Integrating Human Waste with Microbial Fuel Cells to Elevate the Production of Bioelectricity
Abstract
:1. Introduction
2. Changes over Time in Human Waste Treatment
2.1. Conventional Methods
2.2. Modern Methods
2.2.1. Fertilizers
2.2.2. Pathogen Removal
2.2.3. Materials
3. MFC Technology
4. Factors Affecting the Performance of an MFC
4.1. Electron Transfer Mechanism
4.2. Microbial Metabolism and Cell Potential
4.3. Substrate
4.4. Anode
4.5. Cathode
4.6. Operating Conditions
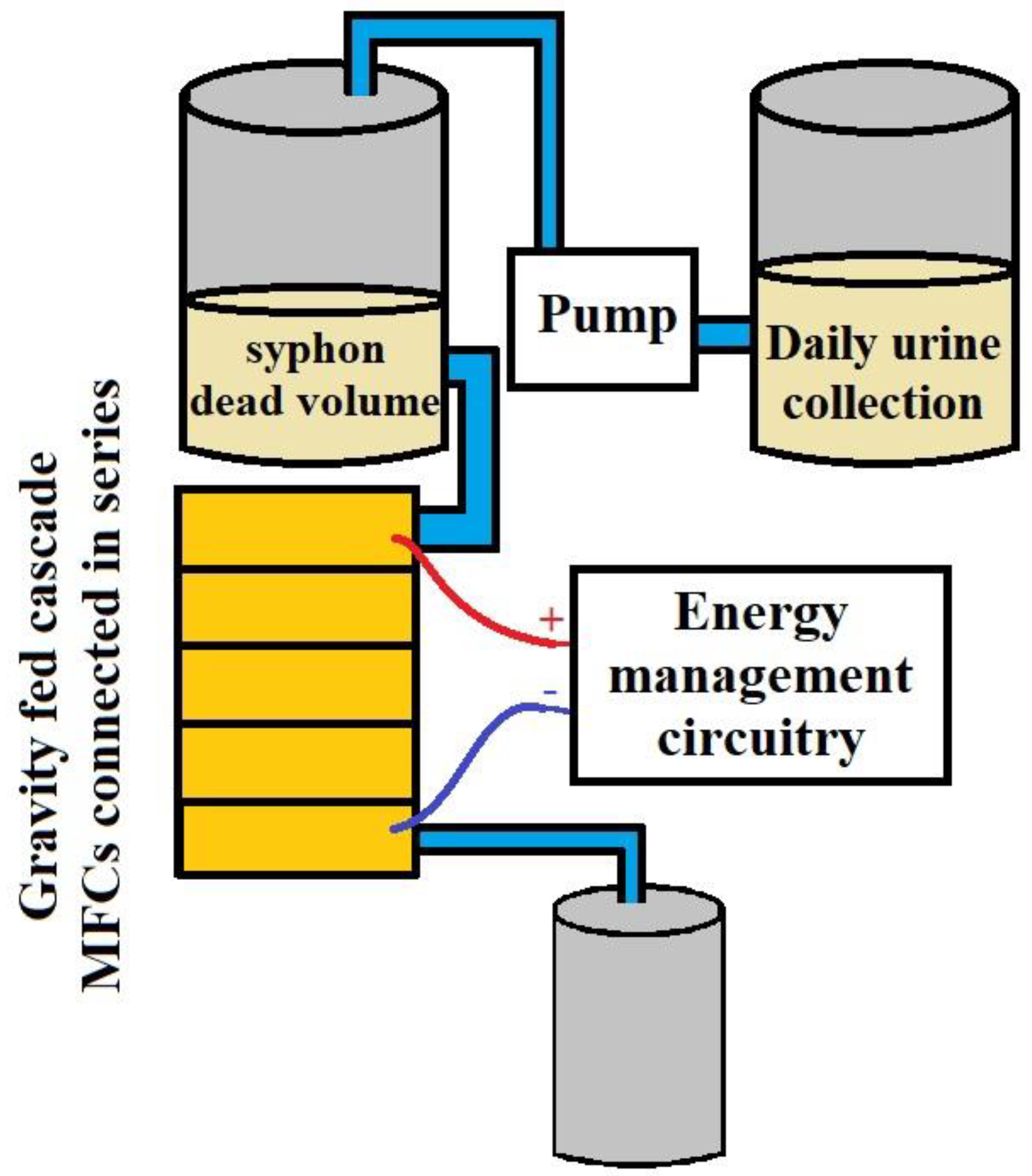
5. Integrating Human Waste with Microbial Fuel Cells
6. Power Production
7. Techno-Economic and Cost Analysis
8. Feasibility Studies
9. Prospects
10. Challenges
11. Strategies for Enhanced Efficiency
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Sample Survey Office. Ministry of Statistics and Programme Implementation Swachhta Status Report; National Sample Survey Office: Delhi, India, 2016.
- Progress on Household Drinking Water, Sanitation and Hygiene, 2000–2020: Five Years into the SDGs. Available online: https://data.unicef.org/resources/progress-on-household-drinking-water-sanitation-and-hygiene-2000-2020/ (accessed on 1 July 2022).
- Jangra, B.; Majra, J.P.; Singh, M. Swachh Bharat Abhiyan (Clean India Mission): SWOT Analysis. Int. J. Community Med. Public Health 2016, 3, 3285–3290. [Google Scholar] [CrossRef] [Green Version]
- Tiquia-Arashiro, S.M.; Pant, D. Microbial Electrochemical Technologies. Available online: https://www.taylorfrancis.com/books/edit/10.1201/9780429487118/microbial-electrochemical-technologies-sonia-tiquia-arashiro-deepak-pant (accessed on 1 July 2022).
