An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives
Abstract
:1. Introduction
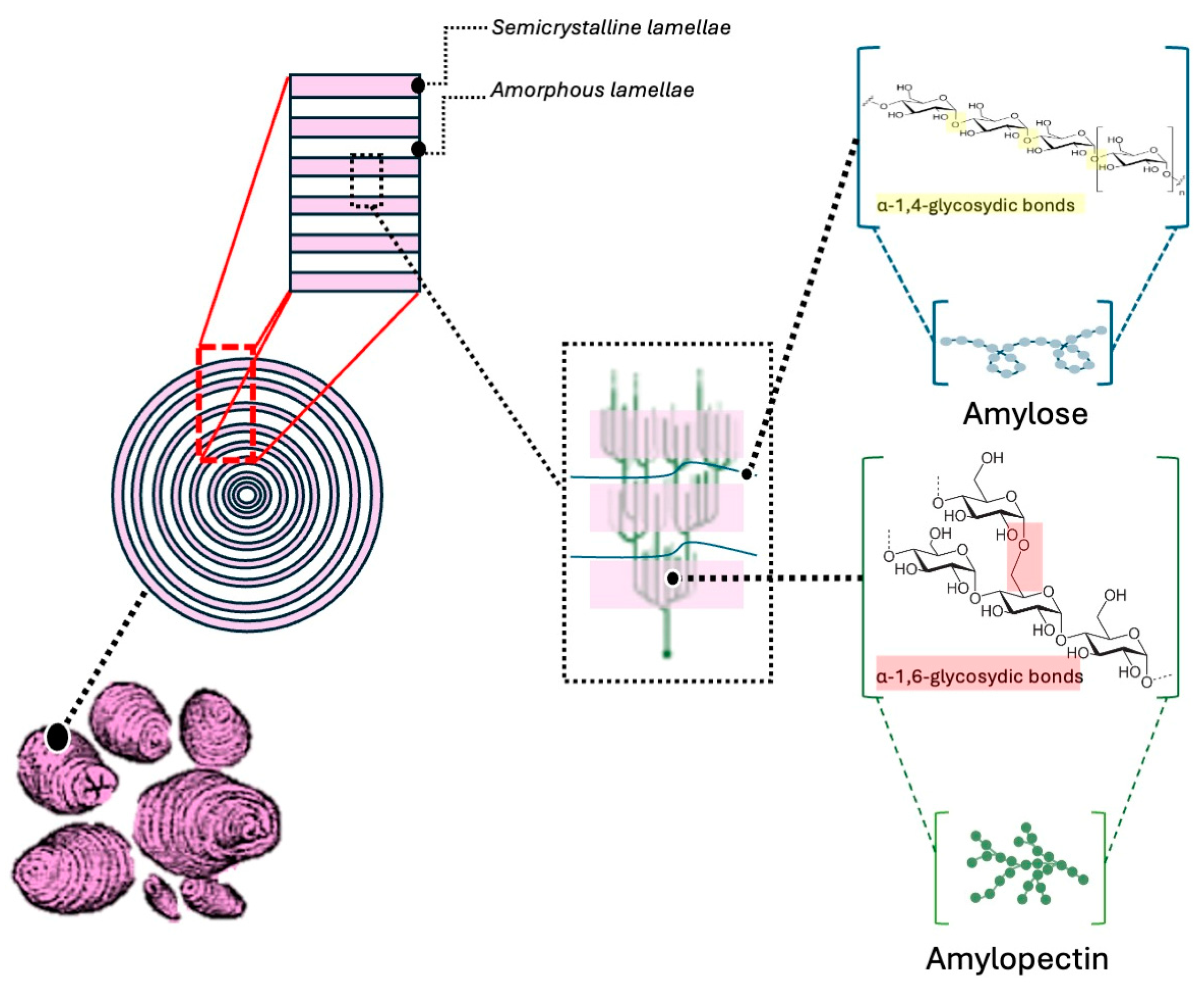
2. Insights into Native Starch Chemistry and Film-Formation Properties
3. Strategies for the Production of Starch-Based Films
| Processes | Advantages | Challenges | Applications | References |
|---|---|---|---|---|
| Extrusion | Continuous; Scalability; Compatibility with additives; Thickness control | Heat sensitivity; Transparency; Homogeneity | Active packaging for meat; Active packaging for bread | [64,65,66] |
| Casting | Simplicity; Homogeneity; Compatibility with additives | Moisture sensitivity; Scalability; Production time | Active packaging for fresh fruits; Active packaging for grapes | [67,68] |
| Electrospinning | Nanometric structure; Morphology control; Compatibility with additives | Precise parameters; Moisture sensitivity | Nanofiber mats; Active packaging | [69,70] |
| Compression molding | Fast process; Good mechanical strength; Compatibility with additives | Moisture sensitivity; Transparency; Heat sensitivity; Homogeneity | Edible fish gelatin film; Active packaging for pork | [71,72] |
4. Advanced Functionalization for Application as a Food Packaging Material
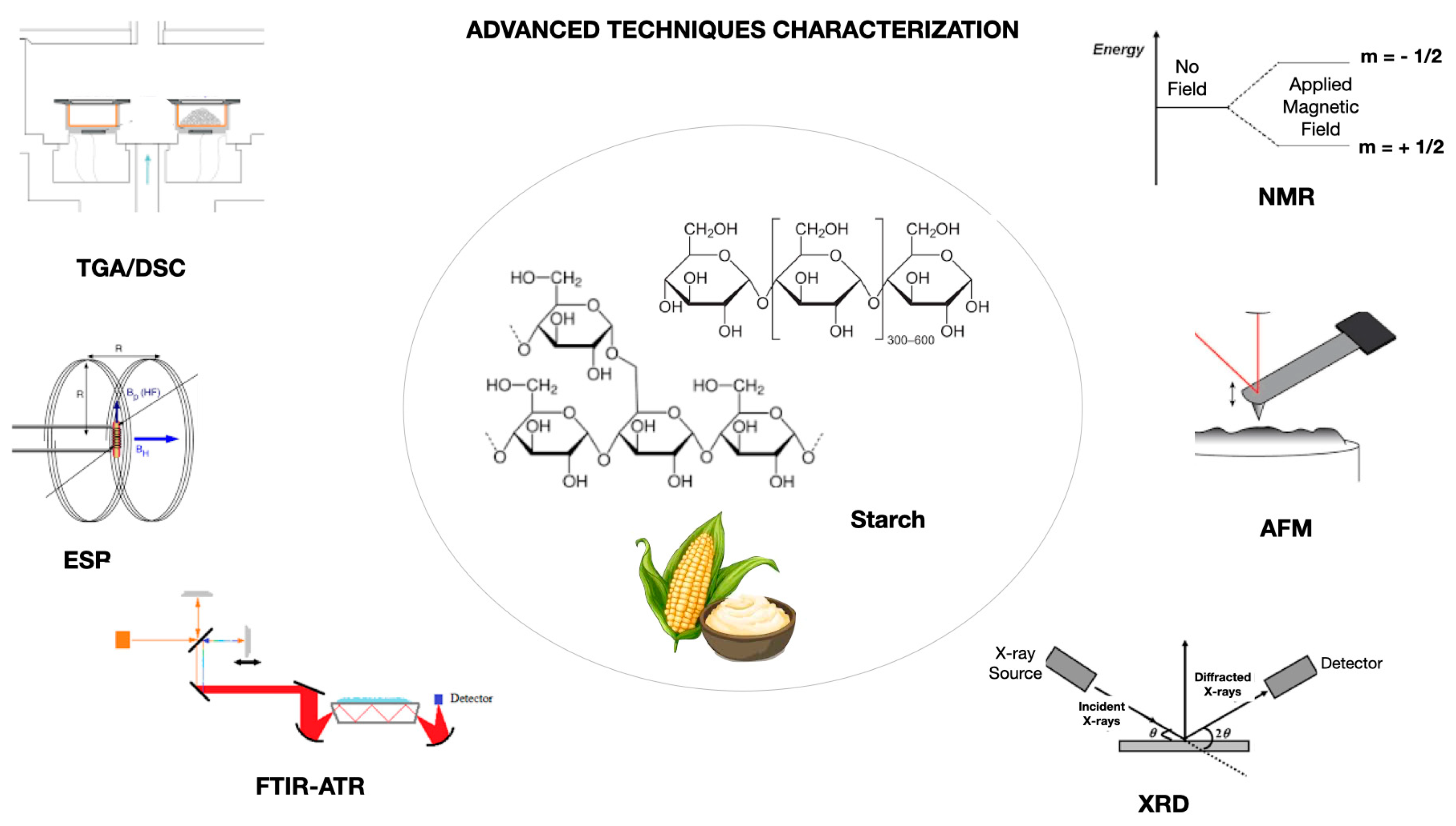
5. Advanced Analytical Techniques for Characterization of Starch-Based Films
5.1. X-Ray Diffraction (XRD)
5.2. Nuclear Magnetic Resonance (NMR)
5.3. Fourier-Transform Infrared Spectrometry (FTIR)
5.4. Thermal Analyses: Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
5.5. Atomic Force Microscopy (AFM)
5.6. Other Analyses
5.7. Monitoring Starch Retrogradation: A Multi-Technique Analytical Approach
5.8. Starch Retrogradation Impact on Practical Applications in Packaging
6. Challenges and Limitations
6.1. Intrinsic Limitations of Starch Films
6.2. Starch for Food Packaging Features and Smart Applications
6.3. Commercial Scalability and Industrial Feasibility
6.4. Scaling up Starch Films: Practical Barriers and Feasibility for Food Packaging
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amar, L.T.D.; Mohanty, K.; Misra, M. Natural Fibers, Biopolymers, and Biocomposites; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; Santos, F.D.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Starch-Based Edible Films and Coatings: An Eco-Friendly Alternative for Food Packaging. In Starches for Food Application; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-conventional starch sources. Curr. Opin. Food Sci. 2021, 39, 93. [Google Scholar] [CrossRef]
- Nagy, E.M.; Coţa, C.; Cioica, N.; Gyorgy, Z.; Todica, M.; Cozar, O. FT-IR investigation of starch based composite reinforced with Miscanthus fibers. AIP Conf. Proc. 2017, 1917, 040009. [Google Scholar] [CrossRef]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953. [Google Scholar] [CrossRef]
- Bof, M.J.; Bordagaray, V.C.; Locaso, D.E.; García, M.A. Chitosan molecular weight effect on starch-composite film properties. Food Hydrocoll 2015, 51, 281. [Google Scholar] [CrossRef]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Nafchi, A.M.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1. [Google Scholar] [CrossRef] [PubMed]
- Bemiller, R.L.; Whistler, J.N. Starch: Chemistry and Technology; Academic Press, Inc.: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Castaño, J.; Rodríguez-Llamazares, S.; Sepúlveda, E.; Giraldo, D.; Bouza, R.; Pozo, C. Morphological and structural changes of starch during processing by melt blending. Starch Staerke 2017, 69, 1600247. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, S.S.; Lim, S.T. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607. [Google Scholar] [CrossRef]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from different botanical sources I: Contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydr. Polym. 2005, 60, 529. [Google Scholar] [CrossRef]
- Young, A.H. Fractionation of starch. Starch Chem. Technol. 1984, 249, 249–283. [Google Scholar]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch Staerke 2016, 68, 1026. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, J.; Duan, Q.; Xie, H.; Dong, X.; Yu, L. Strategies and Methodologies for Improving Toughness of Starch Films. Foods 2024, 13, 4036. [Google Scholar] [CrossRef] [PubMed]
- Jingyi, Y.; Reddy, C.K.; Fan, Z.; Xu, B. Physicochemical and structural properties of starches from non-traditional sources in China. Food Sci. Hum. Wellness 2023, 12, 416. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioproc. Tech. 2012, 5, 2058. [Google Scholar]
- Kupervaser, M.G.; Traffano-Schiffo, M.V.; Dellamea, M.L.; Flores, S.K.; Sosa, C.A. Trends in starch-based edible films and coatings enriched with tropical fruits extracts: A review. Food Hydrocoll. Health 2023, 4, 100138. [Google Scholar] [CrossRef]
- Sissons, M.; Palombieri, S.; Sestili, F.; Lafiandra, D. Impact of Variation in Amylose Content on Durum Wheat cv. Svevo Technological and Starch Properties. Foods 2023, 12, 4112. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zuo, J.; Chen, B.; Fu, Z.; Lin, X.; Wu, J.; Zheng, B.; Lu, X. Structural, properties and digestion in vitro changes of starch subjected to high pressure homogenization: An update review. Int. J. Biol. Macromol. 2024, 282, 137118. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079. [Google Scholar] [CrossRef]
- Morris, V.J. Intense sweeteners for the food industry: An overview. Trends Food Sci. Technol. 1990, 2, 2–6. [Google Scholar] [CrossRef]
- Matignon, A.; Tecante, A. Starch retrogradation: From starch components to cereal products. Food Hydrocoll. 2017, 68, 43. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, J.; Kim, S.K.; Kang, D.H.; Park, H.B.; Shim, J.K. Impact of the Amylose/Amylopectin Ratio of Starch-Based Foams on Foaming Behavior, Mechanical Properties, and Thermal Insulation Performance. ACS Sustain. Chem. Eng. 2023, 11, 2968. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; Santos, F.D.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Starches Food. Appl. Chem. Technol. Health Prop. 2019, 10, 359–420. [Google Scholar]
- Putri, T.R.; Adhitasari, A.; Paramita, V.; Yulianto, M.E.; Ariyanto, H.D. Effect of different starch on the characteristics of edible film as functional packaging in fresh meat or meat products: A review. Mater. Today Proc. 2023, 87, 192–199. [Google Scholar] [CrossRef]
- Ulbrich, M.; Scholz, F.; Braun, B.; Bussert, R.; Flöter, E. High Amylose Corn Starch Gels—Investigation of the Supermolecular Structure. Starch Staerke 2023, 75, 2200138. [Google Scholar] [CrossRef]
- Tian, J.; Qin, L.; Zeng, X.; Ge, P.; Fan, J.; Zhu, Y. The Role of Amylose in Gel Forming of Rice Flour. Foods 2023, 12, 1210. [Google Scholar] [CrossRef]
