Prospects in the Use of Cannabis sativa Extracts in Nanoemulsions
Abstract
1. Introduction
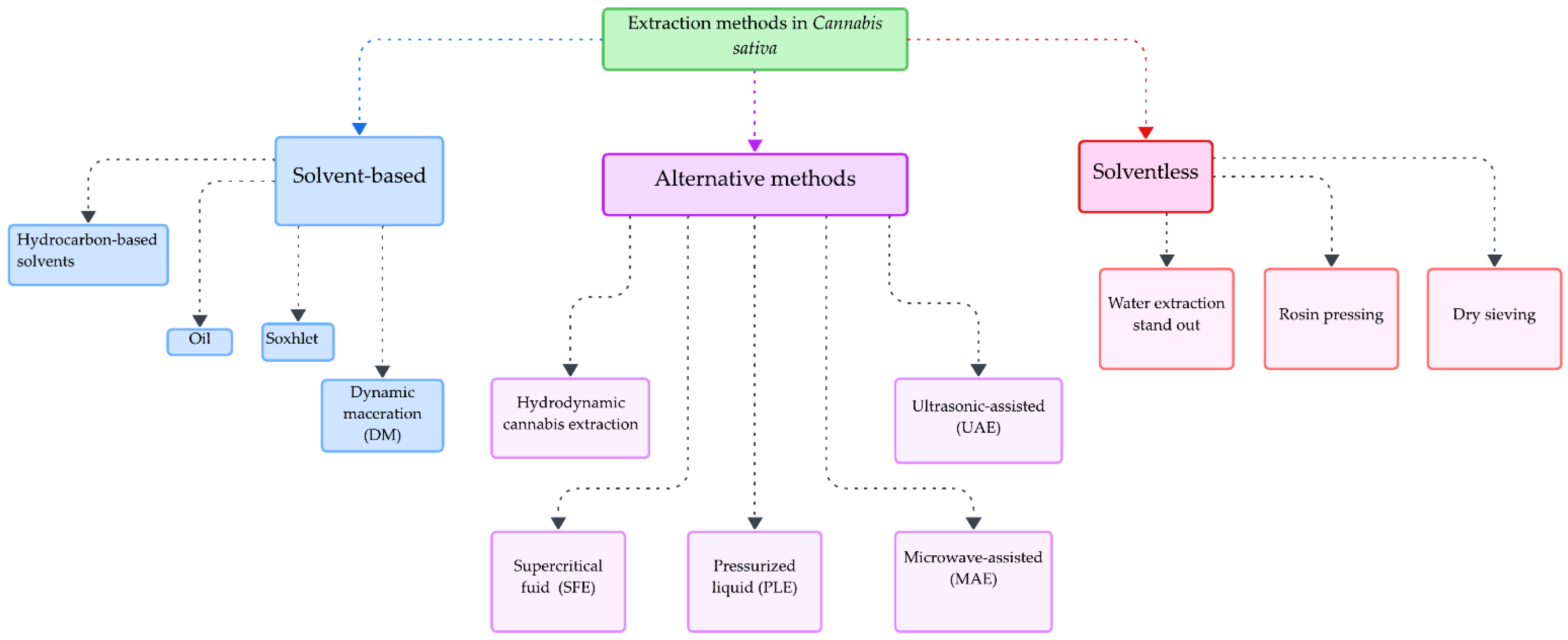
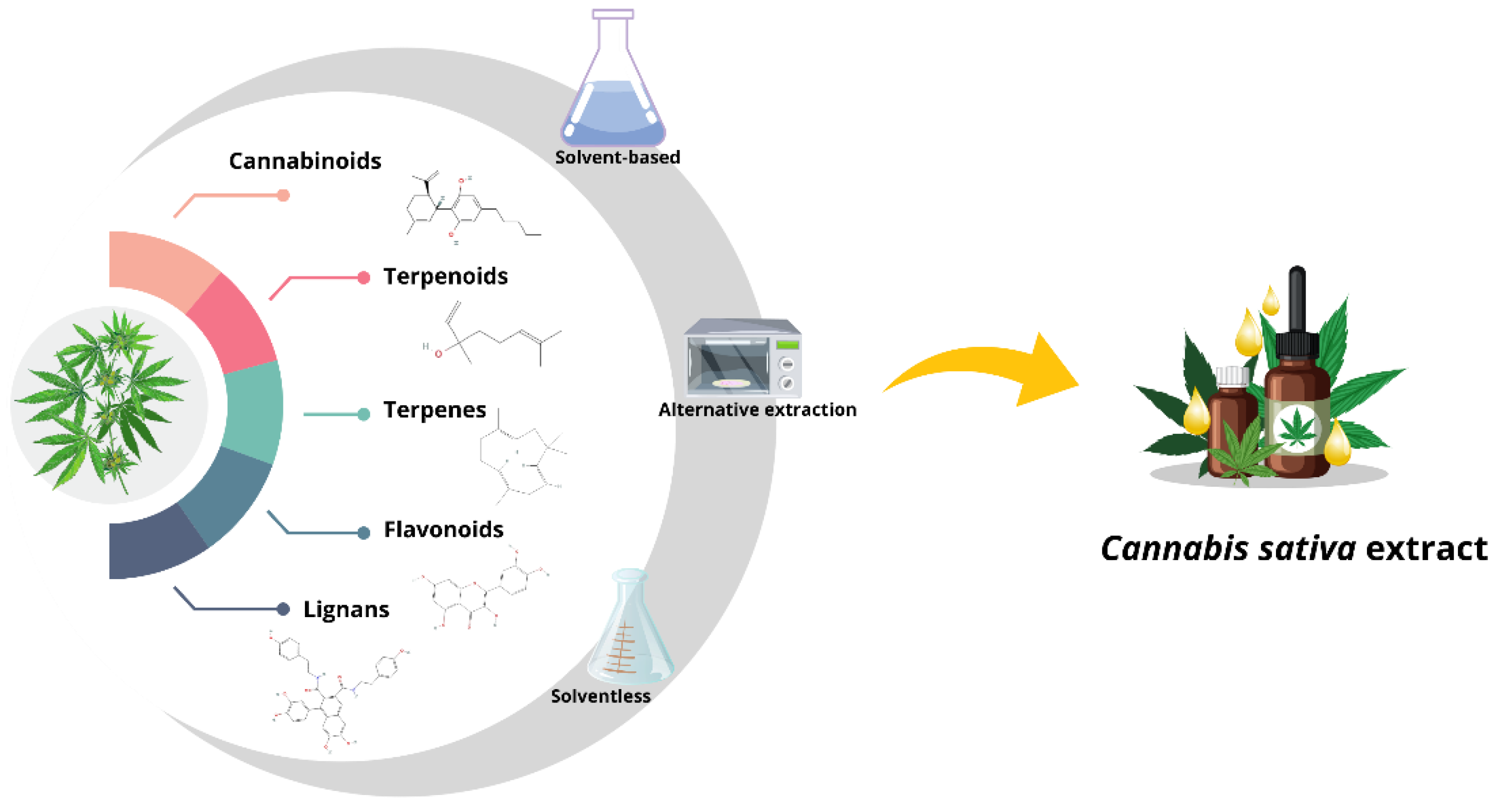
2. Extraction Methods Reported for C. sativa
3. Nanoemulsions: Materials and Synthesis Methods
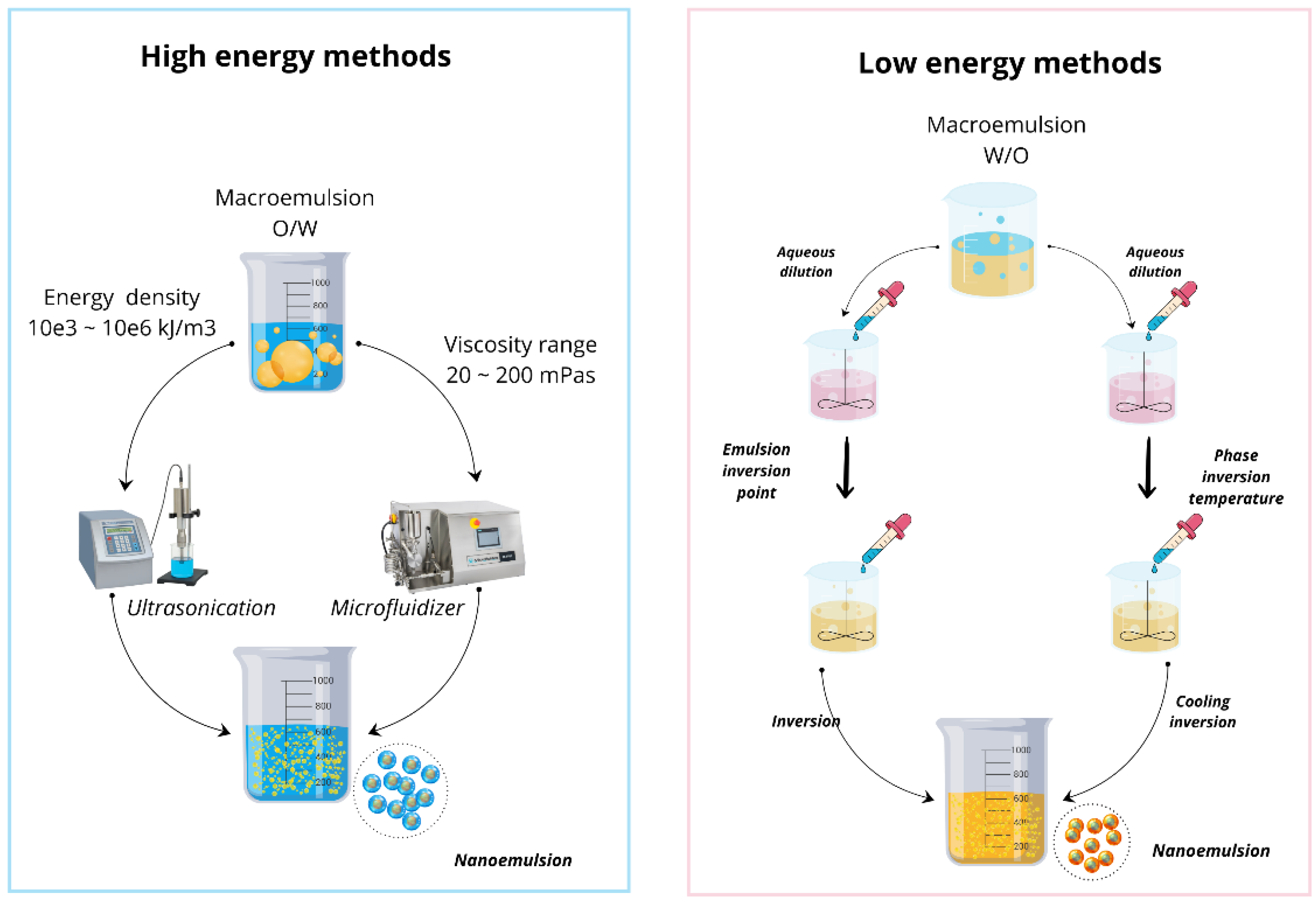
3.1. High-Energy Methods Used for Nanoemulsions
3.2. Low-Energy Methods Used for Nanoemulsions
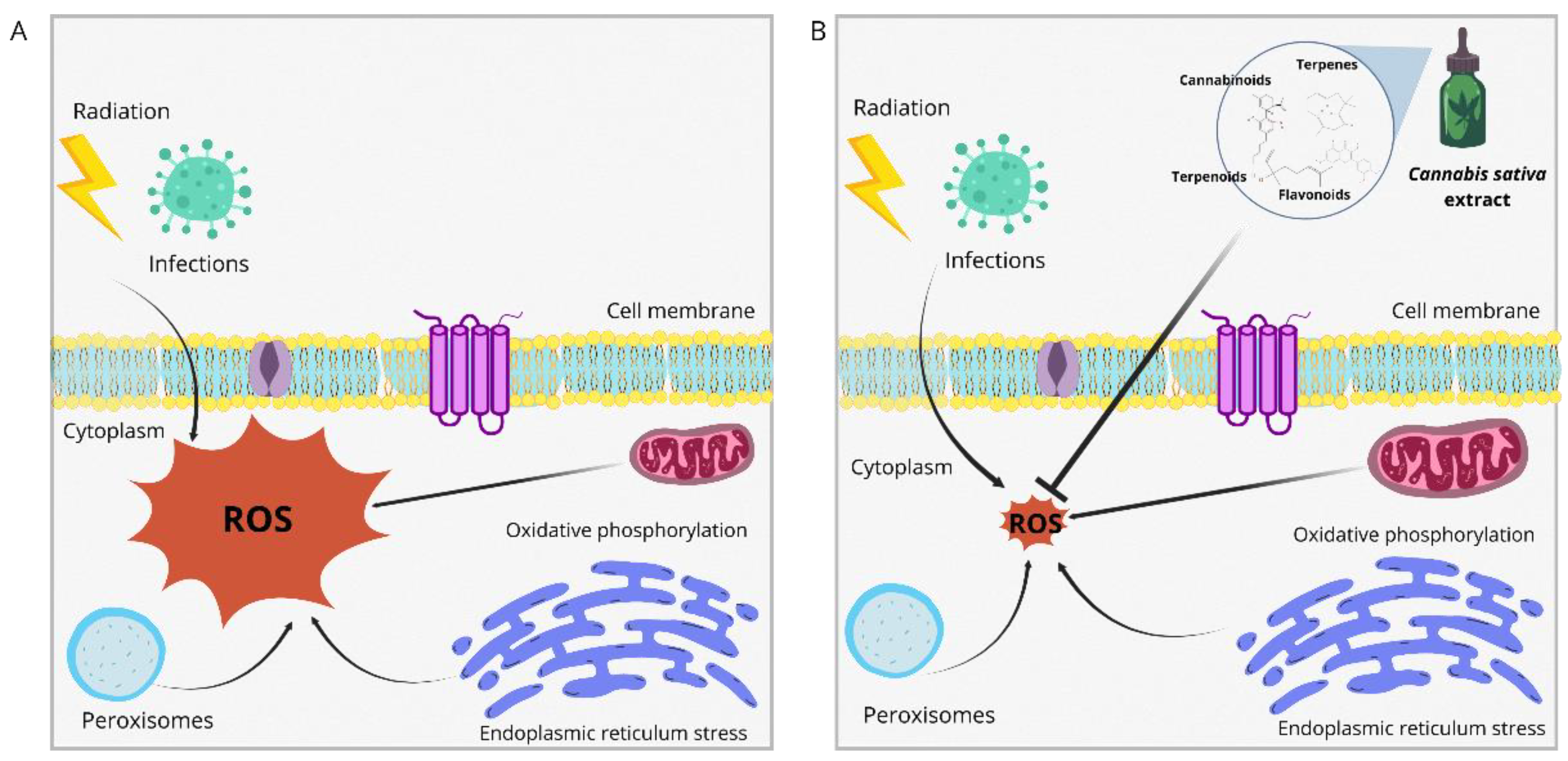
4. Antioxidant Activity of Cannabis sativa Extracts and Nanoemulsions
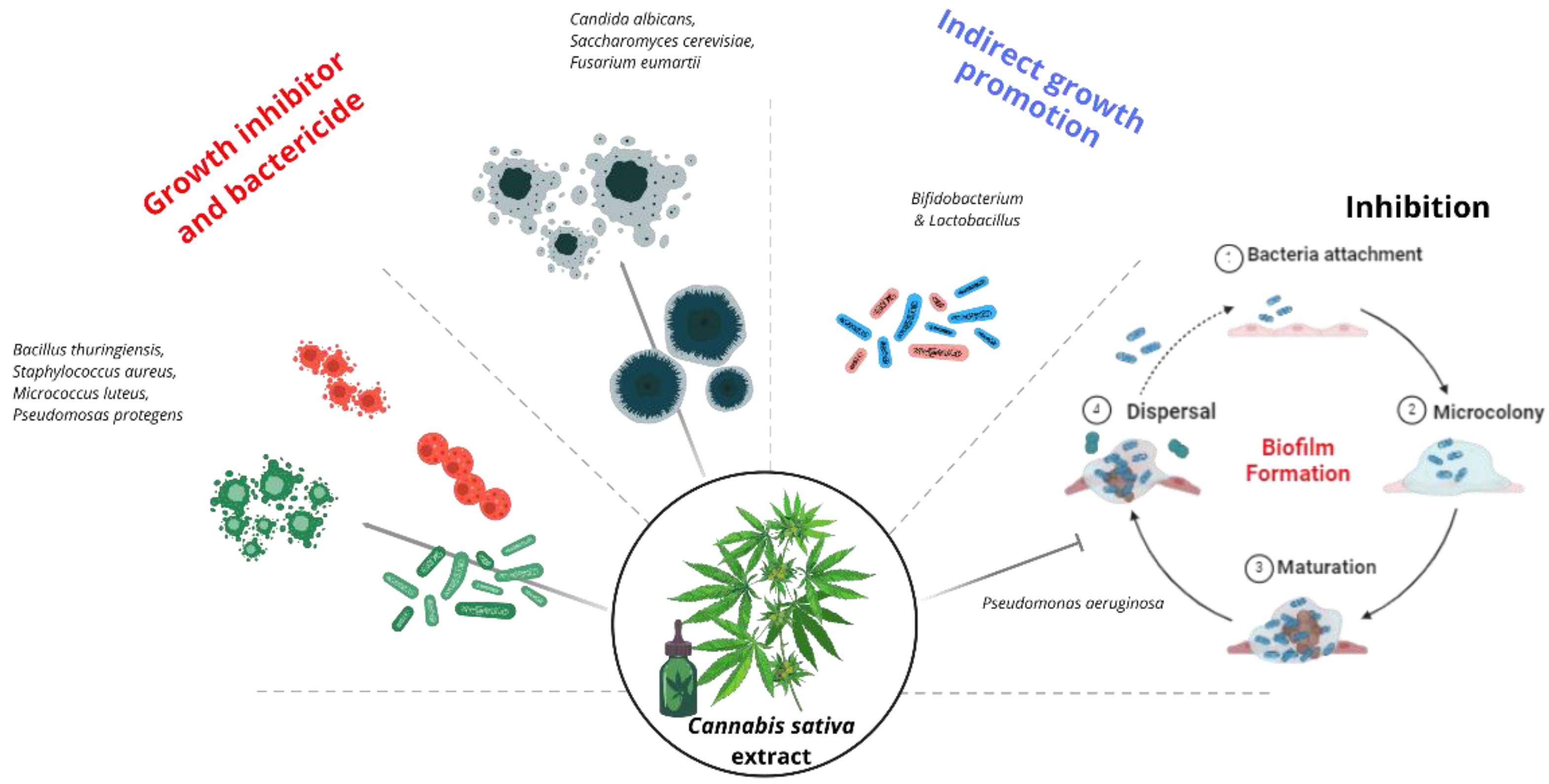
5. Effect of Cannabis sativa Extract and Nanosystems on Antimicrobial Activity
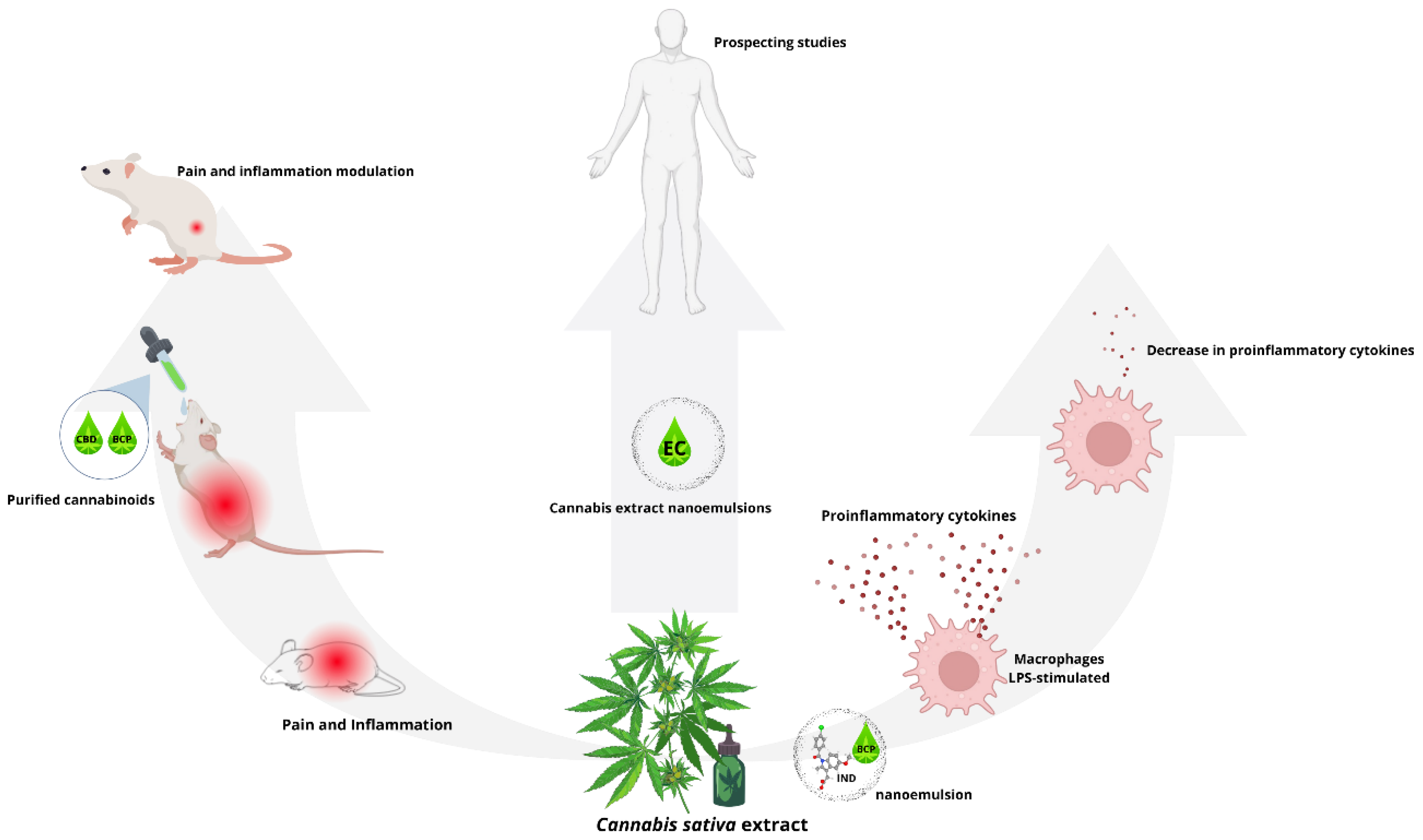
6. Effect of Cannabis sativa Extracts Nanoemulsions on Immunogenicity Activity
7. Computational Analysis of Cannabis sativa Compounds
8. General Critical Points for Cannabis sativa-Based Nanosystems
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leal-Galicia, P.; Betancourt, D.; Gonzalez-Gonzalez, A.; Romo-Parra, H. A brief history of marijuana in the western world. Rev. Neurol. 2018, 67, 133–140. [Google Scholar] [PubMed]
- Xie, Z.; Mi, Y.; Kong, L.; Gao, M.; Chen, S.; Chen, W.; Meng, X.; Sun, W.; Chen, S.; Xu, Z. Cannabis sativa: Origin and History, Glandular Trichome Development, and Cannabinoid Biosynthesis. Hortic. Res. 2023, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content during Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- López Carretero, P.; Pekas, A.; Stubsgaard, L.; Sancho Blanco, G.; Lütken, H.; Sigsgaard, L. Glandular Trichomes Affect Mobility and Predatory Behavior of Two Aphid Predators on Medicinal Cannabis. Biol. Control 2022, 170, 104932. [Google Scholar] [CrossRef]
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef]
- Salamone, S.; Waltl, L.; Pompignan, A.; Grassi, G.; Chianese, G.; Koeberle, A.; Pollastro, F. Phytochemical Characterization of Cannabis sativa L. Chemotype V Reveals Three New Dihydrophenanthrenoids That Favorably Reprogram Lipid Mediator Biosynthesis in Macrophages. Plants 2022, 11, 2130. [Google Scholar] [CrossRef]
- Rodríguez Mesa, X.M.; Moreno Vergara, A.F.; Contreras Bolaños, L.A.; Guevara Moriones, N.; Mejía Piñeros, A.L.; Santander González, S.P. Therapeutic Prospects of Cannabinoids in the Immunomodulation of Prevalent Autoimmune Diseases. Cannabis Cannabinoid Res. 2021, 6, 196–210. [Google Scholar] [CrossRef]
- Kubiliene, A.; Mickute, K.; Baranauskaite, J.; Marksa, M.; Liekis, A.; Sadauskiene, I. The Effects of Cannabis sativa L. Extract on Oxidative Stress Markers In Vivo. Life 2021, 11, 647. [Google Scholar] [CrossRef]
- Ali, E.M.M.; Almagboul, A.Z.I.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial Activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary Metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Leinen, Z.J.; Mohan, R.; Premadasa, L.S.; Acharya, A.; Mohan, M.; Byrareddy, S.N. Therapeutic Potential of Cannabis: A Comprehensive Review of Current and Future Applications. Biomedicines 2023, 11, 2630. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.-J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L.F. CBD Suppression of EAE Is Correlated with Early Inhibition of Splenic IFN-γ + CD8+ T Cells and Modest Inhibition of Neuroinflammation. J. Neuroimmune Pharmacol. 2021, 16, 346–362. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An Entourage Effect: Inactive Endogenous Fatty Acid Glycerol Esters Enhance 2-Arachidonoyl-Glycerol Cannabinoid Activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Opęchowska, A.; Karpiuk, K.; Zahorodnii, A.; Harasim-Symbor, E.; Chabowski, A.; Konstantynowicz-Nowicka, K. Anti-Inflammatory Effects of Cannabidiol in Early Stages of Neuroinflammation Induced by High-Fat Diet in Cerebral Cortex of Rats. Toxicol. Appl. Pharmacol. 2024, 484, 116856. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R. Identification of Psychoactive Metabolites from Cannabis sativa, Its Smoke, and Other Phytocannabinoids Using Machine Learning and Multivariate Methods. ACS Omega 2020, 5, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2020, 25, 19.200. [Google Scholar] [CrossRef]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Chitosan/Cashew Gum Nanogels for Essential Oil Encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; McClements, D.J. Nanoemulsions as Delivery Systems for Lipophilic Nutraceuticals: Strategies for Improving Their Formulation, Stability, Functionality and Bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Cleirec, G.; Desmier, E.; Lacatus, C.; Lesgourgues, S.; Braun, A.; Peloso, C.; Obadia, C. Efficiency of Inhaled Cannabidiol in Cannabis Use Disorder: The Pilot Study Cannavap. Front. Psychiatry 2022, 13, 899221. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The Pharmacokinetics and the Pharmacodynamics of Cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Arianto, A.; Cindy, C. Preparation and Evaluation of Sunflower Oil Nanoemulsion as a Sunscreen. Open Access Maced. J. Med. Sci. 2019, 7, 3757–3761. [Google Scholar] [CrossRef]
- Ren, J.-N.; Dong, M.; Hou, Y.-Y.; Fan, G.; Pan, S.-Y. Effect of Olive Oil on the Preparation of Nanoemulsions and Its Effect on Aroma Release. J. Food Sci. Technol. 2018, 55, 4223–4231. [Google Scholar] [CrossRef]
- He, W.; Tan, Y.; Tian, Z.; Chen, L.; Hu, F.; Wu, W. Food Protein-Stabilized Nanoemulsions as Potential Delivery Systems for Poorly Water-Soluble Drugs: Preparation, in Vitro Characterization, and Pharmacokinetics in Rats. Int. J. Nanomed. 2011, 6, 521–533. [Google Scholar] [CrossRef]
- Kandadi, P.; Syed, M.A.; Goparaboina, S.; Veerabrahma, K. Albumin Coupled Lipid Nanoemulsions of Diclofenac for Targeted Delivery to Inflammation. Nanomedicine 2012, 8, 1162–1171. [Google Scholar] [CrossRef]
- Li, Y.; Jin, H.; Sun, X.; Sun, J.; Liu, C.; Liu, C.; Xu, J. Physicochemical Properties and Storage Stability of Food Protein-Stabilized Nanoemulsions. Nanomaterials 2018, 9, 25. [Google Scholar] [CrossRef]
- Espinosa-Sandoval, L.; Ochoa-Martínez, C.; Ayala-Aponte, A.; Pastrana, L.; Gonçalves, C.; Cerqueira, M.A. Polysaccharide-Based Multilayer Nano-Emulsions Loaded with Oregano Oil: Production, Characterization, and In Vitro Digestion Assessment. Nanomaterials 2021, 11, 878. [Google Scholar] [CrossRef]
- Malik, M.R.; Al-Harbi, F.F.; Nawaz, A.; Amin, A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N. Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo. Molecules 2022, 27, 3183. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef] [PubMed]
- Roda Zitha Vilanculos, J.; Silva de Farias, B.; Inês Engelmann, J.; Silveira Ribeiro, E.; Diaz de Oliveira, P.; Roberto Sant’Anna Cadaval, T.; Antonio de Almeida Pinto, L. Physicochemical Evaluation of Chitosan–Xanthan Gum Nanoemulsions as Polyunsaturated Enriched Lipid–Carrier. J. Mol. Liq. 2023, 386, 122533. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, J.; Ma, R.; Tian, Y. Preparation of Starch-Based Nanoemulsion for Sustained Release and Enhanced Bioaccessibility of Quercetin. Colloids Surf. A Physicochem. Eng. Asp. 2023, 665, 131218. [Google Scholar] [CrossRef]
- Himanath, G.; Shruthy, R.; Preetha, R.; Sreejit, V. Nanoemulsion with Coconut Oil and Soy Lecithin as a Stable Delivery System for Lycopene and Its Incorporation into Yogurt to Enhance Antioxidant Properties and Maintain Quality. ACS Food Sci. Technol. 2021, 1, 1538–1549. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Santamaria-Echart, A.; Ribeiro, A.; Peres, A.M.; Dias, M.M.; Pinho, S.P.; Barreiro, M.F. Formulation and Optimization of Nanoemulsions Using the Natural Surfactant Saponin from Quillaja Bark. Molecules 2020, 25, 1538. [Google Scholar] [CrossRef]
- Rožanc, J.; Kotnik, P.; Milojević, M.; Gradišnik, L.; Knez Hrnčič, M.; Knez, Ž.; Maver, U. Different Cannabis sativa Extraction Methods Result in Different Biological Activities against a Colon Cancer Cell Line and Healthy Colon Cells. Plants 2021, 10, 566. [Google Scholar] [CrossRef]
- Blake, A.; Nahtigal, I. The Evolving Landscape of Cannabis Edibles. Curr. Opin. Food Sci. 2019, 28, 25–31. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic Fingerprinting of Cannabis sativa L., Cannabinoids and Terpenoids for Chemotaxonomic and Drug Standardization Purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef]
