Modern Aspects of Burn Injury Immunopathogenesis and Prognostic Immunobiochemical Markers (Mini-Review)
Abstract
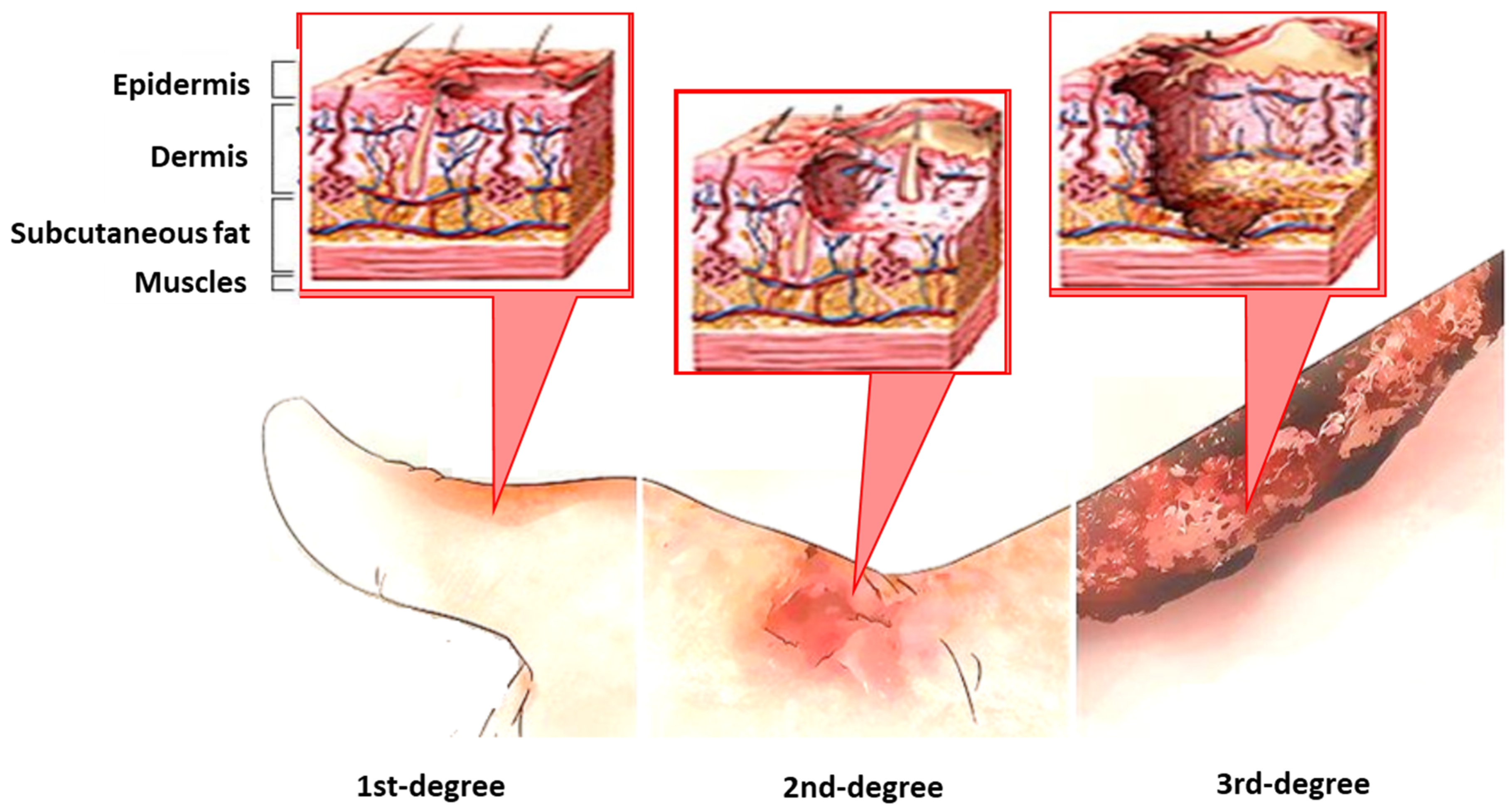
:1. Introduction
2. Methods
3. Results and Discussion
Modern Aspects of the Burn Injury and Burn Sepsis Immunopathogenesis
4. Immunobiochemical Markers of Burn Injury and Burn Sepsis
- Cytokines and growth factors;
- Acute-phase proteins;
- Matrix metalloproteinases;
- Reactive oxygen species;
- Nitric oxide;
4.1. Cytokines
4.2. Growth Factors
4.3. Acute-Phase Proteins
- (1)
- The mechanism of increasing PSP levels is fundamentally different from the mechanism of increasing proinflammatory markers such as TNF-α, IL-6, IL10, PCT, and CRP;
- (2)
- During the induction of systemic inflammation, the increase in PSP occurs earlier and faster than the increase in other markers of sepsis [41].
