Abstract
This work aimed to identify the influence of pH, molarity, w/v fraction, extraction time, agitation, and either a sodium (Na2HPO4·7H2O-NaH2PO4·H2O) or potassium buffer (K2HPO4-KH2PO4) used in the extraction of C-phycoerythrin (C-PE) from a thermotolerant strain of Potamosiphon sp. An experimental design (Minimum Run Resolution V Factorial Design) and a Central Composite Design (CCD) were used. According to the statistical results of the first design, the K-PO4 buffer, pH, molarity, and w/v fraction are vital factors that enhance the extractability of C-PE. The construction of a CCD design of the experiments suggests that the potassium phosphate buffer at pH 5.8, longer extraction times (50 min), and minimal extraction speed (1000 rpm) are ideal for maximizing C-PE concentration, while purity is unaffected by the design conditions. This optimization improves extraction yields and maintains the desired bright purple color of the phycobiliprotein.
Key Contribution:
The correct selection of buffer salt enhances the extraction efficiency and purity of C-PE.
1. Introduction
Cyanobacteria are a group of photosynthetic microorganisms that belong to the bacterial kingdom, although they share some characteristics with algae. Like green plants, they are known for their ability to carry out photosynthesis and produce oxygen. However, unlike plants, cyanobacteria do not have chloroplasts and lack a defined nucleus, distinguishing them as bacteria [1,2,3]. In scientific research and biotechnology, cyanobacteria have also proven helpful because of their ability to produce a variety of valuable compounds, such as biofuels, pharmaceuticals, industrial chemicals, and biofertilizers, through genetic engineering and metabolic modifications [4,5,6,7,8,9].
Phycobiliproteins (PBPs) are abundant water-soluble proteins that cyanobacteria produce to capture the light energy that is transferred to the chlorophyll during photosynthesis [10,11]. PBPs are formed by a complex between covalently bound proteins and phycobilins, which trap light and are the significant components of phycobilisomes [12,13]. The following four categories can be distinguished between phycobiliproteins based on how well they absorb light: Phycoerythrins (490–570 nm), phycocyanins (610–625 nm), phycoerythrocyanins (560–600 nm), and allophycocyanins (650–660 nm) [14,15]. C-phycoerythrin (C-PE) is a water-soluble protein pigment complex found mainly in red algae and, together with phycocyanin and allophycocyanin, belongs to the photosynthetic pigments and forms an accessory light-collecting apparatus [16]. C-phycoerythrin (C-PE) has great potential for applications in the food, pharmaceutical, and cosmetic industries. PE has demonstrated several medical uses in addition to its employment as a synthetic dye substitute in the conventional food sector. For instance, C-PE’s fluorescent qualities make it worthwhile for cancer diagnosis and HIV monitoring. [17].
Among the main functions of C-phycoerythrins (C-PE) is their participation in nitrogen reserve processes; when cyanobacteria are faced with a nitrogen deficiency, they can degrade phycobiliproteins that contain a high concentration of nitrogen, such as C-phycoerythrin, and can also act as a quantum collector for photosynthesis. They absorb light at wavelengths inaccessible to chlorophyll and transfers the energy to the photosynthetic reaction center [18]. Some cyanobacteria can modify the chromophore composition of phycoerythrin by the complementary chromatic adaptation process, allowing them to acclimate to variations in light intensity and quality [19]. Potamosiphon sp. is a novel genus previously isolated in a marine environment in Australia [20]; however, in 2022, our research group was able to isolate a strain from a thermal spring. The strain can grow under high light intensities and high temperatures (30–45 °C) while producing a high concentration of C-PE [21].
The amount of C-phycoerythrin (C-PE) present in cyanobacterial cells varies within different species and culture environments. Cyanobacteria generally maintain cell development and activity in nitrogen deficiency by degrading phycobiliproteins that contain a high nitrogen concentration [22]. When some cyanobacterial strains are acclimated to specific light intensity and wavelength, the complementary chromatic adaptation process allows for the modification of the chromophore composition of C-PE [23,24].
Based on the physicochemical, structural, and spectroscopic properties of phycoerythrin, various biotechnological applications have been proposed, as well as new extraction methods that consider the solubility in water at room temperature and the sensitivity of the pigment to temperatures above 60 °C [25]. Problems associated with large polysaccharides (e.g., agar and cellulose) in the cell wall present a significant obstacle to cell disruption during the primary extraction of metabolites [26], partly because they form a complex matrix. This matrix adds strength and rigidity to the cell, reducing the extractability of biomolecules, and is also due to the strong covalent bond between the xylan bound to the mixture and the glycoprotein complex [27]. Therefore, a suitable cell disruption method is required for extraction. Several methods have been described to extract high-quality products from red cyanobacteria. Several conventional buffers have been tested to extract C-phycoerythrin (C-PE) [28]. The following three extraction solvents have been tested: sodium buffer (Na2HPO4·7H2O-NaH2PO4·H2O), CaCl2 solution, and distilled water. Mittal et al. [16] used a potassium buffer (K2HPO4-KH2PO4, 0.1 M, pH 6.8), and Li et al. [29] used a mixture of cold distilled water with 95% ethanol, followed by hot distilled water to extract PE, lipids, and polysaccharides. Finally, García et al. [30] used PO4 buffer (0.1 M, pH 5.5), followed by precipitation with ammonium sulfate ((NH4)2SO4) at 60% saturation.
Solvent extraction methods are insufficient to achieve optimal metabolite concentrations. Several authors have tried extraction methods using osmotic shock [31], maceration in the presence of liquid nitrogen [32], freeze–trituration [33], freeze–thaw [34], ultrasound [35], and homogenization [36]. These methods may become uneconomical for long extraction times. Therefore, a suitable cell disruption method for extraction should combine different buffers and mechanical processes, such as vortex stirring, and evaluating stirring times, pH, and molarity.
