The Clear Choice: Developing Transparent Cork for Next-Generation Sustainable Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Starting Material
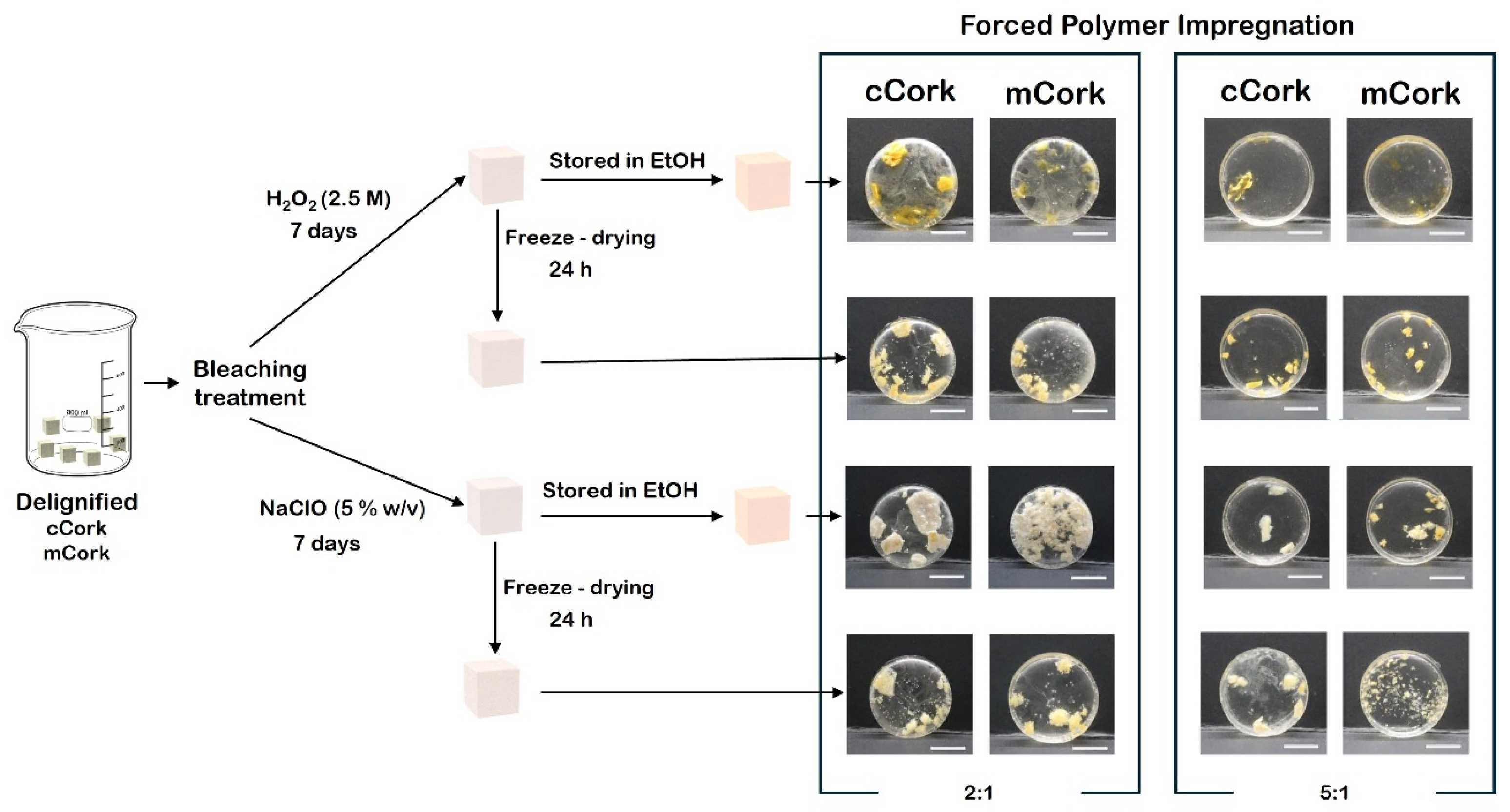
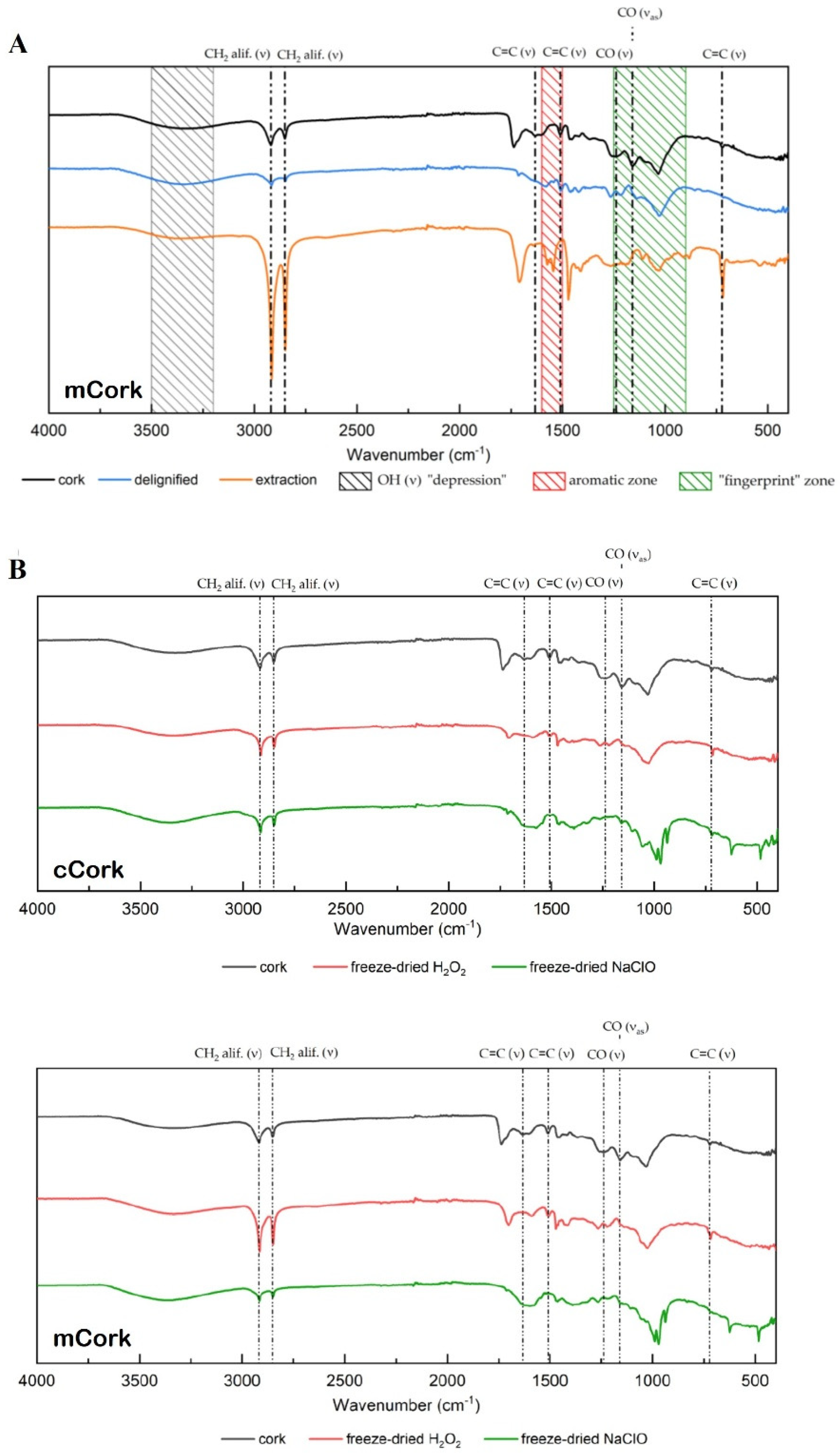
2.2. Delignification Process