- Gong, Y.; Xiao, S.; Yao, Z.; Deng, H.; Chen, X.; Yang, T. Factors and modification techniques enhancing starch gel structure and their applications in foods: A review. Food Chem. X 2024, 24, 102045. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. A comprehensive review of the factors influencing the formation of retrograded starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568. [Google Scholar] [CrossRef]
- Huang, S.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. New insight into starch retrogradation: The effect of short-range molecular order in gelatinized starch. Food Hydrocoll. 2021, 120, 106921. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399. [Google Scholar] [CrossRef]
- Chen, L.; Ren, F.; Zhang, Z.; Tong, Q.; Rashed, M.M.A. Effect of pullulan on the short-term and long-term retrogradation of rice starch. Carbohydr. Polym. 2015, 115, 415. [Google Scholar] [CrossRef]
- Li, C.; Hu, Y. Combination of parallel and sequential digestion kinetics reveals the nature of digestive characteristics of short-termretrograded rice starches. Food Hydrocoll. 2020, 108, 106071. [Google Scholar] [CrossRef]
- Galvis, L.; Bertinetto, C.G.; Putaux, J.L.; Montesanti, N.; Vuorinen, T. Crystallite orientation maps in starch granules from polarized Raman spectroscopy (PRS) data. Carbohydr. Polym. 2016, 154, 70. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, X.; Xiao, Y.; Luo, F.; Lin, Q.; Ding, Y. Structural changes of A-, B- and C-type starches of corn, potato and pea as influenced by sonication temperature and their relationships with digestibility. Food Chem. 2021, 358, 129858. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Zhang, B.; Huang, J.; Xie, F.; Wang, D.K.; Jiang, F.; Zhao, S.; Zhu, J. Hydration-induced crystalline transformation of starch polymer under ambient conditions. Int. J. Biol. Macromol. 2017, 103, 152. [Google Scholar] [CrossRef]
- Imberty, A.; Perez, S. A Revisit to the Three-Dimensional Structure of B-Type Starch. Biopolymers 1988, 27, 1205. [Google Scholar] [CrossRef]
- Lourdin, A.B.D.; Putaux, J.-L.; Potocki-Véronèse, G.; Chevigny, C.; Rolland-Sabaté, A. Crystalline structure in starch. Starch Metab. Struct. 2015, 1, 61–90. [Google Scholar]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Dries, D.M.; Gomand, S.V.; Delcour, J.A.; Goderis, B. V-type crystal formation in starch by aqueous ethanol treatment: The effect of amylose degree of polymerization. Food Hydrocoll. 2016, 61, 649. [Google Scholar] [CrossRef]
- Huang, L.; Li, S.; Tan, C.P.; Feng, Y.; Zhang, B.; Fu, X.; Huang, Q. Solid encapsulation of lauric acid into “empty” V-type starch: Structural characteristics and emulsifying properties. Carbohydr. Polym. 2021, 267, 118181. [Google Scholar] [CrossRef]
- Immel, S.; Lichtenthaler, F.W. The hydrophobic topographies of amylose and its blue iodine complex. Starch Staerke 2000, 52, 1. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, H.; He, K.; McClements, D.J.; Jin, Z.; Xu, Z.; Meng, M.; Peng, X.; Chen, L. Recent progress in the hydrophobic modification of starch-based films. Food Hydrocoll. 2024, 151, 109860. [Google Scholar] [CrossRef]
- Itkor, P.; Singh, A.K.; Lee, M.; Boonsiriwit, A.; Lee, Y.S. Effects of starch–citric acid cross-linking on the fibrous composites using waste paper pulp material for eco-friendly packaging. Biomass Convers. Biorefin. 2024, 14, 14693. [Google Scholar] [CrossRef]
- Min, Y.; Yi, J.; Dai, R.; Liu, W.; Chen, H. A novel efficient wet process for preparing cross-linked starch: Impact of urea on cross-linking performance. Carbohydr. Polym. 2023, 320, 121247. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Fu, J.; Huang, J.; Wang, L. Fish collagen mediated alteration of wheat starch thermal properties during multi-species co-fermentation. Int. J. Biol. Macromol. 2025, 295, 139987. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Kour, M.; Kumar, R. Bioplastic films from starch of Colocasia esculenta and its waste: A smart template for sensing applications. Int. J. Biol. Macromol. 2024, 281, 136218. [Google Scholar] [CrossRef]
- Boetje, L.; Lan, X.; van Dijken, J.; Woortman, A.J.J.; Popken, T.; Polhuis, M.; Loos, K. Starch ester film properties: The role of the casting temperature and starch its molecular weight and amylose content. Carbohydr. Polym. 2023, 316, 121043. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Guembe-Garcia, M.; Cerone, V.; Perugini, P.; Alberti, G.; Biesuz, R. DOE-based multi-criteria optimization of starch/gly/CMC films’ composition and preparation procedure by casting deposition. Chemom. Intell. Lab. Syst. 2024, 244, 105044. [Google Scholar] [CrossRef]
- Devi, N.; Shayoraj; Geeta; Shivani; Ahuja, S.; Dubey, S.K.; Sharma, S.; Kumar, S. Antimicrobial biodegradable packaging films from phosphorylated starch: A sustainable solution for plastic waste. Carbohydr. Res. 2025, 550, 109404. [Google Scholar] [CrossRef]
- Parada-Quinayá, C.; Garces-Porras, K.; Flores, E. Development of biobased films incorporated with an antimicrobial agent and reinforced with Stipa obtusa cellulose microfibers, via tape casting. Results Mater. 2024, 24, 100637. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; da Silva, I.B.; Costa, J.D.S.S.; de Oliveira, J.S.; Oliveira, E.L.; Coutinho, M.L.; de Almeida, M.E.F.; Landim, L.B.; da Silva, N.M.C.; de Oliveira, C.P. Bibliometric study and potential applications in the development of starch films with nanocellulose: A perspective from 2019 to 2023. Int. J. Biol. Macromol. 2024, 277, 133828. [Google Scholar] [CrossRef]
- Li, T.; Guan, L.; Zeng, M.; Li, T.; Lei, S.; Tang, X.; Pan, Y.; Li, S.; Zhou, M.; Yuan, X.; et al. Fabrication of corn starch-based composite films functionalized by Rosa roxburghii Tratt fruit pomace via extrusion compression molding for active food packaging. Int. J. Biol. Macromol. 2025, 284, 138091. [Google Scholar] [CrossRef]
- Wu, H.; Li, W.; Liang, Z.; Gan, T.; Hu, H.; Huang, Z.; Qin, Y.; Zhang, Y. Mechanical activation-enhanced metal-organic coordination strategy to fabricate high-performance starch/polyvinyl alcohol films by extrusion blowing. Carbohydr. Polym. 2024, 333, 121982. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.S.M.; Oliveira, G.H.M.; Talabi, S.I.; Lucas, A.A. Production of thermoplastic starch and poly (butylene adipate-co-terephthalate) films assisted by solid-state shear pulverization. Carbohydr. Polym. 2021, 258, 117732. [Google Scholar] [CrossRef] [PubMed]
- Qing, S.; Weng, W.; Dai, Y.; Li, P.; Ren, Z.; Zhang, Y.; Shi, L.; Li, S. Structural characterization of glutaraldehyde crosslinked starch-based nanofibrous film and adsorption improvement for oyster peptide flavor. Int. J. Biol. Macromol. 2024, 277, 133801. [Google Scholar] [CrossRef]
- Santos, F.N.D.; Fonseca, L.M.; Jansen-Alves, C.; Crizel, R.L.; Pires, J.B.; Kroning, I.S.; de Souza, J.F.; Fajardo, A.R.; Lopes, G.V.; Dias, A.R.G.; et al. Antimicrobial activity of geranium (Pelargonium graveolens) essential oil and its encapsulation in carioca bean starch ultrafine fibers by electrospinning. Int. J. Biol. Macromol. 2024, 265, 130953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, D.; Liu, X.; Ma, T.; He, J.; Dong, Q.; Din, Z.U.; Zhou, J.; Chen, L.; Hu, Z.; et al. Improving the hydrophobicity and mechanical properties of starch nanofibrous films by electrospinning and cross-linking for food packaging applications. LWT 2022, 169, 114005. [Google Scholar] [CrossRef]
