Physiological and Molecular Aspects of Two Thymus Species Differently Sensitive to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
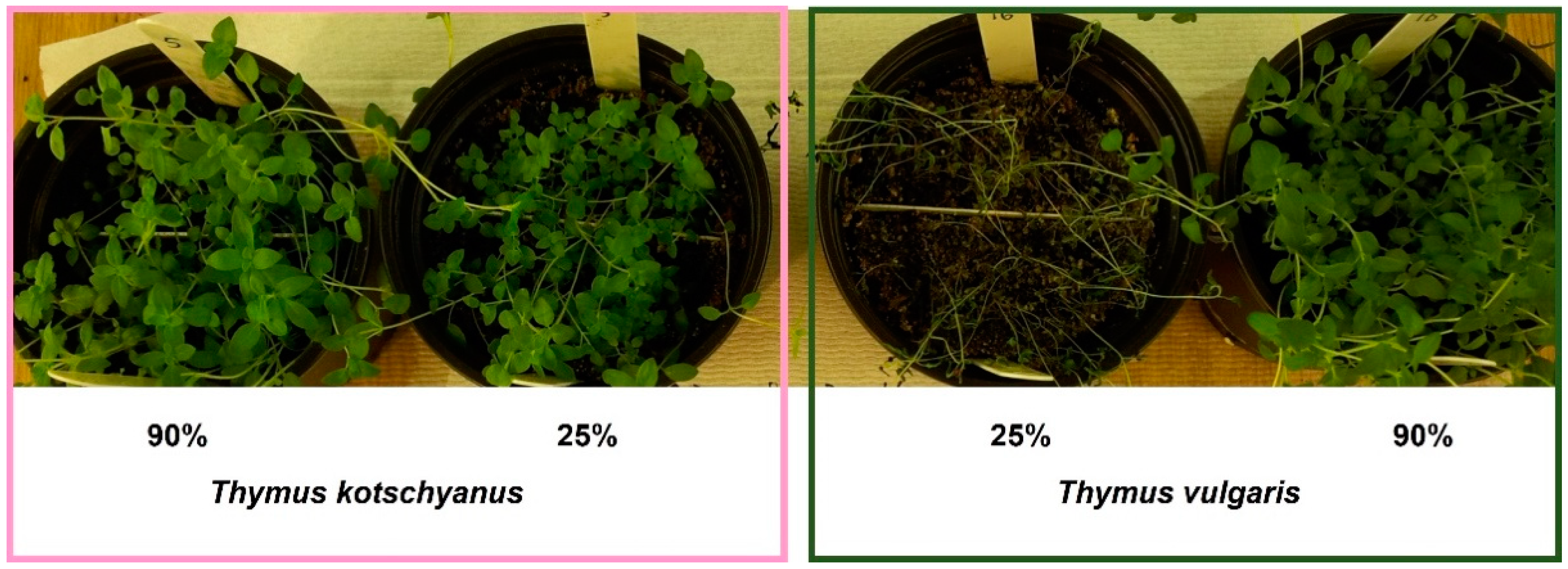
2.1. Plant Material, Growth Condition, and Treatment
2.2. Physiological Measurements
2.3. Total RNA Isolation, Quality Controls, and First Strand cDNA Synthesis
2.4. Gene Selection, Amplification, and Direct Sequencing
2.5. qRT-PCR
2.6. Experimental Design and Statistical Analysis
3. Results
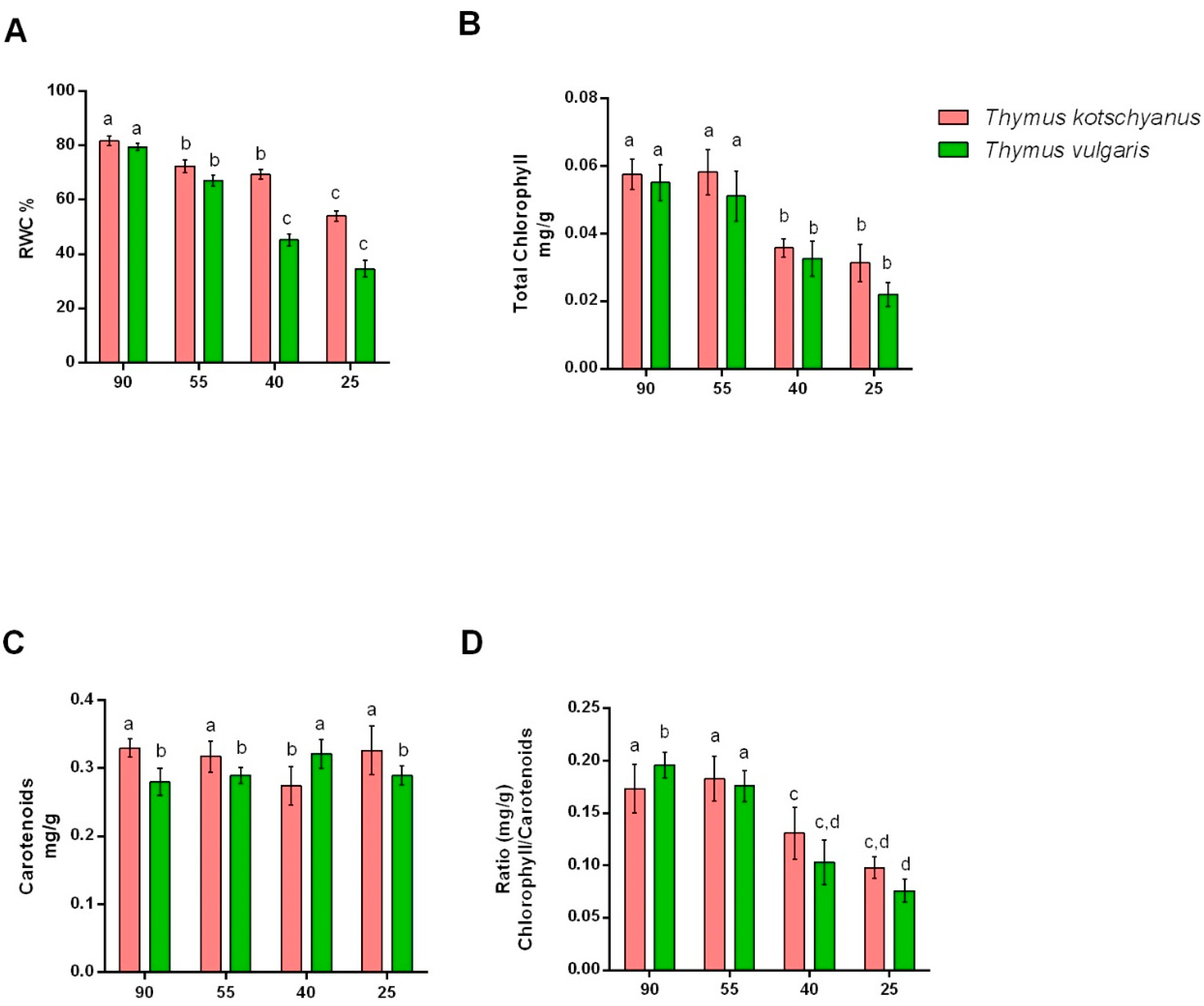
3.1. Physiological Measurements
3.2. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barchet, G.L.H.; Dauwe, R.; Guy, R.D.; Schroeder, W.R.; Soolanayakanahally, R.Y.; Campbell, M.M.; Mansfield, S.D. Investigating the drought-stress response of hybrid poplar genotypes by metabolite profiling. Tree Physiol. 2014, 34, 1203–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moradi, P. Key plant products and common mechanisms utilized by plants in water deficit stress responses. Bot. Sci. 2016, 94, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Amin, B.; Atif, M.J.; Meng, H.W.; Ghani, M.I.; Ali, M.; Wang, X.; Ding, Y.Y.; Li, X.J.; Cheng, Z.H. Biochemical and Physiological Responses of Cucumis sativus Cultivars to Different Combinations of Low-Temperature and High Humidity. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Songsri, P. Photosynthetic and physiological responses to drought of Jerusalem artichoke genotypes differing in drought resistance. Agric. Water Manag. 2022, 259, 107252. [Google Scholar] [CrossRef]
- Alves, F.M.; Joshi, M.; Djidonou, D.; Joshi, V.; Gomes, C.N.; Leskovar, D.I. Physiological and Biochemical Responses of Tomato Plants Grafted onto Solanum pennellii and Solanum peruvianum under Water-Deficit Conditions. Plants 2021, 10, 2236. [Google Scholar] [CrossRef]
- Herrera, J.C.; Calderan, A.; Gambetta, G.A.; Peterlunger, E.; Forneck, A.; Sivilotti, P.; Cochard, H.; Hochberg, U. Stomatal responses in grapevine become increasingly more tolerant to low water potentials throughout the growing season. Plant J. 2021, 109, 804–815. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 2021, 253, 127. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrao, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A.; El-Ansary, D.O.; Mattar, M.A. Effects of Water Stress and Modern Biostimulants on Growth and Quality Characteristics of Mint. Agronomy 2019, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Gharibi, S.; Tabatabaei, B.E.; Saeidi, G.; Goli, S.A. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Ghadyeh Zarrinabadi, I.; Razmjoo, J.; Abdali Mashhadi, A.; Mojeni, H.K.; Boroomand, A. Physiological response and productivity of pot marigold (Calendula officinalis) genotypes under water deficit. Ind. Crops Prod. 2019, 139, 111488. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. Improvement in drought tolerance of lemon balm, Melissa officinalis L. under the pre-treatment of LED lighting. Plant Physiol. Biochem. PPB 2019, 139, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Pohanková, E.; Fischer, M.; Orság, M.; Trnka, M.; Klem, K.; Marek, M. The Evaluation of Radiation Use Efficiency and Leaf Area Index Development for the Estimation of Biomass Accumulation in Short Rotation Poplar and Annual Field Crops. Forests 2018, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Stahl-Biskup, E.; Venskutonis, R.P. Thyme. In Handbook of Herbs and Spices; Woodhead Publishing: Sawston, UK, 2012; pp. 499–525. [Google Scholar]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Thompson, J.; Charpentier, A.; Bouguet, G.; Charmasson, F.; Roset, S.; Buatois, B.; Vernet, P.; Gouyon, P.H. Evolution of a genetic polymorphism with climate change in a Mediterranean landscape. Proc. Natl. Acad. Sci. USA 2013, 110, 2893–2897. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, M.; Azimi-Moqadam, M.R.; Moradi, P.; MohseniFard, E.; Shekari, F.; Kompany-Zareh, M. Effect of drought stress on metabolite adjustments in drought tolerant and sensitive thyme. Plant Physiol. Biochem. PPB 2018, 132, 391–399. [Google Scholar] [CrossRef]
- Mohammadi, H.; Amirikia, F.; Ghorbanpour, M.; Fatehi, F.; Hashempour, H. Salicylic acid induced changes in physiological traits and essential oil constituents in different ecotypes of Thymus kotschyanus and Thymus vulgaris under well-watered and water stress conditions. Ind. Crops Prod. 2019, 129, 561–574. [Google Scholar] [CrossRef]
- Araujo, W.L.; Nunes-Nesi, A.; Osorio, S.; Usadel, B.; Fuentes, D.; Nagy, R.; Balbo, I.; Lehmann, M.; Studart-Witkowski, C.; Tohge, T.; et al. Antisense Inhibition of the Iron-Sulphur Subunit of Succinate Dehydrogenase Enhances Photosynthesis and Growth in Tomato via an Organic Acid-Mediated Effect on Stomatal Aperture. Plant Cell 2011, 23, 600–627. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; To, T.K.; Matsui, A.; Tanoi, K.; Kobayashi, N.I.; Matsuda, F.; Habu, Y.; Ogawa, D.; Sakamoto, T.; Matsunaga, S.; et al. Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 2017, 3, 17097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, F.; Li, Z.; Yang, Z.; Hao, L.; Zhao, H. Comparative Transcriptome Analysis of Cynanchum thesioides Under Drought Stress Reveals Candidate Genes Involved in Succinic Acid Biosynthesis. J. Plant Biol. 2021, 1–13. [Google Scholar] [CrossRef]