This study aimed to develop an efficient method for extracting C-phycoerythrin (C-PE) from a new strain of thermotolerant Potamosiphon sp. An experimental design was used to evaluate the influence of pH, molarity, w/v fraction, extraction time, and agitation on the type of buffer used to extract the highest amount of phycoerythrin (C-PE). We have previously reported the effect of the drying on the extraction efficiency of C-phycoerythrin (C-PE) [21].
2. Materials and Methods
2.1. Strain
Potamosiphon sp. UFPS003 was isolated from a thermal spring near Cucuta (Colombia). The strain was kept in solid BG-11 culture media [37] at the INNOValgae collection (UFPS, Cucuta, Colombia) (https://www.innovalg.com accessed on 5 June 2024). The strain was initially grown in 0.2 L of BG-11 culture media in a 0.5 L Schott GL45 glass flask. The media was aerated by injecting filtered air enriched with 1% (v/v) CO2 at a flow rate of 0.12 L min −1. The culture was exposed to cool-white LED lamps at an intensity of 100 µmol m −2 s −1, 12:12 h photoperiod, and a temperature of 27 ± 1 °C for 15 days.
2.2. Experimental Design
The effect of the critical variables (Table 1) on the purity and concentration of the extracted C-PE was analyzed using a Minimum Run Resolution V Factorial Design with six variables (five numeric and one categoric), coupled with a surface response in Design-Expert® software (version 13.0, Stat-Ease Inc., Minneapolis, MN, USA). The different variables and their levels are presented in Table 1, while Table A1 shows the resolved design with the 22 evaluated experiments.

Table 1.
Variables and their levels for the extraction of C-PE.
2.3. Culture Conditions
The strain was cultured in triplicate (original plus two replicates) for each experiment in 0.5 L Schott GL45 flasks with a working volume of 0.2 L of liquid BG-11 media. Each flask was connected to a compressed air line mixed with CO2 (1% w/w) at 0.12 Lair min−1, 12:12 h photoperiod, at 100 µmol m−2 s−1, and 27 ± 1 °C for 20 days.
2.4. Biomass Drying, C-PE Extraction, and Quantification
Each flask was disconnected from the compressed air and allowed to settle for about twenty minutes. The supernatant was axenically removed, and the precipitated biomass was further concentrated by centrifugation (3600 rpm, 10 °C, 20 min). The harvested biomass was dried according to Vergel-Suarez et al. [21], using a food-grade dehydrator (40 °C, 12 h). The dried biomass was weighted and used for the extraction experiments. A known amount of dried biomass was mixed with a known volume of cold buffer solution (sodium buffer, (Na2HPO4·7H2O-NaH2PO4·H2O) or potassium buffer (K2HPO4-KH2PO4)) until it reached the biomass/solvent ratio, as shown in Table 2. The sample of biomass and buffer was also mixed with a known amount of glass beads (0.5 mm diameter), following the method described by Barajas-Solano [38]. The mixture was mixed using an automatic vortex (Multi Reax, Heidolph, Germany) according to the conditions of each experiment. The mixture was precipitated in a refrigerator overnight. Finally, the deep-purple extract rich in C-PE was separated by centrifugation (3600 rpm, 20 min, 10 °C).

Table 2.
Analysis of variance (ANOVA) of the model obtained for C-PE concentration.
The concentration of C-PE was calculated using Equations (1)–(3), previously described by Bennett and Bogorad [39], while its purity was obtained using Equation (4) [40,41]. The mean obtained for each experiment was used for the ANOVA analysis according to the Design-Expert® software.
2.5. Process Optimization
The most relevant variables to improve the concentration and purity of extracted C-PE were further investigated using a Central Composite Design (CCD). All experiments proposed in the optimization were performed six times (original plus five replicates).
3. Results
3.1. Effect of Multiple Variables on the Concentration and Purity of C-PE
The ANOVA analysis of the experimental data is highlighted in Table 2. According to the results, the variables that significantly affect (p < 0.05) the concentration of C-PE are biomass/buffer ratio (A), pH (B), molarity (C), and the interactions between two variables, such as AC, BE, DE, CE. However, extraction time and speed were not statistically relevant to the process. The model’s F-value of 21.13 implies that the obtained model is significant, with only a 0.01% chance that the F-value obtained could occur due to the noise of the different experiments. On the other hand, the low difference between the Predicted and the Adjusted R2 is less than 0.2, and the Adeq Precision of 17.485 indicates an adequate signal-to-noise ratio. Therefore, the data obtained for the effect of the multiple variables on the concentration of C-PE are enough to improve the content of the extracted protein.
According to the ANOVA for C-PE purity (Table 3), the variables that significantly affect (p < 0.05) the concentration and purity of C-PE are biomass/buffer ratio (A), pH (B), extraction time (D), extraction speed (E), and buffer used (F) and the interactions between two variables, such as AB, AC, BD, BE, CE, CF, DE. However, the molarity of the buffer was not statistically relevant to the process. The model’s F-value of 78.92 implies that the obtained model is significant, with only a 0.01% chance that the F-value obtained could occur due to the noise of the different experiments. On the other hand, the low difference between the Predicted and the Adjusted R2 is less than 0.2, and the Adeq Precision of 40.011 indicates an adequate signal-to-noise ratio. Therefore, the data obtained for the effect of the multiple variables on the concentration of C-PE are enough to improve the purity.

Table 3.
Analysis of variance (ANOVA) of the model obtained for C-PE purity.
3.2. Optimization of Relevant Variables
According to the data obtained, the best possible scenario to enhance the concentration and purity of C-PE requires a lower biomass/buffer ratio, a pH closer to 6.3, a molarity of 0.001, longer extraction times, higher extraction speed, and a potassium phosphate (K2HPO4-KH2PO4) buffer. Based on the obtained results, a new design, specifically a Central Composite Design of three variables (pH, extraction speed, and time), was used to optimize the concentration and purity of the extracted C-PE (Table 4). The resolved design with the experimental data can be found in Table A2.

Table 4.
Variables were evaluated based on their levels for the extraction of C-PE.