- Du, C.; Jiang, Y.; Junejo, S.A.; Jia, X.; Zhang, B.; Huang, Q. Metal-anchored oxidized starch-pullulan nanofiber films enhance ethylene adsorption and banana preservation. Int. J. Biol. Macromol. 2024, 282, 137399. [Google Scholar] [CrossRef]
- Shi, M.; Cheng, L.; Hong, Y.; Li, Z.; Li, C.; Ban, X.; Gu, Z. Effect of Xanthan gum on the structural and functional properties of starch films produced by hot compression molding. Ind. Crops Prod. 2024, 220, 119235. [Google Scholar] [CrossRef]
- Yao, Y.; Hsu, Y.I.; Uyama, H. Enhanced physical properties and water resistance of films using modified starch blend with poly(vinyl alcohol). Ind. Crops Prod. 2024, 222, 119870. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Zhang, S.; Hu, C.Y.; Xu, X. Extrusion-blown oxidized starch/poly(butylene adipate-co-terephthalate) biodegradable active films with adequate material properties and antimicrobial activities for chilled pork preservatio. Int. J. Biol. Macromol. 2023, 253, 127408. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, C.; Zhai, X.; Zhang, R.; Zhang, S.; Sun, C.; Wang, W.; Hou, H. Effect of lipids with different physical state on the physicochemical properties of starch/gelatin edible films prepared by extrusion blowing. Int. J. Biol. Macromol. 2021, 185, 1005. [Google Scholar] [CrossRef] [PubMed]
- Khumkomgool, A.; Saneluksana, T.; Harnkarnsujarit, N. Active meat packaging from thermoplastic cassava starch containing sappan and cinnamon herbal extracts via LLDPE blown-film extrusion. Food Packag. Shelf Life 2020, 26, 100557. [Google Scholar] [CrossRef]
- Chaurasia, S.; Pandey, A. Artocarpus lakoocha seed starch and thymol-based films for extending fresh fruit shelf life: Antioxidant and physicochemical properties. Int. J. Biol. Macromol. 2025, 294, 139556. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Sripahco, T.; Soykeabkaew, N.; Tongdeesoontorn, W. Preparation and characterization of chitosan-rice starch films incorporating Amomum verum Blackw essential oil for grape preservation. Food Biosci. 2024, 62, 105072. [Google Scholar] [CrossRef]
- Lv, H.; Xu, H.; Xu, E.; Jin, Z.; Zhao, H.; Yuan, C.; Zhao, M.; Wu, Z.; He, D.; Cui, B. Improving structural and functional properties of starch-catechin-based green nanofiber mats for active food packaging by electrospinning and crosslinking techniques. Int. J. Biol. Macromol. 2024, 267, 131460. [Google Scholar] [CrossRef]
- Lv, H.; Wang, C.; Xu, E.; Jin, Z.; Zhao, H.; Yuan, C.; Zhao, M.; Yu, B.; Wu, Z.; He, D.; et al. Preparation of starch-based oral fast-disintegrating nanofiber mats for astaxanthin encapsulation and delivery via emulsion electrospinning. Int. J. Biol. Macromol. 2025, 289, 136466. [Google Scholar] [CrossRef]
- Krishna, M.; Nindo, C.I.; Min, S.C. Development of fish gelatin edible films using extrusion and compression molding. J. Food Eng. 2012, 108, 337. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Starch-polyester bilayer films with phenolic acids for pork meat preservation. Food Chem. 2022, 385, 132650. [Google Scholar] [CrossRef]
- Trinh, B.M.; Tadele, D.T.; Mekonnen, T.H. Robust and high barrier thermoplastic starch—PLA blend films using starch-graft-poly(lactic acid) as a compatibilizer. Mater. Adv. 2022, 3, 6208. [Google Scholar] [CrossRef]
- Sohrabi, S.; Hosseini, H.; Shojaee-Aliabadi, S.; Hosseini, S.M.; Mirmoghtadaie, L. Effect of dual modification on physicochemical, structural properties and functional groups distribution of corn starch: A molecular approach. Int. J. Biol. Macromol. 2025, 304, 140792. [Google Scholar] [CrossRef]
- Pooja, N.; Shashank, S.; Singh, B.N.; Mazumder, N. Advancing sustainable bioplastics: Chemical and physical modification of starch films for enhanced thermal and barrier properties. RSC Adv. 2024, 14, 23943. [Google Scholar]
- Shahbazi, M.; Majzoobi, M.; Farahnaky, A. Physical modification of starch by high-pressure homogenization for improving functional properties of κ-carrageenan/starch blend film. Food Hydrocoll. 2018, 85, 204. [Google Scholar] [CrossRef]
- Du, J.; Lu, Z.; Cheng, K.; Li, C.; Wu, H.; Xu, B.; Qian, J.Y. Relationship between starch granule-associated proteins and in vitro digestibility of buckwheat starches: From the perspective of gelatinization degree. Food Chem. 2025, 473, 143115. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, X.; Cui, B.; Wang, B.; El-Aty, A.M.A. Changes in the properties of the corn starch glycerol film in a time-dependent manner during gelatinization. Food Chem. 2024, 458, 140183. [Google Scholar] [CrossRef]
- Sun, K.; Yi, J.; Dai, R.; Chen, H. Highly efficient esterification of waxy maize starch in choline chloride/acetic acid acidic deep eutectic solvent system. Carbohydr. Res. 2025, 548, 109345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rao, Y.; Liu, P.; Han, Z.; Xie, F. Facile fabrication of a starch-based wood adhesive showcasing water resistance, flame retardancy, and antibacterial properties via a dual crosslinking strategy. Int. J. Biol. Macromol. 2024, 282, 137180. [Google Scholar] [CrossRef]
- Xu, K.; Tan, L.; Sun, H.; Chong, C.; Li, L.; Sun, B.; Yao, Z.; Zhuang, Y.; Wang, L. Manipulating gelatinization, retrogradation, and hydrogel properties of potato starch through calcium chloride-controlled crosslinking and crystallization behavior. Carbohydr. Polym. 2025, 357, 123371. [Google Scholar] [CrossRef]
- Yudhistira, B.; Husnayain, N.; Punthi, F.; Gavahian, M.; Chang, C.K.; Hsieh, C.W. Progress in the Application of Emerging Technology for the Improvement of Starch-Based Active Packaging Properties: A Review. ACS Food Sci. Technol. 2024, 4, 1997–2012. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, H. The effect of cold plasma on starch: Structure and performance. Carbohydr. Polym. 2024, 340, 122254. [Google Scholar] [CrossRef]
- Goiana, M.L.; Mattos, A.L.A.; Rosa, M.D.F.; Fernandes, F.A.N. Use of Cold Plasma as an Alternative to Improve Corn Starch-Based Films: Effect of the Plasma Application Strategy. Processes 2024, 12, 1429. [Google Scholar] [CrossRef]
- Okyere, A.Y.; Rajendran, S.; Annor, G.A. Cold plasma technologies: Their effect on starch properties and industrial scale-up for starch modification. Curr. Res. Food Sci. 2022, 5, 451. [Google Scholar] [CrossRef]
- Rahmasari, Y.; Yemiş, G.P. Characterization of ginger starch-based edible films incorporated with coconut shell liquid smoke by ultrasound treatment and application for ground beef. Meat. Sci. 2022, 188, 108799. [Google Scholar] [CrossRef]