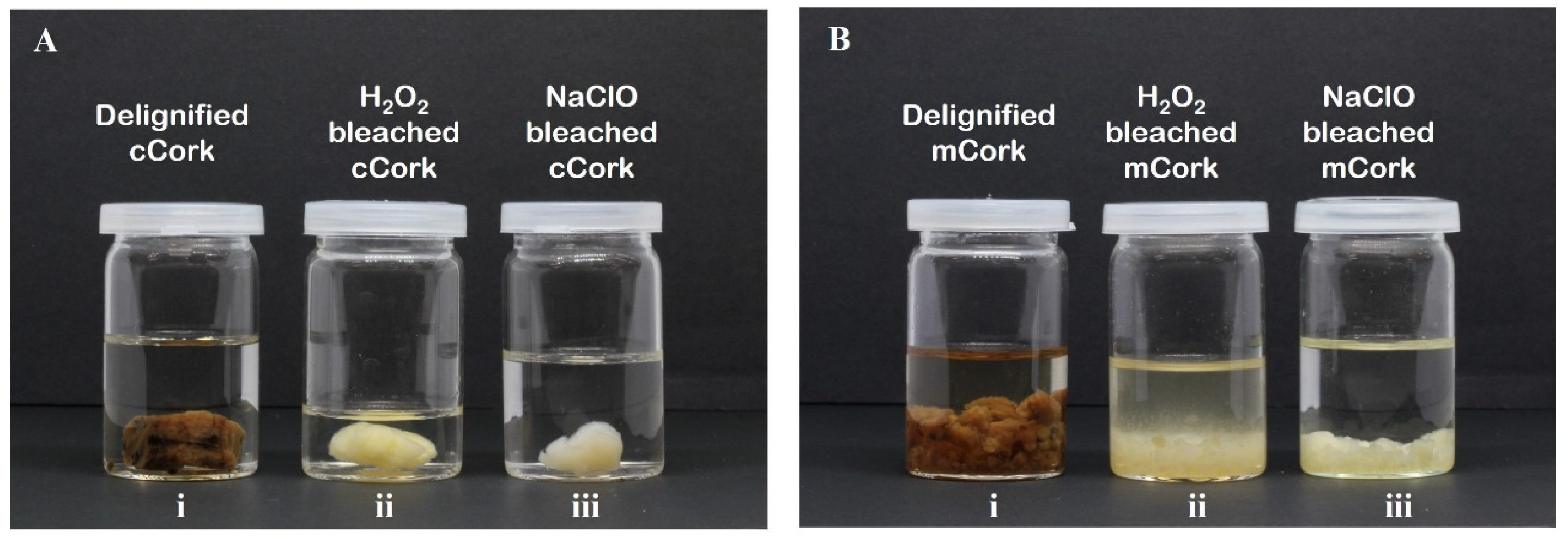
2.3. Bleaching Treatment
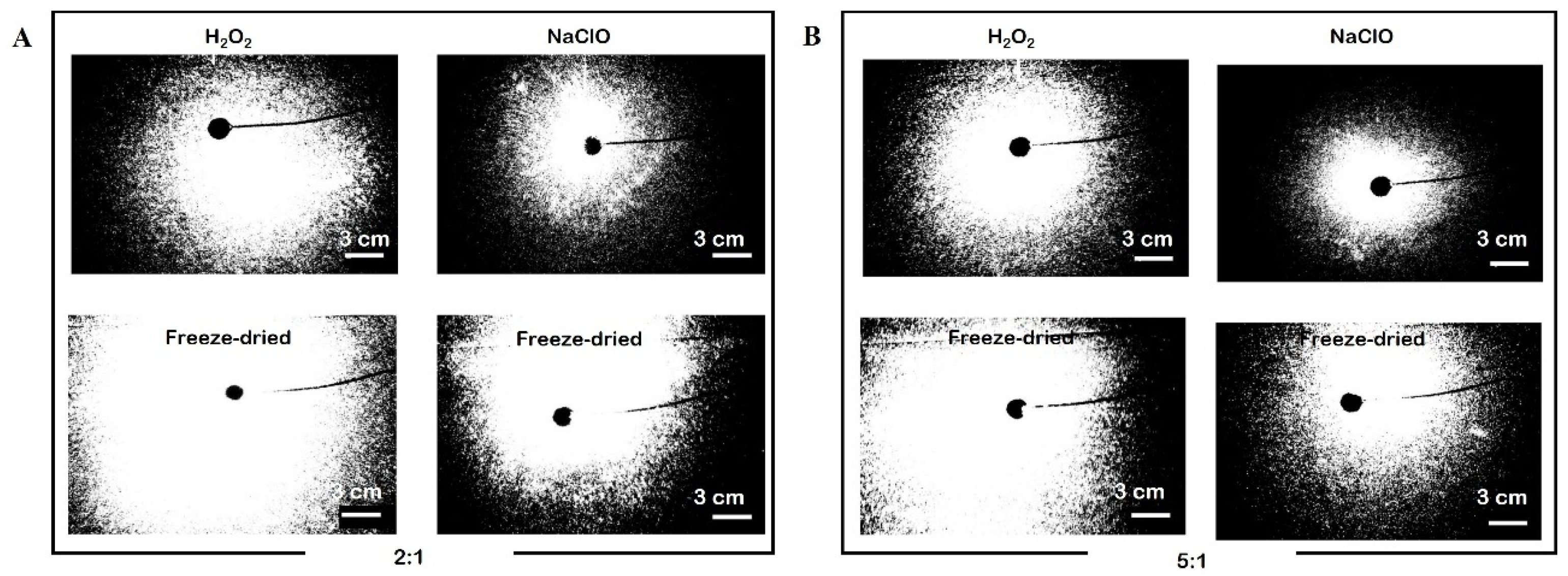
2.4. Forced Polymer Impregnation
2.5. Characterization Techniques
3. Results and Discussion
3.1. Morphology Analysis
3.2. Transmittance Measurements
3.3. Small Angle Light Scattering
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gibson, L.J. Cork: Structure, properties, applications. Arnoldia 2016, 74, 23–27. [Google Scholar] [CrossRef]
- Pullar, R.C.; Novais, R.M. Ecoceramics. Mater. Today 2017, 20, 45–46. [Google Scholar] [CrossRef]
- AmorimCorkComposites. Cork Oak Forest. Available online: https://amorimcorkcomposites.com/en-us/why-cork/facts-and-curiosities/about-oak-forest/ (accessed on 9 June 2023).
- Cortiça, A.P.d. Boletim Estatístico 2020. 2020. Available online: https://apcor.pt/pt/anuario (accessed on 9 June 2023).