4.4. Proteases: Matrix Metalloproteinases
4.5. Reactive Oxygen Species (ROS)
4.6. Nitric Oxide (NO)
4.7. Parameters of the Hemostasis System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Burn Repository. Update, Report of Data from 2009–2018. American Burn Assotiation NBR Advisory Committee. Dataset Version 14.0. Ameriburn.siteym.com.2019. 2019. Available online: https://www.who.int/publications/i/item/9789240011311 (accessed on 14 April 2022).
- Zhang, P.; Zou, B.; Liou, Y.C.; Huang, C. The pathogenesis and diagnosis of sepsis post burn injury. Burn. Trauma 2021, 9, tkaa047. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Herndon, D.N.; Bhattarai, N.; Ogunbileje, J.O.; Szczesny, B.; Szabo, C.; Toliver-Kinsky, T.; Sidossis, L.S. Differential acute and chronic effects of burn trauma on murine skeletal muscle bioenergetics. Burns 2016, 42, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. Sepsis in the burn patient: A different problem than sepsis in the general population. Burn. Trauma 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, R.L.; Keyloun, J.W.; Brummel-Ziedins, K.; Orfeo, T.; Palmieri, T.L.; Johnson, L.S.; Moffatt, L.T.; Pusateri, A.E.; Shupp, J.W. Burn-Induced Coagulopathies: A Comprehensive Review. Shock 2020, 54, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Sherren, P.B.; Hussey, J.; Martin, R.; Kundishora, T.; Parker, M.; Emerson, B. Acute burn induced coagulopathy. Burns 2013, 39, 1157–1161. [Google Scholar] [CrossRef]
- Evers, L.H.; Bhavsar, D.; Mailänder, P. The biology of burn injury. Exp. Dermatol. 2010, 19, 777–783. [Google Scholar] [CrossRef]
- Ravat, F.; Payre, J.; Peslages, P.; Fontaine, M.; Sens, N. Burn: An inflammatory process. Pathol. Biol. 2011, 59, e63–e72. [Google Scholar] [CrossRef]
- Peng, D.Z. Researches in immunological responses after burn injury in China. Chin. J. Burn. 2008, 24, 390–392. [Google Scholar]
- Moins-Teisserenc, H.; Cordeiro, D.J.; Audigier, V.; Ressaire, Q.; Benyamina, M.; Lambert, J.; Maki, G.; Homyrda, L.; Toubert, A.; Legrand, M. Severe altered immune status after burn injury is associated with bacterial infection and septic shock. Front. Immunol. 2021, 12, 586195. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herndon, D.N. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet 2016, 388, 1417–1426. [Google Scholar] [CrossRef] [Green Version]
- Schwacha, M.G. Macrophages and post-burn immune dysfunction. Burns 2003, 29, 1–14. [Google Scholar] [CrossRef]
- Gosain, A.; Gamelli, R.L. A primer in cytokines. J. Burn Care Rehabil. 2005, 26, 7–12. [Google Scholar] [CrossRef]
- Schwacha, M.G.; Ayala, A.; Chaudry, I.H. Insights into the role of γδ T lymphocytes in the immunopathogenic response to thermal injury. J. Leukoc. Biol. 2000, 67, 644–650. [Google Scholar] [CrossRef]
- Kim, A.; Lang, T.; Xue, M.; Wijewardana, A.; Jackson, C.; Vandervord, J. The Role of Th-17 Cells and γδ T-Cells in Modulating the Systemic Inflammatory Response to Severe Burn Injury. Int. J. Mol. Sci. 2017, 18, 758. [Google Scholar] [CrossRef]
- Luo, G.; Peng, D.; Zheng, J.; Chen, X.; Wu, J.; Elster, E.A.; Tadaki, D.K. The role of NO in macrophage dysfunction at early stage after burn injury. Burns 2005, 31, 138–144. [Google Scholar] [CrossRef]
- Laggner, M.; Lingitz, M.T.; Copic, D.; Direder, M.; Klas, K.; Bormann, D.; Gugerell, A.; Moser, B.; Radtke, C.; Hacker, S.; et al. Severity of thermal burn injury is associated with systemic neutrophil activation. Sci. Rep. 2022, 12, 1654. [Google Scholar] [CrossRef]