- Giteru, S.G.; Ali, A.; Oey, I. Understanding the relationship between rheological characteristics of pulsed electric fields treated chitosan-zein-poly(vinyl alcohol)-polyethylene glycol composite dispersions and the structure-function of their resulting thin-films. Food Hydrocoll. 2021, 113, 106452. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Z.; Fan, B. Solo water-gelatinized starch enhances the barrier properties of starch/PBAT. Int. J. Biol. Macromol. 2025, 302, 140621. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zeng, J.; Gao, H.; Qi, J. Barrier properties characterization and release kinetics study of citrus fibers-reinforced functional starch composites. Food Hydrocoll. 2025, 164, 111195. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Wood, D.; Williams, T.G.; Avena-Bustillos, R.J.; McHugh, T.H. Nanocomposite edible films from mango puree reinforced with cellulose nanofibers. J. Food Sci. 2009, 74, 31. [Google Scholar] [CrossRef]
- Curvelo, A.A.S.; De Carvalho, A.J.F.; Agnelli, J.A.M. Thermoplastic starch–cellulosic fibers composites: Preliminary results. Carbohydr. Polym. 2001, 45, 183. [Google Scholar] [CrossRef]
- Lu, Y.; Weng, L.; Cao, X. Biocomposites of Plasticized Starch Reinforced with Cellulose Crystallites from Cottonseed Linter. Macromol. Biosci. 2005, 5, 1101. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer Nanocomposites Reinforced by Cellulose Whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films- A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Krishnamoorti, R. Nanocomposites: Structure, Phase Behavior, and Properties. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Samir, M.A.S.A.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612. [Google Scholar]
- Kuchaiyaphum, P.; Amornsakchai, T.; Chotichayapong, C.; Saengsuwan, N.; Yordsri, V.; Thanachayanont, C.; Batpo, P.; Sotawong, P. Pineapple stem starch-based films incorporated with pineapple leaf carbon dots as functional filler for active food packaging applications. Int. J. Biol. Macromol. 2024, 282, 137224. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Kumari, A.; Kumari, S.; Sharma, R.J. Advanced biodegradable starch-based nanocomposite films incorporating zinc oxide nanoparticles: Synthesis, characterization, and efficacy in antibacterial food packaging applications. Env. Chem. Eng. 2025, 13, 116296. [Google Scholar] [CrossRef]
- Kumar, N.; Khan, A.A.; Pyngrope, D.; Alanazi, A.M.; Upadhyay, A.; Shukla, S. Development and characterization of novel starch (mango kernel and litchi seed) based active edible coatings and films using ultrasonication: Effects on postharvest shelf life of Khasi mandarins. Sustain. Chem. Pharm. 2024, 39, 101610. [Google Scholar] [CrossRef]
- Otero-Herrera, A.; Fuentes-Gaviria, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Development of edible films based on sweet potato (Ipomoea batatas) starch and their application in candy packaging. Int. J. Biol. Macromol. 2025, 299, 140011. [Google Scholar] [CrossRef]
- Mohammadi, M.; Fasihi, M. Eco-friendly polylactic acid/modified thermoplastic starch films enhanced with clove essential oil and cochineal for dual-functional active and intelligent food packaging. Carbohydr. Polym. 2025, 354, 123320. [Google Scholar] [CrossRef]
- Su, M.; Qin, H.; Tang, Q.; Peng, D.; Li, H.; Zou, Z. Colorimetric ammonia-sensing nanocomposite films based on starch/sodium alginate and Cu-Phe nanorods for smart packaging application. Int. J. Biol. Macromol. 2024, 282, 137470. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Development of curcumin integrated smart pH indicator, antibacterial, and antioxidant waste derived Artocarpus lakoocha starch-based packaging film. Int. J. Biol. Macromol. 2024, 275, 133827. [Google Scholar] [CrossRef]
- Sharaby, M.R.; Soliman, E.A.; Khalil, R. Halochromic smart packaging film based on montmorillonite/polyvinyl alcohol-high amylose starch nanocomposite for monitoring chicken meat freshness. Int. J. Biol. Macromol. 2024, 258, 128910. [Google Scholar] [CrossRef] [PubMed]
- Asikkutlu, A.G.; Yildirim-Yalcin, M. Optimization of mechanical and water barrier properties of avocado seed starch based film and its application as smart pH indicator by adding blue butterfly pea flower extract. Food Chem. X 2025, 25, 102155. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Qin, Z.; Luo, Y.; He, Z.; Chen, Q.; Cai, J. Synergistically engineered starch-based composite films: Multifunctional platforms integrating quaternary ammonium chitosan and anthocyanins for intelligent food monitoring and sustainable packaging. Food Chem. 2025, 478, 143560. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR–ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35. [Google Scholar] [CrossRef]
- Bogracheva, T.Y.; Wang, Y.L.; Wang, T.L.; Hedley, C.L. Structural studies of starches with different water contents. Biopolymers 2002, 64, 268. [Google Scholar] [CrossRef] [PubMed]
- Brümmer, T.; Meuser, F.; Van Lengerich, B.; Niemann, C. Expansion and Functional Properties of Corn Starch Extrudates Related to their Molecular Degradation, Product Temperature and Water Content. Starch Staerke 2002, 54, 1. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Shrestha, A.K.; Gidley, M.J.; Gilbert, E.P. Molecular Rearrangement Of Starch During In Vitro Digestion: Toward A Better Understanding Of Enzyme Resistant Starch Formation In Processed Starches. Biomacromolecules 2008, 9, 1951. [Google Scholar] [CrossRef]
- Sahoo, A.R.; Kumari; Sarkhel; Jha; Mukherjee; Jain; Mohan, A. Rice Starch Phase Transition and Detection During Resistant Starch Formation. Food Rev. Int. 2023, 40, 158. [Google Scholar] [CrossRef]
- Kumari, P.; Luthra, A.; Sethi, P.; Banyal, S. Standardized Procedures and Protocols for Starch; Springer: Berlin/Heidelberg, Germany, 2024; pp. 197–220. [Google Scholar]
- Ukkunda, M.J.A.N.S.; Santhoshkumar, P.; Paranthaman, R. X-ray diffraction and its emerging application industry. Taylor Fr. 2024, 15, 37. [Google Scholar] [CrossRef]
- Krishnapriya, B.; Malarselvi, R.I.; Raja, C.R.; Priscilla, R. Elucidation of crystal structures using Bragg’s peaks and Nuclear Magnetic Resonance chemical shifts for polymorphic forms of (E)-4-Bromo-2-[(phenylimino)methyl]phenol. Chem. Phys. Impact 2023, 7, 100340. [Google Scholar] [CrossRef]
- Montoya-Anaya, D.G.; Madera-Santana, T.J.; Aguirre-Mancilla, C.L.; Grijalva-Verdugo, C.; Gonzales-Garcia, G.; Nuñez-Colín, C.A.; Rodriguez-Nuñez, J.R. Physicochemical characterization of residual potato (Solanum tuberosum) starch recovered from the potato chips industry in Mexico. Biotecnia 2023, 25, 60. [Google Scholar]
- Man, J.; Yang, Y.; Huang, J.; Zhang, C.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q.; Wei, C.J. Effect of simultaneous inhibition of starch branching enzymes I and IIb on the crystalline structure of rice starches with different amylose contents. Agric. Food Chem. 2013, 61, 9930. [Google Scholar] [CrossRef]