- Gil, L. New Cork-Based Materials and Applications. Materials 2015, 8, 625–637. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, H.; Yoon, B.; Michaud, V.; Nam, J.-D.; Suhr, J. All natural cork composites with suberin-based polyester and lignocellulosic residue. Ind. Crops Prod. 2017, 109, 843–849. [Google Scholar] [CrossRef]
- Marques, A.V.; Rencoret, J.; Gutiérrez, A.; Río, J.C.d.; Pereira, H. Ferulates and lignin structural composition in cork. Holzforschung 2016, 70, 275–289. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork from Quercus suber L. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Gil, L.; Moiteiro, C. Cork. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Pereira, H.; Emília Rosa, M.; Fortes, M.A. The cellular structure of cork from Quercus suber L. IAWA J. 1987, 8, 213–218. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Şen, A.; Miranda, I.; Pereira, H. Temperature-induced structural and chemical changes in cork from Quercus cerris. Ind. Crops Prod. 2012, 37, 508–513. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Silva, E.A.A.; do Carmo, S.N.; Steffens, F.; Souto, A.P.G.d.V. Functionalization of natural cork composite with microcapsules after plasma treatment. Adv. Mater. Sci. Eng. 2014, 2014, 685829. [Google Scholar] [CrossRef]
- Leal, A.I.; Correia, R.A.; Palmeirim, J.M.; Bugalho, M.N. Is research supporting sustainable management in a changing world? Insights from a mediterranean silvopastoral system. Agrofor. Syst. 2018, 93, 355–368. [Google Scholar] [CrossRef]
- Zhang, J.; Koubaa, A.; Tao, Y.; Li, P.; Xing, D. The emerging development of transparent wood: Materials, characteristics, and applications. Curr. For. Rep. 2022, 8, 333–345. [Google Scholar] [CrossRef]
- Li, Y.; Vasileva, E.; Sychugov, I.; Popov, S.; Berglund, L. Optically transparent wood: Recent progress, opportunities, and challenges. Adv. Opt. Mater. 2018, 6, 1800059. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Müller, U.; Rätzsch, M.; Schwanninger, M.; Steiner, M.; Zöbl, H. Yellowing and IR-changes of spruce wood as result of UV-irradiation. J. Photochem. Photobiol. B Biol. 2003, 69, 97–105. [Google Scholar] [CrossRef]
- Zhu, M.; Song, J.; Li, T.; Gong, A.; Wang, Y.; Dai, J.; Yao, Y.; Luo, W.; Henderson, D.; Hu, L. Highly anisotropic, highly transparent wood composites. Adv. Mater. 2016, 28, 5181–5187. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, Q.; Yu, S.; Yan, M.; Berglund, L. Optically transparent wood from a nanoporous cellulosic template: Combining functional and structural performance. Biomacromolecules 2016, 17, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, T.; Davis, C.S.; Yao, Y.; Dai, J.; Wang, Y.; AlQatari, F.; Gilman, J.W.; Hu, L. Transparent and haze wood composites for highly efficient broadband light management in solar cells. Nano Energy 2016, 26, 332–339. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Júnior, S.V.; Jameel, H.; Chang, H.-M.; Narron, R.; Jiang, X.; Colodette, J.L. Chemical study of kraft lignin during alkaline delignification of E. urophylla x E. grandis hybrid in low and high residual effective alkali. ACS Sustain. Chem. Eng. 2019, 7, 10274–10282. [Google Scholar] [CrossRef]
- Kumar, A.; Jyske, T.; Petrič, M. Delignified wood from understanding the hierarchically aligned cellulosic structures to creating novel functional materials: A review. Adv. Sustain. Syst. 2021, 5, 2000251. [Google Scholar] [CrossRef]
- Fearon, O.; Kuitunen, S.; Ruuttunen, K.; Alopaeus, V.; Vuorinen, T. Detailed modeling of kraft pulping chemistry. Delignification. Ind. Eng. Chem. Res. 2020, 59, 12977–12985. [Google Scholar] [CrossRef]