The results for the concentration and purity of the extracted C-PE from the optimization design are presented in Table 5. According to the results obtained for the concentration of C-PE, the model’s F-value (339.73) is significant, and the noise does not affect the data. The most significant terms (p < 0.05) were the extraction time and speed, their quadratic counterparts (A2, B2, C2), and several of the interactions (AB, AC, BC). The R2 obtained shows a good fit (0.9974). Also, the difference between the adjusted R2 (0.9945) and the predicted R2 (0.9830) was less than 0.2. In the case of the data obtained for the purity of the extracted C-PE, the model’s F-value is also significant (53.46). Unlike the concentration, the pH of the buffer only affects the purity. Finally, the R2 obtained shows a good fit (0.9197). Also, the difference between the adjusted R2 (0.9025) and the predicted R2 (0.8389) is less than 0.2.

Table 5.
Analysis of variance (ANOVA) of the optimization model for concentration and purity of extracted C-PE.
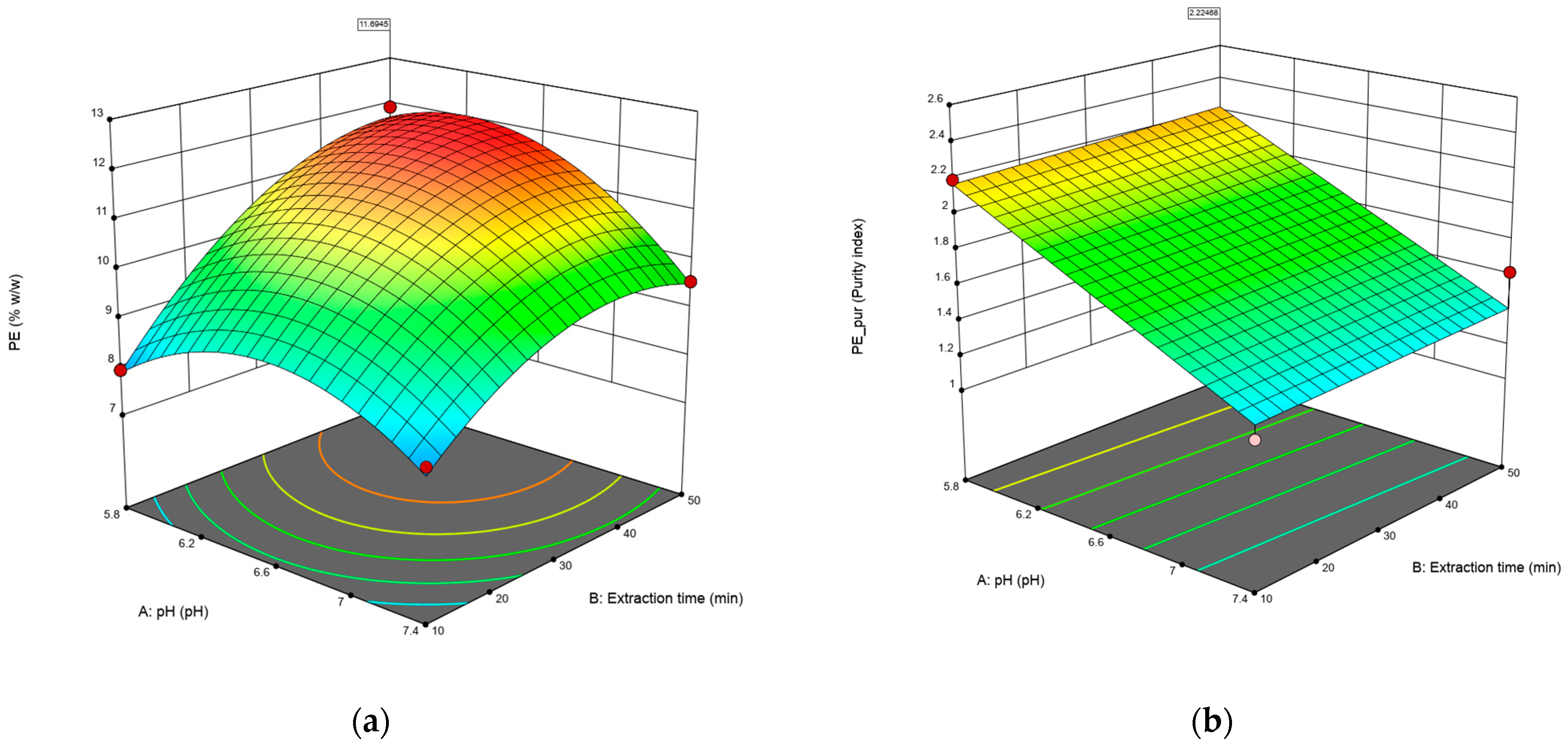
Figure 1 shows the response surfaces for the concentration (Figure 1a) and purity (Figure 1b) of C-PE. In the case of the concentration of C-PE, longer times and lower pH significantly improve the final content of C-PE; however, on the purity of the extract, there is no curvature, and only the pH increases the result, while the time does not affect the outcome. It should be noted that the purity index obtained is higher than 0.7, which is considered food-grade.
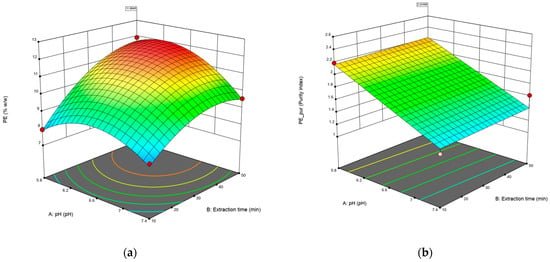
Figure 1.
The surface response of the model was fitted to the data on concentration (% w/w) (a) and purity of C-PE (b). Red dots in figures represents design points used to create the surface.
Using the optimization feature of Design-Expert® software, the best scenario that maximizes C-PE concentration and purity is presented in Table 6. Those variables were tested using fresh biomass (under the same conditions as the other experiments).

Table 6.
Best conditions to improve concentration and purity of extracted C-PE.