- Ameh, E.S. A review of basic crystallography and x-ray diffraction applications. Int. J. Adv. Manuf. Technol. 2019, 105, 3289. [Google Scholar] [CrossRef]
- Zhong, Y.; Tian, Y.; Liu, X.; Ding, L.; Kirkensgaard, J.J.K.; Hebelstrup, K.; Putaux, J.L.; Blennow, A. Influence of microwave treatment on the structure and functionality of pure amylose and amylopectin systems. Food Hydrocoll. 2021, 119, 106856. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Zhang, W.; Xue, B.; Luo, Z. Production and Applications ofAmylose-Lipid Complexes as Resistant Starch: Recent Approaches. Starch Staerke 2021, 73, 2000249. [Google Scholar] [CrossRef]
- Yi, D.; Maike, W.; Yi, S.; Xiaoli, S.; Dianxing, W.; Wenjian, S. Physiochemical Properties of Resistant Starch and Its Enhancement Approaches in Rice. Rice. Sci. 2021, 28, 31. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, Y.C.; Herrera, A.; Prakash, O. Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr. Polym. 2011, 83, 407. [Google Scholar] [CrossRef]
- Zhu, F. NMR spectroscopy of starch systems. Food Hydrocoll. 2017, 63, 611. [Google Scholar] [CrossRef]
- Farhat, I.A.; Belton Peter, S.; Webb, G. Magnetic Resonance in Food Science: From Molecules to Man; Royal Society of Chemistry: London, UK, 2007; ISBN 978-0-85404-340-8. [Google Scholar]
- Halley, P.; Avérous, L. Starch polymers: From genetic engineering to green applications. Starch Polym. 2014, 1, 64023. [Google Scholar]
- Lawas, A.J.D.D.; Bitter, H.-M.L. Solid-State NMR Spectroscopic Methods in Chemistry. Angew. Chem. Int. Ed. Engl. 2002, 41, 3096. [Google Scholar]
- Spyros, A.; Dais, P. NMR Spectroscopy in Food Analysis; RCS Publishing: Cambridge, UK, 2012. [Google Scholar]
- Flanagan, B.M.; Gidley, M.J.; Warren, F.J. Rapid quantification of starch molecular order through multivariate modelling of 13C CP/MAS NMR spectra. Chem. Commun. 2015, 51, 14856. [Google Scholar] [CrossRef] [PubMed]
- Veregin, R.P.; Fyfe, C.A.; Marchessault, R.H.; Taylor, M.G. Characterization of the crystalline A and B starch polymorphs and investigation of starch crystallization by high-resolution carbon-13 CP/MAS NMR. Macromolecules 1986, 19, 1030. [Google Scholar] [CrossRef]
- Gidley, M.J. Quantification of the structural features of starch polysaccharides by n.m.r. spectroscopy. Carbohydr. Res. 1985, 139, 85. [Google Scholar] [CrossRef]
- Paris, M.; Bizot, H.; Emery, J.; Buzaré, J.Y.; Buléon, A. Crystallinity and structuring role of water in native and recrystallized starches by 13C CP-MAS NMR spectroscopy: 1: Spectral decomposition. Carbohydr. Polym. 1999, 39, 327. [Google Scholar] [CrossRef]
- Paris, M.; Bizot, H.; Emery, J.; Buzaré, J.Y.; Buléon, A. NMR local range investigations in amorphous starchy substrates: II-Dynamical heterogeneity probed by 1H/13C magnetization transfer and 2D WISE solid state NMR. Int. J. Biol. Macromol. 2001, 29, 137. [Google Scholar] [CrossRef]
- Kalichevsky, M.T.; Jaroszkiewicz, E.M.; Ablett, S.; Blanshard, J.M.V.; Lillford, P.J. A study of the effect of water on the glass transition of 1:1 mixtures of amylopectin, casein and gluten using DSC and DMTA. Carbohydr. Polym. 1992, 18, 77. [Google Scholar] [CrossRef]
- Dunn, L.B.; Krueger, W.J. Branching Ratios of Starch via Proton Nuclear Magnetic Resonance and Their Use in Determining Amylose/Amylopectin Content: Evidence for Three Types of Amylopectin. Macromol. Symp. 1999, 140, 179. [Google Scholar] [CrossRef]
- Molina-boisseau, S.; Belgacem, M.N.; Dufresne, A. Surface Chemical Modification of Waxy Maize Starch Nanocrystals H. Langmuir 2005, 23, 2425–2433. [Google Scholar]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch Staerke 2013, 65, 48. [Google Scholar] [CrossRef]
- Capron, I.; Robert, P.; Colonna, P.; Brogly, M.; Planchot, V. Starch in rubbery and glassy states by FTIR spectroscopy. Carbohydr. Polym. 2007, 68, 249. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Vliegenthart, J.F.G. Crystallinity in starch plastics: Consequences for material properties. Trends Biotechnol. 1997, 15, 208. [Google Scholar] [CrossRef]
- Wilson, R.H.; Kalichevsky, M.T.; Ring, S.G.; Belton, P.S. A fourier-transform infrared study of the gelation and retrogradation of waxy-maize starch. Carbohydr. Res. 1987, 166, 162. [Google Scholar] [CrossRef]
- Lu, H.; Ma, R.; Chang, R.; Tian, Y. Evaluation of starch retrogradation by infrared spectroscopy. Food Hydrocoll. 2021, 120, 106975. [Google Scholar] [CrossRef]
- Schick, C. Differential scanning calorimetry (DSC) of semicrystalline polymers. Differential scanning calorimetry (DSC) of semicrystalline polymers. Anal. Bioanal. Chem. 2009, 395, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, L.; Xie, F.; Chen, L. Gelatinization of cornstarch with different amylose/amylopectin content. Carbohydr. Polym. 2006, 65, 357. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Tong, Z.; Chen, L. Retrogradation of waxy cornstarch studied by DSC. Starch Staerke 2010, 62, 524. [Google Scholar] [CrossRef]
- Liu, P.; Yu, L.; Wang, X.; Li, D.; Chen, L.; Li, X.J. Glass transition temperature of starches with different amylose/amylopectin ratios. Cereal Sci. 2010, 51, 388. [Google Scholar] [CrossRef]
- Bogracheva, T.Y.; Meares, C.; Hedley, C.L. The effect of heating on the thermodynamic characteristics of potato starch. Carbohydr. Polym. 2006, 63, 323. [Google Scholar] [CrossRef]
- Cooke, D.; Gidley, M.J. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydr. Res. 1992, 227, 103. [Google Scholar] [CrossRef]
- Alvani, K.; Qi, X.; Tester, R.F.; Snape, C.E. Physico-chemical properties of potato starches. Food Chem. 2011, 125, 958. [Google Scholar] [CrossRef]
- Li, Z.; Wei, C. Morphology, structure, properties and applications of starch ghost: A review. Int. J. Biol. Macromol. 2020, 163, 2084. [Google Scholar] [CrossRef]
- Zhang, H.; He, F.; Wang, T.; Chen, G. Thermal, pasting, and rheological properties of potato starch dual-treated with CaCl2 and dry heat. LWT 2021, 146, 111467. [Google Scholar] [CrossRef]
- Gonçalves, I.; Lopes, J.; Barra, A.; Hernández, D.; Nunes, C.; Kapusniak, K.; Kapusniak, J.; Evtyugin, D.V.; da Silva, J.A.L.; Ferreira, P.; et al. Tailoring the surface properties and flexibility of starch-based films using oil and waxes recovered from potato chips byproducts. Int. J. Biol. Macromol. 2020, 163, 251. [Google Scholar] [CrossRef]
- Genkina, N.K.; Kozlov, S.S.; Martirosyan, V.V.; Kiseleva, V.I. Effects of treatment pressure, holding time, and starch content on gelatinization and retrogradation properties of potato starch–water mixtures treated with high hydrostatic pressure. Starch Staerke 2014, 66, 700. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90. [Google Scholar] [CrossRef]
- Ridout, M.J.; Gunning, A.P.; Parker, M.L.; Wilson, R.H.; Morris, V.J. Using AFM to image the internal structure of starch granules. Carbohydr. Polym. 2002, 50, 123. [Google Scholar] [CrossRef]
