Enhancing Bovine Embryo Development In Vitro Using Oil-in-Water Nanoemulsions as Specific Carriers for Essential Lipids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Nanoemulsions
2.3. Experimental Design
2.4. Preparation of Nanoemulsions by the Emulsification/Evaporation Method
2.5. Characterization of Nanoemulsions
2.5.1. Droplet Size and Polydispersity Index (Span)
2.5.2. Physical Stability
2.6. Characterization of NEs Produced under the Best Conditions
2.6.1. Size Distribution and Droplet Concentration of Nanoemulsions
2.6.2. Morphology
2.6.3. Surface Tension
2.6.4. Density and pH
2.6.5. Rheological Behavior
2.6.6. Application of NEs in In Vitro Embryo Production
- (1)
- Control (C; without nanoemulsion);
- (2)
- F1 (nanoemulsion composed of 5.0% phosphatidylcholine plus 0.0% cholesterol);
- (3)
- F2 (nanoemulsion composed of 4.0% phosphatidylcholine plus 1.0% cholesterol);
- (4)
- F3 (nanoemulsion composed of 4.5% phosphatidylcholine plus 0.5% cholesterol).
2.7. Statistical Analysis
3. Results
3.1. Results of the Experimental Design
3.2. Characterization of NEs for Embryo Culture Supplementation
3.2.1. pH, Density, and Surface Tension of Nanoemulsions
3.2.2. Rheological behavior
3.2.3. Morphology of NEs
3.2.4. Droplet Diameter Distribution
3.2.5. Physical stability of NEs
3.3. Applying Nanoemulsions for Bovine Embryonic Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo, C.; Abuelo, A.; Hernandez, J. Biotechnological Approaches to Improve Sustainable Milk and Meat Yield in Bovines. In Reference Module in Food Sciences; Elsevier: New York, NY, USA, 2016; pp. 1–27. [Google Scholar]
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/ (accessed on 16 October 2023).