- Radoiu, M.; Kaur, H.; Bakowska-Barczak, A.; Splinter, S. Microwave-Assisted Industrial Scale Cannabis Extraction. Technologies 2020, 8, 45. [Google Scholar] [CrossRef]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of Delta9-THCA-A from Hemp and Analytical Aspects Concerning the Determination of Delta9-THC in Cannabis Products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of Bioactive Compounds from Cannabis sativa L. Flowers and/or Leaves Using Deep Eutectic Solvents. Front. Nutr. 2022, 9, 892314. [Google Scholar] [CrossRef]
- Madia, V.N.; Di Santo, R.; Costi, R. Chapter 2—Medical Cannabis and Cannabinoids: How Best to Extract Components from Plant Material. In Medicinal Usage of Cannabis and Cannabinoids; Preedy, V.R., Patel, V.B., Martin, C.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 15–23. ISBN 978-0-323-90036-2. [Google Scholar]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Ultrasonic-Assisted Virgin Coconut Oil Based Extraction for Maximizing Polyphenol Recovery and Bioactivities of Mangosteen Peels. J. Food Sci. Technol. 2020, 57, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.; Trifunovska, M.; Antunes, M.; Miranda, I.; Moldão, M.; Alves, V.; Vidrih, R.; Lopes, P.A.; Aparicio, L.; Neves, M.; et al. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Pelvetia canaliculata to Sunflower Oil. Foods 2021, 10, 1732. [Google Scholar] [CrossRef]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franzè, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P.; et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.-S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods 2022, 11, 3412. [Google Scholar] [CrossRef]
- Koina, I.M.; Sarigiannis, Y.; Hapeshi, E. Green Extraction Techniques for the Determination of Active Ingredients in Tea: Current State, Challenges, and Future Perspectives. Separations 2023, 10, 121. [Google Scholar] [CrossRef]
- Discover Advanced Hydrodynamic Cannabis Extraction—Cannabis Tech. 2018. Available online: https://cannabistech.com/articles/hydrodynamic-cannabis-extraction/ (accessed on 5 March 2024).
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized Liquid Extraction in the Analysis of Food and Biological Samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Kraujalis, P.; Tamkutė, L.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Recovery of Bioactive Substances from Rowanberry Pomace by Consecutive Extraction with Supercritical Carbon Dioxide and Pressurized Solvents. J. Ind. Eng. Chem. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Pilařová, V.; Hadysová, Z.; Švec, F.; Nováková, L. Supercritical Fluids in Analysis of Cannabinoids in Various Cannabis Products. Anal. Chim. Acta 2022, 1232, 340452. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; McClements, D. Nanoemulsions: Formulation, Applications, and Characterization; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Addo, P.W.; Sagili, S.U.K.R.; Bilodeau, S.E.; Gladu-Gallant, F.-A.; MacKenzie, D.A.; Bates, J.; McRae, G.; MacPherson, S.; Paris, M.; Raghavan, V.; et al. Microwave- and Ultrasound-Assisted Extraction of Cannabinoids and Terpenes from Cannabis Using Response Surface Methodology. Molecules 2022, 27, 8803. [Google Scholar] [CrossRef] [PubMed]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and Extraction Methods of Medicinal Cannabis: A Narrative Review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, O.Y.; Ardila, R.; Orjuela, A.; Santaella, M.A.; Arturo, D.E.; Hurtado, A. Affordable Method for Batch Supercritical Extraction Using Solid Carbon Dioxide–Extraction of Cannabis Threshing Residues. Chem. Eng. Process.—Process Intensif. 2024, 198, 109721. [Google Scholar] [CrossRef]
- Naveira-Pazos, C.; Veiga, M.C.; Mussagy, C.U.; Farias, F.O.; Kennes, C.; Pereira, J.F.B. Carotenoids Production and Extraction from Yarrowia Lipolytica Cells: A Biocompatible Approach Using Biosolvents. Sep. Purif. Technol. 2024, 343, 127136. [Google Scholar] [CrossRef]