- Szymońska, J.; Krok, F. Potato starch granule nanostructure studied by high resolution non-contact AFM. Int. J. Biol. Macromol. 2003, 33, 1. [Google Scholar] [CrossRef]
- Krupska, A.; Wieckowski, A.B.; Słomińska, L.; Jarosławski, L.; Zielonka, R. Influence of heating time and pressure treatment of potato starch on the generation of radicals: EPR studies. Carbohydr. Polym. 2012, 89, 54. [Google Scholar] [CrossRef]
- Dyrek, K.; Bidzinska, E.; ŁAbanowska, M.; Fortuna, T.; Przetaczek, I.; Pietrzyk, S. EPR Study of Radicals Generated in Starch by Microwaves or by Conventional Heating. Starch Staerke 2007, 59, 318. [Google Scholar] [CrossRef]
- Schaich, K.M.; Rebello, C.A. Extrusion Chemistry of Wheat Flour Proteins: I. Free Radical Formation. Cereal Chem. 1999, 76, 748. [Google Scholar] [CrossRef]
- Kameya, H.; Nakamura, H.; Ukai, M.; Shimoyama, Y. Electron Spin Resonance Spectroscopy of Gamma-Irradiated Glucose Polymers. Appl. Magn. Reson. 2011, 40, 395. [Google Scholar] [CrossRef]
- Ciesielski, W.; Tomasik, P. Starch radicals. Part I. Thermolysis of plain starch. Carbohydr. Polym. 1996, 31, 205. [Google Scholar] [CrossRef]
- Food and Drug Administration. Code Fed. Regul. 2023, 21. Available online: https://www.ecfr.gov/current/title-21 (accessed on 19 February 2025).
- Masina, N.; Choonara, Y.E.; Kumar, P.; Toit, L.C.D.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226. [Google Scholar] [CrossRef]
- Santhosh, R.; Ahmed, J.; Thakur, R.; Sarkar, P. Starch-based edible packaging: Rheological, thermal, mechanical, microstructural, and barrier properties—A review. Sustain. Food Technol. 2024, 2, 307. [Google Scholar] [CrossRef]
- Tang, M.; Hong, Y.; Gu, Z.; Zhang, Y.; Cai, X. The effect of xanthan on short and long-term retrogradation of rice starch. Starch Staerke 2013, 65, 702. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Noomhorm, A. Effect of Plasticizers on Mechanical and Barrier Properties of Rice Starch Film. Starch Staerke 2004, 56, 348. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. III. Structural aspects provide insight in amylopectin retrogradation properties and gel texture. J. Cereal Sci. 2003, 38, 61. [Google Scholar] [CrossRef]
- Canevarolo, S.V.J. Ciência Dos Polímeros; Artiliber: São Paulo, Brazil, 2002. [Google Scholar]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; de Soares, F.F. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci 2021, 42, 101202. [Google Scholar] [CrossRef]
- Silva, R.R.A.; de Freitas, P.A.V.; Teixeira, S.C.; de Oliveira, T.V.; Marques, C.S.; Stringheta, P.C.; Pires, A.C.D.S.; Ferreira, S.O.N.; de Soares, F.F. Plasticizer effect and ionic cross-linking: The impact of incorporating divalent salts in methylcellulose films for colorimetric detection of volatile ammonia. Food Biophys. 2022, 17, 59–74. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V.; Stringheta, P.C.; Pires, A.C.D.S.; de Soares, F.F. Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application. Polysaccharides 2022, 3, 426. [Google Scholar] [CrossRef]
- Subroto, E.; Cahyana, Y.; Indiarto, R.; Rahmah, T.A. Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications. Polymers 2023, 15, 2990. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of acetylation on the properties of corn starch. Food Chem. 2008, 106, 923. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Wang, X.L.; Zhao, G.M.; Wang, Y.Z. Preparation and properties of oxidized starch with high degree of oxidation. Carbohydr. Polym. 2012, 87, 2554. [Google Scholar] [CrossRef]
- Zamudio-Flores, L.A.B.-P.P.B.; Torres, A.V.; Salgado-Delgado, R. Influence of the Oxidation and Acetylation of Banana Starch on the Mechanical and Water Barrier Properties of Modified Starch and Modified Starch/Chitosan Blend Films. J. Appl. Polym. Sci. 2010, 116, 2658. [Google Scholar] [CrossRef]
- Koo, S.H.; Lee, K.Y.; Lee, H.G. Effect of cross-linking on the physicochemical and physiological properties of corn starch. Food Hydrocoll. 2010, 24, 619. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Moorthy, S.N.; Rajasekharan, K.N. Effect of Cross-linking with Epichlorohydrin on the Properties of Cassava (Manihot esculenta Crantz) Starch. Starch Staerke 2006, 58, 292. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch Staerke 2018, 70, 1. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W.; Dufresne, A. Polymer Grafting onto Starch Nanocrystals. Biomacromolecules 2007, 8, 2916. [Google Scholar] [CrossRef] [PubMed]
- Athawale, V.D.; Rathi, S.C. Graft Polymerization: Starch as a Model Substrate. J. Macromol. Sci. 2007, 39, 445–480. [Google Scholar] [CrossRef]
- Yan, X.; Wen, W.; Li, M.; Luo, S.; Ye, J.; Liu, C. Effect of chemically modified starch on retrogradation and quality characteristics of semi-dry rice noodles. Grain Oil Sci. Technol. 2025, 8, 13–20. [Google Scholar] [CrossRef]
- Zieba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations: Part 1. Structure, susceptibility to amylase, and pasting characteristics. LWT 2011, 44, 1314. [Google Scholar] [CrossRef]
- Shaikh, M.; Haider, S.; Ali, T.M.; Hasnain, A. Physical, thermal, mechanical and barrier properties of pearl millet starch films as affected by levels of acetylation and hydroxypropylation. Int. J. Biol. Macromol. 2019, 124, 209. [Google Scholar] [CrossRef]
- González-Torres, B.; Robles-García, M.Á.; Gutiérrez-Lomelí, M.; Padilla-Frausto, J.J.; Navarro-Villarruel, C.L.; Del-Toro-sánchez, C.L.; Rodríguez-Félix, F.; Barrera-Rodríguez, A.; Reyna-Villela, M.Z.; Avila-Novoa, M.G.; et al. Combination of Sorbitol and Glycerol, as Plasticizers, and Oxidized Starch Improves the Physicochemical Characteristics of Films for Food Preservation. Polymers 2021, 13, 3356. [Google Scholar] [CrossRef]
- Gupta, V.; Thakur, R.; Das, A.B. Effect of natural deep eutectic solvents on thermal stability, syneresis, and viscoelastic properties of high amylose starch. Int. J. Biol. Macromol. 2021, 187, 575. [Google Scholar] [CrossRef]
- Skowrońska, D.; Wilpiszewska, K. Deep Eutectic Solvents for Starch Treatment. Polymers 2022, 14, 220. [Google Scholar] [CrossRef]
- Zdanowicz, M. Starch treatment with deep eutectic solvents, ionic liquids and glycerol. A comparative study. Carbohydr. Polym. 2020, 229, 115574. [Google Scholar] [CrossRef]
- Samarakoon, E.R.J.; Waduge, R.; Liu, Q.; Shahidi, F.; Banoub, J.H. Impact of annealing on the hierarchical structure and physicochemical properties of waxy starches of different botanical origins. Food Chem. 2020, 303, 125344. [Google Scholar] [CrossRef]
- Dias, A.R.G.; da Rosa Zavareze, E.; Spier, F.; de Castro, L.A.S.; Gutkoski, L.C. Effects of annealing on the physicochemical properties and enzymatic susceptibility of rice starches with different amylose contents. Food Chem. 2010, 123, 711. [Google Scholar] [CrossRef]
- Asranudin; Holilah; Syarifin, A.N.K.; Purnomo, A.S.; Ansharullah; Fudholi, A. The effect of heat moisture treatment on crystallinity and physicochemical-digestibility properties of purple yam flour. Food Hydrocoll. 2021, 120, 106889. [Google Scholar] [CrossRef]