- Rizos, D.; Ward, F.; Duffy, P.; Boland, P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Develop. 2002, 61, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Melo-Sterza, A.; Poehland, R. Lipid metabolism in bovine oocytes and early embryos under in vivo, in vitro, and stress conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- Roy, A.; Patra, S.K. Lipid raft facilitated receptor organization and signaling: A functional rheostat in embryonic development, stem cell biology and cancer. Stem Cell Rev. Rep. 2023, 19, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zeng, X.; Cai, S.; Qiao, S.; Zeng, X. Mechanisms of lipid metabolism in uterine receptivity and embryo development. Trends Endocrinol. Metab. 2021, 32, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Sudano, M.J.; Paschoal, D.M.; Da Silva, R.T.; Magalhães, L.C.; Crocomo, L.F.; de Lima-Neto, J.F.; Landim-Alvarenga, F.C. Lipid content and apoptosis of in vitro-produced bovine embryos as determinants of susceptibility to vitrification. Theriogenology 2011, 75, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Norman, R.J.; Robker, R.L. The impact of obesity on oocytes: Evidence for lipotoxicity mechanisms. Reprod. Fertil. Dev. 2011, 24, 29–34. [Google Scholar] [CrossRef]
- Murillo, A.; Muñoz, M.; Martín-González, D.; Carrocera, S.; Martínez-Nistal, A.; Gómez, E. Low serum concentration in bovine embryo culture enhances early blastocyst rates on Day-6 with quality traits in the expanded blastocyst stage similar to BSA-cultured embryos. Reprod. Biol. 2017, 17, 162–171. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P.J.; Aquino, A.; Neves, M.A.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.; Meleson, K.; Chang, C.; Graves, S. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 18, R635. [Google Scholar] [CrossRef]
- Choi, S.J.; Mc Clements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Watanabe, T.; Tokumitsu, H.; Fukumori, Y. Formulation Considerations of Gadolinium Lipid Nanoemulsion for Intravenous Delivery to Tumors in Neutron-Capture Therapy. Curr. Drug Deliv. 