Fungicide Resistance in Botrytis spp. and Regional Strategies for Its Management in Northern European Strawberry Production
Abstract
1. Introduction
2. Fungicides and Fungicide Resistance
2.1. Multi-Site and Single-Site Fungicides
2.2. Resistance to Historic Single-Site Fungicides
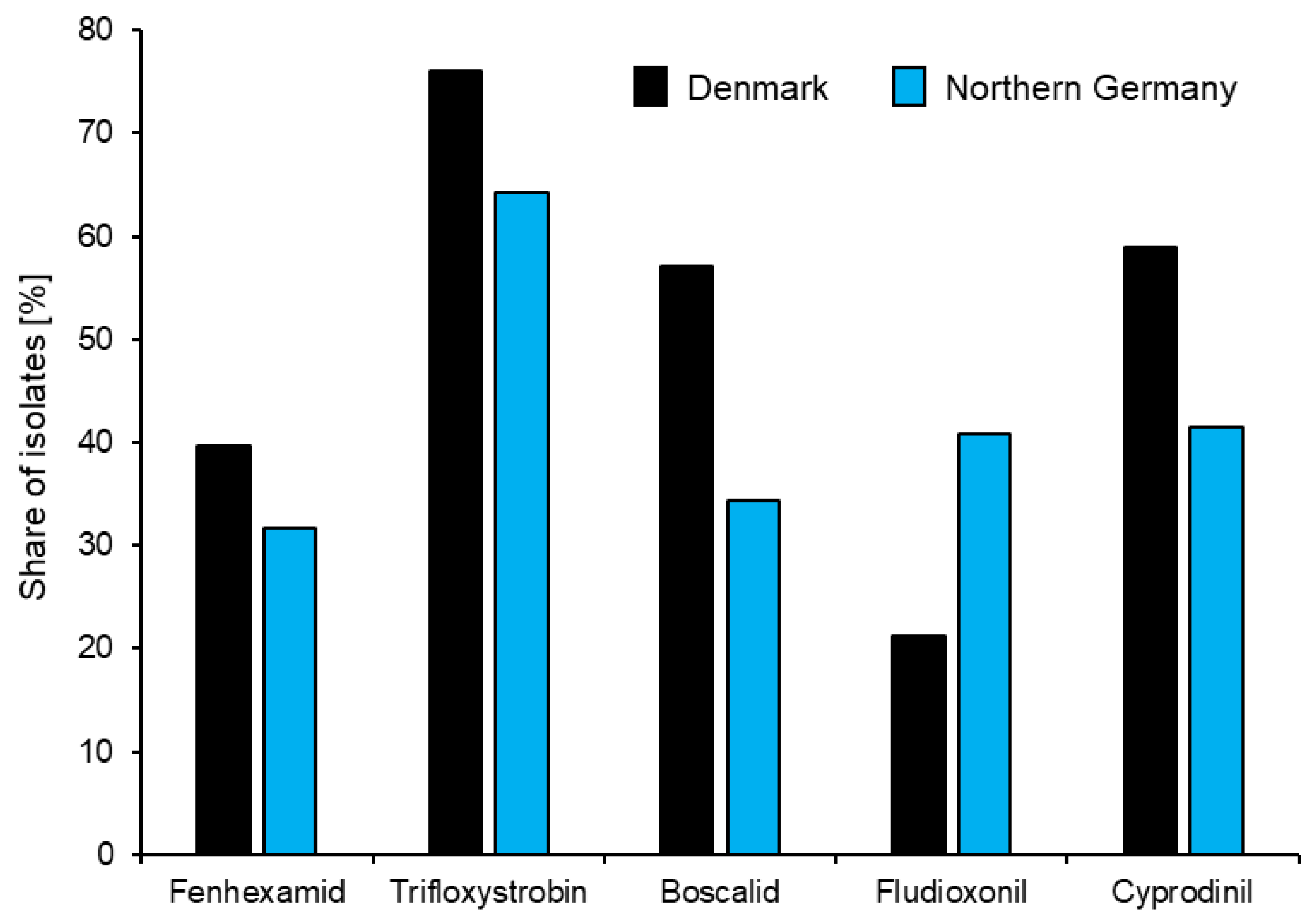
2.3. Resistance to Currently Used Single-Site Fungicides
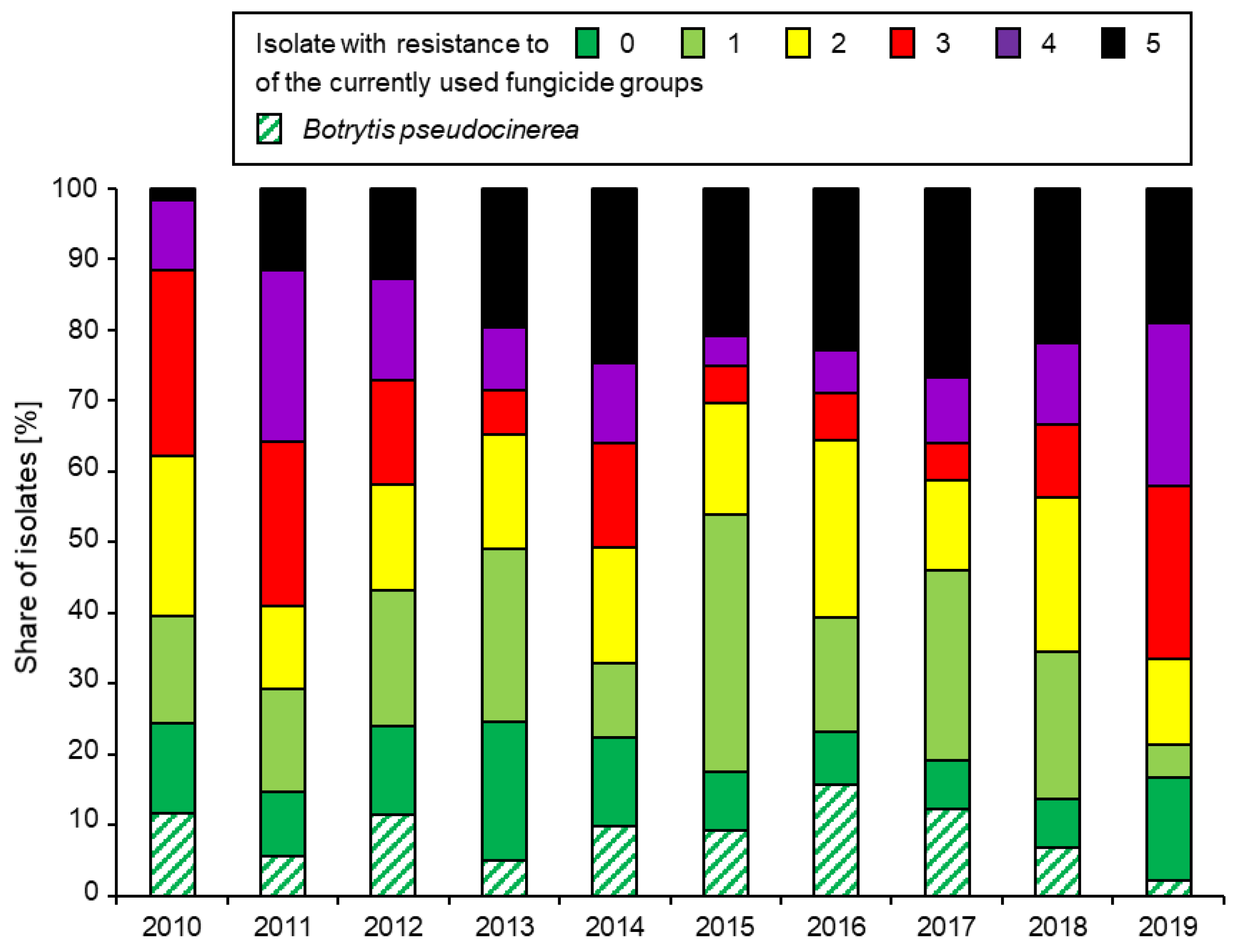
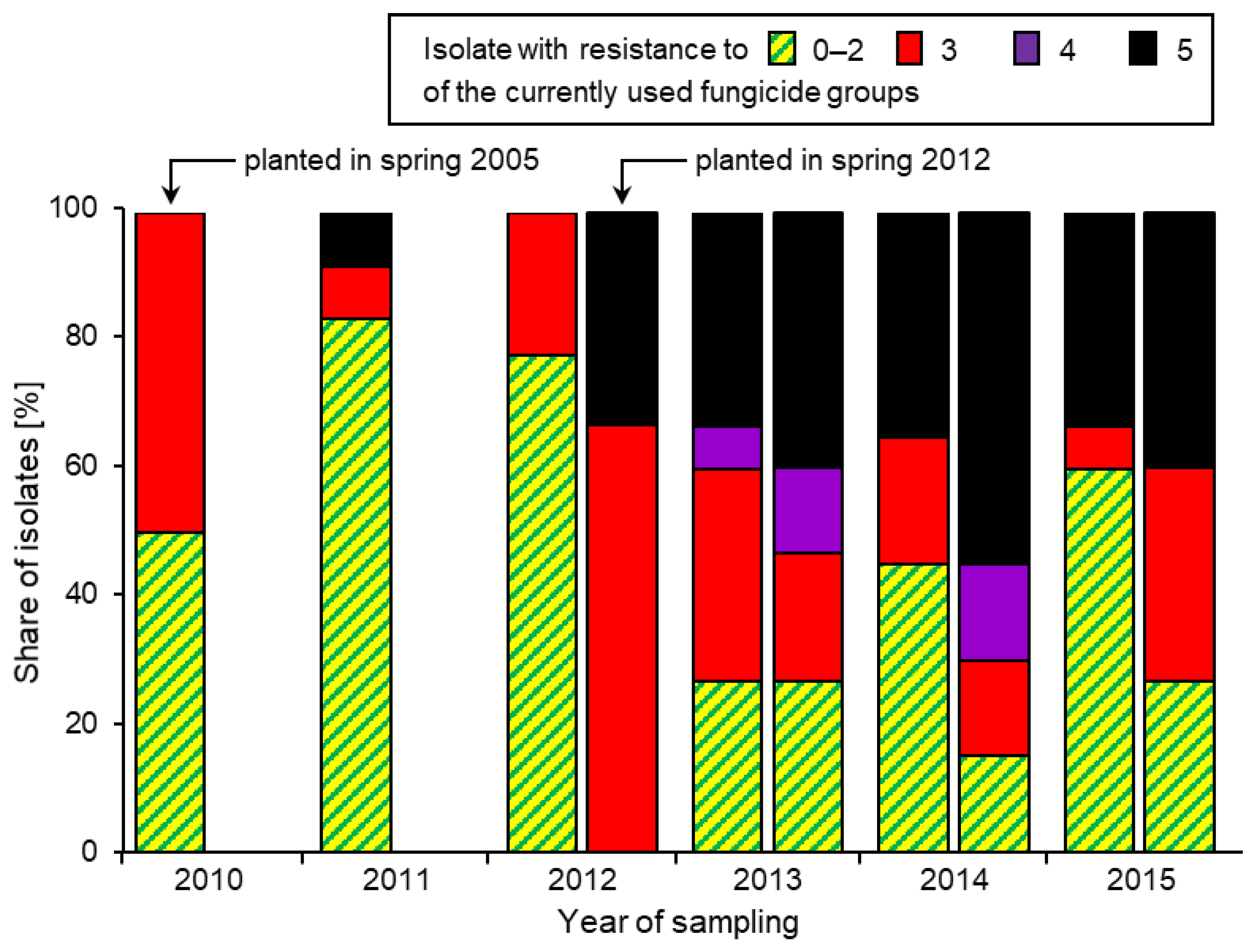
3. Resistance Development over Time
3.1. Overall Frequencies of Fungicide Resistance
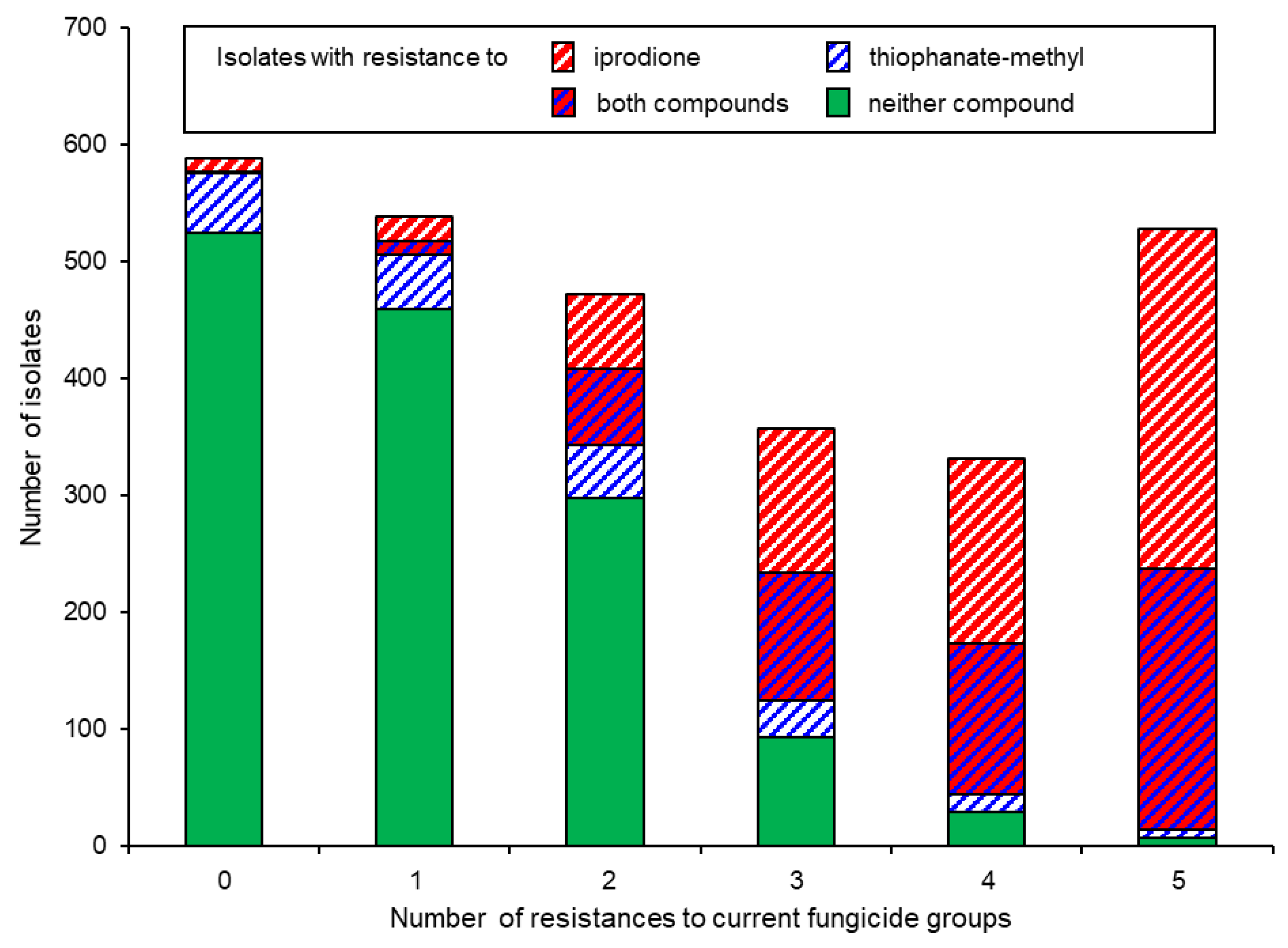
3.2. Combinations of Fungicide Resistance
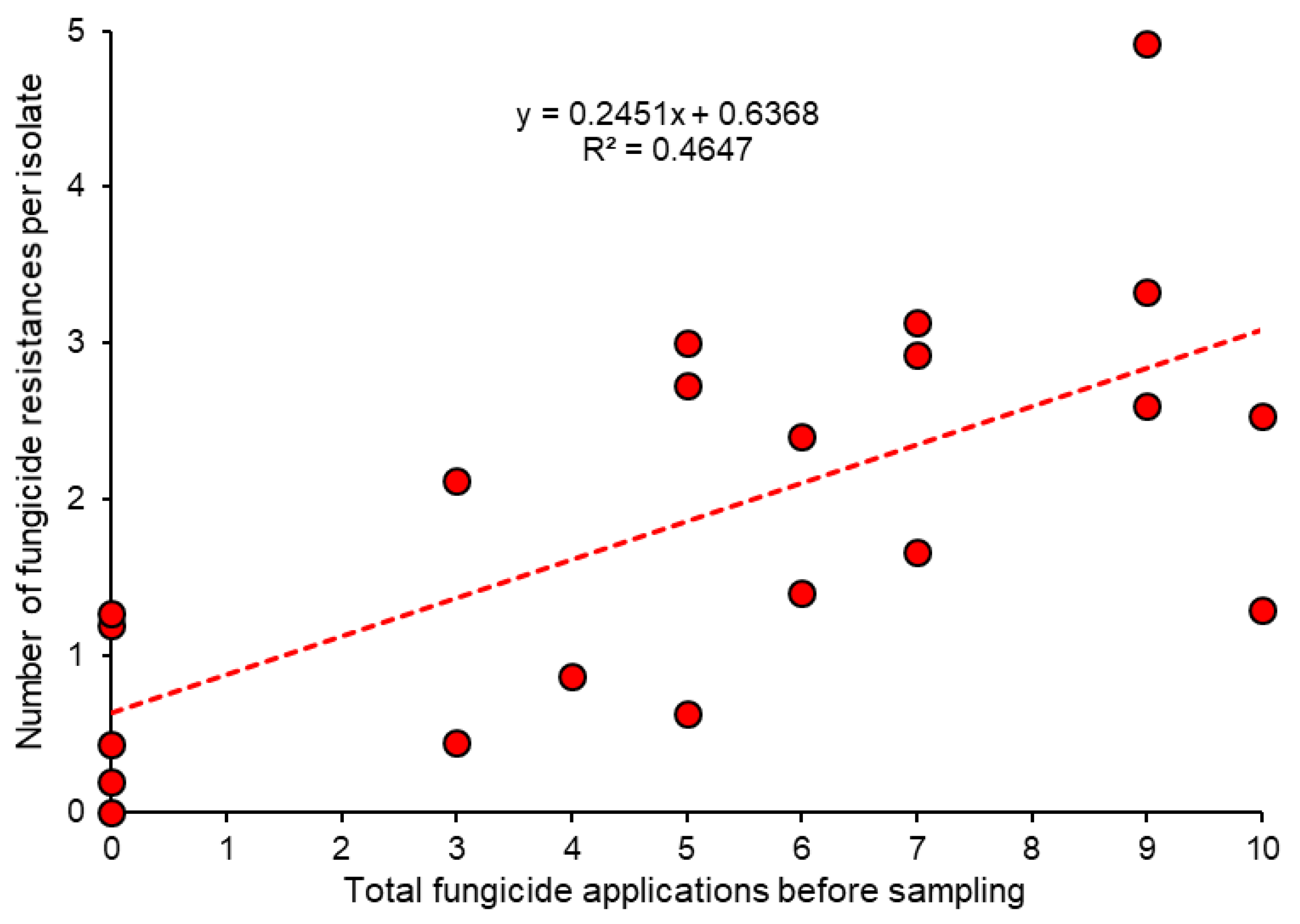
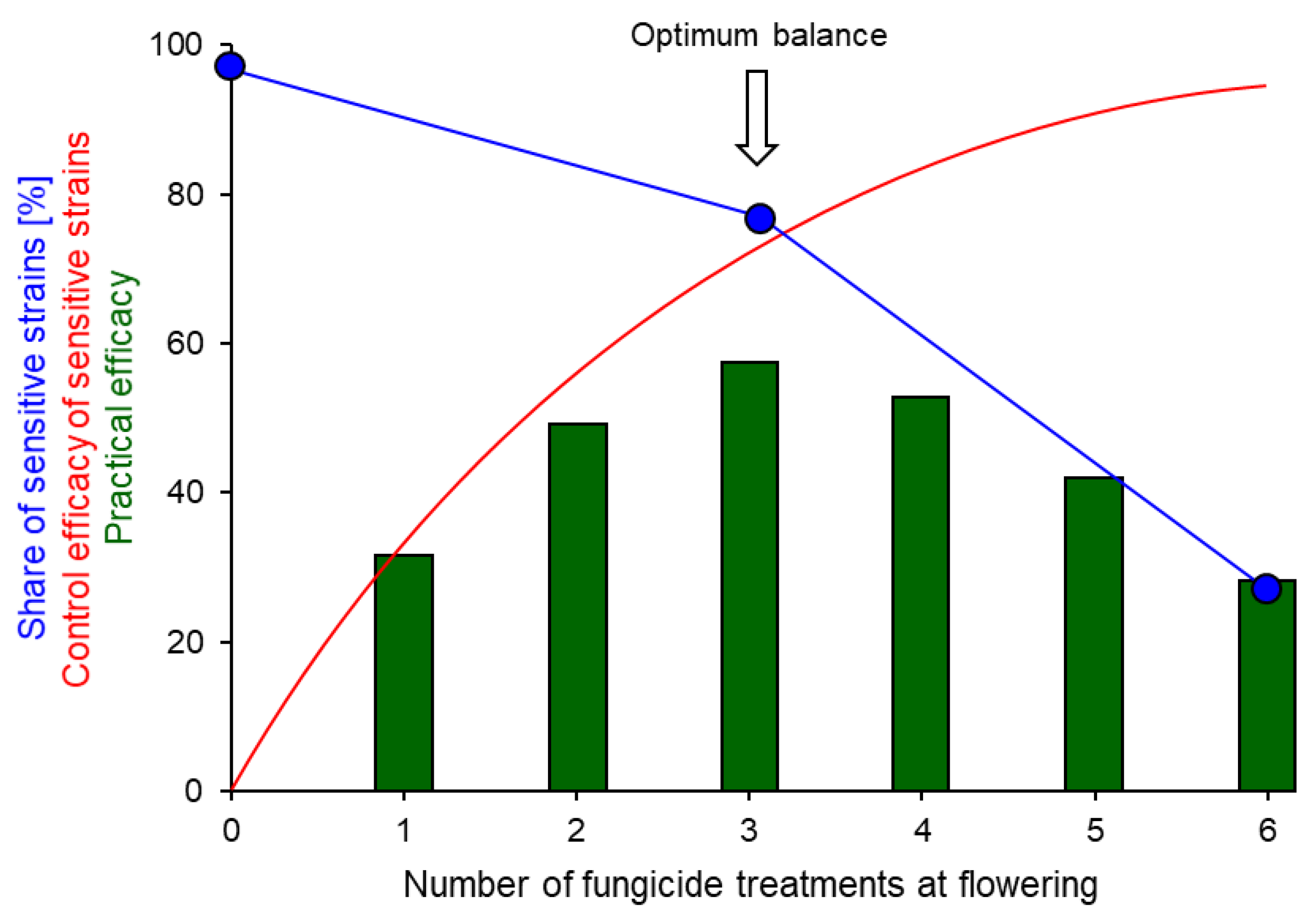
4. Selection of Fungicide Resistance in the Field
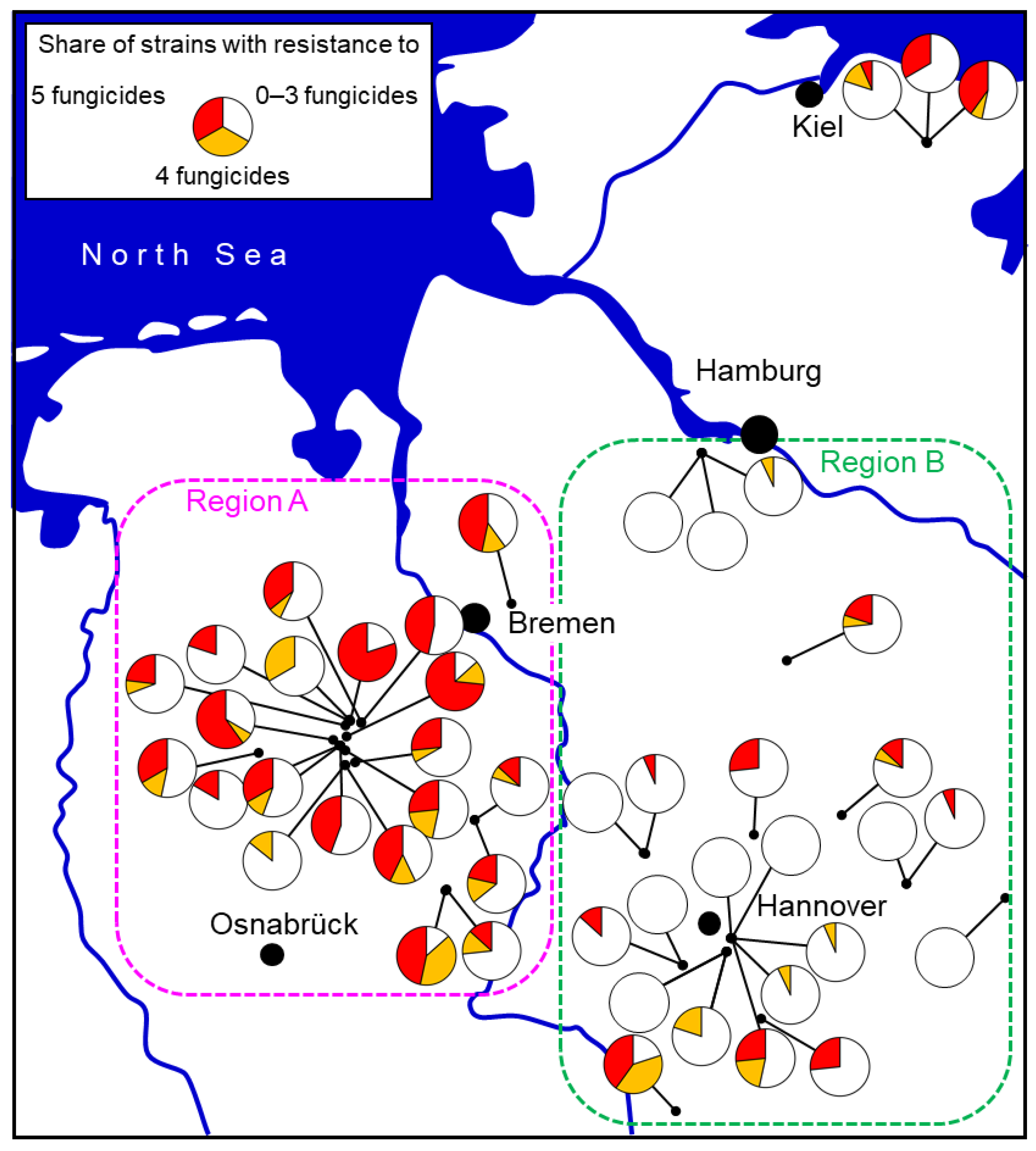
5. The Origin, Spread, and Further Fate of MR Strains