- Ogawa, D.; Suzuki, Y.; Yokoo, T.; Katoh, E.; Teruya, M.; Muramatsu, M.; Ma, J.F.; Yoshida, Y.; Isaji, S.; Ogo, Y.; et al. Acetic-acid-induced jasmonate signaling in root enhances drought avoidance in rice. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, Y.; Utsumi, C.; Tanaka, M.; Ha, C.V.; Takahashi, S.; Matsui, A.; Matsunaga, T.M.; Matsunaga, S.; Kanno, Y.; Seo, M.; et al. Acetic Acid Treatment Enhances Drought Avoidance in Cassava (Manihot esculenta Crantz). Front. Plant Sci. 2019, 10, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baulcombe, D.C.; Dean, C. Epigenetic regulation in plant responses to the environment. Cold Spring Harb. Perspect. Biol. 2014, 6, a019471. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic Mechanisms of Plant Adaptation to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef]
- Rasheed, S.; Bashir, K.; Kim, J.M.; Ando, M.; Tanaka, M.; Seki, M. The modulation of acetic acid pathway genes in Arabidopsis improves survival under drought stress. Sci. Rep. 2018, 8, 7831. [Google Scholar] [CrossRef]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-Step Method of Rna Isolation by Acid Guanidinium Thiocyanate Phenol Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Gideon Onyekachi, O.; Ogbonnaya Boniface, O.; Felix Gemlack, N.; Nicholas, N. The Effect of Climate Change on Abiotic Plant Stress: A Review. Abiotic Biot. Stress Plants 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.; Kumar, A.; Malla, M.A.; Chowdhary, K.; Singh, G.; Ravikanth, G.; Harish; Sharma, S.; Saati-Santamaria, Z.; Menendez, E.; et al. Approaches for the amelioration of adverse effects of drought stress on crop plants. Front. Biosci. 2021, 26, 928–947. [Google Scholar] [CrossRef]
- Wijewardene, I.; Mishra, N.; Sun, L.; Smith, J.; Zhu, X.L.; Payton, P.; Shen, G.X.; Zhang, H. Improving drought-, salinity-, and heat-tolerance in transgenic plants by co-overexpressing Arabidopsis vacuolar pyrophosphatase gene AVP1 and Larrea Rubisco activase gene RCA. Plant Sci. 2020, 296, 110499. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Trindade, H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crops Prod. 2019, 134, 89–99. [Google Scholar] [CrossRef]
- Moradi, P.; Mahdavi, A.; Khoshkam, M.; Iriti, M. Lipidomics Unravels the Role of Leaf Lipids in Thyme Plant Response to Drought Stress. Int. J. Mol. Sci. 2017, 18, 2067. [Google Scholar] [CrossRef] [Green Version]