- Han, Q.-H.; Liu, W.; Li, H.-Y.; He, J.-L.; Guo, H.; Lin, S.; Zhao, L.; Chen, H.; Liu, Y.-W.; Wu, D.-T.; et al. Extraction Optimization, Physicochemical Characteristics, and Antioxidant Activities of Polysaccharides from Kiwifruit (Actinidia chinensis Planch.). Molecules 2019, 24, 461. [Google Scholar] [CrossRef]
- Chouhan, K.B.S.; Tandey, R.; Sen, K.K.; Mehta, R.; Mandal, V. A Unique Model of Gravity Assisted Solvent Free Microwave Based Extraction of Essential Oil from Mentha Leaves Ensuring Biorefinery of Leftover Waste Biomass for Extraction of Nutraceuticals: Towards Cleaner and Greener Technology. J. Clean. Prod. 2019, 225, 587–598. [Google Scholar] [CrossRef]
- Sorita, G.D.; Favaro, S.P.; Ambrosi, A.; Luccio, M.D. Aqueous Extraction Processing: An Innovative and Sustainable Approach for Recovery of Unconventional Oils. Trends Food Sci. Technol. 2023, 133, 99–113. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-429-12389-4. [Google Scholar]
- Gan, L.; Cui, D.; Ali, N.; Zhang, Q.; Zhang, D.; Jiang, W.; Zhang, W. Phase Behavior of Polyglycerol Ester-Based Nanoemulsions. J. Nanosci. Nanotechnol. 2021, 21, 6188–6195. [Google Scholar] [CrossRef]
- Zeeb, B.; Herz, E.; McClements, D.; Weiss, J. Impact of Alcohols on the Formation and Stability of Protein-Stabilized Nanoemulsions. J. Colloid. Interface Sci. 2014, 433, 196–203. [Google Scholar] [CrossRef]
- Bleoanca, I.; Lanciu, A.; Patrașcu, L.; Ceoromila, A.; Borda, D. Efficacy of Two Stabilizers in Nanoemulsions with Whey Proteins and Thyme Essential Oil as Edible Coatings for Zucchini. Membranes 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Aminzare, M.; Hassanzad Azar, H.; Rostamizadeh, K. Effect of Corn Starch Coating Incorporated with Nanoemulsion of Zataria Multiflora Essential Oil Fortified with Cinnamaldehyde on Microbial Quality of Fresh Chicken Meat and Fate of Inoculated Listeria Monocytogenes. J. Food Sci. Technol. 2021, 58, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Agame-Lagunes, B.; Grube-Pagola, P.; García-Varela, R.; Alexander-Aguilera, A.; García, H.S. Effect of Curcumin Nanoemulsions Stabilized with MAG and DAG-MCFAs in a Fructose-Induced Hepatic Steatosis Rat Model. Pharmaceutics 2021, 13, 509. [Google Scholar] [CrossRef]
- Vega-Hernández, L.C.; Serrano-Niño, J.C.; Velázquez-Carriles, C.A.; Martínez-Preciado, A.H.; Cavazos-Garduño, A.; Silva-Jara, J.M. Improving Foodborne Pathogen Control Using Green Nanosized Emulsions of Plectranthus hadiensis Phytochemicals. Colloids Interfaces 2024, 8, 3. [Google Scholar] [CrossRef]
- Kim, T.-I.; Kim, T.-G.; Lim, D.-H.; Kim, S.-B.; Park, S.-M.; Hur, T.-Y.; Ki, K.-S.; Kwon, E.-G.; Vijayakumar, M.; Kim, Y.-J. Preparation of Nanoemulsions of Vitamin A and C by Microfluidization: Efficacy on the Expression Pattern of Milk-Specific Proteins in MAC-T Cells. Molecules 2019, 24, 2566. [Google Scholar] [CrossRef]
- Knoke, S.; Bunjes, H. Transfer of Lipophilic Drugs from Nanoemulsions into Lipid-Containing Alginate Microspheres. Pharmaceutics 2021, 13, 173. [Google Scholar] [CrossRef]
- Vater, C.; Bosch, L.; Mitter, A.; Göls, T.; Seiser, S.; Heiss, E.; Elbe-Bürger, A.; Wirth, M.; Valenta, C.; Klang, V. Lecithin-Based Nanoemulsions of Traditional Herbal Wound Healing Agents and Their Effect on Human Skin Cells. Eur. J. Pharm. Biopharm. 2022, 170, 1–9. [Google Scholar] [CrossRef]
- Ali, A.; Mekhloufi, G.; Huang, N.; Agnely, F. β-Lactoglobulin Stabilized Nanemulsions--Formulation and Process Factors Affecting Droplet Size and Nanoemulsion Stability. Int. J. Pharm. 2016, 500, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Abbasian Chaleshtari, Z.; Salimi-Kenari, H.; Foudazi, R. Glassy and Compressed Nanoemulsions Stabilized with Sodium Dodecyl Sulfate in the Presence of Poly(Ethylene Glycol)-Diacrylate. Soft Matter 2023, 19, 5989–6004. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Investigation of Factors Influencing Formation of Nanoemulsion by Spontaneous Emulsification: Impact on Droplet Size, Polydispersity Index, and Stability. Bioengineering 2022, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, Q.; Yuan, Y.; Guo, X.; Pan, D.; Xie, R.; Ju, X.; Liu, Z.; Wang, W.; Chu, L. Functional Nanoemulsions: Controllable Low-Energy Nanoemulsification and Advanced Biomedical Application. Chin. Chem. Lett. 2024, 35, 108710. [Google Scholar] [CrossRef]