- Rani, M.; Zhang, Q.; Schwacha, M.G. Burn wound γδ T-cells support a Th2 and Th17 immune response. J. Burn Care Res. 2014, 35, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Hanschen, M.; Tajima, G.; O’Leary, F.; Ikeda, K.; Lederer, J.A. Injury induces early activation of T-cell receptor signaling pathways in CD4+ regulatory T cells. Shock 2011, 35, 252–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.F.; Yao, Y.M.; Dong, N.; Yu, Y.; He, L.; Sheng, Z. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: A prospective, observational study. Crit. Care 2010, 14, R3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlton, M.; Voisey, J.; Parker, T.J.; Punyadeera, C.; Cuttle, L. A review of potential biomarkers for assessing physical and psychological trauma in paediatric burns. Burn. Trauma 2021, 9, tkaa049. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, L.; Boldeanu, M.V.; Bogdan, M.; Meca, A.D.; Coman, C.G.; Buca, B.R.; Tartau, C.G.; Tartau, L.M. Immunological approaches and therapy in burns (Review). Exp. Ther. Med. 2020, 20, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Mohd, J.; Shah, Y.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Science, medicine and the future: Healing chronic wounds. BMJ 2002, 324, 160–163. [Google Scholar] [CrossRef]
- Vinaik, R.; Abdullahi, A.; Barayan, D.; Jeschke, M.G. NLRP3 inflammasome activity is required for wound healing after burns. Transl. Res. 2020, 217, 47–60. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Herndon, D.N.; Chinkes, D.L.; Jeschke, M.G. Serum cytokine differences in severely burned children with and without sepsis. Shock 2007, 27, 4–9. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-term persistence of the pathophysiologic response to severe burn injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, M.G.; Barrow, R.E.; Herndon, D.N. Extended hypermetabolic response of the liver in severely burned pediatric patients. Arch. Surg. 2004, 139, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hafez, N.M.; Saleh Hassan, Y.; El-Metwally, T.H. A study on biomarkers, cytokines, and growth factors in children with burn injuries. Ann. Burn. Fire Disasters 2007, 20, 89–100. [Google Scholar]
- Sherbet, G.V. (Ed.) The epidermal growth factor (EGF) family. In Growth Factors and Their Receptors in Cell Differentiation, Cancer and Cancer Therapy; Elsevier: London, UK, 2011; pp. 173–198. [Google Scholar]
- Jeschke, M.G.; Finnerty, C.C.; Kulp, G.A.; Kraft, R.; Herndon, D.N. Can we use C-reactive protein levels to predict severe infection or sepsis in severely burned patients? Int. J. Burn. Trauma 2013, 3, 137–143. [Google Scholar]
- Joby, J.; Meer, M.; Krishnakumar, G. C-reactive protein: An early predictor of sepsis in patients with thermal burns. Int. Surg. J. 2017, 4, 628–632. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Kaneki, M.; Andreas, J.; Tompkins, R.G.; Martyn, J.A. Novel mitochondria-targeted antioxidant peptide ameliorates burn-induced apoptosis and endoplasmic reticulum stress in the skeletal muscle of mice. Shock 2011, 36, 580–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, L.; Afreixo, V.; Almeida, L.; Paiva, J.A. The Use of Procalcitonin (PCT) for Diagnosis of Sepsis in Burn Patients: A Meta-Analysis. PLoS ONE 2016, 11, e0168475. [Google Scholar] [CrossRef] [Green Version]