- Chuwech, M.; Rakariyatham, N.; Tinoi, J.; Suwitchayanon, P.; Chandet, N. Effect of Heat–Moisture Treatment on Crystallinity, Digestibility Properties, Bioactive Compounds, and Antioxidant Activity of Purple Rice (Oryza sativa L. indica) Flour. Processes 2023, 11, 969. [Google Scholar] [CrossRef]
- Li, G.; Ge, X.; Guo, C.; Liu, B. Effect of Ultrasonic Treatment on Structure and Physicochemical Properties of Pea Starch. Foods 2023, 12, 2620. [Google Scholar] [CrossRef]
- Ke, T.; Sun, X.S. Blending of poly(lactic acids) and starches containing varying amylose content. J. Polym. Environ. 2003, 11, 7. [Google Scholar] [CrossRef]
- Ke, T.; Sun, X. Physical Properties of Poly(Lactic Acid) and Starch Composites with Various Blending Ratios. Cereal Chem. 2000, 77, 761. [Google Scholar] [CrossRef]
- Avella, M.; Errico, M.E.; Laurienzo, P.; Martuscelli, E.; Raimo, M.; Rimedio, R. Preparation and characterisation of compatibilised polycaprolactone/starch composites. Polymer 2000, 41, 3875. [Google Scholar] [CrossRef]
- Wu, C.S. Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym. Degrad. Stab. 2003, 80, 127. [Google Scholar] [CrossRef]
- Kampeerapappun, P.; Aht-ong, D.; Pentrakoon, D.; Srikulkit, K. Preparation of cassava starch/montmorillonite composite film. Carbohydr. Polym. 2007, 67, 155. [Google Scholar] [CrossRef]
- Xiao, C. Current advances of chemical and physical starch-based hydrogels. Starch Staerke 2013, 65, 82. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 2017, 63, 561. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Oxygen absorbing food packaging made by extrusion compounding of thermoplastic cassava starch with gallic acid. Food Control 2022, 142, 109273. [Google Scholar] [CrossRef]
- Cervera, M.F.; Karjalainen, M.; Airaksinen, S.; Rantanen, J.; Krogars, K.; Heinämäki, J.; Colarte, A.I.; Yliruusi, J. Physical stability and moisture sorption of aqueous chitosan-amylose starch films plasticized with polyols. Eur. J. Pharm. Biopharm. 2004, 58, 69. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Mur. Int. J. Biol. Macromol. 2019, 134, 80. [Google Scholar] [CrossRef]
- Wu, X.; Yan, X.; Zhang, J.; Wu, X.; Luan, M.; Zhang, Q. Preparation and characterization of pH-sensitive intelligent packaging films based on cassava starch/polyvinyl alcohol matrices containing Aronia melanocarpa anthocyanins. LWT 2024, 194, 115818. [Google Scholar] [CrossRef]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Silva, R.R.A.; de Oliveira, T.V.; Soares, R.R.A.; Junior, N.S.; Moraes, A.R.F.; Pires, A.C.D.S.; Soares, N.F.F. Development and characterization of intelligent cellulose acetate-based films using red cabbage extract for visual detection of volatile bases. LWT 2020, 132, 109780. [Google Scholar] [CrossRef]
- Teixeira, S.C.; de Oliveira, T.V.; Batista, L.F.; Silva, R.R.A.; de Lopes, M.P.; Ribeiro, A.R.C.; Rigolon, T.C.B.; Stringheta, P.C.; de Soares, F.F. Anthocyanins of Açaí Applied as a Colorimetric Indicator of Milk Spoilage: A Study Using Agar-Agar and Cellulose Acetate as Solid Support to Be Applied in Packaging. Polysaccharides 2022, 3, 715–727. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; de Oliveira, T.V.; Silva, R.R.A.; Moraes, A.R.F.E.; Pires, A.C.D.S.; Soares, R.R.A.; Junior, N.S.; Soares, N.F.F. Effect of pH on the intelligent film-forming solutions produced with red cabbage extract and hydroxypropylmethylcellulose. Food Packag. Shelf Life 2020, 26, 100604. [Google Scholar] [CrossRef]
- Duan, A.; Yang, J.; Wu, L.; Wang, T.; Liu, Q.; Liu, Y. Preparation, physicochemical and application evaluation of raspberry anthocyanin and curcumin based on chitosan/starch/gelatin film. Int. J. Biol. Macromol. 2022, 220, 147. [Google Scholar] [CrossRef] [PubMed]
- Boonkanon, C.; Phatthanawiwat, K.; Wongniramaikul, W.; Choodum, A. Curcumin nanoparticle doped starch thin film as a green colorimetric sensor for detection of boron. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117351. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.A.; de Freitas, P.A.V.; de Oliveira, T.V.; Teixeira, S.C.; Rigolon, T.C.B.; Stringheta, P.C.; Otoni, C.G.; de Soares, F.F. Fraud-proof methylcellulose-based fish freshness indicator: Reversibility in halochromic sensing of basic volatiles is tailored by ionic strength. Int. J. Biol. Macromol. 2024, 277, 134486. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.S.; Oliveira, M.; De Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial nanostructured starch based films for packaging. Carbohydr. Polym. 2015, 129, 127. [Google Scholar] [CrossRef]
- Zhai, X.; Zhou, S.; Zhang, R.; Wang, W.; Hou, H. Antimicrobial starch/poly(butylene adipate-co-terephthalate) nanocomposite films loaded with a combination of silver and zinc oxide nanoparticles for food packaging. Int. J. Biol. Macromol. 2022, 206, 298. [Google Scholar] [CrossRef]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the Properties of Edible Starch-Based Films by the Incorporation of Additives: A Review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef]
- Krishnan, K.R.; Babuskin, S.; Rakhavan, K.R.; Tharavin, R.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M.J. Potential application of corn starch edible films with spice essential oils for the shelf life extension of red meat. Appl. Microbiol. 2015, 119, 1613. [Google Scholar] [CrossRef]
- Baysal, G.; Doğan, F.J. Investigation and preparation of biodegradable starch-based nanofilms for potential use of curcumin and garlic in food packaging applications. Biomater. Sci. Polym. Ed. 2020, 31, 1127. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch Staerke 2013, 65, 61. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic starch processing and characteristics-a review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353. [Google Scholar] [CrossRef]
- Siyamak, S.; Laycock, B.; Luckman, P. Synthesis of starch graft-copolymers via reactive extrusion: Process development and structural analysis. Carbohydr. Polym. 2020, 227, 115066. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, R.A.; Broekroelofs, A.; Janssen, L.P.B.M. The acetylation of starch by reactive extrusion. Starch Staerke 1998, 50, 198. [Google Scholar] [CrossRef]
- Hablot, E.; Dewasthale, S.; Zhao, Y.; Zhiguan, Y.; Shi, X.; Graiver, D.; Narayan, R. Reactive extrusion of glycerylated starch and starch-polyester graft copolymers. Eur. Polym. J. 2013, 49, 873. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Deri, F. Thermoplastic starch blends: A review of recent works. Polym. Sci. Ser. A 2012, 54, 165. [Google Scholar] [CrossRef]
- Tábi, T.; Kovács, J.G. Examination of injection moulded thermoplastic maize starch. Express Polym. Lett. 2007, 1, 804. [Google Scholar] [CrossRef]
- Jeevahan, J.J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Joseph, G.B.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210. [Google Scholar] [CrossRef]