- Hadžiabdić, J.; Džana, O.; Vranić, E.; Ognjenka, R. Preparation of nanoemulsions by high-energy and lowenergy emulsification methods. In IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2017; pp. 317–322. [Google Scholar]
- Song, R.; Lin, Y.; Li, Z. Ultrasonic-Assisted Preparation of Eucalyptus Oil Nanoemulsion: Process Optimization, In Vitro Digestive Stability, and Anti-Escherichia Coli Activity. Ultrason. Sonochem. 2021, 82, 105904. [Google Scholar] [CrossRef]
- Ochoa, A.A.; Hernández-Becerra, J.A.; Cavazos-Garduño, A.; Vernon-Carter, E.J.; García, H.S.; Ochoa, A.A.; Hernández-Becerra, J.A.; Cavazos-Garduño, A.; Vernon-Carter, E.J.; García, H.S. Preparation and Characterization of Curcumin Nanoemulsions Obtained by Thin-Film Hydration Emulsification and Ultrasonication Methods. Rev. Mex. Ing. Química 2016, 15, 79–90. [Google Scholar]
- Sharma, S.; Sahni, J.K.; Ali, J.; Baboota, S. Effect of High-Pressure Homogenization on Formulation of TPGS Loaded Nanoemulsion of Rutin—Pharmacodynamic and Antioxidant Studies. Drug Deliv. 2015, 22, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Ochoa-Martínez, L.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Long-Term Stability of Food-Grade Nanoemulsions from High Methoxyl Pectin Containing Essential Oils. Food Hydrocoll. 2016, 52, 438–446. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.; Coco, R.; Aude, L.B.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S. Bunjes Preparation of Nanoemulsions and Solid Lipid Nanoparticles by Premix Membrane Emulsification. J. Pharm. Sci. 2012, 101, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, A.; McBain, J.W. Spontaneous Emulsification of Pure Xylene in an Aqueous Solution through Mere Adsorption of a Detergent in the Interface. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1997, 198, 447–454. [Google Scholar] [CrossRef]
- McBain, J.W.; Woo, T. Spontaneous Emulsification, and Reactions Overshooting Equilibrium. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1997, 163, 182–188. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant Functionalized Nanoparticles: A Combat against Oxidative Stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol Prevents LPS-Induced Microglial Inflammation by Inhibiting ROS/NF-κB-Dependent Signaling and Glucose Consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Raja, A.; Ahmadi, S.; de Costa, F.; Li, N.; Kerman, K. Attenuation of Oxidative Stress by Cannabinoids and Cannabis Extracts in Differentiated Neuronal Cells. Pharmaceuticals 2020, 13, 328. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and Their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- Berardo, M.E.V.; Mendieta, J.R.; Villamonte, M.D.; Colman, S.L.; Nercessian, D. Antifungal and Antibacterial Activities of Cannabis sativa L. Resins. J. Ethnopharmacol. 2024, 318, 116839. [Google Scholar] [CrossRef]
- Demisli, S.; Galani, E.; Goulielmaki, M.; Kyrilis, F.L.; Ilić, T.; Hamdi, F.; Crevar, M.; Kastritis, P.L.; Pletsa, V.; Nallet, F.; et al. Encapsulation of Cannabidiol in Oil-in-Water Nanoemulsions and Nanoemulsion-Filled Hydrogels: A Structure and Biological Assessment Study. J. Colloid Interface Sci. 2023, 634, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Suganthy, N.; Chau, T.P.; Sharma, A.; Unpaprom, Y.; Ramaraj, R.; Karuppusamy, I.; Brindhadevi, K. Cannabinoids as Anticancer and Neuroprotective Drugs: Structural Insights and Pharmacological Interactions—A Review. Process Biochem. 2021, 111, 9–31. [Google Scholar] [CrossRef]
- Rebibo, L.; Frušić-Zlotkin, M.; Ofri, R.; Nassar, T.; Benita, S. The Dose-Dependent Effect of a Stabilized Cannabidiol Nanoemulsion on Ocular Surface Inflammation and Intraocular Pressure. Int. J. Pharm. 2022, 617, 121627. [Google Scholar] [CrossRef]