- Cakir, M.Ö.; Yakupoglu, S.B.N.; Yücel, N.; Akbaba, O.K.D. Evaluation of soluble CD14 subtype (presepsin) in burn sepsis. Burns 2014, 40, 664–669. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Kontakiotis, T.; Lazaridis, L.; Tsotsolis, N.; Koumis, J.; Kyriazis, G.; Bitzani, M. Inflammatory markers in patients with severe burn injury: What is the best indicator of sepsis? Burns 2007, 33, 189–194. [Google Scholar] [CrossRef]
- Mokline, A.; Garsallah, L.; Rahmani, I.; Jerbi, K.; Oueslati, H.; Tlaili, S.; Hammouda, R.; Gasri, B.; Messadi, A.A. Procalcitonin: A diagnostic and prognostic biomarker of sepsis in burned patients. Ann. Burn. Fire Disasters 2015, 28, 116–120. [Google Scholar]
- Velissaris, D.; Zareifopoulos, N.; Karamouzos, V.; Karanikolas, E.; Pierrakos, C.; Koniari, I.; Karanikolas, M. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus 2021, 13, e15019. [Google Scholar] [CrossRef]
- Masson, S.; Caironi, P.; Spanuth, E.; Thomae, R.; Panigada, M.; Sangiorgi, G.; Fumagalli, R.; Mauri, T.; Isgrò, S.; Fanizza, C.; et al. Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: Data from the Albumin Italian Outcome Sepsis trial. Crit. Care 2014, 18, R6. [Google Scholar] [CrossRef] [Green Version]
- Egea-Guerrero, J.J.; Martínez-Fernández, C.; Rodríguez-Rodríguez, A.; Bohórquez-López, A.; Vilches-Arenas, A.; Pacheco-Sánchez, M.; Guerrero, J.M.; Murillo-Cabezas, F. The utility of C-reactive protein and procalcitonin for sepsis diagnosis in critically burned patients: A preliminary study. Plast. Surg. 2015, 23, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Utz, E.R.; Elster, E.A.; Tadaki, D.K.; Gage, F.; Perdue, P.W.; Forsberg, J.A.; Stojadinovic, A.; Hawksworth, J.S.; Brown, T.S. Metalloproteinase expression is associated with traumatic wound failure. J. Surg. Res. 2010, 159, 633–639. [Google Scholar] [CrossRef]
- Weremijewicz, A.; Matuszczak, E.; Sankiewicz, A.; Tylicka, M.; Komarowska, M.; Tokarzewicz, A. Matrix metalloproteinase-2 and its correlation with basal membrane components laminin-5 and collagen type IV in paediatric burn patients measured with surface Plasmon resonance imaging (SPRI) biosensors. Burns 2018, 44, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Spies, M.; Barrow, R.E.; Herndon, D.N. Matrix metalloproteinases and their tissue inhibitors in severely burned children. Wound Repair Regen. 2003, 11, 177–180. [Google Scholar] [CrossRef]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef]
- Horton, J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology 2003, 189, 75–88. [Google Scholar] [CrossRef]
- Auger, C.; Samadi, O.; Jeschke, M.G. The biochemical alterations underlying post-burn hypermetabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2633–2644. [Google Scholar] [CrossRef]
- Lestaevel, P.; Agay, D.; Peinnequin, A.; Cruz, C.; Cespuglio, R.; Clarençon, D.; Multon, E.; Chancerelle, Y. Effects of a thermal injury on brain and blood nitric oxide (NO) content in the rat. Burns 2003, 29, 557–562. [Google Scholar] [CrossRef]
- Haycock, J.W.; Ralston, D.R.; Morris, B.; Freedlander, E.; MacNeil, S. Oxidative damage to protein and alterations to antioxidant levels in human cutaneous thermal injury. Burns 1997, 23, 533–540. [Google Scholar] [CrossRef]
- Privett, B.J.; Shinb, J.H.; Schoenfisch, M.H. Tutorial Review: Electrochemical Nitric Oxide Sensors for Physiological Measurements. Chem. Soc. Rev. 2010, 39, 1925–1935. [Google Scholar] [CrossRef] [Green Version]