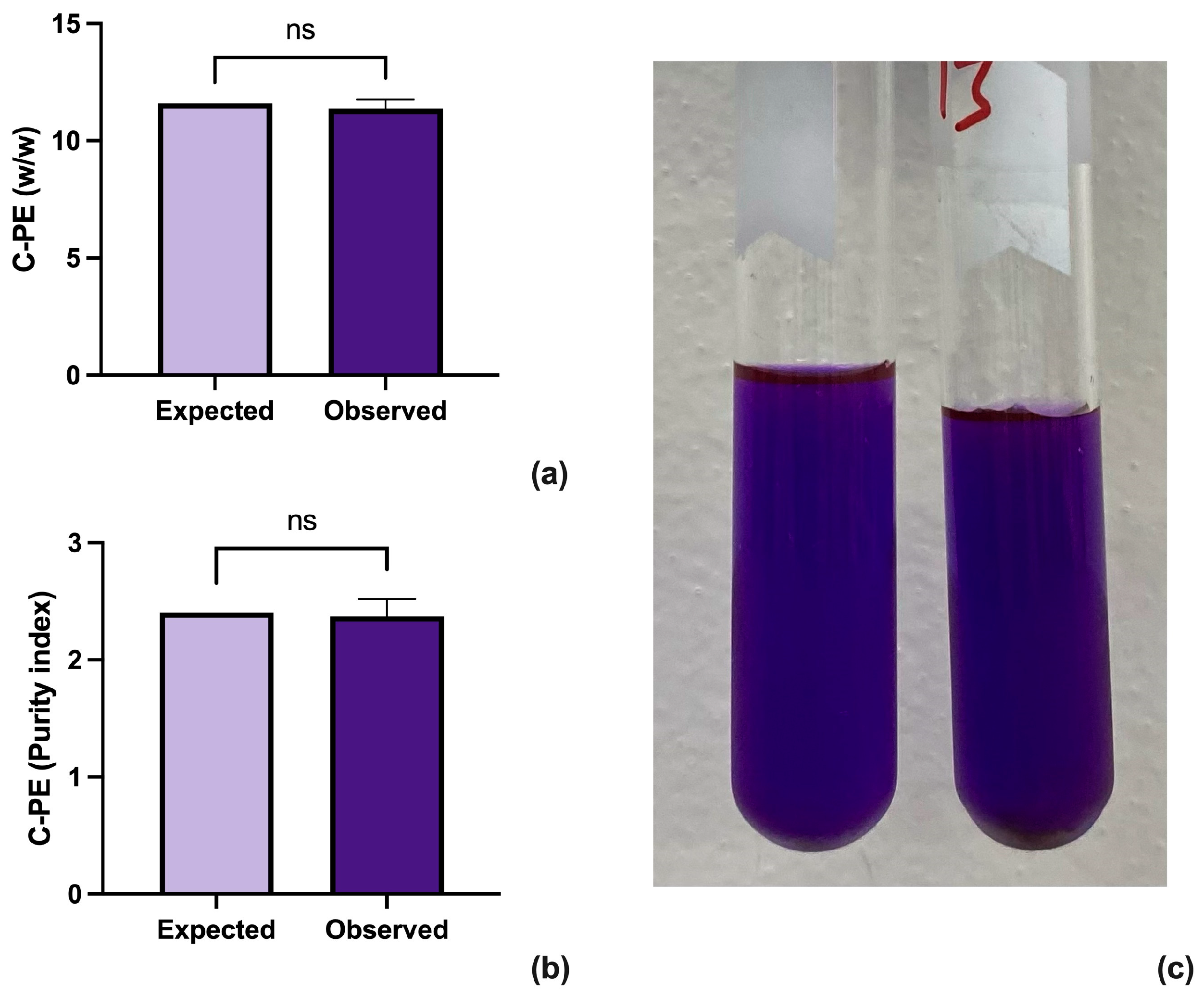
The results were analyzed through a one-sample t-test using GraphPad Prism version 10.2.3 for Mac (GraphPad Software, www.graphpad.com (accessed on 10 June 2024)) (Figure 2a,b). The analysis shows that the proposed method improves both the concentration and purity of the extracted C-PE while retaining a brilliant purple color (Figure 2c).
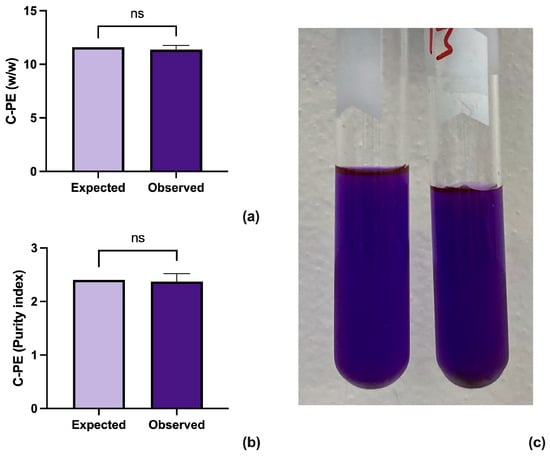
Figure 2.
Results from the one sample t-test between the expected and observed results for concentration (a) and purity (b) of C-PE. A sample of highly concentrated sample of C-PE (c).
4. Discussion
Due to their thermolability, extracting any phycobiliprotein is a critical step that requires fine-tuning according to the specific requirements of the target protein. Multiple variables such as extraction time, speed pH, and the selected buffer are the most used to improve the extraction of phycobiliproteins [42,43]. In the case of extraction time and speed, it is always preferable to obtain the conditions that transmit the least energy to the sample, which can generate significant losses due to the rapid degradation of specific proteins at temperatures above 60 °C. According to the literature, the most common extraction times range between 15 and 20 min [43,44,45], while the reported speed used was from 2000 to 10,000 rpm. Now, most of those conditions have been adjusted to multiple cycles of freeze–thaw of the biomass, such as those reported by Ji et al. [46] However, in this case, the biomass was first dehydrated to reduce the cellular moisture as much as possible while improving the extracted content of C-PE [21].
