polymers via Matrix Assisted Laser Desorption Ionization Time of Flight (MALDI-TOF) Mass Spectrometry. J. Vis. Exp. 2018, 136, e57174. [Google Scholar] [CrossRef]
- Nielen, M.W.F. Maldi time-of-flight mass spectrometry of synthetic polymers. Mass Spectrom. Rev. 1999, 18, 309–344. [Google Scholar] [CrossRef]
- Charles, L. MALDI of synthetic polymers with labile end-groups. Mass Spectrom. Rev. 2014, 33, 523–543. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Kim, D.-H.; Shin, D.J.; Oh, Y.S.; Lee, S.J.; Lee, J.Y.; Choi, Y.-J.; Lee, S.H.; Lee, K.-S.; Kim, Y.S.; et al. Recent developments in pre-treatment and analytical techniques for synthetic polymers by MALDI-TOF mass spectrometry. Anal. Methods 2020, 12, 5767–5800. [Google Scholar] [CrossRef]
- Abbasi, A.; Nasef, M.M.; Yahya, W.Z.N. Sulfur-based polymers by inverse vulcanization: A novel path to foster green chemistry. Green Mater. 2020, 8, 172–180. [Google Scholar] [CrossRef]
- Wang, M.; Huang, W.J.; Kondev, F.G.; Audi, G.; Naimi, S. The AME 2020 atomic mass evaluation(II). Tables, graphs and references. Chin. Phys. C 2021, 45, 030003. [Google Scholar] [CrossRef]
- Frenkel, M.; Heller-Kallai, L. Aromatization of limonene—A geochemical model. Org. Geochem. 1977, 1, 3–5. [Google Scholar] [CrossRef]
- Weitkamp, A.W. The Action of Sulfur on Terpenes. The Limonene Sulfides. J. Am. Chem. Soc. 1959, 81, 3430–3434. [Google Scholar] [CrossRef]
- Tarasova, N.; Zanin, A.; Krivoborodov, E.; Toropygin, I.; Pascal, E.; Mezhuev, Y. The New Approach to the Preparation of Polyacrylamide-Based Hydrogels: Initiation of Polymerization of Acrylamide with 1,3-Dimethylimidazolium (Phosphonooxy-)Oligosulphanide under Drying Aqueous Solutions. Polymers 2021, 13, 1806. [Google Scholar] [CrossRef]
- Bahr, U.; Karas, M.; Hillenkamp, F. Analysis of biopolymers by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Z. Anal. Chem. 1994, 348, 783–791. [Google Scholar] [CrossRef]
- Drzeżdżon, J.; Jacewicz, D.; Sielicka, A.; Chmurzyński, L. MALDI-MS for polymer characterization–Recent developments and future prospects. TrAC Trends Anal. Chem. 2019, 115, 121–128. [Google Scholar] [CrossRef]
- Nechaeva, A.; Artyukhov, A.; Luss, A.; Shtilman, M.; Gritskova, I.; Shulgin, A.; Motyakin, M.; Levina, I.; Krivoborodov, E.; Toropygin, I.; et al. Synthesis of Amphiphilic Copolymers of N-Vinyl-2-pyrrolidone and Allyl Glycidyl Ether for Co-Delivery of Doxorubicin and Paclitaxel. Polymers 2022, 14, 1727. [Google Scholar] [CrossRef]
- Tarasova, N.P.; Krivoborodov, E.G.; Mezhuev, Y.O. Nucleophilic activation of the sulfur S8 cyclic form as a green chemistry tool. Russ. Chem. Bull. 2023, 72, 415–424. [Google Scholar] [CrossRef]
- Purohit, V.B.; Pięta, M.; Pietrasik, J.; Plummer, C.M. Recent advances in the ring-opening polymerization of sulfur-containing monomers. Polym. Chem. 2022, 13, 4858–4878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).