- Glas, G.J.; Levi, M.; Schultz, M.J. Coagulopathy and its management in patients with severe burns. J. Thromb. Haemost. 2016, 14, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, R.H.; Fong, S.W.; Marrujo, G.; Hardeman, J.; Anderson, W. Coagulation and platelet changes after thermal injury in man. Burns 1981, 7, 370–377. [Google Scholar] [CrossRef]
- Hayakawa, M.; Maekawa, K.; Kushimoto, S.; Kato, H.; Sasaki, J.; Ogura, H.; Matauoka, T.; Uejima, T.; Morimura, N.; Ishikura, H.; et al. High D-Dimer levels predict a poor outcome in patients with severe trauma, even with high fibrinogen levels on arrival: A multicenter retrospective study. Shock 2016, 45, 308–314. [Google Scholar] [CrossRef]
- Kowal-Vern, A.; Walenga, J.M.; McGill, V.; Gamelli, R.L. The impact of antithrombin (H) concentrate infusions on pulmonary function in the acute phase of thermal injury. Burns 2001, 27, 52–60. [Google Scholar] [CrossRef]
- King, D.R.; Namias, N.; Andrews, D.M. Coagulation abnormalities following thermal injury. Blood Coagul. Fibrinolysis 2010, 21, 666–669. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Kontakiotis, T.; Bitzani, M.; Papaioannou-Gaki, G.; Parlapani, A.; Thomareis, O.; Tsotsolis, N.; Giala, M.A. Early coagulation disorders after severe burn injury: Impact on mortality. Intensive Care Med. 2008, 34, 700–706. [Google Scholar] [CrossRef]
- Garcia-Avello, A.; Lorente, J.A.; Cesar-Perez, J.; Garcia-Frade, L.J.; Alvarado, R.; Arevalo, J.M.; Navarro, J.L.; Esteban, A. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thromb. Res. 1998, 89, 59–64. [Google Scholar] [CrossRef]
- Lu, R.P.; Ni, A.; Lin, F.C.; Ortiz-Pujols, S.M.; Adams, S.D.; Monroe, D.M., 3rd; Whinna, H.C.; Cairns, B.A.; Key, N.S. Major burn injury is not associated with acute traumatic coagulopathy. J. Trauma Acute Care Surg. 2013, 74, 1474–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, R.; Herndon, D.N.; Finnerty, C.C.; Cox, R.A.; Song, J.; Jeschke, M.G. Predictive Value of IL-8 for Sepsis and Severe Infections After Burn Injury: A Clinical Study. Shock 2015, 43, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Chester, S.J.; Stockton, K.; De Young, A.; Kipping, B.; Tyack, Z.; Griffin, B.; Chester, R.L.; Kimble, R.M. Effectiveness of medical hypnosis for pain reduction and faster wound healing in pediatric acute burn injury: Study protocol for a randomized controlled trial. Trials 2016, 17, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, M.G.; Finnerty, C.C.; Herndon, D.N.; Song, J.; Boehning, D.; Tompkins, R.G.; Baker, H.V.; Gauglitz, G.G. Severe injury is associated with insulin resistance, endoplasmic reticulum stress response, and unfolded protein response. Ann. Surg. 2012, 255, 370–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, R.; Kulp, G.A.; Herndon, D.N.; Emdad, F.; Williams, F.N.; Hawkins, H.K.; Leonard, K.R.; Jeschke, M.G. Is there a difference in clinical outcomes, inflammation, and hypermetabolism between scald and flame burn? Pediatr. Crit. Care Med. 2011, 12, e275–e281. [Google Scholar] [CrossRef] [Green Version]
- Zang, T.; Broszczak, D.A.; Broadbent, J.A.; Cuttle, L.; Lu, H.; Parker, T.J. The biochemistry of blister fluid from pediatric burn injuries: Proteomics and metabolomics aspects. Expert Rev. Proteomics. 2016, 13, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Voutsas, V.; Konoglou, M.; Karali, V.; Koukiasa, P.; Loridas, N.; Papaioannou, M.; Vasileiadou, G.; Bitzani, M. Determinants of Outcome in Burn ICU Patients with Septic Shock. J. Burn Care Res. 2017, 38, e172–e179. [Google Scholar] [CrossRef] [PubMed]