5.1. Routes of Entry of MR Strains into Strawberry Fields
5.2. Competitive Fitness of MR Strains
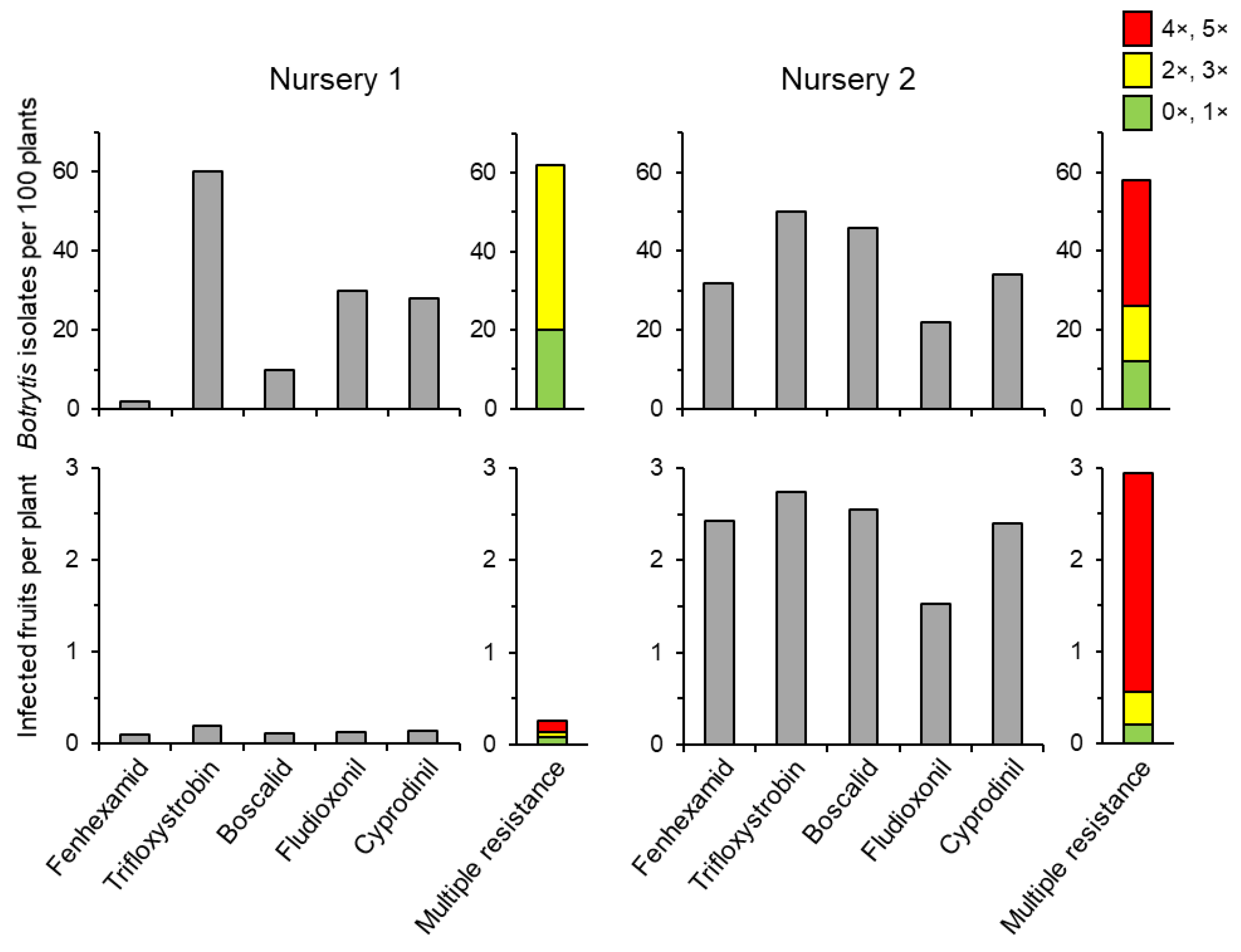
6. The Practical Relevance of Clean Nursery Material
7. Synthesis: A Practicable Concept for Managing Fungicide Resistance
7.1. Nursery Material
7.2. Intensity of Fungicide Use
7.3. Disease Forecasting and Precision Agriculture
8. The Contribution of Non-Chemical Approaches
8.1. Cultivar Susceptibility
8.2. Protected Cultivation
8.3. Aspects of Cultivation and Sanitation
8.4. Non-Chemical Crop Protection
8.5. Storage
9. Potential Environmental Impact
10. Conclusions and Further Work
- 1.
- Nursery material should be free from MR strains. Ideally a quality control scheme for nurseries should be established, especially where few businesses supply large numbers of farms or even entire production regions.