Over the years, several buffers have been tested as viable solvents for extracting all phycobiliproteins. Buffers such as sodium phosphate (NaHPO4-Na2HPO4), potassium phosphate (K2HPO4-KH2PO4), Tris-HCl, and even CaCl2 have been tested [47,48]. However, according to Pez Jaeschke et al. [47], the preferred extraction buffer should be acidic (between 5 and 6); in this case, the results show that an acidic potassium phosphate buffer (pH 5.8) allows for a good extraction of C-PE from the biomass. Other variables used in the initial design showed no statistical significance in concentration and purity extraction.
An interesting result is that the buffer type only affects the purity rather than the concentration of C-PE extracted. The latter is relevant since most of the literature shows that the potassium phosphate buffer is preferred; however, to the author’s knowledge, no study has proven such a specific difference between the sodium phosphate (NaHPO4-Na2HPO4) and potassium phosphate (K2HPO4-KH2PO4) buffers.
In the case of the first design, it was found that the type of buffer used only affects the purity index and does not affect the concentration. Although in the literature, it is possible to see the use of different buffers, including sodium phosphate, potassium phosphate, Tris-HCl, and even CaCl2, the potassium phosphate buffer is perhaps the most used for both phycocyanins and phycoerythrins; however, as far as the authors know, no report explains why the potassium phosphate buffer is better than the others mentioned above. This is a significant result because it allows us to identify that in the case of C-PE, the potassium phosphate buffer, in comparison with the sodium phosphate buffer, selectively avoids secondary proteins, accessory or globular proteins, which maximizes both the concentration of C-PE and the purity of the crude extract.
In the case of C-PE concentration, the results indicate that the most important factors are the biomass–solvent ratio, pH, and molarity. Those factors that have almost no effect are extraction and time. In contrast, all the factors studied significantly influenced the purity index. In the specific case of this study, the biomass–solvent ratio and molarity factors were not explored in the optimization because the design aimed to use low concentrations of these two variables. A low biomass–solvent ratio will dilute the concentration of C-PE, significantly reducing its final content; similarly, a very low molarity (such as 0.001 mM) is close to zero, so it is not technologically relevant. This is why speed, extraction time, and pH were chosen to be optimized, as they are factors that can be increased and have a more significant effect. Optimization was performed in this case, and the results show that obtaining a considerable concentration and purity is possible. Similar conditions were obtained using Lyngbya sp. CCNM 2053 [49]; however, the final content of C-PE was higher in Potamosiphon sp. than in Lyngbya sp. CCNM 2053 (5% w/w) and Microcoleus autumnalis PACC 5522 [50].
Although statistical tools were used, such as in the design of experiments, the response surface for purity shows that, unlike the C-PE concentration, its purity is far from presenting results that can be considered optimal. It should be noted that the test results show that adjusting the extraction conditions makes it possible to obtain an extract with a considerable concentration of extracted C-PE and a purity of higher than 0.7 (considered as food-grade) [51,52]. At the same time, it is assumed that the conditions are to bring out as much protein as possible. In the case of purity, forcing a higher efficiency, i.e., a possible optimization at a more extended time and higher speed, is very complicated. All the conditions are given for as many of the water-soluble metabolites as possible, such as carbohydrates, glycoconjugates, accessory proteins, and other phycobiliproteins present in the phycobilisome such as C-PC and APC, to come out. So, we found that there is no possible optimization in purity, but there is a possible optimization in concentration.
5. Conclusions
The optimized extraction conditions suggest that a potassium phosphate buffer at pH 5.8, longer extraction times, and minimal extraction speed are ideal for maximizing C-PE concentration. At the same time, purity is unaffected by the design conditions studied. This optimization improves extraction yields and maintains the desired bright-purple color of the phycobiliprotein.
Author Contributions
Conceptualization, J.B.G.-M. and N.A.U.-S.; methodology, A.H.V.-S. and G.L.L.-B.; software, A.F.B.-S. and J.B.G.-M.; validation, N.A.U.-S. and A.H.V.-S.; formal analysis, A.H.V.-S., N.A.U.-S. and J.B.G.-M.; investigation, A.H.V.-S.; resources, A.F.B.-S. and J.B.G.-M.; data curation, N.A.U.-S. and G.L.L.-B.; writing—original draft preparation, A.F.B.-S. and A.H.V.-S.; writing—review and editing, J.B.G.-M. and N.A.U.-S.; visualization, A.F.B.-S.; supervision, G.L.L.-B.; project administration, J.B.G.-M.; funding acquisition, A.F.B.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from Universidad Francisco de Paula Santander (Colombia) (FINU 011-2023, FINU 013-2023), the Ministry of Science and Technology of Colombia, and the Colombian Institute of Educational Credit and Technical Studies Abroad (MINCIENCIAS-ICETEX) under the project titled “FOTOLIX” with ID 81829-2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article.
Acknowledgments
We would like to express our sincere gratitude to S Universidad Francisco de Paula Santander (Colombia) for providing the equipment for this research. We also thank the Colombian Ministry of Science, Technology, and Innovation MINCIENCIAS for supporting national Ph.D. Doctorates through the Francisco José de Caldas scholarship program.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Resolved Minimum Run Resolution V Factorial Design for extracting C-PE and their experimental data used in Design-Expert® software.
Table A1.