- Liu; X.; Qin; Z.; Ma; Y.; Liu; H.; Wang, X.J. Cellulose-Based Films for Food Packaging Applications: Review of Preparation, Properties, and Prospects. Renew. Mater. 2023, 11, 3203. [Google Scholar] [CrossRef]
| Method | Type of Analysis | Measured Properties | Advantages | Limitations | Measurement Purpose |
|---|---|---|---|---|---|
| Differential Scanning Calorimetry (DSC) | Thermal | Melting temperature, enthalpy of recrystallization | Highly sensitive; quantitative | Requires controlled moisture; only detects crystalline phase | Quantifies retrogradation by measuring energy changes in stored starch gels |
| Thermogravimetric Analysis (TGA) | Thermal | Moisture loss, decomposition profile | Simple; real-time measurement | Limited sensitivity to structural changes | Evaluates water retention and stability of retrograded starch |
| Rapid Visco Analyzer (RVA) | Rheological | Setback viscosity, pasting profile | Fast; mimics food processing | Shear disrupts structure; limited to short-term retrogradation | Ranks starches by retrogradation tendency via setback viscosity |
| Texture Profile Analysis (TPA) | Mechanical | Gel hardness, adhesiveness, cohesiveness | Directly relates to texture | End-point measurement; sample-dependent | Assesses firmness increase in starch gels during storage |
| Fourier Transform Infrared Spectroscopy (FTIR) | Spectroscopic | Molecular order (1047/1022 cm−1 ratio), hydrogen bonding | Non-destructive; rapid | Water interference; qualitative without calibration | Tracks short-range order changes in retrograded starch |
| Raman Spectroscopy | Spectroscopic | Backbone conformation, glycosidic bonds | Water has minimal interference | Weaker signals; fluorescence issues | Complements FTIR to analyse starch molecular changes |
| Nuclear Magnetic Resonance (NMR) | Spectroscopic | Water mobility (T2 relaxation), molecular conformation | In situ monitoring; differentiates bound vs. free water | Requires specialized equipment; complex interpretation | Monitors retrogradation kinetics via water dynamics and starch crystallization |
| X-Ray Diffraction (XRD) | Structural | Crystallinity, polymorphic transitions (A, B, V) | Identifies crystal forms; quantifies order | Requires dried samples; low sensitivity to amorphous regions | Confirms amylopectin retrogradation by detecting B-type crystals |
| Small-Angle X-ray Scattering (SAXS) | Structural | Lamellar structure (5–20 nm scale) | Sensitive to nanoscale ordering | Requires advanced modelling; not routine | Analyses amylopectin rearrangement during retrogradation |
| Scanning Electron Microscopy (SEM) | Microscopy | Gel morphology, phase separation, granule remnants | High resolution; visual confirmation | Sample preparation can introduce artifacts | Examines gel structure changes due to retrogradation |
| Turbidity Measurement | Physical | Light transmittance loss (paste cloudiness) | Simple, fast | Non-specific; requires consistency | Monitors aggregation of retrograded starch in pastes |
| Syneresis Test | Physical | Water separation from gels | Directly relevant to food stability | Destructive; semi-quantitative | Measures water expulsion due to retrogradation |
| Iodine Binding (Blue Value) | Chemical | Amylose retrogradation (iodine complex formation) | Quick, inexpensive | Affects amylose only; influenced by branching | Estimates extent of amylose retrogradation |
| Resistant Starch (Enzymatic Digestibility Test) | Chemical | Resistance of starch to enzymatic hydrolysis | Nutritional relevance | Multi-step procedure; indirect measurement | Determines digestibility reduction due to retrogradation |
| Properties | Starch-Based Films | Poly (Lactic Acid) | Poly (Hydroxyalkanoates) | Protein-Based Films |
|---|---|---|---|---|
| Fundamental Characteristics | ||||
| Film Transparency | ++ (clear films) | ++ (transparent) | ± (varies by crystallinity) | ++ (glossy, clear) |
| Edibility & Food Safety | ++ (safe, edible, GRAS **) | + (safe but not edible) | + (safe, but not edible) | ++ (safe, edible) |
| Performance and Processing Features | ||||
| Mechanical Strength | - (brittle, weak alone) | ++ (strong, durable) | + (flexible, varies by type) | ± (moderate, varies by moisture) |
| Oxygen Barrier | ++ (very low O2 permeability) | + (moderate O2 barrier) | + (good O2 barrier) | ++ (excellent O2 barrier when dry) |
| Moisture Barrier | -- (high water absorption) | ± (moderate resistance) | ++ (highly water-resistant) | - (poor, absorbs moisture) |
| Processability | ± (requires blending/modification) | ++ (easily extruded/molded) | ± (thermal instability in some PHAs) | - (not thermoplastic, solution-cast only) |
| Environmental and Economic Aspects | ||||
| Biodegradability | ++ (fast, all environments) | ± (only industrial composting) | ++ (biodegrades everywhere) | ++ (fast, edible) |
| Scalability & Cost | ++ (low cost, abundant) | ++ (commercially established) | - (expensive) | - (more costly, variable availability) |
| Active and Smart Packaging Features | ||||
| Antimicrobial Suitability | ++ (high compatibility with bioactives) | + (can hold antimicrobials) | + (less explored, but promising) | ++ (strong binding to antimicrobials) |
| Oxygen Scavenging | + (can host O2 absorbers) | ± (requires additives) | ± (not widely studied) | ± (requires additives) |
| pH-Indicator Use | ++ (excellent for color changes) | - (difficult for hydrophilic dyes) | - (limited research) | ++ (works well with pH dyes) |
| Moisture Regulation | + (can absorb/release moisture) | - (not suited for moisture control) | ++ (best for wet conditions) | ± (moisture-sensitive, needs coating) |
| Scalability & Cost | ++ (low cost, abundant) | ++ (commercially established) | - (expensive) | - (more costly, variable availability) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, T.R.; Machado, G.d.O.; Marques, C.S.; Souza, A.L.d.; Pelissari, F.M.; Oliveira, T.V.d.; Silva, R.R.A. An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives. Macromol 2025, 5, 19. https://doi.org/10.3390/macromol5020019
Arruda TR, Machado GdO, Marques CS, Souza ALd, Pelissari FM, Oliveira TVd, Silva RRA. An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives. Macromol. 2025; 5(2):19. https://doi.org/10.3390/macromol5020019
Chicago/Turabian StyleArruda, Tarsila Rodrigues, Gabriela de Oliveira Machado, Clara Suprani Marques, Amanda Lelis de Souza, Franciele Maria Pelissari, Taíla Veloso de Oliveira, and Rafael Resende Assis Silva. 2025. "An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives" Macromol 5, no. 2: 19. https://doi.org/10.3390/macromol5020019
APA StyleArruda, T. R., Machado, G. d. O., Marques, C. S., Souza, A. L. d., Pelissari, F. M., Oliveira, T. V. d., & Silva, R. R. A. (2025). An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives. Macromol, 5(2), 19. https://doi.org/10.3390/macromol5020019