2007, 4, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Heo, W.; Kim, J.H.; Pan, J.H.; Kim, Y.J. Lecithin-Based Nanoemulsification Improves the Bioavailability of Conjugated Linoleic Acid. J. Agric. Food Chem. 2016, 64, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Prévot, G.; Mousli, Y.; Barthélémy, P.; Crauste-Manciet, S.; Dehay, B.; Desvergnes, V. Synthesis and Intracellular Uptake of Rhodamine–Nucleolipid Conjugates into a Nanoemulsion Vehicle. ACS Omega 2020, 5, 5815–5823. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Van Dommelen, S.M.; Vader, P.; Lakhal, S.; Kooijmans, S.A.; Van Solinge, W.W.; Wood, M.J.; Schiffelers, R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release 2012, 161, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or cell membrane-based drug delivery systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Duong, T.; Nguyen, T.; Marasini, R.; Santosh, A. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Pitchaimani, A.; Nguyen, T.D.; Aryal, S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 2018, 160, 124–137. [Google Scholar] [CrossRef]
- Pitchaimani, A.; Nguyen, R.; Marasini, A.; Eliyapura, T.; Azizi, M.; Douraki, J.; Aryal, S. Biomimetic natural killer membrane camouflaged polymeric nanoparticle for targeted bioimaging. Adv. Funct. Mater. 2019, 29, 1806817. [Google Scholar] [CrossRef]
- Roux, C.; Wolf, C.; Mulliez, N.; Gaoua, W.; Cormier, V.; Chevy, F.; Citadelle, D. Role of cholesterol in embryonic development. Am. J. Clin. Nutr. 2000, 71, S1270–S1279. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Sobral, P.J. Formulation optimization of lecithin-enhanced pickering emulsions stabilized by chitosan nanoparticles for hesperidin encapsulation. J. Food Eng. 2018, 229, 2–11. [Google Scholar] [CrossRef]