Resolved Minimum Run Resolution V Factorial Design for extracting C-PE and their experimental data used in Design-Expert® software.
| Factor A | Factor B | Factor C | Factor D | Factor E | Factor F | Response 1 | Response 2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Std | Run | Biomass/Buffer Ratio (% w/v) | pH | Molarity (mM) | Extraction Time (min) | Extraction Speed (rpm) | Buffer | C-PE_Purity (Purity Index) | C-PE (% w/w) |
| 17 | 1 | 1 | 7.4 | 0.1 | 10 | 500 | Sodium phosphate | 0.7606 | 1.48095531 |
| 16 | 2 | 10 | 5.8 | 0.001 | 10 | 1500 | Potassium phosphate | 0.76680159 | 0.49353519 |
| 4 | 3 | 10 | 7.4 | 0.1 | 30 | 500 | Sodium phosphate | 0.74754367 | 0.98112929 |
| 9 | 4 | 10 | 5.8 | 0.1 | 10 | 500 | Sodium phosphate | 1.27514269 | 4.39665455 |
| 22 | 5 | 1 | 5.8 | 0.1 | 10 | 1500 | Potassium phosphate | 0.54055468 | 0.93936676 |
| 19 | 6 | 1 | 5.8 | 0.001 | 30 | 500 | Sodium phosphate | 1.12154758 | 3.91775927 |
| 5 | 7 | 1 | 7.4 | 0.001 | 30 | 500 | Potassium phosphate | 1.09045083 | 1.79881353 |
| 3 | 8 | 1 | 5.8 | 0.001 | 10 | 1500 | Sodium phosphate | 1.04191554 | 3.9589539 |
| 8 | 9 | 1 | 7.4 | 0.001 | 30 | 1500 | Sodium phosphate | 1.41099453 | 6.90128049 |
| 14 | 10 | 10 | 7.4 | 0.1 | 10 | 500 | Sodium phosphate | 1.16715 | 1.39814374 |
| 21 | 11 | 10 | 7.4 | 0.1 | 10 | 1500 | Sodium phosphate | 0.447544 | 0.98112929 |
| 11 | 12 | 10 | 5.8 | 0.001 | 30 | 1500 | Sodium phosphate | 1.83422485 | 1.23462747 |
| 2 | 13 | 10 | 7.4 | 0.001 | 10 | 500 | Sodium phosphate | 0.165782 | 0.37727198 |
| 18 | 14 | 1 | 5.8 | 0.001 | 10 | 500 | Sodium phosphate | 1.21558923 | 4.37041458 |
| 7 | 15 | 10 | 5.8 | 0.001 | 30 | 500 | Potassium phosphate | 0.84726926 | 1.13063171 |
| 15 | 16 | 1 | 5.8 | 0.1 | 30 | 1500 | Sodium phosphate | 1.79609 | 1.05074815 |
| 13 | 17 | 1 | 7.4 | 0.001 | 10 | 1500 | Potassium phosphate | 1.12034 | 4.21950129 |
| 6 | 18 | 10 | 7.4 | 0.1 | 10 | 500 | Potassium phosphate | 1.04725735 | 1.05317935 |
| 1 | 19 | 1 | 5.8 | 0.1 | 30 | 500 | Potassium phosphate | 0.68369843 | 1.86514947 |
| 20 | 20 | 1 | 7.4 | 0.1 | 30 | 1500 | Potassium phosphate | 0.87808136 | 2.47663117 |
| 10 | 21 | 10 | 5.8 | 0.1 | 30 | 1500 | Potassium phosphate | 2.45712 | 1.57674675 |
| 12 | 22 | 10 | 7.4 | 0.001 | 30 | 1500 | Potassium phosphate | 1.03431253 | 2.45301365 |

Table A2.
Resolved Central Composite Design for the optimization of extraction of C-PE and their experimental data used in Design-Expert® software.
Table A2.
Resolved Central Composite Design for the optimization of extraction of C-PE and their experimental data used in Design-Expert® software.
| Factor A | Factor B | Factor C | Response 1 | Response 2 | |||
|---|---|---|---|---|---|---|---|
| Std | Block | Run | pH | Extraction Time (min) | Extraction Speed (rpm) | C-PE_Purity (Purity Index) | C-PE (% w/w) |
| 6 | Block 1 | 1 | 7.4 | 10 | 2000 | 1.18785 | 10.47209 |
| 4 | 2 | 7.4 | 50 | 1000 | 1.61976 | 9.55415 | |
| 7 | 3 | 5.8 | 50 | 2000 | 2.0816 | 8.49407 | |
| 9 | 4 | 6.6 | 30 | 1500 | 1.93423 | 11.0031 | |
| 10 | 5 | 6.6 | 30 | 1500 | 1.90554 | 11.09339 | |
| 1 | 6 | 5.8 | 10 | 1000 | 2.1911 | 7.9383 | |
| 2 | Block 2 | 7 | 7.4 | 10 | 1000 | 1.27555 | 8.021 |
| 12 | 8 | 6.6 | 30 | 1500 | 1.8204 | 11.0031 | |
| 5 | 9 | 5.8 | 10 | 2000 | 2.27758 | 8.67546 | |
| 3 | 10 | 5.8 | 50 | 1000 | 2.04976 | 11.91282 | |
| 8 | 11 | 7.4 | 50 | 2000 | 1.17745 | 8.42667 | |
| 11 | 12 | 6.6 | 30 | 1500 | 1.95816 | 11.09339 | |
| 16 | Block 3 | 13 | 6.6 | 63.64 | 1500 | 1.71559 | 8.92233 |
| 13 | 14 | 5.25 | 30 | 1500 | 2.46082 | 7.56443 | |
| 15 | 15 | 6.6 | −3.63 | 1500 | 1.6 | 7.63122 | |
| 14 | 16 | 7.95 | 30 | 1500 | 1.023975 | 7.44745 | |
| 19 | 17 | 6.6 | 30 | 1500 | 1.82696 | 11.0031 | |
| 17 | 18 | 6.6 | 30 | 659.10 | 1.65863 | 11.4593 | |
| 18 | 19 | 6.6 | 30 | 2340.90 | 1.5425 | 11.0417 | |
| 20 | 20 | 6.6 | 30 | 1500 | 1.8564 | 11.09339 |
References
- Datta, D.; Weiss, E.L.; Wangpraseurt, D.; Hild, E.; Chen, S.; Golden, J.W.; Golden, S.S.; Pokorski, J.K. Phenotypically Complex Living Materials Containing Engineered Cyanobacteria. Nat. Commun. 2023, 14, 4742. [Google Scholar] [CrossRef] [PubMed]
- Babele, P.K.; Srivastava, A.; Young, J.D. Metabolic Flux Phenotyping of Secondary Metabolism in Cyanobacteria. Trends Microbiol. 2023, 31, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Del Mondo, A.; Smerilli, A.; Corato, F.; Sansone, C.; Brunet, C. Biotechnological Response Curve of the Cyanobacterium Spirulina subsalsa to Light Energy Gradient. Biotechnol. Biofuels Bioprod. 2023, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Thajuddin, N.; Subramanian, G. Cyanobacterial Biodiversity and Potential Applications in Biotechnology. Curr. Sci. Assoc. 2005, 89, 47–57. [Google Scholar]
- Shrivastav, A.; Mishra, S.K.; Mishra, S. Polyhydroxyalkanoate (PHA) Synthesis by Spirulina subsalsa from Gujarat Coast of India. Int. J. Biol. Macromol. 2010, 46, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Nowruzi, B.; Jalil, B.S.; Metcalf, J.S. Antifungal Screening of Selenium Nanoparticles Biosynthesized by Microcystin-Producing Desmonostoc Alborizicum. BMC Biotechnol. 2023, 23, 41. [Google Scholar] [CrossRef]