- Jadhav, D.A.; Jain, S.C.; Ghangrekar, M.M. Simultaneous Wastewater Treatment, Algal Biomass Production and Electricity Generation in Clayware Microbial Carbon Capture Cells. Appl. Biochem. Biotechnol. 2017, 183, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Deshpande, P.A.; Ghangrekar, M.M. Enhancing the Performance of Single-Chambered Microbial Fuel Cell Using Manganese/Palladium and Zirconium/Palladium Composite Cathode Catalysts. Bioresour. Technol. 2017, 238, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Chendake, A.D.; Schievano, A.; Pant, D. Suppressing Methanogens and Enriching Electrogens in Bioelectrochemical Systems. Bioresour. Technol. 2019, 277, 148–156. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghangrekar, M.M. Effective Ammonium Removal by Anaerobic Oxidation in Microbial Fuel Cells. Environ. Technol. 2015, 36, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, D.A.; Ghosh Ray, S.; Ghangrekar, M.M. Third Generation in Bio-Electrochemical System Research—A Systematic Review on Mechanisms for Recovery of Valuable by-Products from Wastewater. Renew. Sustain. Energy Rev. 2017, 76, 1022–1031. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A Review of the Substrates Used in Microbial Fuel Cells (MFCs) for Sustainable Energy Production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent Advances in the Use of Different Substrates in Microbial Fuel Cells toward Wastewater Treatment and Simultaneous Energy Recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Colombo, A.; Marzorati, S.; Lucchini, G.; Cristiani, P.; Pant, D.; Schievano, A. Assisting Cultivation of Photosynthetic Microorganisms by Microbial Fuel Cells to Enhance Nutrients Recovery from Wastewater. Bioresour. Technol. 2017, 237, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Sevda, S.; Sreekishnan, T.R.; Pous, N.; Puig, S.; Pant, D. Bioelectroremediation of Perchlorate and Nitrate Contaminated Water: A Review. Bioresour. Technol. 2018, 255, 331–339. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Jain, S.C.; Ghangrekar, M.M. Cow’s Urine as a Yellow Gold for Bioelectricity Generation in Low Cost Clayware Microbial Fuel Cell. Energy 2016, 113, 76–84. [Google Scholar] [CrossRef]
- Ghadge, A.N.; Jadhav, D.A.; Ghangrekar, M.M. Wastewater Treatment in Pilot-Scale Microbial Fuel Cell Using Multielectrode Assembly with Ceramic Separator Suitable for Field Applications. Environ. Prog. Sustain. Energy 2016, 35, 1809–1817. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghadge, A.N.; Ghangrekar, M.M. Simultaneous Organic Matter Removal and Disinfection of Wastewater with Enhanced Power Generation in Microbial Fuel Cell. Bioresour. Technol. 2014, 163, 328–334. [Google Scholar] [CrossRef]
- Rossi, R.; Jones, D.; Myung, J.; Zikmund, E.; Yang, W.; Gallego, Y.A.; Pant, D.; Evans, P.J.; Page, M.A.; Cropek, D.M.; et al. Evaluating a Multi-Panel Air Cathode through Electrochemical and Biotic Tests. Water Res. 2019, 148, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hiegemann, H.; Littfinski, T.; Krimmler, S.; Lübken, M.; Klein, D.; Schmelz, K.-G.; Ooms, K.; Pant, D.; Wichern, M. Performance and Inorganic Fouling of a Submergible 255 L Prototype Microbial Fuel Cell Module during Continuous Long-Term Operation with Real Municipal Wastewater under Practical Conditions. Bioresour. Technol. 2019, 294, 122227. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Greenman, J.; Ieropoulos, I.A. Microbial Fuel Cells in the House: A Study on Real Household Wastewater Samples for Treatment and Power. Sustain. Energy Technol. Assess. 2021, 48, 101618. [Google Scholar] [CrossRef]
- Hu, H.; Zhou, X. Biochemical Treatment and Utilization of Fecal Wastewater. Open Access Libr. J. 2020, 7, 104324. [Google Scholar] [CrossRef]