- 2.
- The use of chemical fungicides against Botrytis must be restricted to flowering, and the number of sprays should be limited. The use of disease forecasting models can further reduce the number of sprays and optimise fungicide efficacy by pinpointing suitable spray dates. The main aims are to retard the spread of MR strains, thereby maintaining fungicide efficacy at a high level.
- 3.
- There is still some way to go before effective biological control measures are ready for large-scale strawberry production in open-field culture. Further research under given regional conditions is necessary. Disease forecasting systems may help to optimise application dates. It is expected that the efficacy of BCAs will improve if the available inoculum can be reduced or infection conditions can be altered.
- 4.
- Hygiene and sanitation measures, such as the removal of dead leaves and rotting fruits at harvest, may reduce inoculum, but critical experiments on their efficacy are necessary under northern European conditions before labour-intensive measures can be recommended on a large scale.
- 5.
- The effects of cultural measures such as reduced plant spacing or reduced nitrogen fertilisation also need to be critically evaluated under the cultivation conditions prevalent in any given area.
- 6.
- Protected cultivation presents a major advance against grey mould. Little is known about the number and types of fungicide applications. Presumably, there is potential to reduce sprays. Market forces will dictate to which extent this form of cultivation is profitable in any given region. It is possible that the withdrawal of fungicides from registration will favour protected cultivation.