- Tran, V.N.; Strnad, O.; Šuman, J.; Veverková, T.; Sukupová, A.; Cejnar, P.; Hynek, R.; Kronusová, O.; Šach, J.; Kaštánek, P.; et al. Cannabidiol Nanoemulsion for Eye Treatment—Anti-Inflammatory, Wound Healing Activity and Its Bioavailability Using In Vitro Human Corneal Substitute. Int. J. Pharm. 2023, 643, 123202. [Google Scholar] [CrossRef]
- Pagano, C.; Savarese, B.; Coppola, L.; Navarra, G.; Avilia, G.; Laezza, C.; Bifulco, M. Cannabinoids in the Modulation of Oxidative Signaling. Int. J. Mol. Sci. 2023, 24, 2513. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Gioia, D.D. Antimicrobial and Antibiofilm Activity of Cannabis sativa L. Seeds Extract against Staphylococcus Aureus and Growth Effects on Probiotic Lactobacillus spp. LWT 2020, 124, 109149. [Google Scholar] [CrossRef]
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; ElSohly, M.A. Chemical and Biological Studies of Cannabis sativa Roots. Med. Cannabis Cannabinoids 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Menossi, M.; Tejada, G.; Colman, S.L.; Nercessian, D.; Mendieta, J.R.; Islan, G.A.; Alvarez, V.A. Cannabis Extract-Loaded Lipid and Chitosan-Coated Lipid Nanoparticles with Antifungal Activity. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133207. [Google Scholar] [CrossRef]
- Michailidu, J.; Miškovská, A.; Jarošová, I.; Čejková, A.; Mat’átková, O. Bimetallic Nanoparticle Production Using Cannabis sativa and Vitis Vinifera Waste Extracts. RSC Adv. 2024, 14, 5309–5318. [Google Scholar] [CrossRef]
- Friedman, H.; Klein, T.W.; Newton, C.; Daaka, Y. Marijuana, Receptors and Immunomodulation. Adv. Exp. Med. Biol. 1995, 373, 103–113. [Google Scholar] [CrossRef]
- Blevins, L.K.; Bach, A.P.; Crawford, R.B.; Zhou, J.; Henriquez, J.E.; Rizzo, M.D.; Sermet, S.; Khan, D.M.I.O.; Turner, H.; Small-Howard, A.L.; et al. Evaluation of the Anti-Inflammatory Effects of Selected Cannabinoids and Terpenes from Cannabis sativa Employing Human Primary Leukocytes. Food Chem. Toxicol. 2022, 170, 113458. [Google Scholar] [CrossRef]
- Nayak, A.P.; Loblundo, C.; Bielory, L. Immunomodulatory Actions of Cannabinoids: Clinical Correlates and Therapeutic Opportunities for Allergic Inflammation. J. Allergy Clin. Immunol. Pr. 2023, 11, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Stella, N. THC and CBD: Similarities and Differences between Siblings. Neuron 2023, 111, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Blanton, H.; Yin, L.; Duong, J.; Benamar, K. Cannabidiol and Beta-Caryophyllene in Combination: A Therapeutic Functional Interaction. Int. J. Mol. Sci. 2022, 23, 15470. [Google Scholar] [CrossRef]
- De Oliveira Carvalho, H.; de Melo Santos, A.; de Lima Teixeira dos Santos, A.V.T.; Gonçalves, D.E.S.; Picanço, K.R.T.; Souza, B.S.F.e.; Carvalho, J.C.T. Cannabis sativa L. Fixed Oil and Its Nanoemulsion: Effect on Diabetes and Dyslipidemia Induced in Rats. Pharmacogn. Mag. 2024, 20, 908–920. [Google Scholar] [CrossRef]
- Weimer, P.; Kirsten, C.N.; de Araújo Lock, G.; Nunes, K.A.A.; Rossi, R.C.; Koester, L.S. Co-Delivery of Beta-Caryophyllene and Indomethacin in the Oily Core of Nanoemulsions Potentiates the Anti-Inflammatory Effect in LPS-Stimulated Macrophage Model. Eur. J. Pharm. Biopharm. 2023, 191, 114–123. [Google Scholar] [CrossRef]
- Acharya, N.; Penukonda, S.; Shcheglova, T.; Hagymasi, A.T.; Basu, S.; Srivastava, P.K. Endocannabinoid System Acts as a Regulator of Immune Homeostasis in the Gut. Proc. Natl. Acad. Sci. USA 2017, 114, 5005–5010. [Google Scholar] [CrossRef]
- Brown, K.J.; Laun, A.S.; Song, Z.-H. Cannabidiol, a Novel Inverse Agonist for GPR12. Biochem. Biophys. Res. Commun. 2017, 493, 451–454. [Google Scholar] [CrossRef]
- Murphy, K.; Mowat, A.; Weaver, C. Janeway’s Immunobiology; Garland Science, Taylor & Francis Group: New York, NY, USA, 2012; ISBN 978-0-8153-4243-4. [Google Scholar]
- Cai, S.; Zhang, Z.; Huang, S.; Bai, X.; Huang, Z.; Zhang, Y.J.; Huang, L.; Tang, W.; Haughn, G.; You, S.; et al. CannabisGDB: A Comprehensive Genomic Database for Cannabis sativa L. Plant Biotechnol. J. 2021, 19, 857–859. [Google Scholar] [CrossRef]
- Salha, M.; Adenusi, H.; Dupuis, J.H.; Bodo, E.; Botta, B.; McKenzie, I.; Yada, R.Y.; Farrar, D.H.; Magolan, J.; Tian, K.V.; et al. Bioactivity of the Cannabigerol Cannabinoid and Its Analogues—The Role of 3-Dimensional conformation. Org. Biomol. Chem. 2023, 21, 4683–4693. [Google Scholar] [CrossRef]