- Pérez-Córdoba, L.J. Filmes à base de blenda gelatina-quitosana com agentes ativos nanoemulsificados: Desenvolvimento, caracterização e aplicação na conservação de mortadela fatiada refrigerada. 222 f. Ph.D. Thesis, Faculdade de Zootecnia e Engenharia de Alimentos, Universidade de São Paulo, Pirassununga, Brazil, 2018. [Google Scholar]
- Sangalli, J.R.; Nociti, R.P.; del Collado, M.; Sampaio, R.V.; da Silveira, J.C.; Perecin, F.; Smith, L.C.; Ross, P.J.; Meirelles, F.V. Characterization of histone lysine β-hydroxybutyrylation in bovine tissues, cells, and cumulus–oocyte complexes. Mol. Reprod. Dev. 2022, 89, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Vanroose, G.; Nauwynck, H.; Soom, A.V.; Ysebaert, M.T.; Charlier, G.; Oostveldt, P.V.; de Kruif, A. Structural aspects of the zona pellucida of in vitro-produced bovine embryos: A scanning electron and confocal laser scanning microscopic study. Biol. Reprod. 2000, 62, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Artiga-Artigas, M.; Montoliu-Boneu, J.; Salvia-Trujillo, L.; Martín-Belloso, O. Factors affecting the formation of highly concentrated emulsions and nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123577. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P. Investigation into the physicochemical stability and rheological properties of rutin emulsions stabilized by chitosan and lecithin. J. Food Eng. 2018, 229, 12–20. [Google Scholar] [CrossRef]
- Róg, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 97–121. [Google Scholar] [CrossRef]
- Mardešić, I.; Boban, Z.; Subczynski, W.K.; Raguz, M. Membrane Models and Experiments Suitable for Studies of the Cholesterol Bilayer Domains. Membranes 2023, 13, 320. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Michigan Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Dammak, I.; Sobral, P. Curcumin nanoemulsions stabilized with natural plant-based emulsifiers. Food Biosci. 2021, 43, 101335. [Google Scholar] [CrossRef]