- 7.
- The need to achieve rapid cooling of strawberries at harvest is paramount for post-harvest stability. Especially on hot days, the fruits should be collected several times per day and placed in the cold store. There is a need for research on long-term storage conditions for northern European cultivars and marketing situations.
- 8.
- The breeding of more robust cultivars is a long-term goal. Such cultivars must be tested under regional conditions. Unfortunately, the decision about their introduction into the market is not taken by plant pathologists.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elad, Y.; Pertot, I.; Prado, A.M.C.; Stewart, A. Plant hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar] [CrossRef]
- Sutton, J.C. Botrytis fruit rot (gray mold) and blossom blight. In Compendium of Strawberry Diseases, 2nd ed.; Maas, J.L., Ed.; APS Press: St. Paul, MN, USA, 1998; pp. 28–31. [Google Scholar] [CrossRef]
- Holz, G.; Coertze, S.; Williamson, B. The ecology of Botrytis on plant host surfaces. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 9–27. [Google Scholar] [CrossRef]
- Sutton, J.C. Alternative methods for managing grey mold of strawberry. In The Strawberry into the 21st Century; Dale, A., Luby, J.S., Eds.; Timber Press: Portland, OR, USA, 1991; pp. 183–190. [Google Scholar]
- Strømeng, G.M.; Hjeljord, L.G.; Stensvand, A. Relative contribution of various sources of Botrytis cinerea inoculum in strawberry fields in Norway. Plant Dis. 2009, 93, 1305–1310. [Google Scholar] [CrossRef]
- Bulger, M.A.; Ellis, M.A.; Madden, L.V. Influence of temperature and wetness duration on infection of strawberry flowers by Botrytis cinerea and disease incidence of fruit originating from infected flowers. Phytopathology 1987, 77, 1225–1230. [Google Scholar] [CrossRef]
- Wilcox, W.F.; Seem, R.C. Relationship between strawberry gray mold incidence, environmental variables, and fungicide applications during different periods of the fruiting season. Phytopathology 1994, 84, 264–270. [Google Scholar] [CrossRef]
- Boff, P.; de Kraker, J.; Gerlagh, M.; Köhl, J. The role of petals in development of grey mould in strawberries. Fitopatol. Bras. 2003, 28, 76–83. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; Van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, W.R. The infection of strawberry and raspberry fruits by Botrytis cinerea Fr. Ann. Appl. Biol. 1962, 50, 569–575. [Google Scholar] [CrossRef]
- Walker, A.-S.; Gautier, A.; Confais, J.; Martinho, D.; Viaud, M.; Le Pêcheur, P.; Dupont, J.; Fournier, E. Botrytis pseudocinerea, a new cryptic species causing gray mold in French vineyards in sympatry with Botrytis cinerea. Phytopathology 2011, 101, 1433–1445. [Google Scholar] [CrossRef]
- Plesken, C.; Weber, R.W.S.; Rupp, S.; Leroch, M.; Hahn, M. Botrytis pseudocinerea is a significant pathogen of several crop plants but susceptible to displacement by fungicide-resistant B. cinerea strains. Appl. Environ. Microbiol. 2015, 81, 7048–7056. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Raddatz, C.; Kutz, S. Relative abundance and fungicide resistance patterns of Botrytis cinerea and B. pseudocinerea on apple in Northern Germany. J. Plant Dis. Prot. 2018, 125, 501–504. [Google Scholar] [CrossRef]
- Hauschildt, M.; Steinkellner, S.; Weber, R.W.S. Grey mould populations in Northern German sweet cherry and plum orchards: Selection of fungicide-resistant Botrytis cinerea strains over sensitive B. pseudocinerea by fungicide treatments. Eur. J. Plant Pathol. 2020, 157, 615–623. [Google Scholar] [CrossRef]
- Weber, R.W.S. Zehn Jahre Fungizidresistenztests bei Botrytis im norddeutschen Erdbeeranbau. Erw.-Obstb. 2020, 62, 155–161. [Google Scholar] [CrossRef]
- Nielsen, B.J.; Jensen, N.L.; Hartvig, P.; Hjelmroth, L.; Weber, R.W.S. Fungicide resistance in Botrytis in Danish strawberry production. Erw.-Obstb. 2021, 63, 1–6. [Google Scholar] [CrossRef]
- Rupp, S.; Plesken, C.; Rumsey, S.; Dowling, M.; Schnabel, G.; Weber, R.W.S.; Hahn, M. Botrytis fragariae, a new species causing gray mold on strawberries, shows high frequencies of specific and efflux-based fungicide resistance. Appl. Environ. Microbiol. 2017, 83, e00269-17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fernández-Ortuño, D.; Chai, W.; Wang, F.; Schnabel, G. Identification and prevalence of Botrytis spp. from blackberry and strawberry fields of the Carolinas. Plant Dis. 2012, 96, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Leroch, M.; Plesken, C.; Weber, R.W.S.; Kauff, F.; Scalliet, G.; Hahn, M. Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef]
- Walker, A.-S. Diversity within and between species of Botrytis. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 91–125. [Google Scholar] [CrossRef]
- Døving, A.; Måge, F. Prediction of strawberry fruit yield. Acta Agric. Scand. Sect. B Soil Plant Sci. 2001, 51, 35–42. [Google Scholar] [CrossRef]
- Cordova, L.G.; Amiri, A.; Peres, N.A. Effectiveness of fungicide treatments following the Strawberry Advisory System for control of Botrytis fruit rot in Florida. Crop Prot. 2017, 100, 163–167. [Google Scholar] [CrossRef]
- Leroux, P. Chemical control of Botrytis and its resistance to chemical fungicides. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 195–222. [Google Scholar] [CrossRef]
- Fillinger, S.; Walker, A.-S. Chemical control and resistance management of Botrytis diseases. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 165–187. [Google Scholar] [CrossRef]
- Shao, W.; Zhao, Y.; Ma, Z. Advances in understanding fungicide resistance in Botrytis cinerea in China. Phytopathology 2021, 111, 455–463. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Entrop, A.-P. Multiple fungicide resistance in Botrytis: A growing problem in German soft-fruit production. In Fungicides—Beneficial and Harmful Aspects; Thajuddin, N., Ed.; Intech Publishing: Rijeka, Croatia, 2011; pp. 45–60. [Google Scholar] [CrossRef][Green Version]
- Weber, R.W.S.; Hahn, M. Grey mould disease of strawberry in northern Germany: Causal agents, fungicide resistance and management strategies. Appl. Microbiol. Biotechnol. 2019, 103, 1589–1597. [Google Scholar] [CrossRef]
- Amiri, A.; Zuniga, A.I.; Peres, N.A. Potential impact of populations drift on Botrytis occurrence and resistance to multi- and single-site fungicides in Florida southern highbush blueberry fields. Plant Dis. 2018, 102, 2142–2148. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bollen, G.J.; Scholten, G. Acquired resistance to benomyl and some other systemic fungicides in a strain of Botrytis cinerea in cyclamen. Neth. J. Plant Pathol. 1971, 77, 83–90. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Scholefield, S.M. Benomyl tolerance in isolates of Botrytis cinerea from tomato plants. Ann. Appl. Biol. 1976, 82, 529–536. [Google Scholar] [CrossRef]
- Davidse, L.C.; Ishii, H. Biochemical and molecular aspects of the mechanisms of action of benzimidazoles, N-phenylcarbamates and N-phenylformamidoximes and the mechanisms of resistance to these compounds in fungi. In Modern Selective Fungicides; Lyr, H., Ed.; Gustav Fischer Verlag: Jena, Germany, 1995; pp. 305–322. [Google Scholar]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Tzavella-Klonari, K. Resistance of Botrytis cinerea isolates from vegetable crops to anilinopyrimidine, phenylpyrrole, hydroxyanilide, benzimidazole, and dicarboximide fungicides. Plant Dis. 2007, 91, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.C.; Cooke, B.K.; Jordan, V.W.L. Insensitivity of Botrytis cinerea to iprodione, procymidone and vinclozolin and their uptake by the fungus. Plant Pathol. 1979, 28, 71–76. [Google Scholar] [CrossRef]