- Martignago, D.; Rico-Medina, A.; Blasco-Escámez, D.; Fontanet-Manzaneque, J.B.; Caño-Delgado, A.I. Drought Resistance by Engineering Plant Tissue-Specific Responses. Front. Plant Sci. 2020, 10, 1676. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Kaur, H.; Kohli, S.K.; Khanna, K.; Bhardwaj, R. Scrutinizing the impact of water deficit in plants: Transcriptional regulation, signaling, photosynthetic efficacy, and management. Physiol. Plant. 2021, 172, 935–962. [Google Scholar] [CrossRef]
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A.; Centritto, M. Impaired Stomatal Control Is Associated with Reduced Photosynthetic Physiology in Crop Species Grown at Elevated [CO2]. Front. Plant Sci. 2016, 7, 1568. [Google Scholar] [CrossRef] [Green Version]
- Tátrai, Z.A.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress inThymus citriodorus. Int. J. Agron. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pirzad, A.; Mohammadzadeh, S. Water use efficiency of three mycorrhizal Lamiaceae species (Lavandula officinalis, Rosmarinus officinalis and Thymus vulgaris). Agric. Water Manag. 2018, 204, 1–10. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.-M.; Mastinu, A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Wang, Y.-H.; Li, T.; Tan, G.-F.; Tao, J.-P.; Su, X.-J.; Xu, Z.-S.; Tian, Y.-S.; Xiong, A.-S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2020, 258, 379–390. [Google Scholar] [CrossRef]
- Luo, M.; Cheng, K.; Xu, Y.C.; Yang, S.G.; Wu, K.Q. Plant Responses to Abiotic Stress Regulated by Histone Deacetylases. Front. Plant Sci. 2017, 8, 2147. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, L.; Gupta, S.; Mishra, S.K.; Pandey, G.; Kumar, S.; Chauhan, P.S.; Chakrabarty, D.; Nautiyal, C.S. Elucidation of Complex Nature of PEG Induced Drought-Stress Response in Rice Root Using Comparative Proteomics Approach. Front. Plant Sci. 2016, 7, 1466. [Google Scholar] [CrossRef] [Green Version]
- Duca, M. Plant Respiration. Biol. Med. Phys. Biomed. 2015, 123–148. [Google Scholar] [CrossRef]
- Cavalcanti, J.H.F.; Esteves-Ferreira, A.A.; Quinhones, C.G.S.; Pereira-Lima, I.A.; Nunes-Nesi, A.; Fernie, A.R.; Araujo, W.L. Evolution and Functional Implications of the Tricarboxylic Acid Cycle as Revealed by Phylogenetic Analysis. Genome Biol. Evol. 2014, 6, 2830–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Gene | Primer Sequence (5′ → 3′) | Accession Id | Product Length | Ta * | |
|---|---|---|---|---|---|
| SCL | Forward Reverse | TCTATGTHCCTCCDCCWTTTGC TCTGCYGTRCCACCRATYTCAC | NM_001324746.1 XM_006350317.2 XM_015211494.1 NM_001247645.2 XM_019302845.1 | 436 | 53 |
| PDC | Forward Reverse | CAAACTGTBACTTGCTAYCAGG GCCCRTCRTGGATYTCTACTTC | JF775376.1 XM_023031688.1 XM_006362311.2 XM_019399015.1 | 1060 | 52 |