- Sabin, J.M.; Leverenz, H.; Bischel, H.N. Microbial Fuel Cell Treatment Energy-Offset for Fertilizer Production from Human Urine. Chemosphere 2022, 294, 133594. [Google Scholar] [CrossRef]
- Fangzhou, D.; Zhenglong, L.; Shaoqiang, Y.; Beizhen, X.; Hong, L. Electricity Generation Directly Using Human Feces Wastewater for Life Support System. Acta Astronaut. 2011, 68, 1537–1547. [Google Scholar] [CrossRef]
- Kretzschmar, J.; Riedl, S.; Brown, R.K.; Schröder, U.; Harnisch, F. ELatrine: Lessons Learned from the Development of a Low-Tech MFC Based on Cardboard Electrodes for the Treatment of Human Feces. J. Electrochem. Soc. 2016, 164, H3065. [Google Scholar] [CrossRef]
- Castro, C. The Green Latrine: Development of a Large Scale Microbial Fuel Cell for the Treatment of Human Waste in Developing Areas. Environ. Water Resour. Eng. Masters Proj. 2014. [Google Scholar] [CrossRef]
- Fahad, A.; Radin Mohamed, R.M.S.; Saphira, M.; Radhi, B.; Al-Sahari, M. Wastewater and Its Treatment Techniques: An Ample Review. Indian J. Sci. Technol. 2019, 12, 13. [Google Scholar] [CrossRef]
- Austin, D.; Liu, L.; Dong, R. Performance of Integrated Household Constructed Wetland for Domestic Wastewater Treatment in Rural Areas. Ecol. Eng. 2011, 37, 948–954. [Google Scholar] [CrossRef]
- Badejo, A.A.; Omole, D.; Ndambuki, J. Municipal Wastewater Management Using Vetiveria Zizanioides Planted in Vertical Flow Constructed Wetland. Appl. Water Sci. 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Yang, X.; Spinosa, L. Development of Sludge-Based Adsorbents: Preparation, Characterization, Utilization and Its Feasibility Assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef]
- Badejo, A.A.; Omole, D.; Ndambuki, J.; Kupolati, W. Municipal Wastewater Treatment Using Sequential Activated Sludgereactor and Vegetated Submerged Bed Constructed Wetland Plantedwith Vetiveria Zizanioides. Ecol. Eng. 2017, 99, 525–529. [Google Scholar] [CrossRef]
- Kaur, T. Vermicomposting: An Effective Option for Recycling Organic Wastes. In Organic Agriculture; IntechOpen: London, UK, 2020. [Google Scholar]
- Tang, Z.; Li, W.; Tam, V.; Xue, C. Advanced Progress in Recycling Municipal and Construction Solid Wastes for Manufacturing Sustainable Construction Materials. Resour. Conserv. Recycl. 2020, 6, 100036. [Google Scholar] [CrossRef]
- Andersson, K.; Dickin, S.; Rosemarin, A. Towards “Sustainable” Sanitation: Challenges and Opportunities in Urban Areas. Sustainability 2016, 8, 1289. [Google Scholar] [CrossRef] [Green Version]
- Bhat, M.; Adil, A.; Sikander, B.; Lone, Y.; Malik, J. Waste Management Technology for Sustainable Agriculture: Waste Management. In Research Anthology on Strategies for Achieving Agricultural Sustainability; IGI Global: Hershey, PA, USA, 2022; pp. 379–398. ISBN 978-1-66845-352-0. [Google Scholar]
- Malav, L.; Yadav, K.K.; Gupta, N.; Kumar, S.; Sharma, G.; Krishnan, S.; Rezania, S.; Kamyab, H.; Pham, Q.; Yadav, S.; et al. A Review on Municipal Solid Waste as a Renewable Source for Waste-to-Energy Project in India: Current Practices, Challenges, and Future Opportunities. J. Clean. Prod. 2020, 277, 123227. [Google Scholar] [CrossRef]
- Adesanya, A. Africa in a Globalised World; Evaluation of Globalization and Development in Africa. Int. J. Innov. Soc. Sci. Humanit. Res. 2022, 10, 73–78. [Google Scholar]
- Kandel, A. On a Report On E-Waste Management E-Waste Management; 2022. [Google Scholar]
- Eghtesadifard, M.; Afkhami, P.; Bazyar, A. An Integrated Approach to the Selection of Municipal Solid Waste Landfills through GIS, K-Means and Multi-Criteria Decision Analysis. Environ. Res. 2020, 185, 109348. [Google Scholar] [CrossRef]