- Northover, J.; Matteoni, J.A. Resistance of Botrytis cinerea to benomyl and iprodione in vineyards and greenhouses after exposure to the fungicides alone or mixed with captan. Plant Dis. 1986, 70, 398–402. [Google Scholar] [CrossRef]
- Faretra, F.; Pollastro, S. Genetic basis of resistance to benzimidazole and dicarboximide fungicides in Botryotinia fuckeliana (Botrytis cinerea). Mycol. Res. 1991, 95, 943–951. [Google Scholar] [CrossRef]
- Ma, Z.H.; Yan, L.Y.; Luo, Y.; Michailides, T.J. Sequence variation in the two-component histidine kinase gene of Botrytis cinerea associated with resistance to dicarboximide fungicides. Pestic. Biochem. Physiol. 2007, 88, 300–306. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Hahn, M. A rapid and simple method for determining fungicide resistance in Botrytis. J. Plant Dis. Prot. 2011, 118, 17–25. [Google Scholar] [CrossRef]
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.-S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of Botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010, 76, 6615–6630. [Google Scholar] [CrossRef]
- Markoglou, A.N.; Malandrakis, A.A.; Vitoratos, A.G.; Ziogas, B.N. Characterization of laboratory mutants of Botrytis cinerea resistant to QoI fungicides. Eur. J. Plant Pathol. 2006, 115, 149–162. [Google Scholar] [CrossRef]
- Veloukas, T.; Kalogeropoulou, P.; Markoglou, A.N.; Karaoglanidis, G.S. Fitness and competitive ability of Botrytis cinerea field isolates with dual resistance to SDHI and QoI fungicides, associated with several sdhB and the cytb G143A mutations. Phytopathology 2014, 104, 347–356. [Google Scholar] [CrossRef]
- Veloukas, T.; Markoglou, A.N.; Karaoglanidis, G.S. Differential effect of SdhB gene mutations on the sensitivity to SDHI fungicides in Botrytis cinerea. Plant Dis. 2013, 97, 118–122. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Entrop, A.-P.; Goertz, A.; Mehl, A. Status of sensitivity of Northern German Botrytis populations to the new SDHI fungicide fluopyram prior to its release as a commercial fungicide. J. Plant Dis. Prot. 2015, 122, 81–90. [Google Scholar] [CrossRef]
- Lalève, A.; Fillinger, S.; Walker, A.-S. Fitness measurement reveals contrasting costs in homologous recombinant mutants of Botrytis cinerea resistant to succinate dehydrogenase inhibitors. Fungal Genet. Biol. 2014, 67, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Heath, S.M.; Peres, N.A. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. 2014, 98, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.X.; Adnan, M.; Shang, Y.; Lin, Y.; Luo, C.X. Sensitivity of Botrytis cinerea from nectarine/cherry in China to six fungicides and characterization of resistant isolates. Plant Dis. 2018, 102, 2578–2585. [Google Scholar] [CrossRef]
- Rosslenbroich, H.-J.; Stuebler, D. Botrytis cinerea—History of chemical control and novel fungicides for its management. Crop Prot. 2000, 19, 557–561. [Google Scholar] [CrossRef]
- Billard, A.; Fillinger, S.; Leroux, P.; Lachaise, H.; Beffa, R.; Debieu, D. Strong resistance to the fungicide fenhexamid entails a fitness cost in Botrytis cinerea, as shown by comparisons of isogenic strains. Pest Manag. Sci. 2012, 68, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Debieu, D.; Bach, J.; Montesinos, E.; Fillinger, S.; Leroux, P. Role of sterol 3-ketoreductase sensitivity in susceptibility to the fungicide fenhexamid in Botrytis cinerea and other phytopathogenic fungi. Pest Manag. Sci. 2013, 69, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Esterio, M.; Osorio-Navarro, C.; Azócar, M.; Copier, C.; Rubilar, M.; Pizarro, L.; Auger, J. Reduced fitness cost and increased aggressiveness in fenhexamid-resistant Botrytis cinerea field isolates from Chile. Phytopathol. Mediterr. 2021, 60, 69–77. [Google Scholar] [CrossRef]
- Fan, F.; Zhu, Y.-X.; Wu, M.-Y.; Yin, W.-X.; Li, G.-Q.; Hahn, M.; Hamada, M.S.; Luo, C.-X. Mitochondrial inner membrane ABC transporter Bcmdl1 is involved in conidial germination, virulence, and resistance to anilinopyrimidine fungicides in Botrytis cinerea. Microbiol. Spectr. 2023, 11, e00108-23. [Google Scholar] [CrossRef]
- Hilber, U.W.; Schüepp, H. A reliable method for testing the sensitivity of Botryotinia fuckeliana to anilinopyrimidines in vitro. Pest Manag. Sci. 1996, 47, 241–247. [Google Scholar] [CrossRef]
- Latorre, B.A.; Spadaro, I.; Rioja, M.E. Occurrence of resistant strains of Botrytis cinerea to anilinopyrimidine fungicides in table grapes in Chile. Crop Prot. 2002, 21, 957–961. [Google Scholar] [CrossRef]
- Bardas, G.A.; Myresiotis, C.K.; Karaoglanidis, G.S. Stability and fitness of anilinopyrimidine-resistant strains of Botrytis cinerea. Phytopathology 2008, 98, 443–450. [Google Scholar] [CrossRef]
- Sang, C.; Ren, W.; Wang, J.; Xu, H.; Zhang, Z.; Zhou, M.; Chen, C.; Wang, K. Detection and fitness comparison of target-based highly fludioxonil-resistant isolates of Botrytis cinerea from strawberry and cucumber in China. Pestic. Biochem. Physiol. 2018, 147, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Shao, W.; Han, X.; Zhou, M.; Chen, C. Molecular and biochemical characterization of laboratory and field mutants of Botrytis cinerea resistant to fludioxonil. Plant Dis. 2016, 100, 1414–1423. [Google Scholar] [CrossRef]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef]
- Kretschmer, M.; Leroch, M.; Mosbach, A.; Walker, A.-S.; Fillinger, S.; Mernke, D.; Schoonbeek, H.-J.; Pradier, J.-M.; Leroux, P.; de Waard, M.A.; et al. Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathog. 2009, 5, e1000696. [Google Scholar] [CrossRef]
- Weber, R.W.S. Resistance of Botrytis cinerea to multiple fungicides in Northern German small fruit production. Plant Dis. 2011, 95, 1263–1269. [Google Scholar] [CrossRef]
- Rupp, S.; Weber, R.W.S.; Rieger, D.; Detzel, P.; Hahn, M. Spread of Botrytis cinerea strains with multiple fungicide resistance in German horticulture. Front. Microbiol. 2017, 7, 2075. [Google Scholar] [CrossRef]
- Latorre, B.A.; Torres, R. Prevalence of isolates of Botrytis cinerea resistant to multiple fungicides in Chilean vineyards. Crop Prot. 2012, 40, 49–52. [Google Scholar] [CrossRef]
- Cosseboom, S.; Hu, M. Identification and characterization of fungicide resistance in Botrytis populations from small fruit fields in the mid-Atlantic United States. Plant Dis. 2021, 105, 2366–2373. [Google Scholar] [CrossRef]
- Cosseboom, S.D.; Ivors, K.L.; Schnabel, G.; Bryson, P.K.; Holmes, G.J. Within-season shift in fungicide resistance profiles of Botrytis cinerea in California strawberry fields. Plant Dis. 2019, 103, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.N.; Beger, G.; Pereira, W.V.; de Mio, L.L.M.; Duarte, H.D.S.S. Gray mold in strawberries in the Paraná state of Brazil is caused by Botrytis cinerea and its isolates exhibit multiple-fungicide resistance. Crop Prot. 2021, 140, 105415. [Google Scholar] [CrossRef]
- Nielsen, K.A.G.; Skårn, M.N.; Strømeng, G.M.; Brurberg, M.B.; Stensvand, A. Pervasive fungicide resistance in Botrytis from strawberry in Norway: Identification of the grey mould pathogen and mutations. Plant Pathol. 2022, 71, 1392–1403. [Google Scholar] [CrossRef]
- Li, X.; Fernández-Ortuño, D.; Chen, S.; Grabke, A.; Luo, C.-X.; Bridges, W.C.; Schnabel, G. Location-specific fungicide resistance profiles and evidence for stepwise accumulation of resistance in Botrytis cinerea. Plant Dis. 2014, 98, 1066–1074. [Google Scholar] [CrossRef]