- Alotaiby, S.; Zhao, X.; Boesch, C.; Sergeeva, N.N. Sustainable Approach towards Isolation of Photosynthetic Pigments from Spirulina and the Assessment of Their Prooxidant and Antioxidant Properties. Food Chem. 2024, 436, 137653. [Google Scholar] [CrossRef] [PubMed]
- Akmukhanova, N.R.; Leong, Y.K.; Seiilbek, S.N.; Konysbay, A.; Zayadan, B.K.; Sadvakasova, A.K.; Sarsekeyeva, F.K.; Bauenova, M.O.; Bolatkhan, K.; Alharby, H.F.; et al. Eco-Friendly Biopesticides Derived from CO2-Fixing Cyanobacteria. Environ. Res. 2023, 239, 117419. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Estupiñan, M.A.; Carrillo-Botello, A.M.; Rozo-Granados, L.S.; Becerra-Moreno, D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; López-Barrera, G.L.; Barajas-Solano, A.F.; Bryan, S.J.; Zuorro, A. Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria. Water 2022, 14, 558. [Google Scholar] [CrossRef]
- Malairaj, S.; Muthu, S.; Gopal, V.B.; Perumal, P.; Ramasamy, R. Qualitative and Quantitative Determination of R-Phycoerythrin from Halymenia Floresia (Clemente) C. Agardh by Polyacrylamide Gel Using Electrophoretic Elution Technique. J. Chromatogr. A 2016, 1454, 120–126. [Google Scholar] [CrossRef]
- Xiao, C.; Guo, N.; Liang, Z.; Huang, Z.; Li, W.; Xie, M.; Zhao, F. Ultrafast Energy Transfer Dynamics in a Cyanobacterial Light-Harvesting Phycobilisome. Processes 2023, 11, 1656. [Google Scholar] [CrossRef]
- Adir, N. Elucidation of the Molecular Structures of Components of the Phycobilisome: Reconstructing a Giant. Photosynth. Res. 2005, 85, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, W.; Lei, J.; Chen, H.; Wang, Q. The Unique Light-Harvesting System of the Algal Phycobilisome: Structure, Assembly Components, and Functions. Int. J. Mol. Sci. 2023, 24, 9733. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, Purification and Characteristics of R-Phycoerythrin from a Marine Macroalga Heterosiphonia Japonica. Protein Expr. Purif. 2009, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Cyanobacterial Phycobilisome Allostery as Revealed by Quantitative Mass Spectrometry. Biochemistry 2023, 62, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Sharma, R.; Raghavarao, K.S.M.S. Novel Adsorption Approach for the Enrichment of R-Phycoerythrin from Marine Macroalga Gelidium pusillum. Algal Res. 2022, 62, 102605. [Google Scholar] [CrossRef]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J. Variation in the Biochemical Composition of the Edible Seaweed Grateloupia turuturu Yamada Harvested from Two Sampling Sites on the Brittany Coast (France): The Influence of Storage Method on the Extraction of the Seaweed Pigment r-Phycoerythrin. J. Chem. 2013, 2013, 568548. [Google Scholar] [CrossRef]
- Wyman, M.; Gregory, R.P.F.; Carr, N.G. Novel Role for Phycoerythrin in a Marine Cyanobacterium, Synechococcus Strain DC2. Science 1985, 230, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.A. Phycoerythrocyanin and Phycoerythrin: Properties and Occurrence in Cyanobacteria. J. Gen. Microbiol. 1982, 128, 835–844. [Google Scholar] [CrossRef]
- McGregor, G.B.; Sendall, B.C. Potamosiphon Australiensis Gen. Nov., Sp Nov. (Oscillatoriales), a New Filamentous Cyanobacterium from Subtropical North-Eastern Australia. Phytotaxa 2019, 387, 77–93. [Google Scholar] [CrossRef]
- Vergel-Suarez, A.H.; García-Martínez, J.B.; López-Barrera, G.L.; Barajas-Solano, A.F.; Zuorro, A. Impact of Biomass Drying Process on the Extraction Efficiency of C-Phycoerythrin. BioTech 2023, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Thoisen, C.; Hansen, B.W.; Nielsen, S.L. A Simple and Fast Method for Extraction and Quantification of Cryptophyte Phycoerythrin. MethodsX 2017, 4, 209–213. [Google Scholar] [CrossRef]
- Tan, H.T.; Yusoff, F.M.; Khaw, Y.S.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Katayama, T.; Ahmad, S.A. A Review on a Hidden Gem: Phycoerythrin from Blue-Green Algae. Mar. Drugs 2023, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ropero, J.E.; Lidueñez-Ballesteros, V.S.; Rodríguez-Bohórquez, A.D.; García-Martínez, J.B.; Urbina-Suarez, N.A.; López-Barrera, G.L.; Barajas-Solano, A.F.; Bryan, S.J.; Zuorro, A. The Effect of LEDs on Biomass and Phycobiliproteins Production in Thermotolerant Oscillatoria sp. Appl. Sci. 2022, 12, 11664. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Jagatheesan, A.; Perumal, K.; Arunkumar, K. Methods of Phycobiliprotein Extraction from Gracilaria crassa and Its Applications in Food Colourants. Algal Res. 2015, 8, 115–120. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound Assisted Methods for Enhanced Extraction of Phycobiliproteins from Marine Macro-Algae, Gelidium Pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Raghavarao, K.S.M.S. Extraction of R-Phycoerythrin from Marine Macro-Algae, Gelidium pusillum, Employing Consortia of Enzymes. Algal Res. 2018, 34, 1–11. [Google Scholar] [CrossRef]