- Negrón-Pérez, V.M.; Fausnacht, D.W.; Rhoads, M.L. Invited review: Management strategies capable of improving the reproductive performance of heat-stressed dairy cattle. J. Dairy Sci. 2019, 102, 10695–10710. [Google Scholar] [CrossRef]
- Pickering, N.K.; Oddy, V.H.; Basarab, J.; Cammack, K.; Hayes, B.; Hegarty, R.S.; Lassen, J.; McEwan, J.C.; Miller, S.; Pinares-Patiño, C.S.; et al. Animal board invited review: Genetic possibilities to reduce enteric methane emissions from ruminants. Animal 2015, 9, 1431–1440. [Google Scholar] [CrossRef]
- Berry, D.P.; Conroy, S.B.; Pabiou, T.; Cromie, A.R. Animal breeding strategies can improve meat quality attributes within entire populations. Meat Sci. 2017, 132, 6–18. [Google Scholar] [CrossRef]

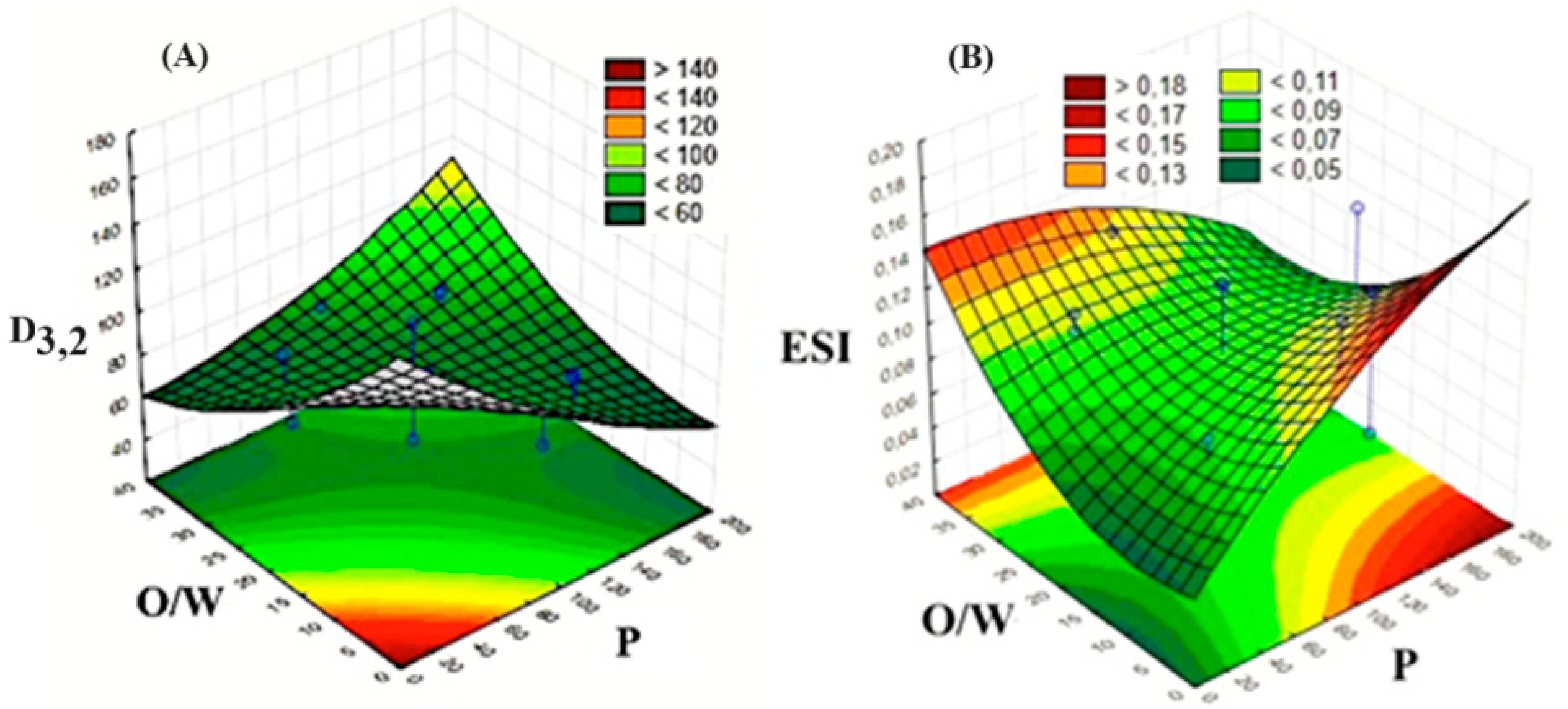


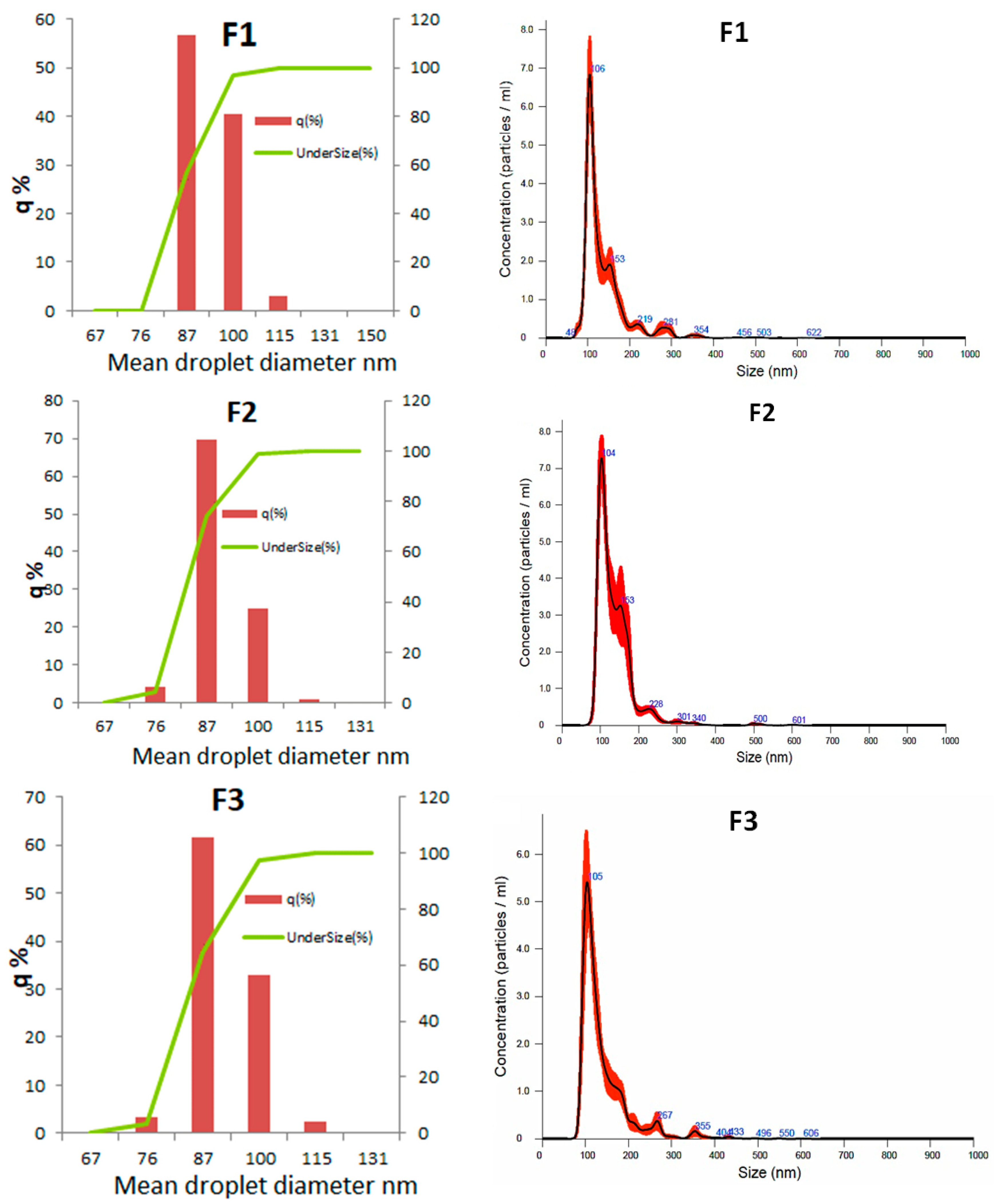

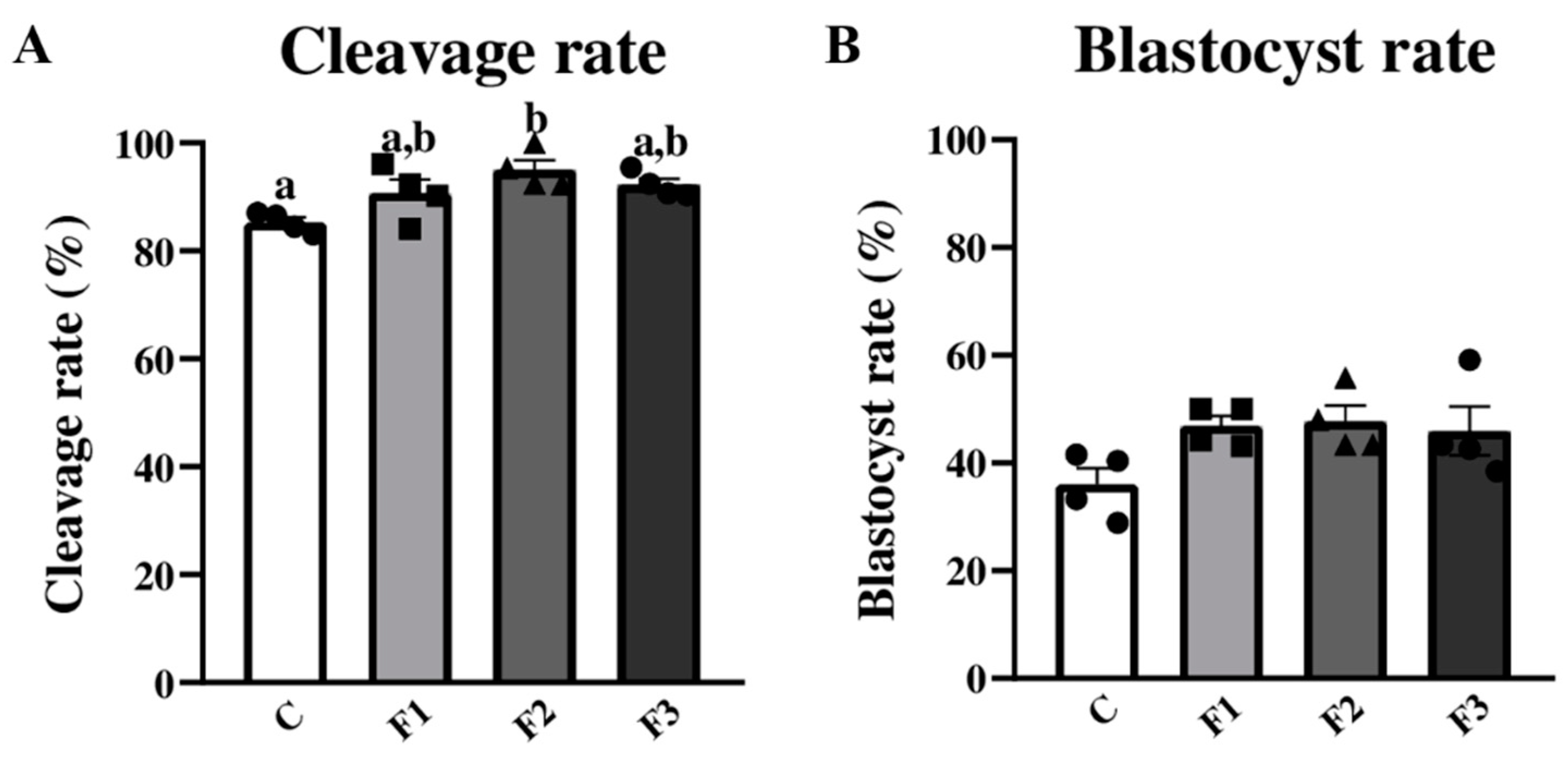
| Variables | Code | Levels | ||||
|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||
| Pressure (MPa) | X1 | 16 | 50 | 100 | 150 | 184 |
| O/W ratio | X2 | 3/97 | 10/90 | 20/80 | 30/70 | 37/63 |
| Concentration of Lecithin (% w/w) | X3 | 0.16 | 0.5 | 1.0 | 1.5 | 1.84 |
| Run | D3,2 (nm) | ESI |
|---|---|---|
| 1 | 85.0 ± 0.2 | 0.087 ± 0.002 |
| 2 | 138.0 ± 0.4 | 0.058 ± 0.001 |
| 3 | 83.2 ± 0.5 | 0.099 ± 0.001 |
| 4 | 64.8 ± 0.4 | 0.106 ± 0.001 |
| 5 | 80.2 ± 0.1 | 0.055 ± 0.001 |
| 6 | 80.1 ± 0.9 | 0.131 ± 0.013 |
| 7 | 80.1 ± 0.0 | 0.063 ± 0.000 |
| 8 | 79.5 ± 0.4 | 0.077 ± 0.001 |
| 9 | 84.6 ± 0.7 | 0.052 ± 0.001 |
| 10 | 64.6 ± 0.6 | 0.139 ± 0.003 |
| 11 | 79.2 ± 0.1 | 0.146 ± 0.007 |
| 12 | 75.9 ± 0.1 | 0.124 ± 0.001 |
| 13 | 74.0 ± 0.6 | 0.127 ± 0.016 |
| 14 | 78.1 ± 0.0 | 0.081 ± 0.008 |
| 15 | 77.7 ± 0.3 | 0.128 ± 0.004 |
| 16 | 75.8 ± 0.1 | 0.078 ± 0.004 |
| 17 | 68.0 ± 9.0 | 0.061 ± 0.001 |
| Treatment → Emulsion Production Conditions | F1 | F2 | F3 |
|---|---|---|---|
| O/W ratio | 20/80 | 20/80 | 20/80 |
| Concentration of Lecitin (%) | 0.7 | 0.7 | 0.7 |
| Homogenization pressure (MPa) | 100 | 100 | 100 |