- Millan-Linares, M.C.; Rivero-Pino, F.; la Rosa, T.G.; Villanueva, A.; Montserrat-de la Paz, S. Identification, Characterization, and Molecular Docking of Immunomodulatory Oligopeptides from Bioavailable Hempseed Protein Hydrolysates. Food Res. Int. 2024, 176, 113712. [Google Scholar] [CrossRef]
- Nwonuma, C.O.; Atanu, F.O.; Okonkwo, N.C.; Egharevba, G.O.; Udofia, I.A.; Evbuomwan, I.O.; Alejolowo, O.O.; Osemwegie, O.O.; Adelani-Akande, T.; Dogunro, F.A. Evaluation of Anti-Malarial Activity and GC–MS Finger Printing of Cannabis: An In-Vivo and In Silico Approach. Sci. Afr. 2022, 15, e01108. [Google Scholar] [CrossRef]
- Verma, R.P.; Matthews, E.J. Estimation of the Chemical-Induced Eye Injury Using a Weight-of-Evidence (WoE) Battery of 21 Artificial Neural Network (ANN) c-QSAR Models (QSAR-21): Part II: Corrosion Potential. Regul. Toxicol. Pharmacol. 2015, 71, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Toropova, A.P.; Toropov, A.A. (Eds.) QSPR/QSAR Analysis Using SMILES and Quasi-SMILES; Challenges and Advances in Computational Chemistry and Physics; Springer International Publishing: Cham, Switzerland, 2023; Volume 33, ISBN 978-3-031-28400-7. [Google Scholar]
- Li, S.; Yi, H.; Leng, Q.; Wu, Y.; Mao, Y. New Perspectives on Cancer Clinical Research in the Era of Big Data and Machine Learning. Surg. Oncol. 2024, 52, 102009. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Roy, A.; Tobin, J.M. Artificial Intelligence and Machine Learning: Definition of Terms and Current Concepts in Critical Care Research. J. Crit. Care 2024, 82, 154792. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, L.; Yuan, J.; Cui, J.; Zhang, Z.; Zhuo, W.; Lin, D. Construction Safety Management in the Data-Rich Era: A Hybrid Review Based upon Three Perspectives of Nature of Dataset, Machine Learning Approach, and Research Topic. Adv. Eng. Inform. 2023, 58, 102144. [Google Scholar] [CrossRef]
- Hernán Pérez de la Ossa, D.; Lorente, M.; Gil-Alegre, M.E.; Torres, S.; García-Taboada, E.; Aberturas, M.D.R.; Molpeceres, J.; Velasco, G.; Torres-Suárez, A.I. Local Delivery of Cannabinoid-Loaded Microparticles Inhibits Tumor Growth in a Murine Xenograft Model of Glioblastoma Multiforme. PLoS ONE 2013, 8, e54795. [Google Scholar] [CrossRef]
- Mobaleghol Eslam, H.; Hataminia, F.; Esmaeili, F.; Salami, S.A.; Ghanbari, H.; Amani, A. Preparation of a Nanoemulsion Containing Active Ingredients of Cannabis Extract and Its Application for Glioblastoma: In Vitro and In Vivo Studies. BMC Pharmacol. Toxicol. 2024, 25, 73. [Google Scholar] [CrossRef]
- Reddy, T.S.; Zomer, R.; Mantri, N. Nanoformulations as a Strategy to Overcome the Delivery Limitations of Cannabinoids. Phytother. Res. 2023, 37, 1526–1538. [Google Scholar] [CrossRef]
- Baek, E.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of Thermal and UV-Light Stability of β-Carotene-Loaded Nanoemulsions by Water-Soluble Chitosan Coating. Int. J. Biol. Macromol. 2020, 165, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shu, B.; Lou, X.; Wang, K.; Zhai, Y.; Qu, Y.; Song, R.; Liu, F.; Dong, X.; Xu, H. Improvement of Stability and in Vitro Bioaccessibility of Nervonic Acid by Nonionic Surfactant in Protein-Based Nanoemulsions. Food Biosci. 2023, 51, 102299. [Google Scholar] [CrossRef]
- Rave, M.C.; Echeverri, J.D.; Salamanca, C.H. Improvement of the Physical Stability of Oil-in-Water Nanoemulsions Elaborated with Sacha Inchi Oil Employing Ultra-High-Pressure Homogenization. J. Food Eng. 2020, 273, 109801. [Google Scholar] [CrossRef]
- Yao, X.; Teng, W.; Wang, J.; Wang, Y.; Zhang, Y.; Cao, J. Polyglycerol Polyricinoleate and Lecithin Stabilized Water in Oil Nanoemulsions for Sugaring Beijing Roast Duck: Preparation, Stability Mechanisms and Color Improvement. Food Chem. 2024, 447, 138979. [Google Scholar] [CrossRef] [PubMed]