- Powell, J.; Chertow, M.; Esty, D. Where Is Global Waste Management Heading? An Analysis of Solid Waste Sector Commitments from Nationally-Determined Contributions. Waste Manag. 2018, 80, 137–143. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance Evaluation of Anaerobic Digestion Technology for Energy Recovery from Organic Fraction of Municipal Solid Waste: A Review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Maqsoom, A.; Ullah, F.; Aslam, B.; Sami Ur Rehman, M.; Albattah, M.; Diaz, G. Solid Waste Management; Mittal Publications: Chandigarh, India, 2022. [Google Scholar]
- Dong, Y.; Wang, Y.; Tian, Z.; Li, Y.; Lin, Y.; Oloman, C.; Gyenge, E.; Su, J.; Chen, L. Enhanced ORR Performance of Pt Catalysts in Acidic Media by Coupling with Topological Carbon Defects. Innovation 2021, 2, 100161. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Raheem, H.; Korri-Youssoufi, H.; Hassan, R. Biofilm and the Electron Transfer Mechanism in Bioelectrochemical Systems. In Bio-Electrochemical Systems; CRC Press: Boca Raton, FL, USA, 2022; pp. 65–91. ISBN 978-1-00-322543-0. [Google Scholar]
- Philips, J.; Verbeeck, K.; Rabaey, K.; Arends, J. Electron Transfer Mechanisms in Biofilms. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing: Sawston, CA, USA, 2015; pp. 67–113. ISBN 978-1-78242-375-1. [Google Scholar]
- Montaño López, J.; Duran, L.; Avalos, J.L. Physiological Limitations and Opportunities in Microbial Metabolic Engineering. Nat. Rev. Microbiol. 2022, 20, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kong, T.; Wang, Q.; Jiang, J.; Zhou, Q.; Li, P.; Zhu, B.; Gu, Q. Regulation of Microbial Metabolism on the Formation of Characteristic Flavor and Quality Formation in the Traditional Fish Sauce during Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, S.; Seelam, J.; Mohanakrishna, G. Anodic Electron Transfer Mechanism in Bioelectrochemical Systems. In Microbial Fuel Cell: A Bioelectrochemical System that Converts Waste to Watts; Springer International Publishing: Cham, Switzerland; Capital Publishung Company: New Delhi, India, 2018; pp. 87–100. ISBN 978-3-319-66792-8. [Google Scholar]
- Bhagchandanii, D.D.; Babu, R.P.; Sonawane, J.M.; Khanna, N.; Pandit, S.; Jadhav, D.A.; Khilari, S.; Prasad, R. A Comprehensive Understanding of Electro. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Deng, X.; Okamoto, A. Electrode Potential Dependency of Single-Cell Activity Identifies the Energetics of Slow Microbial Electron Uptake Process. Front. Microbiol. 2018, 9, 2744. [Google Scholar] [CrossRef] [Green Version]
- Grandrath, R.; Bohrmann-Linde, C. Teaching Sustainability in the Chemistry Classroom: Exploring Fuel Cells in Simple Hands-on Experiments with Hydrogen, Sugar and Alcohol. World J. Chem. Educ. 2019, 7, 172–178. [Google Scholar] [CrossRef]
- Wu, Y.; Jing, X.; Gao, C.; Huang, Q.; Cai, P. Recent Advances in Microbial Electrochemical System for Soil Bioremediation. Chemosphere 2018, 211, 156–163. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Simoes, M.; Santos, J.G.; Peixoto, L.; Martins, C.R.; Silva, B.P.; Neto, A.O.; Brito, A.G.; Maiorano, A.E. Application of Microbial Fuel Cell Technology for Vinasse Treatment and Bioelectricity Generation. Biotechnol. Lett. 2018, 41, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.; Chen, B.-Y.; Hong, J. Unraveling Interactive Characteristics of Microbial Community Associated with Bioelectric Energy Production in Sludge Fermentation Fluid-Fed Microbial Fuel Cells. Bioresour. Technol. 2019, 289, 121652. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.; Logan, B.E. Production of Electricity from Acetate or Butyrate Using a Single-Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hatzell, M.C.; Cusick, R.D.; Logan, B.E. Microbial Reverse-Electrodialysis Chemical-Production Cell for Acid and Alkali Production. Electrochem. Commun. 