| Solvent (mL) | 15 | 15 | 15 |
| Phosphatidylcholine (%) | 5.0 | 4.0 | 4.5 |
| Cholesterol (%) | 0 | 1 | 0.5 |
| Treatment → | F1 | F2 | F3 |
|---|---|---|---|
| pH | 6.3 ± 0.0 a | 6.3 ± 0.1 a | 6.3 ± 0.0 a |
| Density (kg/m3) | 1.006 ± 0.0 a | 1.005 ± 0.0 a | 0.997 ± 0.0 a |
| Surface tension (mN/m) | 35.91 ± 2.67 a | 31.31 ± 1.82 b | 31.80 ± 0.50 b |
| Viscosity (mPa.s) | 2.1 ± 0.2 a | 2.0 ± 0.0 a | 2.0 ± 0.1 a |
| Mean droplet diameter (nm) | 86.4 ± 1.0 a | 83.8 ± 0.7 a | 86.0 ± 0.4 a |
| Polidispersion index | 0.224 ± 0.003 a | 0.214 ± 0.004 a | 0.228 ± 0.004 a |
| Emulsion stability index | 0.083 ± 0.003 a | 0.047 ± 0.001 b | 0.057 ± 0.004 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Angulo, D.; Lourenço, R.V.; Bridi, A.; Chaves, M.A.; da Silveira, J.C.; Sobral, P.J.d.A. Enhancing Bovine Embryo Development In Vitro Using Oil-in-Water Nanoemulsions as Specific Carriers for Essential Lipids. BioTech 2024, 13, 19. https://doi.org/10.3390/biotech13020019
López Angulo D, Lourenço RV, Bridi A, Chaves MA, da Silveira JC, Sobral PJdA. Enhancing Bovine Embryo Development In Vitro Using Oil-in-Water Nanoemulsions as Specific Carriers for Essential Lipids. BioTech. 2024; 13(2):19. https://doi.org/10.3390/biotech13020019
Chicago/Turabian StyleLópez Angulo, Daniel, Rodrigo Vinicius Lourenço, Alessandra Bridi, Matheus Andrade Chaves, Juliano Coelho da Silveira, and Paulo José do Amaral Sobral. 2024. "Enhancing Bovine Embryo Development In Vitro Using Oil-in-Water Nanoemulsions as Specific Carriers for Essential Lipids" BioTech 13, no. 2: 19. https://doi.org/10.3390/biotech13020019
APA StyleLópez Angulo, D., Lourenço, R. V., Bridi, A., Chaves, M. A., da Silveira, J. C., & Sobral, P. J. d. A. (2024). Enhancing Bovine Embryo Development In Vitro Using Oil-in-Water Nanoemulsions as Specific Carriers for Essential Lipids. BioTech, 13(2), 19. https://doi.org/10.3390/biotech13020019