- Köller, W.; Wilcox, W.F. Evidence for the predisposition of fungicide-resistant phenotypes of Venturia inaequalis to a preferential selection for resistance to other fungicides. Phytopathology 2001, 91, 776–781. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Petridis, A.; Jensen, N.L.; Christensen, D.H. Mindre gråskimmel fungicidresistens i danske jordbær. Gart. Tid. 2022, 138, 28–29. [Google Scholar]
- Wedge, D.E.; Smith, B.J.; Quebedeaux, J.P.; Constantin, R.J. Fungicide management strategies for control of strawberry fruit rot diseases in Louisiana and Mississippi. Crop Prot. 2007, 26, 1449–1458. [Google Scholar] [CrossRef]
- Hu, M.-J.; Cox, K.D.; Schnabel, G. Resistance to increasing chemical classes of fungicides by virtue of “selection by association” in Botrytis cinerea. Phytopathology 2016, 106, 1513–1520. [Google Scholar] [CrossRef]
- Mernke, D.; Dahm, S.; Walker, A.-S.; Lalève, A.; Fillinger, S.; Leroch, M.; Hahn, M. Two promoter rearrangements in a drug efflux transporter gene are responsible for the appearance and spread of multidrug resistance phenotype MDR2 in Botrytis cinerea isolates in French and German vineyards. Phytopathology 2011, 101, 1176–1183. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Amiri, A.; Zuniga, A.I.; Peres, N.A. Sources of primary inoculum of Botrytis cinerea and their impact on fungicide resistance development in commercial strawberry fields. Plant Dis. 2017, 101, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.W.S.; Entrop, A.-P. Recovery of Botrytis strains with multiple fungicide resistance from raspberry nursery plants. Eur. J. Plant Pathol. 2017, 147, 933–936. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Entrop, A.-P. Recovery of fungicide-resistant Botrytis isolates from strawberry nursery plants. Eur. J. Plant Pathol. 2017, 149, 739–742. [Google Scholar] [CrossRef]
- Fan, F.; Hamada, M.S.; Li, N.; Li, G.Q.; Luo, C.X. Multiple fungicide resistance in Botrytis cinerea from greenhouse strawberries in Hubei Province, China. Phytopathology 2017, 101, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Cosseboom, S.D.; Schnabel, G.; Hu, M. Competitive ability of multi-fungicide resistant Botrytis cinerea in a blackberry planting over three years. Pestic. Biochem. Physiol. 2020, 163, 1–7. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Grabke, A.; Li, X.; Schnabel, G. Independent emergence of resistance to seven chemical classes of fungicides in Botrytis cinerea. Phytopathology 2015, 105, 424–432. [Google Scholar] [CrossRef]
- Chen, S.N.; Luo, C.X.; Hu, M.J.; Schnabel, G. Fitness and competitive ability of Botrytis cinerea isolates with resistance to multiple chemical classes of fungicides. Phytopathology 2016, 106, 997–1005. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Petridis, A. Rent plantemateriale til nye jordbærarealer. Gart. Tid. 2022, 138, 46–48. [Google Scholar]
- Gahatraj, B.; Nielsen, K.A.G.; Le, V.H.; Sønsteby, A.; Stensvand, A. Steam thermotherapy strongly reduces Botrytis in strawberry transplants with no or minor negative effects on plant growth and yield. Eur. J. Plant Pathol. 2023, 166, 109–121. [Google Scholar] [CrossRef]
- Zuniga, A.I.; Wang, N.-Y.; Peres, N.A. Heat treatment as a possible means to reduce Botrytis inoculum on strawberry transplants. Plant Health Progr. 2023, 24, 345–352. [Google Scholar] [CrossRef]
- Weber, R.W.S. Resistent gråskimmel i danske jordbær. Gart. Tid. 2016, 132, 20–21. [Google Scholar]
- Weber, R.W.S. Occurrence of Hyd R3 fenhexamid resistance among Botrytis isolates in Northern German soft fruit production. J. Plant Dis. Prot. 2010, 117, 177–179. [Google Scholar] [CrossRef]
- Ellis, M.A.; Madden, L.V. Progress in the development of disease forecasting systems for strawberry fruit rots in Ohio. In The Strawberry into the 21st Century; Dale, A., Luby, J.S., Eds.; Timber Press: Portland, OR, USA, 1991; pp. 244–251. [Google Scholar]
- MacKenzie, S.J.; Peres, N.A. Use of leaf wetness and temperature to time fungicide applications to control Botrytis fruit rot of strawberry in Florida. Plant Dis. 2012, 96, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikova, E.; Borisova, T.; Van Sickle, J.J. Evaluation of the profitability of a new precision fungicide application system for strawberry production. Agric. Syst. 2014, 130, 77–88. [Google Scholar] [CrossRef]
- Swett, C.L.; Butler, B.B.; Peres, N.A.; Koivunen, E.E.; Hellman, E.M.; Beaulieu, J.R. Using model-based fungicide programing to effectively control Botrytis and Anthracnose fruit rots in Mid-Atlantic strawberry fields and co-manage strawberry sap beetle (Stelidota geminate). Crop Prot. 2020, 134, 105175. [Google Scholar] [CrossRef]
- Petrasch, S.; Mesquida-Pasci, S.D.; Pincot, D.D.A.; Feldmann, M.J.; López, C.M.; Famula, R.; Hardigan, M.A.; Cole, G.S.; Knapp, S.J.; Blanco-Ulate, B. Genomic prediction of strawberry resistance to postharvest fruit decay caused by the fungal pathogen Botrytis cinerea. G3—Genes Genom. Genet. 2022, 12, jkab378. [Google Scholar] [CrossRef]
- Hortynski, J.A. The problem of grey mold in strawberry breeding. In The Strawberry into the 21st Century; Dale, A., Luby, J.S., Eds.; Timber Press: Portland, OR, USA, 1991; pp. 54–56. [Google Scholar]
- Bestfleisch, M.; Luderer-Pflimpfl, M.; Höfer, M.; Schulte, E.; Wünsche, J.N.; Hanke, M.-V.; Flachowsky, H. Evaluation of strawberry (Fragaria L.) genetic resources for resistance to Botrytis cinerea. Plant Pathol. 2015, 64, 396–405. [Google Scholar] [CrossRef]
- Li, H.; Larsen, D.H.; Cao, R.; van de Peppel, A.C.; Tikunov, Y.M.; Marcelis, L.F.M.; Woltering, E.J.; van Kan, J.A.L.; Schouten, R.E. The association between the susceptibility to Botrytis cinerea and the levels of volatile and non-volatile metabolites in red ripe strawberry genotypes. Food Chem. 2022, 393, 133252. [Google Scholar] [CrossRef]
- Fredericks, C.H.; Fanning, K.J.; Gidley, M.J.; Netzel, G.; Zabaras, D.; Herrington, M.; Netzel, M. High-anthocyanin strawberries through cultivar selection. J. Sci. Food Agric. 2013, 93, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Demchak, K. Small fruit production in high tunnels. HortTechnol. 2009, 19, 44–49. [Google Scholar] [CrossRef]
- Xu, X.; Wedgwood, E.; Berrie, A.M.; Allen, J.; O’Neill, T.M. Management of raspberry and strawberry grey mould in open field and under protection. A review. Agron. Sustain. Dev. 2012, 32, 531–543. [Google Scholar] [CrossRef]
- Daugovish, O.; Larson, K.D. Strawberry production with protected culture in Southern California. Acta Hortic. 2009, 842, 163–166. [Google Scholar] [CrossRef]
- Xiao, C.L.; Chandler, C.K.; Price, J.F.; Duval, J.R.; Mertely, J.C.; Legard, D.E. Comparison of epidemics of Botrytis fruit rot and powdery mildew of strawberry in large plastic tunnel and field production systems. Plant Dis. 2001, 85, 901–909. [Google Scholar] [CrossRef]
- Burlakoti, R.R.; Zandstra, J.; Jackson, K. Comparison of epidemiology of gray mold, anthracnose fruit rot, and powdery mildew in day-neutral strawberries in field and high-tunnel conditions in Ontario. Int. J. Fruit Sci. 2013, 13, 19–27. [Google Scholar] [CrossRef]
- Trandem, N. Greenhouse production of strawberries and blackberries in Norway—Arthropod pests and biological control. IOBC/WPRS Bull. 2003, 26, 45–50. [Google Scholar]
- Daugaard, H. Cultural methods for controlling Botrytis cinerea Pers. in strawberry. Biol. Agric. Hortic. 1999, 16, 351–361. [Google Scholar] [CrossRef]