2013, 31, 52–55. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; He, Z. Graphene-Modified Electrodes for Enhancing the Performance of Microbial Fuel Cells. Nanoscale 2015, 7, 7022–7029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clauwaert, P.; Van der Ha, D.; Boon, N.; Verbeken, K.; Verhaege, M.; Rabaey, K.; Verstraete, W. Open Air Biocathode Enables Effective Electricity Generation with Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 7564–7569. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Lei, Y.; Li, B.; Cristiani, P. Power Generation from Wastewater Using Single Chamber Microbial Fuel Cells (MFCs) with Platinum-Free Cathodes and Pre-Colonized Anodes. Biochem. Eng. J. 2012, 62, 8–16. [Google Scholar] [CrossRef]
- Rezaei, F.; Xing, D.; Wagner, R.; Regan, J.M.; Richard, T.L.; Logan, B.E. Simultaneous Cellulose Degradation and Electricity Production by Enterobacter Cloacae in a Microbial Fuel Cell. Appl. Environ. Microbiol. 2009, 75, 3673–3678. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Liu, G.; Zhang, R.; Jin, S. Phenol Degradation in Microbial Fuel Cells. Chem. Eng. J. 2009, 147, 259–264. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Ren, N.; Wang, H.; Lee, H.; Li, N.; Zhao, Q. Accelerated Start-up of Two-Chambered Microbial Fuel Cells: Effect of Anodic Positive Poised Potential. Electrochim. Acta 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Ichihashi, O.; Hirooka, K. Removal and Recovery of Phosphorus as Struvite from Swine Wastewater Using Microbial Fuel Cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef]
- Simeon, M.I.; Asoiro, F.U.; Aliyu, M.; Raji, O.A.; Freitag, R. Polarization and Power Density Trends of a Soil-Based Microbial Fuel Cell Treated with Human Urine. Int. J. Energy Res. 2020, 44, 5968–5976. [Google Scholar] [CrossRef]
- Vogl, A.; Bischof, F.; Wichern, M. Single Chamber Microbial Fuel Cells for High Strength Wastewater and Blackwater Treatment—A Comparison of Idealized Wastewater, Synthetic Human Blackwater, and Diluted Pig Manure. Biochem. Eng. J. 2016, 115, 64–71. [Google Scholar] [CrossRef]
- Yumpu.com Field Testing of Various Microbial Fuel Cell…—Mortenson Center. Available online: https://www.yumpu.com/en/document/view/17885689/field-testing-of-various-microbial-fuel-cell-mortenson-center (accessed on 1 July 2022).
- Yazdi, H.; Alzate-Gaviria, L.; Ren, Z.J. Pluggable Microbial Fuel Cell Stacks for Septic Wastewater Treatment and Electricity Production. Bioresour. Technol. 2015, 180, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Leton, T.; Momoh, Y.; Akatah, B. Utilization of Multistage Microbial Fuel Cell for Septic Wastewater Treatment. J. Mech. Civ. Eng. 2019, 13, 80–86. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Nainamohamed, S.; Ebenezer Samuel, J.O.; Soorangkattan, S.; Muthuramalingam, J.; Kulanthaisamy, M.; Balasubramani, R.; Nguyen, D.D.; Chang, S.W.; Bolan, N.; et al. Comparative Study on Cronobacter Sakazakii and Pseudomonas Otitidis Isolated from Septic Tank Wastewater in Microbial Fuel Cell for Bioelectricity Generation. Fuel 2019, 248, 47–55. [Google Scholar] [CrossRef]
- Dacewicz, E. Waste Assessment Decision Support Systems Used for Domestic Sewage Treatment. J. Water Process Eng. 2019, 31, 100885. [Google Scholar] [CrossRef]
- Zhi, Y.; Liu, H.; Yao, L. The Effect of Suspended Sludge on Electricity Generation in Microbial Fuel Cells. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 2923–2927. [Google Scholar]