- Huschek, G.; Rawel, H.M.; Schweikert, T.; Henkel-Oberländer, J.; Sagu, S.T. Characterization and Optimization of Microwave-Assisted Extraction of B-Phycoerythrin from Porphyridium purpureum Using Response Surface Methodology and Doehlert Design. Bioresour. Technol. Rep. 2022, 19, 101212. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wang, W.; Chen, Z.; Li, C.; Wu, H.; Wu, H.; Xiang, W. A Novel Three-Step Extraction Strategy for High-Value Products from Red Algae Porphyridium purpureum. Foods 2021, 10, 2164. [Google Scholar] [CrossRef]
- García, A.B.; Longo, E.; Murillo, M.C.; Bermejo, R. Using a B-Phycoerythrin Extract as a Natural Colorant: Application in Milk-Based Products. Molecules 2021, 26, 297. [Google Scholar] [CrossRef]
- Fleurence, O.G.J. Contribution of Electrophoresis of Red Algae Seaweeds (Gracilaria sp.) Used as Food Ingredients. Sci. Aliments 1995, 15, 43–48. [Google Scholar]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morançais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical Factors Affecting the Stability of Two Pigments: R-Phycoerythrin of Grateloupia turuturu and B-Phycoerythrin of Porphyridium cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Galland-Irmouli, A.V.; Pons, L.; Luçon, M.; Villaume, C.; Mrabet, N.T.; Guéant, J.L.; Fleurence, J. One-Step Purification of R-Phycoerythrin from the Red Macroalga Palmaria palmata Using Preparative Polyacrylamide Gel Electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Kurinjimalar, C.; Thangam, R.; Suresh, V.; Kavitha, G.; Gunasekaran, P.; Rengasamy, R. Further Studies and Biological Activities of Macromolecular Protein R-Phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol. 2013, 62, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.Y.; Fleurence, J.; Bergé, J.P. Ultrasound-Assisted Extraction of R-Phycoerythrin from Grateloupia turuturu with and without Enzyme Addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of Protein from the Macroalga Palmaria palmata. LWT-Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Andersen, R.; Berges, J.; Harrison, P.; Watanabe, M. Recipes for Freshwater and Seawater Media. In Algal Culture Techniques, 1st ed.; Elsevier: London, UK, 2005. [Google Scholar]
- Barajas-Solano, A.F. Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp. Processes 2022, 10, 836. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Raghavarao, K.S.M.S. Aqueous Two Phase Extraction for Purification of C-Phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- Antelo, F.; Anschau, A.; Costa, J.; Kalil, S. Extraction and Purification of C-Phycocyanin from Spirulina platensis in Conventional and Integrated Aqueous Two-Phase Systems. J. Braz. Chem. Soc. 2010, 21, 921–926. [Google Scholar] [CrossRef]
- Xie, J.; Chen, S.; Wen, Z. Effects of Light Intensity on the Production of Phycoerythrin and Polyunsaturated Fatty Acid by Microalga Rhodomonas salina. Algal Res. 2021, 58, 102397. [Google Scholar] [CrossRef]
- Lee, M.-C.; Yeh, H.-Y.; Jhang, F.-J.; Lee, P.-T.; Lin, Y.-K.; Nan, F.-H. Enhancing Growth, Phycoerythrin Production, and Pigment Composition in the Red Alga Colaconema sp. Through Optimal Environmental Conditions in an Indoor System. Bioresour. Technol. 2021, 333, 125199. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and Efficient Method for Extraction of C-Phycocyanin from Dry Biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Sukwong, P.; Sunwoo, I.Y.; Nguyen, T.H.; Jeong, G.-T.; Kim, S.-K. R-Phycoerythrin, R-Phycocyanin and ABE Production from Gelidium amansii by Clostridium acetobutylicum. Process Biochem. 2019, 81, 139–147. [Google Scholar] [CrossRef]
- Ji, L.; Liu, Y.; Luo, J.; Fan, J. Freeze-Thaw-Assisted Aqueous Two-Phase System as a Green and Low-Cost Option for Analytical Grade B-Phycoerythrin Production from Unicellular Microalgae Porphyridium purpureum. Algal Res. 2022, 67, 102831. [Google Scholar] [CrossRef]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A Review of Extraction Methods and Stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Ng, I.-S. Production, Isolation and Characterization of C-Phycocyanin from a New Halo-Tolerant Cyanobacterium aponinum Using Seawater. Bioresour. Technol. 2021, 342, 125946. [Google Scholar] [CrossRef]
- Ghosh, T.; Mishra, S. Studies on Extraction and Stability of C-Phycoerythrin from a Marine Cyanobacterium. Front. Sustain. Food Syst. 2020, 4, 102. [Google Scholar] [CrossRef]
- Basheva, D.; Moten, D.; Stoyanov, P.; Belkinova, D.; Mladenov, R.; Teneva, I. Content of Phycoerythrin, Phycocyanin, Alophycocyanin and Phycoerythrocyanin in Some Cyanobacterial Strains: Applications. Eng. Life Sci. 2018, 18, 861–866. [Google Scholar] [CrossRef]
- Hemlata; Afreen, S.; Fatma, T. Extraction, Purification and Characterization of Phycoerythrin from Michrochaete and Its Biological Activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar] [CrossRef]
- Ismail, M.M.; El-Fakharany, E.M.; Hegazy, G.E. Purification and Fractionation of Phycobiliproteins from Arthrospira platensis and Corallina officinalis with Evaluating Their Biological Activities. Sci. Rep. 2023, 13, 14270. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).