- Bonhivers, J.-C.; Stuart, P.R. 25—Applications of process integration methodologies in the pulp and paper industry. In Handbook of Process Integration (PI); Klemeš, J.J., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 765–798. [Google Scholar]
- Jardim, J.M.; Hart, P.W.; Lucia, L.A.; Jameel, H.; Chang, H.-m. The effect of the kraft pulping process, wood species, and pH on lignin recovery from black liquor. Fibers 2022, 10, 16. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmér, A.; Tomani, P.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M.-M. From waste to wealth: From kraft lignin to free-standing supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Mariani, A.; Malucelli, G. Transparent wood-based materials: Current state-of-the-art and future perspectives. Materials 2022, 15, 9069. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E. 1.5. Reflection and refraction of a plane wave. In Principles of Optics, 7th ed.; Cambridge University Press: Cambridge, UK, 1999; pp. 36–37. [Google Scholar]
- von Hagens, G.; Tiedemann, K.; Kriz, W. The current potential of plastination. Anat. Embryol. 1987, 175, 411–421. [Google Scholar] [CrossRef]
- von Hagens, G. Animal and vegetal tissues permanently preserved by synthetic resin impregnation. U.S. Patent US-4205059-A, 14 July 1981. [Google Scholar]
- Chen, H.; Baitenov, A.; Li, Y.; Vasileva, E.; Popov, S.; Sychugov, I.; Yan, M.; Berglund, L. Thickness dependence of optical transmittance of transparent wood: Chemical modification effects. ACS Appl. Mater. Interfaces 2019, 11, 35451–35457. [Google Scholar] [CrossRef]
- Tirpak, A.; Young, R. Accurate Transmission measurements of translucent materials. Photonics Spectra 2008, 42, 66–69. [Google Scholar]
- Stamm, A.J. Wood and Cellulose Science; Ronald Press Company: New York, NY, USA, 1964. [Google Scholar]
- Borrega, M.; Ahvenainen, P.; Serimaa, R.; Gibson, L. Composition and structure of balsa (Ochroma pyramidale) wood. Wood Sci. Technol. 2015, 49, 403–420. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Fu, Q.; Rojas, R.; Yan, M.; Berglund, L. Towards centimeter thick transparent wood through interface manipulation. J. Mater. Chem. A 2018, 6, 1094–1101. [Google Scholar] [CrossRef]
- Fink, S. Transparent Wood—A New Approach in the functional study of wood structure. Holzforschung 1992, 46, 403–408. [Google Scholar] [CrossRef]
- Okahisa, Y.; Yoshida, A.; Miyaguchi, S.; Yano, H. Optically transparent wood–cellulose nanocomposite as a base substrate for flexible organic light-emitting diode displays. Compos. Sci. Technol. 2009, 69, 1958–1961. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Veinot, J.G.C.; Linnros, J.; Berglund, L.; Sychugov, I. Luminescent transparent wood. Adv. Opt. Mater. 2017, 5, 1600834. [Google Scholar] [CrossRef]
- Vasileva, E.; Li, Y.; Sychugov, I.; Mensi, M.; Berglund, L.; Popov, S. Lasing from organic dye molecules embedded in transparent wood. Adv. Opt. Mater. 2017, 5, 1700057. [Google Scholar] [CrossRef]
- Höglund, M.; Johansson, M.; Sychugov, I.; Berglund, L.A. Transparent wood biocomposites by fast UV-curing for reduced light-scattering through wood/thiol–ene interface design. ACS Appl. Mater. Interfaces 2020, 12, 46914–46922. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, M.; Jungstedt, E.; Xu, B.; Sun, L.; Berglund, L. Optically transparent wood substrate for perovskite solar cells. ACS Sustain. Chem. Eng. 2019, 7, 6061–6067. [Google Scholar] [CrossRef]