- Ghangrekar, M.M.; Shinde, V.B. Simultaneous Sewage Treatment and Electricity Generation in Membrane-Less Microbial Fuel Cell. Water Sci. Technol. 2008, 58, 37–43. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghangrekar, M.M. Optimising the Proportion of Pure and Mixed Culture in Inoculum to Enhance the Performance of Microbial Fuel Cells. Int. J. Environ. Technol. Manag. 2020, 23, 50–67. [Google Scholar] [CrossRef]
- Kuntke, P.; Śmiech, K.M.; Bruning, H.; Zeeman, G.; Saakes, M.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Ammonium Recovery and Energy Production from Urine by a Microbial Fuel Cell. Water Res. 2012, 46, 2627–2636. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Urine Utilisation by Microbial Fuel Cells; Energy Fuel for the Future. Phys. Chem. Chem. Phys. 2011, 14, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zang, G.-L.; Sheng, G.-P.; Li, W.-W.; Tong, Z.-H.; Zeng, R.J.; Shi, C.; Yu, H.-Q. Nutrient Removal and Energy Production in a Urine Treatment Process Using Magnesium Ammonium Phosphate Precipitation and a Microbial Fuel Cell Technique. Phys. Chem. Chem. Phys. 2012, 14, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee Power Urinal—Microbial Fuel Cell Technology Field Trials in the Context of Sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Kuntke, P.; Geleji, M.; Bruning, H.; Zeeman, G.; Hamelers, H.V.M.; Buisman, C.J.N. Effects of Ammonium Concentration and Charge Exchange on Ammonium Recovery from High Strength Wastewater Using a Microbial Fuel Cell. Bioresour. Technol. 2011, 102, 4376–4382. [Google Scholar] [CrossRef]
- Srivastava, P.; Abbassi, R.; Garaniya, V.; Lewis, T.; Yadav, A.K. Performance of Pilot-Scale Horizontal Subsurface Flow Constructed Wetland Coupled with a Microbial Fuel Cell for Treating Wastewater. J. Water Process Eng. 2020, 33, 100994. [Google Scholar] [CrossRef]
- Meehan, A.; Gao, H.; Lewandowski, Z. Energy Harvesting With Microbial Fuel Cell and Power Management System. IEEE Trans. Power Electron. 2011, 26, 176–181. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghadge, A.N.; Mondal, D.; Ghangrekar, M.M. Comparison of Oxygen and Hypochlorite as Cathodic Electron Acceptor in Microbial Fuel Cells. Bioresour. Technol. 2014, 154, 330–335. [Google Scholar] [CrossRef]
- Das, I.; Ghangrekar, M.M.; Satyakam, R.; Srivastava, P.; Khan, S.; Pandey, H.N. On-Site Sanitary Wastewater Treatment System Using 720-L Stacked Microbial Fuel Cell: Case Study. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020025. [Google Scholar] [CrossRef]
- Walter, X.A.; Stinchcombe, A.; Greenman, J.; Ieropoulos, I. Urine Transduction to Usable Energy: A Modular MFC Approach for Smartphone and Remote System Charging. Appl. Energy 2017, 192, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Cheng, S. Microbial Fuel Cells for Energy Production from Wastewaters: The Way toward Practical Application. J. Zhejiang Univ. Sci. A 2014, 15, 841–861. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.T.; Kim, J.R.; Premier, G.C.; Lee, T.H.; Kim, C.; Sloan, W.T. Sustainable Wastewater Treatment: How Might Microbial Fuel Cells Contribute. Biotechnol. Adv. 2010, 28, 871–881. [Google Scholar] [CrossRef]
- Katuri, K.P.; Kalathil, S.; Ragab, A.; Bian, B.; Alqahtani, M.F.; Pant, D.; Saikaly, P.E. Dual-Function Electrocatalytic and Macroporous Hollow-Fiber Cathode for Converting Waste Streams to Valuable Resources Using Microbial Electrochemical Systems. Adv. Mater. 2018, 30, 1707072. [Google Scholar] [CrossRef] [PubMed]
- Trapero, J.R.; Horcajada, L.; Linares, J.J.; Lobato, J. Is Microbial Fuel Cell Technology Ready? An Economic Answer towards Industrial Commercialization. Appl. Energy 2017, 185, 698–707. [Google Scholar] [CrossRef]
- Lefebvre, O.; Uzabiaga, A.; Chang, I.S.; Kim, B.-H.; Ng, H.Y. Microbial Fuel Cells for Energy Self-Sufficient Domestic Wastewater Treatment—A Review and Discussion from Energetic Consideration. Appl. Microbiol. Biotechnol. 2011, 89, 259–270. [Google Scholar] [CrossRef]