| ACS | Forward Reverse | MABATAGAGTGGTTCAARGGTG DAARGTAGCAGAGCCAGGTTTC | XM_012988613.1 XM_020698950.1 KJ531400.1 XM_022985897.1 XM_019315775.1 | 1143 | 52 |
| HDA-6 | Forward Reverse | ATCGGCGAYTACTACTACGG ATGACYTTYTGGATKATGGGAC | XM_023030385.1 XM_011102990.2 XR_002286330.1 XM_020691311.1 XM_012973837.1 | 698 | 52 |
| Gene | Primer Sequence (5′ → 3′) | Product Length nt | Efficiency | TA * | |
|---|---|---|---|---|---|
| SCL | Forward | CTGGTTTGTGAATGTATCCC | 105 | 1.9 | 55 |
| Reverse | TGAATCAGCAGAAAAAGACTC | ||||
| PDC | Forward | CCGATGAAATGAGGGTGA | 123 | 2.03 | 55 |
| Reverse | GAAACTGTGTGTGGCGAAAG | ||||
| ACS | Forward | TCAAGCAAACATCTACGACTG | 99 | 2.06 | 55 |
| Reverse | CTGTAAAACGAGGACCCAAG | ||||
| HDA-6 | Forward | GTTTCAATGTTGGAGAGGACTG | 165 | 2 | 55 |
| Reverse | AGGACTCGCTCTTCTTCGC | ||||
| Act | Forward | AGCAACTGGGATGATATGGAG | 111 | 1.92 | 55 |
| Reverse | CTTGGGGTTAAGAGGAGCC | ||||
| GAPDH | Forward | AACGGAAAGTTGACTGGTATG | 126 | 1.98 | 55 |
| Reverse | TGACTCCTCCTTGATGGCA | ||||
| EF-1A | Forward | AGATCGGAAATGGTTATGCTC | 94 | 1.95 | 55 |
| Reverse | GACCTCCTGTCAATCTTCGT | ||||
| Source of Variation | Degrees Freedom | Mean Squares | |||
|---|---|---|---|---|---|
| RWC | Total Chlorophyll | Carotenoids | Chlorophyll/Carotenoids | ||
| Species (T. vulgaris and T. kotschyanus) | 1 | 235.61 ** | 0.004972 ** | 0.019777 ** | 0.025516 ** |
| Treatment (four irrigation regimes) | 3 | 1088.79 ** | 0.001462 ** | 0.002133 ns | 0.012725 ** |
| Species × Treatment | 3 | 2.15 ns | 0.000382 ns | 0.004442 ns | 0.001099 ns |
| Residuals | 24 | 14.82 | 0.000147 | 0.001748 | 0.001015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafi, M.; Azimi-Moqadam, M.-R.; MohseniFard, E.; Shekari, F.; Jafary, H.; Moradi, P.; Pucci, M.; Abate, G.; Mastinu, A. Physiological and Molecular Aspects of Two Thymus Species Differently Sensitive to Drought Stress. BioTech 2022, 11, 8. https://doi.org/10.3390/biotech11020008
Ashrafi M, Azimi-Moqadam M-R, MohseniFard E, Shekari F, Jafary H, Moradi P, Pucci M, Abate G, Mastinu A. Physiological and Molecular Aspects of Two Thymus Species Differently Sensitive to Drought Stress. BioTech. 2022; 11(2):8. https://doi.org/10.3390/biotech11020008
Chicago/Turabian StyleAshrafi, Mohsen, Mohammad-Reza Azimi-Moqadam, Ehsan MohseniFard, Farid Shekari, Hossein Jafary, Parviz Moradi, Mariachiara Pucci, Giulia Abate, and Andrea Mastinu. 2022. "Physiological and Molecular Aspects of Two Thymus Species Differently Sensitive to Drought Stress" BioTech 11, no. 2: 8. https://doi.org/10.3390/biotech11020008