- Mi, R.; Li, T.; Dalgo, D.; Chen, C.; Kuang, Y.; He, S.; Zhao, X.; Xie, W.; Gan, W.; Zhu, J.; et al. A clear, strong, and thermally insulated transparent wood for energy efficient windows. Adv. Funct. Mater. 2020, 30, 1907511. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Zhu, T.; Chen, Z.; Wu, Y.; Gao, Y. Transparent wood composites fabricated by impregnation of epoxy resin and W-Doped VO2 nanoparticles for application in energy-saving windows. ACS Appl. Mater. Interfaces 2020, 12, 34777–34783. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, C.; Li, T.; He, S.; Gao, J.; Wang, X.; Hu, L. Solar-assisted fabrication of large-scale, patternable transparent wood. Sci. Adv. 2021, 7, eabd7342. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.; He, Y.; Zheng, R. House model with 2–5 cm thick translucent wood walls and its indoor light performance. Eur. J. Wood Wood Prod. 2019, 77, 843–851. [Google Scholar] [CrossRef]
- Wang, X.; Zhan, T.; Liu, Y.; Shi, J.; Pan, B.; Zhang, Y.; Cai, L.; Shi, S.Q. Large-size transparent wood for energy-saving building applications. ChemSusChem 2018, 11, 4086–4093. [Google Scholar] [CrossRef]
- Jia, C.; Chen, C.; Mi, R.; Li, T.; Dai, J.; Yang, Z.; Pei, Y.; He, S.; Bian, H.; Jang, S.-H.; et al. Clear wood toward high-performance building materials. ACS Nano 2019, 13, 9993–10001. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Querciagrossa, L.; Silva, P.E.S.; Gonçalves, F.; Canejo, J.P.; Almeida, P.L.; Godinho, M.H.; Zannoni, C. Reversible water driven chirality inversion in cellulose-based helices isolated from Erodium awns. Soft Matter 2019, 15, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.E.; Pereira, H.; Fortes, M.A. Effect of hot water treatment on the structure and properties of cork. Wood Fiber Sci. 1990, 22, 149–164. [Google Scholar]
- Berlanga-Del Pozo, M.; Gallardo-Guerrero, L.; Gandul-Rojas, B. Influence of alkaline treatment on structural modifications of clorophyll pigments in NaOH-treated table olives preserved without fermentation. Foods 2020, 9, 701. [Google Scholar] [CrossRef]
- Herstedt, L.; Herstedt, M. Chemical bleaching of wood: An investigation into the bleaching of mahogany, walnut, rosewood, padauk, and purpleheart. Stud. Conserv. 2017, 62, 162–172. [Google Scholar] [CrossRef]
- Potucek, F.; Milichovsky, M. Kraft pulp bleaching with hydrogen peroxide and pancreatic acid. Chem. Pap. 2000, 54, 406–411. [Google Scholar]
- Abbott, T.P.; Palmer, D.M.; Gordon, S.H.; Bagby, M.O. Solid state analysis of plant polymers by FTIR. J. Wood Chem. Technol. 1988, 8, 1–28. [Google Scholar] [CrossRef]
- Lopes, M.H.; Neto, C.P.; Barros, A.S.; Rutledge, D.; Delgadillo, I.; Gil, A.M. Quantitation of aliphatic suberin in Quercus suber L. cork by FTIR spectroscopy and solid-state 13C-NMR spectroscopy. Biopolymers 2000, 57, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Debye, P.; Bueche, A.M. Scattering by an inhomogeneous solid. J. Appl. Phys. 1949, 20, 518–525. [Google Scholar] [CrossRef]
- Beaucage, G. 2.14—Combined small-angle scattering for characterization of hierarchically structured polymer systems over nano-to-micron meter: Part II theory. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 399–409. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, P.; Almeida, P.L.; Godinho, M.H.; Almeida, A.P.C. The Clear Choice: Developing Transparent Cork for Next-Generation Sustainable Materials. Macromol 2025, 5, 17. https://doi.org/10.3390/macromol5020017
Gil P, Almeida PL, Godinho MH, Almeida APC. The Clear Choice: Developing Transparent Cork for Next-Generation Sustainable Materials. Macromol. 2025; 5(2):17. https://doi.org/10.3390/macromol5020017
Chicago/Turabian StyleGil, Pedro, Pedro L. Almeida, Maria H. Godinho, and Ana P. C. Almeida. 2025. "The Clear Choice: Developing Transparent Cork for Next-Generation Sustainable Materials" Macromol 5, no. 2: 17. https://doi.org/10.3390/macromol5020017
APA StyleGil, P., Almeida, P. L., Godinho, M. H., & Almeida, A. P. C. (2025). The Clear Choice: Developing Transparent Cork for Next-Generation Sustainable Materials. Macromol, 5(2), 17. https://doi.org/10.3390/macromol5020017