- Watanabe, K. Recent Developments in Microbial Fuel Cell Technologies for Sustainable Bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ghadge, A.N.; Ghangrekar, M.M. Enhancing the Power Generation in Microbial Fuel Cells with Effective Utilization of Goethite Recovered from Mining Mud as Anodic Catalyst. Bioresour. Technol. 2015, 191, 110–116. [Google Scholar] [CrossRef]
- Alzate-Gaviria, L.; García-Rodríguez, O.; Flota-Bañuelos, M.; Del Rio Jorge-Rivera, F.; Cámara-Chalé, G.; Domínguez-Maldonado, J. Stacked-MFC into a Typical Septic Tank Used in Public Housing. Biofuels 2016, 7, 79–86. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lay, C.-H.; Sen, B.; Chu, C.-Y.; Kumar, G.; Chen, C.-C.; Chang, J.-S. Fermentative Hydrogen Production from Wastewaters: A Review and Prognosis. Int. J. Hydrogen Energy 2012, 37, 15632–15642. [Google Scholar] [CrossRef]
- James Perlow Field Testing of Various Microbial Fuel Cell Designs Kampala, Uganda. Available online: https://www.yumpu.com/en/document/read/17885689/field-testing-of-various-microbial-fuel-cell-mortenson-center (accessed on 4 August 2022).
- Taghavi, M.; Stinchcombe, A.; Greenman, J.; Mattoli, V.; Beccai, L.; Mazzolai, B.; Melhuish, C.; Ieropoulos, I.A. Wearable Self Sufficient MFC Communication System Powered by Urine. In Proceedings of the Advances in Autonomous Robotics Systems, Birmingham, UK, 1–3 September 2014; Mistry, M., Leonardis, A., Witkowski, M., Melhuish, C., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 131–138. [Google Scholar]
- Santoro, C.; Ieropoulos, I.; Greenman, J.; Cristiani, P.; Vadas, T.; Mackay, A.; Li, B. Power Generation and Contaminant Removal in Single Chamber Microbial Fuel Cells (SCMFCs) Treating Human Urine. Int. J. Hydrogen Energy 2013, 38, 11543–11551. [Google Scholar] [CrossRef]
- Kumar, P. Handbook on IR-DRDO Bio-Toilets for Open Lien Maintenance; Indian Railways Centre for Advanced Maintenance Technology: Gwalior, India, 2012.
- Nazari, S.; Zinatizadeh, A.A.; Mirghorayshi, M.; Loosdrecht, M.C.M. van Waste or Gold? Bioelectrochemical Resource Recovery in Source-Separated Urine. Trends Biotechnol. 2020, 38, 990–1006. [Google Scholar] [CrossRef] [PubMed]
- Ashok, J.D. Performance Enhancement of Microbial Fuel Cells through Electrode Modifications along with Development of Bioelectric Toilet. Ph.D. Thesis, Indian Institutes of Technology, Kharagpur, India, 2017. [Google Scholar]
- Kalagbor, I. Electricity Generation from Septic Waste Water Using Septic Tank as Microbial Fuel Cell. Sustain. Energy 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Das, I.; Jadhav, D.; Ghangrekar, M. Scaling up of Microbial Fuel Cell for Treatment of Human Waste to Develop Bioelectric Toilet. In Proceedings of the International Conference on Sustainable Technologies for Intelligent Water Management, Kharagpur, India, 16–19 February 2018. [Google Scholar]

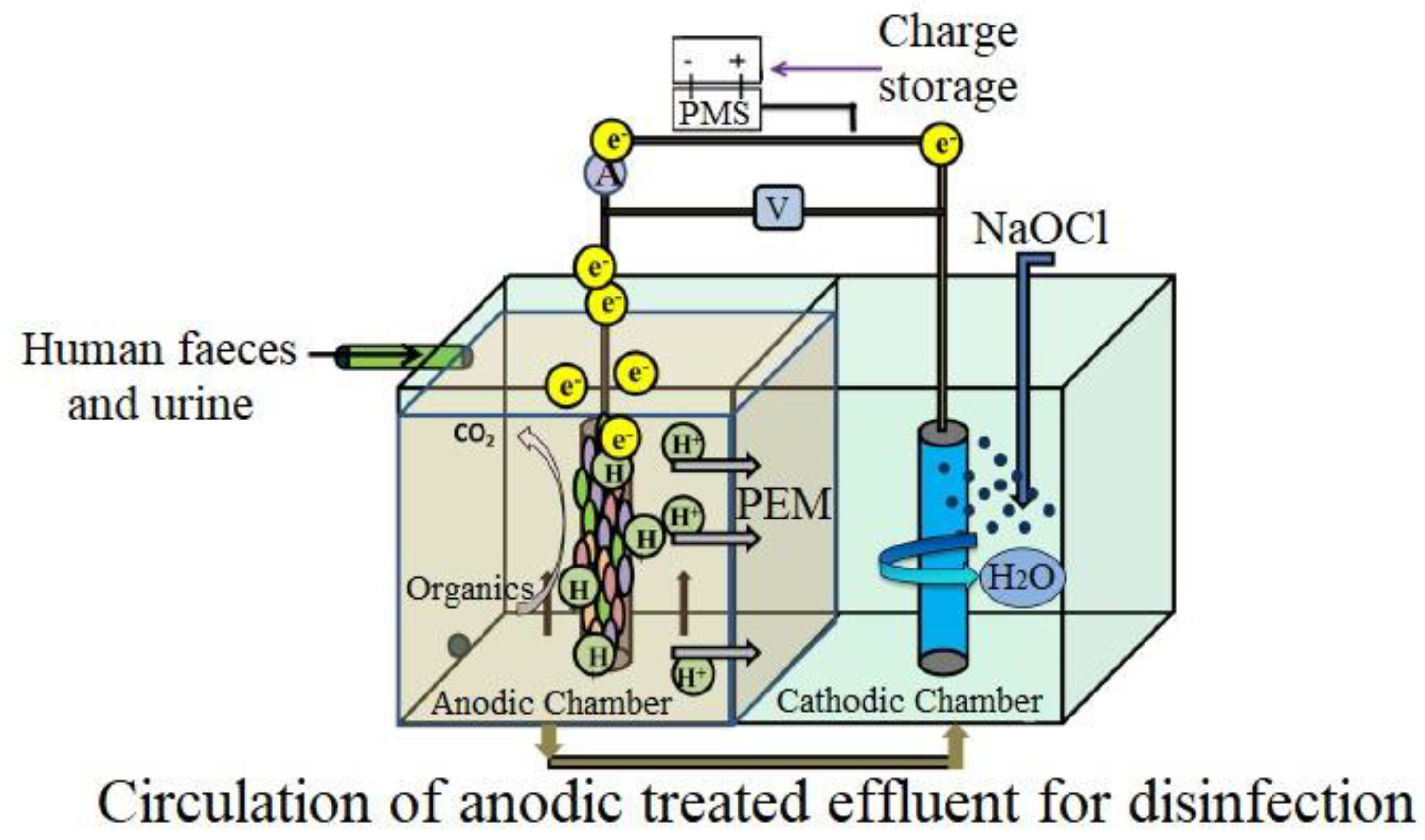
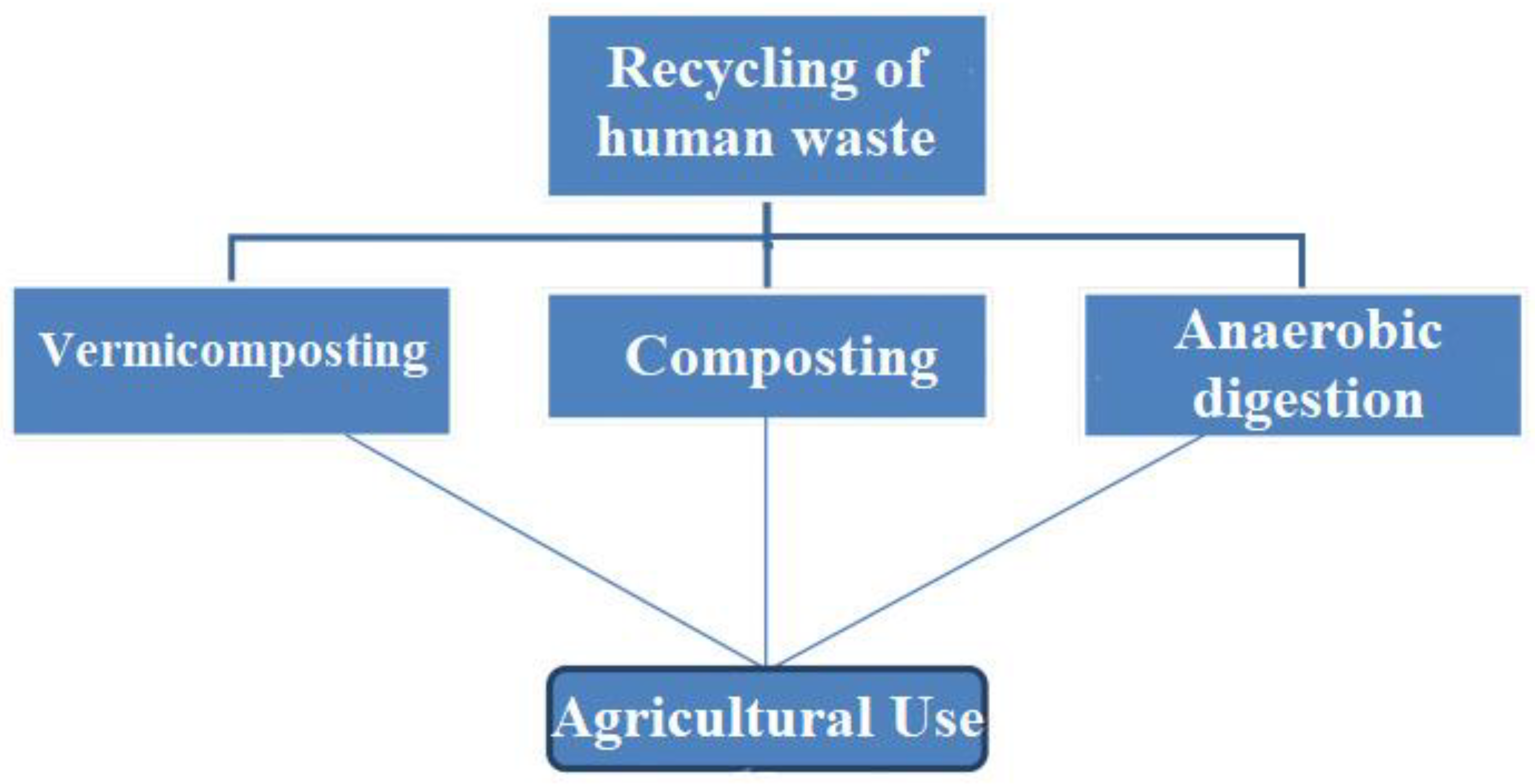
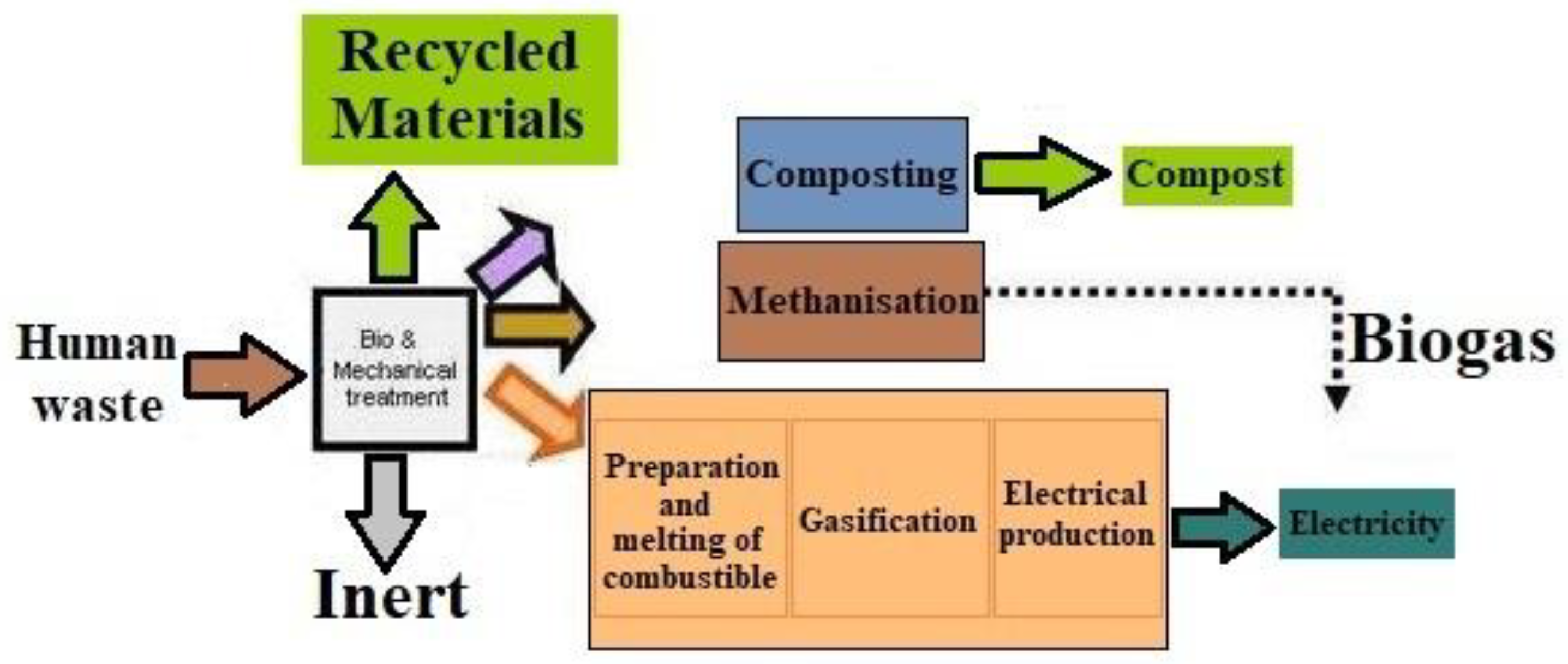
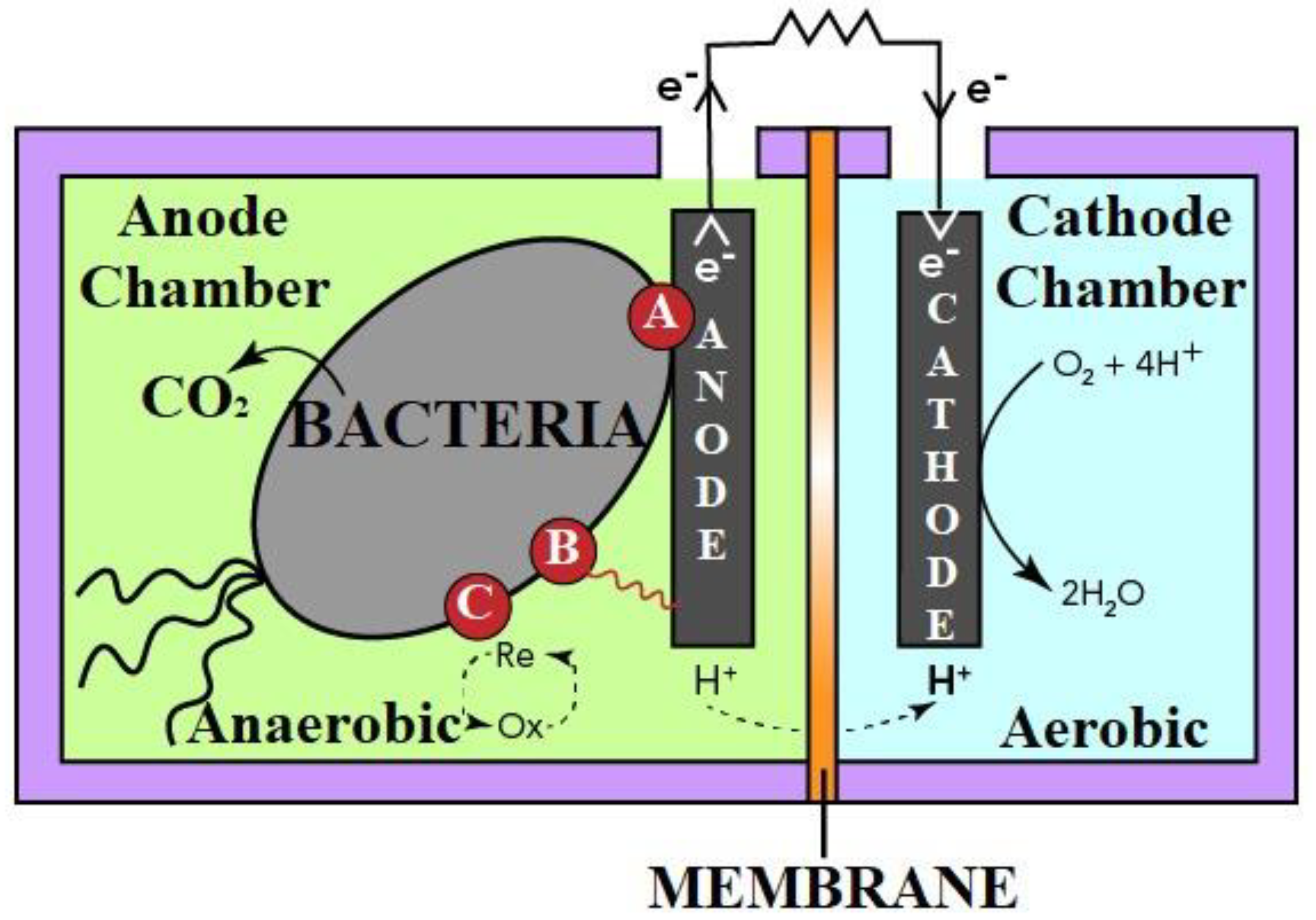
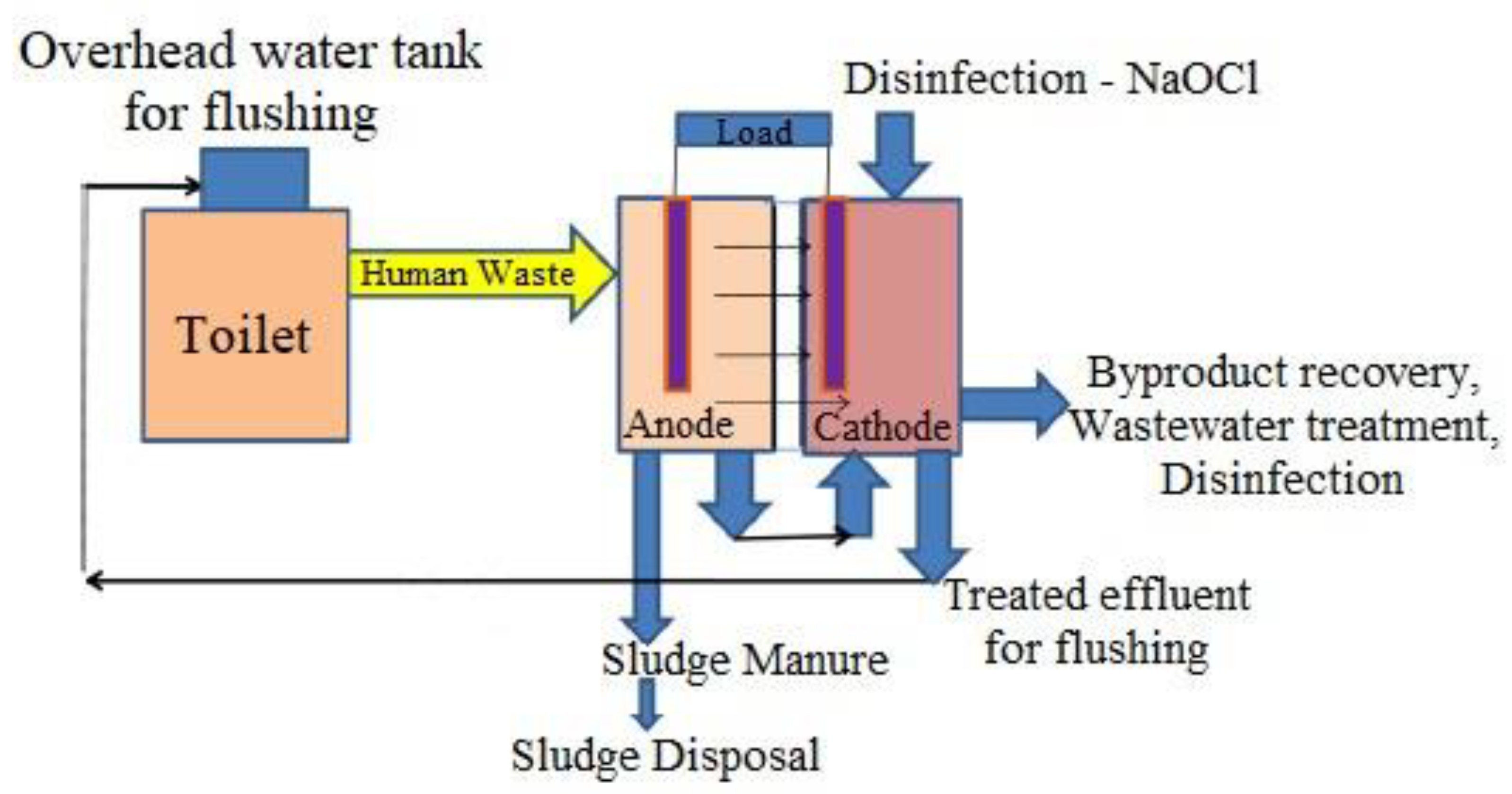
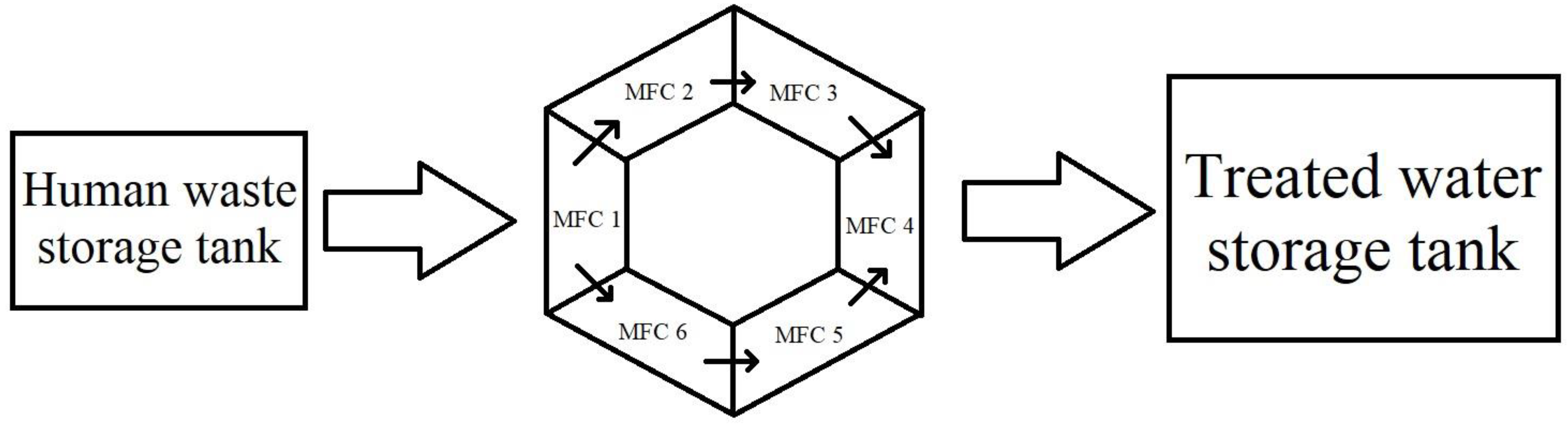
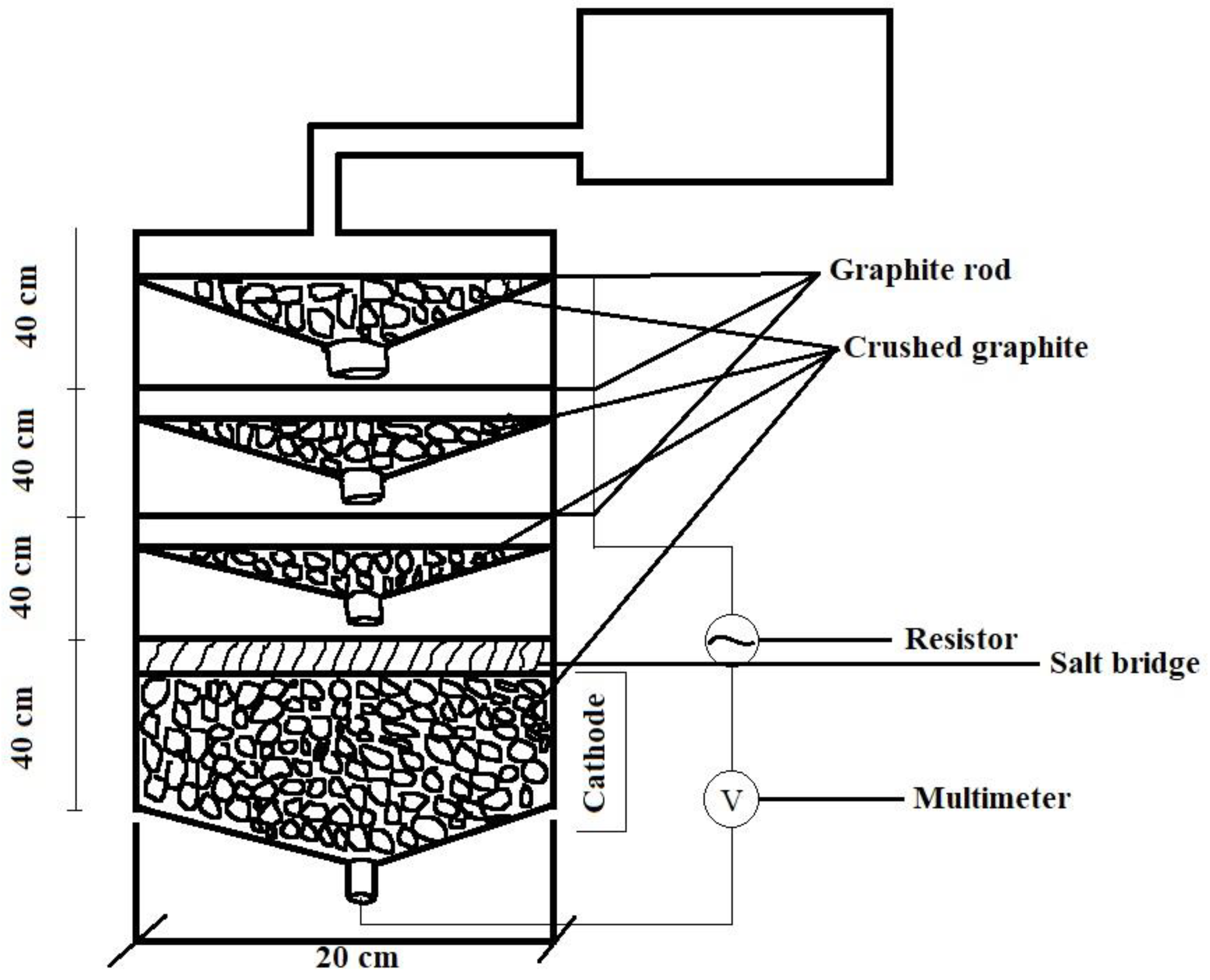
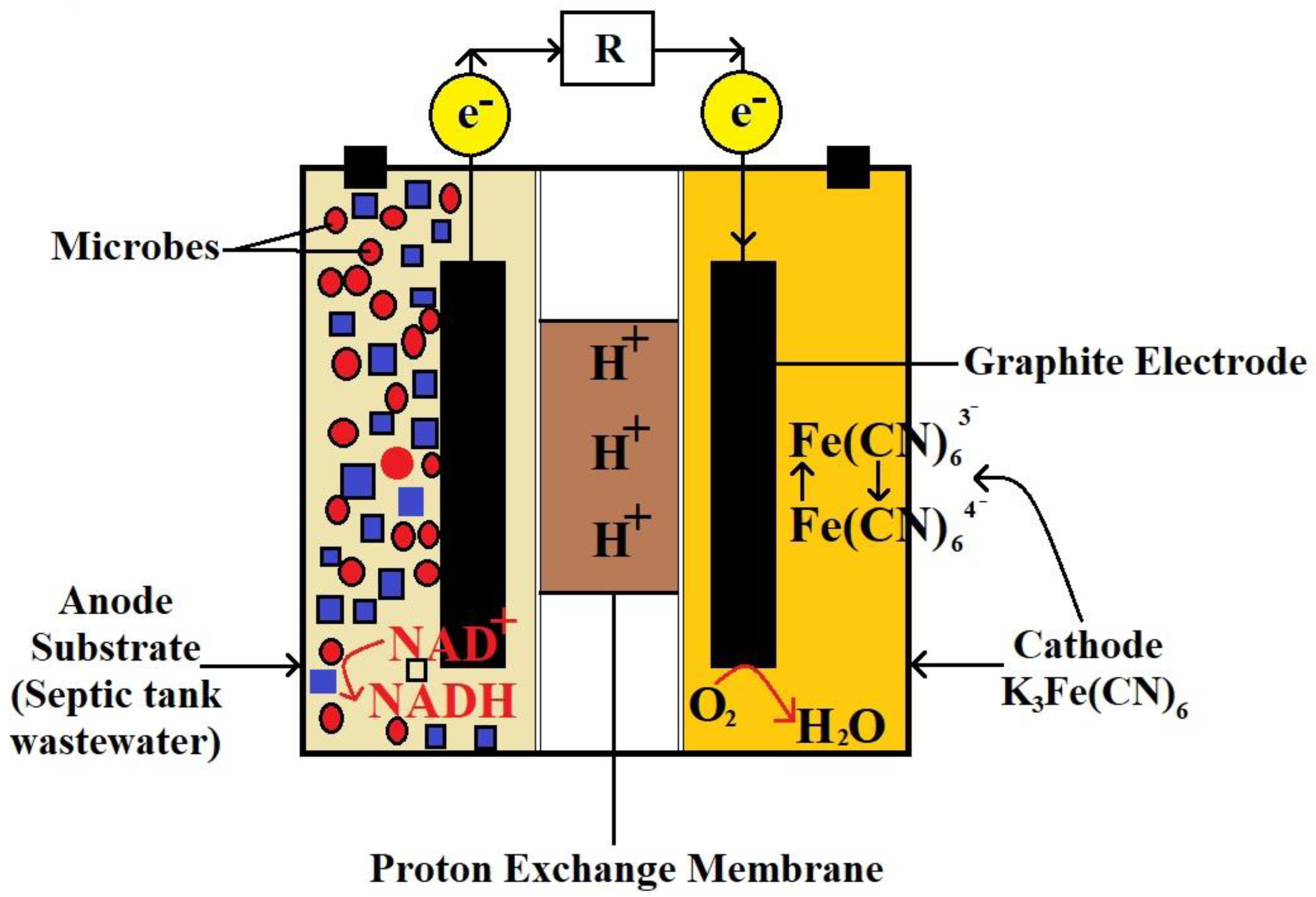
| S. No. | Technique | Working | Disadvantages |
|---|---|---|---|
| 1. | Incineration Technology | Thermal treatment technology reduces the volume of waste requiring final disposal. Incineration can typically reduce the waste volume by over 90%. | Pollution during the incineration process is a potential risk to human health, and living or working near an incineration facility can have social, economic, and psychological effects. |