- Matwiyoff, G.N.; Prahl, J.D.; Miller, R.J.; Carmichael, J.J.; Amundson, D.E.; Seda, G.; Daheshia, M. Immune regulation of procalcitonin: A biomarker and mediator of infection. Inflamm. Res. 2012, 61, 401–409. [Google Scholar] [CrossRef]
- Tan, J.; Wu, J. Current progress in understanding the molecular pathogenesis of burn scar contracture. Burn. Trauma 2017, 5, 14. [Google Scholar] [CrossRef]
- Sierawska, O.; Małkowska, P.; Taskin, C.; Hrynkiewicz, R.; Mertowska, P.; Grywalska, E.; Korzeniowski, T.; Torres, K.; Surowiecka, A.; Niedźwiedzka-Rystwej, P.; et al. Innate Immune System Response to Burn Damage-Focus on Cytokine Alteration. Int. J. Mol. Sci. 2022, 23, 716. [Google Scholar] [CrossRef]

| Markers | Biomarker Level (Parameter) | Ref. |
|---|---|---|
| Cytokines (proinflammatory IL-1α, IL-6, TNFα) | Levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) increase within 24–48 h after a burn Levels of anti-inflammatory cytokines (IL-1 receptor antagonist, IL-4, IL-10, IL-11, and IL-13) are reduced (in cases with sepsis levels that are significantly higher than cases without sepsis) | [27,30] |
| C-reactive protein (CRP) | Levels increased in cases with sepsis | [34,35,36] |
| Procalcitonin (PCT) and presepsin | Levels increased in cases with sepsis | [32,38,39,40,41,42,43] |
| Growth factors (IGFBR-1, IGFBR-3, YGF, bFGF, IGF-1, TGF) | Levels increased | [30,31,32,33] |
| Matrix metalloproteinases (MMP-1, 2, 3, 9, etc.) | Activity increased | [44,45,46,47,48] |
| Reactive oxygen species (ROS) | Levels increased due to oxidative stress | [49,50,51,52] |
| Nitric oxide (NO) | Levels increased | [19,53] |
| Parameters of the hemostasis system (platelets, fibrinogen, D-dimer, protamine sulfate, fibrin degradation products, activated partial thromboplastin time, prothrombin time, and thrombin time) | Abnormal coagulation parameters: - thrombocytopenia 24–48 h after burn; - hypercoagulability and was attributed to high levels of fibrinogen and thromboplastin due to tissue lysis; Signs of coagulopathy and DIC syndrome | [54,55,56,57,58,59,60,61] |
| Inflammatory markers (IL-1β, Il-6, IL-8, TNFα, INFχ, IL12h70, Il-17, Il 2, Il-4, IL-5, IL-10, IL-13, IL-7 | Levels increased | [61,62] |
| Stress marker (adrenaline, noradrenaline, dopamine, cortisol) | Levels increased | [63,64,65] |
| Hormones (leptin, progesterone, insulin, thyroid hormones) | Levels increased; levels of thyroid hormones lowered | [61,66] |
| Structural proteins (proteasome, type IV collagen, lfminin-5, pyrodinoline, deoxypyrodinoline) | Levels vary depending on the severity of the burn injury and sepsis | [67,68,69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N. Modern Aspects of Burn Injury Immunopathogenesis and Prognostic Immunobiochemical Markers (Mini-Review). BioTech 2022, 11, 18. https://doi.org/10.3390/biotech11020018
Kuznetsova TA, Andryukov BG, Besednova NN. Modern Aspects of Burn Injury Immunopathogenesis and Prognostic Immunobiochemical Markers (Mini-Review). BioTech. 2022; 11(2):18. https://doi.org/10.3390/biotech11020018
Chicago/Turabian StyleKuznetsova, Tatyana A., Boris G. Andryukov, and Natalia N. Besednova. 2022. "Modern Aspects of Burn Injury Immunopathogenesis and Prognostic Immunobiochemical Markers (Mini-Review)" BioTech 11, no. 2: 18. https://doi.org/10.3390/biotech11020018
APA StyleKuznetsova, T. A., Andryukov, B. G., & Besednova, N. N. (2022). Modern Aspects of Burn Injury Immunopathogenesis and Prognostic Immunobiochemical Markers (Mini-Review). BioTech, 11(2), 18. https://doi.org/10.3390/biotech11020018