- Ceprián, M.; Jiménez-Sánchez, L.; Vargas, C.; Barata, L.; Hind, W.; Martínez-Orgado, J. Cannabidiol Reduces Brain Damage and Improves Functional Recovery in a Neonatal Rat Model of Arterial Ischemic Stroke. Neuropharmacology 2017, 116, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Victor, T.R.; Hage, Z.; Tsirka, S.E. Prophylactic Administration of Cannabidiol Reduces Microglial Inflammatory Response to Kainate-Induced Seizures and Neurogenesis. Neuroscience 2022, 500, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vilela, L.R.; Lima, I.V.; Kunsch, É.B.; Pinto, H.P.P.; de Miranda, A.S.; Vieira, É.L.M.; de Oliveira, A.C.P.; Moraes, M.F.D.; Teixeira, A.L.; Moreira, F.A. Anticonvulsant Effect of Cannabidiol in the Pentylenetetrazole Model: Pharmacological Mechanisms, Electroencephalographic Profile, and Brain Cytokine Levels. Epilepsy Behav. 2017, 75, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lv, Y.; Tian, X.; Lou, J.; An, R.; Zhang, Q.; Li, M.; Xu, L.; Dong, Z. Neuroprotective Effect of β-Caryophyllene on Cerebral Ischemia-Reperfusion Injury via Regulation of Necroptotic Neuronal Death and Inflammation: In Vivo and In Vitro. Front. Neurosci. 2017, 11, 583. [Google Scholar] [CrossRef]
- Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Huestis, M.A. The Cannabinoid CB1 Receptor Antagonist Rimonabant Attenuates the Hypotensive Effect of Smoked Marijuana in Male Smokers. Am. Heart J. 2006, 151, 754.e1–754.e5. [Google Scholar] [CrossRef]
- Hodcroft, C.J.; Rossiter, M.C.; Buch, A.N. Cannabis-Associated Myocardial Infarction in a Young Man with Normal Coronary Arteries. J. Emerg. Med. 2014, 47, 277–281. [Google Scholar] [CrossRef]
- Jeffers, A.M.; Glantz, S.; Byers, A.L.; Keyhani, S. Association of Cannabis Use With Cardiovascular Outcomes among US Adults. J. Am. Heart Assoc. 2024, 13, e030178. [Google Scholar] [CrossRef]
- Bashashati, M.; Storr, M.A.; Nikas, S.P.; Wood, J.T.; Godlewski, G.; Liu, J.; Ho, W.; Keenan, C.M.; Zhang, H.; Alapafuja, S.O.; et al. Inhibiting Fatty Acid Amide Hydrolase Normalizes Endotoxin-Induced Enhanced Gastrointestinal Motility in Mice. Br. J. Pharmacol. 2012, 165, 1556–1571. [Google Scholar] [CrossRef]
- Duncan, M.; Mouihate, A.; Mackie, K.; Keenan, C.M.; Buckley, N.E.; Davison, J.S.; Patel, K.D.; Pittman, Q.J.; Sharkey, K.A. Cannabinoid CB2 Receptors in the Enteric Nervous System Modulate Gastrointestinal Contractility in Lipopolysaccharide-Treated Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G78. [Google Scholar] [CrossRef]
- Jamontt, J.M.; Molleman, A.; Pertwee, R.G.; Parsons, M.E. The Effects of Δ9-Tetrahydrocannabinol and Cannabidiol Alone and in Combination on Damage, Inflammation and In Vitro Motility Disturbances in Rat Colitis. Br. J. Pharmacol. 2010, 160, 712. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.M.; Nookaew, I.; Ewing, L.E.; Wongsurawat, T.; Jenjaroenpun, P.; Quick, C.M.; Yee, E.U.; Piccolo, B.D.; ElSohly, M.; Walker, L.A.; et al. Potential Probiotic or Trigger of Gut Inflammation—The Janus-Faced Nature of Cannabidiol-Rich Cannabis Extract. J. Diet. Suppl. 2020, 17, 543–560. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitola, I.; Angulo, C.; Baptista-Rosas, R.C.; Anaya-Esparza, L.M.; Escalante-García, Z.Y.; Villarruel-López, A.; Silva-Jara, J.M. Prospects in the Use of Cannabis sativa Extracts in Nanoemulsions. BioTech 2024, 13, 53. https://doi.org/10.3390/biotech13040053
Vitola I, Angulo C, Baptista-Rosas RC, Anaya-Esparza LM, Escalante-García ZY, Villarruel-López A, Silva-Jara JM. Prospects in the Use of Cannabis sativa Extracts in Nanoemulsions. BioTech. 2024; 13(4):53. https://doi.org/10.3390/biotech13040053
Chicago/Turabian StyleVitola, Ian, Carlos Angulo, Raul C. Baptista-Rosas, Luis Miguel Anaya-Esparza, Zazil Yadel Escalante-García, Angélica Villarruel-López, and Jorge Manuel Silva-Jara. 2024. "Prospects in the Use of Cannabis sativa Extracts in Nanoemulsions" BioTech 13, no. 4: 53. https://doi.org/10.3390/biotech13040053
APA StyleVitola, I., Angulo, C., Baptista-Rosas, R. C., Anaya-Esparza, L. M., Escalante-García, Z. Y., Villarruel-López, A., & Silva-Jara, J. M. (2024). Prospects in the Use of Cannabis sativa Extracts in Nanoemulsions. BioTech, 13(4), 53. https://doi.org/10.3390/biotech13040053