| 2. | Autoclaving | Autoclaving is typically used for healthcare or industrial applications. An autoclave is a machine that uses steam under pressure to kill harmful bacteria, viruses, fungi, and spores on items placed inside a pressure vessel. | Use only limited quantities of waste. |
| 3. | Microwaving | An alternative technology to the incinerator is a steam-based process and electromagnetic waves with frequencies between radio and infrared waves that use moisture inside the waste or additional steam to sterilize waste and destroy infectious agents and pathogenic organisms in the waste. | Not as effective as other sterilizing methods at killing bacteria; heat tends to be lower. |
| 4. | Landfilling | The landfill is permanently capped with a plastic liner when it is full. After it is capped, the landfill is covered with two feet of soil. Then, vegetation (normally grass and plants without penetrating roots) is planted on top to prevent soil erosion due to rainfall and wind. | It can increase human health risks and environmental pollution if not handled carefully and properly. |
| 5. | Plasma Pyrolysis | High temperature is produced using a plasma torch in an oxygen-starved environment to convert waste efficiently and in an eco-friendly manner. | High operation cost, large initial investment and low net energy production are some of its bottlenecks. |
| Source (Substrate) | Concentration of Feed (kg COD/m3) | Resistance (Ω) | Power Density (mW/m2) | Reference |
|---|---|---|---|---|
| Cellulose | 0.4 | 10 | 0.02 | [60] |
| Phenol | 0.004 | 15 | 0.1 | [61] |
| Domestic Wastewater | 0.006 | 50 | 0.06 | [62] |
| Swine Wastewater | 0.009 | 10 | 0.7 | [63] |
| Urine | 0.25 | 10 | 4.508 | [64] |
| Human feces | 1 | 50 | 2.4 | [22] |
| Cow urine | 3 | 50 | 5.23 | [14] |
| Reactor Details | Volume (L) | Design Aspects | Power (mA) | COD Removal (%) | Reference |
|---|---|---|---|---|---|
| Single chamber MFC | 0.13 | Pt based catalyst | 0.23 | 75 | [95] |
| MPC stack of 24 MFCs | 0.0063 | Cathode with a microporous layer | 1–1.2 | - | [86] |
| Modular MFCs of 432 units | 25 | Field testing of Pee power urinals | 800 | 95 | [96] |
| Air cathode, Nafion PEM | 2 | 15 cartridges of MFCs | 124 | 89.67 | [97] |
| Pluggable flow MFC | 3 | Column air-cathode MFC | 142 | - | [65] |
| Bioelectric toilet MFC | 100 | 36 stacked MEAs | 36 | 91 | [98] |
| Hexagonal MFC | 720 | 6 chambers | 247 | - | [82] |
| Multistage cylindrical MFC | 20 | 5 sections | - | 86.4 | [68] |
| 4 chambered concrete MFC | 648 | 4 chambers | 3 | 94 | [99] |
| Bioelectric toilet | 1500 | 6 chambers | 239 | 95 | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandit, C.; Thapa, B.S.; Srivastava, B.; Mathuriya, A.S.; Toor, U.-A.; Pant, M.; Pandit, S.; Jadhav, D.-A. Integrating Human Waste with Microbial Fuel Cells to Elevate the Production of Bioelectricity. BioTech 2022, 11, 36. https://doi.org/10.3390/biotech11030036
Pandit C, Thapa BS, Srivastava B, Mathuriya AS, Toor U-A, Pant M, Pandit S, Jadhav D-A. Integrating Human Waste with Microbial Fuel Cells to Elevate the Production of Bioelectricity. BioTech. 2022; 11(3):36. https://doi.org/10.3390/biotech11030036
Chicago/Turabian StylePandit, Chetan, Bhim Sen Thapa, Bhagyashree Srivastava, Abhilasha Singh Mathuriya, Umair-Ali Toor, Manu Pant, Soumya Pandit, and Deepak-A. Jadhav. 2022. "Integrating Human Waste with Microbial Fuel Cells to Elevate the Production of Bioelectricity" BioTech 11, no. 3: 36. https://doi.org/10.3390/biotech11030036
APA StylePandit, C., Thapa, B. S., Srivastava, B., Mathuriya, A. S., Toor, U.-A., Pant, M., Pandit, S., & Jadhav, D.-A. (2022). Integrating Human Waste with Microbial Fuel Cells to Elevate the Production of Bioelectricity. BioTech, 11(3), 36. https://doi.org/10.3390/biotech11030036