- Mertely, J.C.; Chandler, C.K.; Xiao, C.L.; Legard, D.E. Comparison of sanitation and fungicides for management of Botrytis fruit rot of strawberry. Plant Dis. 2000, 84, 1197–1202. [Google Scholar] [CrossRef]
- Legard, D.E.; Xiao, C.L.; Mertely, J.C.; Chandler, C.K. Effects of plant spacing and cultivar on incidence of Botrytis fruit rot in annual strawberry. Plant Dis. 2000, 84, 531–538. [Google Scholar] [CrossRef]
- Dara, S.K.; Sandoval-Solis, S.; Peck, D. Improving strawberry irrigation with micro-sprinklers and their impact on pest management. Agric. Sci. 2016, 7, 859–868. [Google Scholar] [CrossRef]
- Weber, R.W.S.; Fried, A. Fungizid-Resistenzen bei Botrytis im Beerenobst: Wie gut schützen wir unsere Kulturen und unsere Pflanzenschutzmittel? Obstbau 2011, 36, 144–171. [Google Scholar]
- Elad, Y. Cultural and integrated control of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 149–164. [Google Scholar] [CrossRef]
- Nicot, P.C.; Stewart, A.; Bardin, M.; Elad, Y. Biological control and biopesticide suppression of Botrytis-incited diseases. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 165–187. [Google Scholar] [CrossRef]
- Elad, Y.; Stewart, A. Microbial control of Botrytis. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 223–241. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Adikaram, N.K.B.; Joyce, D.C.; Terryc, L.A. Biocontrol activity and induced resistance as a possible mode of action for Aureobasidium pullulans against grey mould of strawberry fruit. Australas. Plant Pathol. 2002, 31, 223–229. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Fischer, E.; Dinoor, A. Improving biological control by combining biocontrol agents each with several mechanisms of disease suppression. Phytopathology 2002, 92, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Peng, G.; Gossen, B.D.; McGregor, L.; Yu, F.Q.; Hynes, R.K.; Hwang, S.F.; McDonald, M.R.; Boyetchko, S.M. Evidence that the biofungicide Serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology 2013, 103, 245–254. [Google Scholar] [CrossRef]
- Xu, X.; Robinson, J.; Jeger, M.; Jeffries, P. Using combinations of biocontrol agents to control Botrytis cinerea on strawberry leaves under fluctuating temperatures. Biocontrol Sci. Technol. 2010, 20, 359–373. [Google Scholar] [CrossRef]
- Peng, G.; Sutton, J.C.; Kevan, P.G. Effectiveness of honey bees for applying the biocontrol agent Gliocladium roseum to strawberry flowers to suppress Botrytis cinerea. Can. J. Plant Pathol. 1992, 14, 117–129. [Google Scholar] [CrossRef]
- Kovach, J.; Petzold, R.; Harman, G.E. Use of honey bees and bumble bees to disseminate Trichoderma harzianum 1295-22 to strawberries for Botrytis control. Biol. Control 2000, 18, 235–242. [Google Scholar] [CrossRef]
- Peng, G.; Sutton, J.C. Evaluation of microorganisms for biocontrol of Botrytis cinerea in strawberry. Can. J. Plant. Pathol. 1991, 13, 247–257. [Google Scholar] [CrossRef]
- Freeman, S.; Minz, D.; Kolesnik, I.; Barbul, O.; Zveibil, A.; Maymon, M.; Nitzani, Y.; Kirshner, B.; Rav-David, D.; Bilu, A.; et al. Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. Eur. J. Plant Pathol. 2004, 110, 361–370. [Google Scholar] [CrossRef]
- Sylla, J.; Alsanius, B.W.; Krüger, E.; Wohanka, W. Control of Botrytis cinerea in strawberries by biological control agents applied as single or combined treatments. Eur. J. Plant Pathol. 2015, 143, 461–471. [Google Scholar] [CrossRef]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Dinoor, A. Combining biocontrol agents to reduce the variability of biological control. Phytopathology 2001, 91, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Boyer, L.; Jeger, M.J.; Xu, X.-M.; Jeffries, P. Management of strawberry grey mould using mixtures of biocontrol agents with different mechanisms of action. Biocontrol Sci. Technol. 2009, 19, 1051–1065. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Belkacemi, K.; Corcuff, R.; Castaigne, F.; Arul, J. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharv. Biol. Technol. 2000, 20, 39–51. [Google Scholar] [CrossRef]
- Nicot, P.C.; Bardin, M.; Debruyne, F.; Duffaud, M.; Lecompte, F.; Neu, L.; Pascal, M. Effect of nitrogen fertilisation of strawberry plants on the efficacy of defence-stimulating biocontrol products against Botrytis cinerea. IOBC-WPRS Bull. 2013, 88, 39–42. [Google Scholar]
- Nunes, M.C.N.; Morais, A.M.M.B.; Brecht, J.K.; Sargent, S.A.; Bartz, J.A. Prompt cooling reduces incidence and severity of decay caused by Botrytis cinerea and Rhizopus stolonifer in strawberry. HortTechnol. 2005, 15, 153–156. [Google Scholar] [CrossRef]
- Romanazzi, G.; Droby, S. Control strategies for postharvest grey mould on fruit crops. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 217–228. [Google Scholar] [CrossRef]
- Kader, A. Quality and its maintenance in relation to the postharvest physiology of strawberry. In The Strawberry into the 21st Century; Dale, A., Luby, J.S., Eds.; Timber Press: Portland, OR, USA, 1991; pp. 145–152. [Google Scholar]
- El-Kazzaz, M.K.; Sommer, N.F.; Fortlage, R.J. Effect of different atmospheres on postharvest decay and quality of fresh strawberries. Phytopathology 1983, 73, 282–285. [Google Scholar] [CrossRef]
- Nadas, A.; Olmo, M.; García, J.M. Growth of Botrytis cinerea and strawberry quality in ozone-enriched atmospheres. J. Food Sci. 2003, 68, 1798–1802. [Google Scholar] [CrossRef]
- Tsortsakis, N.; Singleton, I.; Barnes, J. Deployment of low-level ozone-enrichment for the preservation of chilled fresh produce. Postharv. Biol. Technol. 2007, 43, 261–270. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Maxin, P.; Williams, M.; Weber, R.W.S. Control of fungal storage rots of apple by hot-water treatments: A Northern European perspective. Erw.-Obstb. 2014, 56, 25–34. [Google Scholar] [CrossRef]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharv. Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- Romero-Gámez, M.; Suárez-Rey, E.M. Environmental footprint of cultivating strawberry in Spain. Int. J. Life Cycle Assess. 2020, 25, 719–732. [Google Scholar] [CrossRef]
- Valiante, D.; Sirtori, I.; Cossa, S.; Corengia, L.; Pedretti, M.; Cavallaro, L.; Vignoli, L.; Galvagni, A.; Gomarasca, S.; Pesce, G.R.; et al. Environmental impact of strawberry production in Italy and Switzerland with different cultivation practices. Sci. Total Environ. 2019, 664, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Soode-Schimonsky, E.; Richter, K.; Weber-Blaschke, G. Product environmental footprint of strawberries: Case studies in Estonia and Germany. J. Environ. Manag. 2017, 203, 564–577. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, R.W.S.; Petridis, A. Fungicide Resistance in Botrytis spp. and Regional Strategies for Its Management in Northern European Strawberry Production. BioTech 2023, 12, 64. https://doi.org/10.3390/biotech12040064
Weber RWS, Petridis A. Fungicide Resistance in Botrytis spp. and Regional Strategies for Its Management in Northern European Strawberry Production. BioTech. 2023; 12(4):64. https://doi.org/10.3390/biotech12040064
Chicago/Turabian StyleWeber, Roland W. S., and Antonios Petridis. 2023. "Fungicide Resistance in Botrytis spp. and Regional Strategies for Its Management in Northern European Strawberry Production" BioTech 12, no. 4: 64. https://doi.org/10.3390/biotech12040064
APA StyleWeber, R. W. S., & Petridis, A. (2023). Fungicide Resistance in Botrytis spp. and Regional Strategies for Its Management in Northern European Strawberry Production. BioTech, 12(4), 64. https://doi.org/10.3390/biotech12040064





