Matrix Metalloproteinases in Glioma: Drivers of Invasion and Therapeutic Targets
Abstract
:1. Introduction
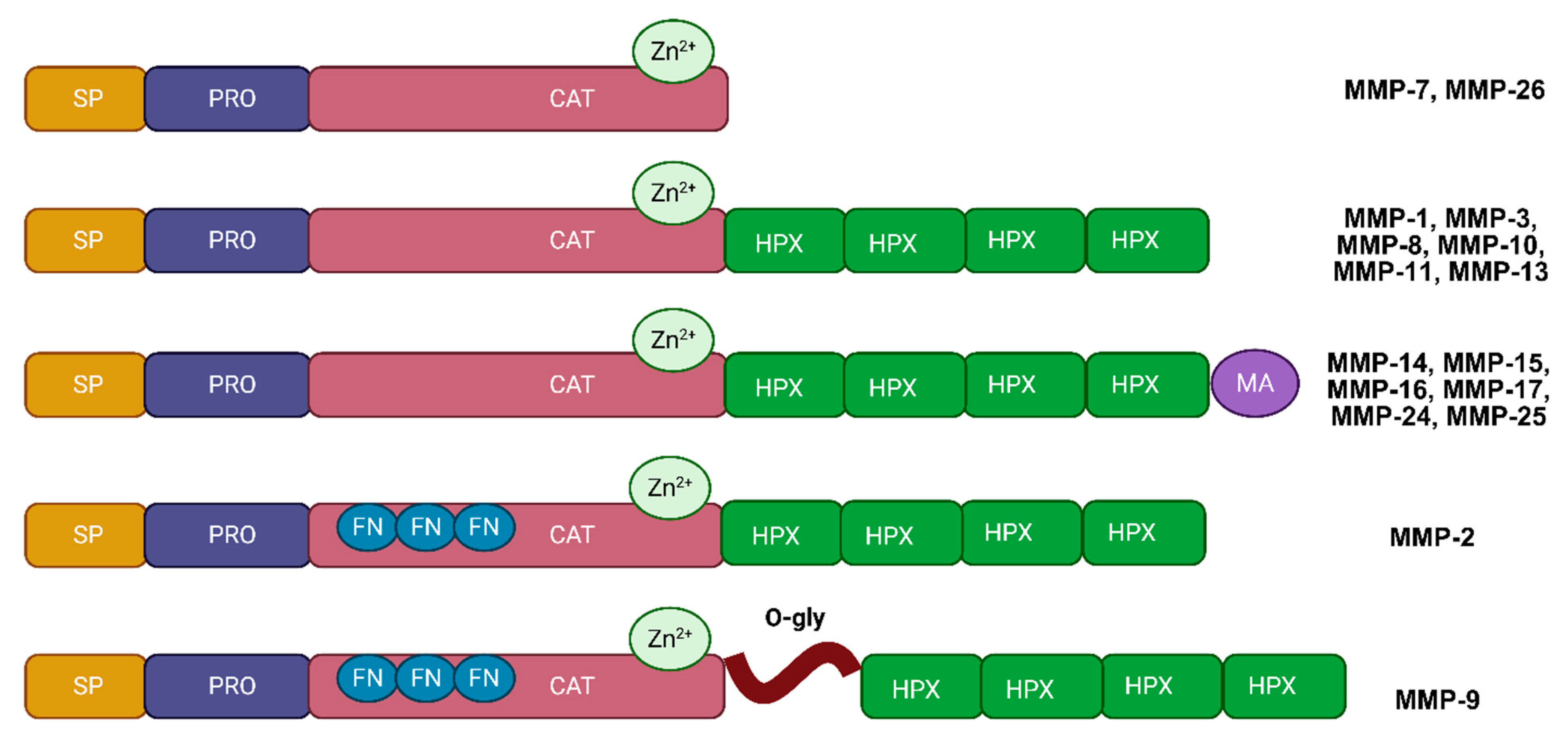
2. Structure and Function of MMPs
| MMP Family | Cell Source | Tissue Specificity | Function | Clinical Associations | Ref. |
|---|---|---|---|---|---|
| MMP-1 (Collagenase-1) | Fibroblasts, keratinocytes | Tumor cells, stromal fibroblasts | Degrades interstitial collagen (types I, II, III), ECM remodeling | Correlates with invasiveness, metastasis, poor prognosis | [38] |
| MMP-2 (Gelatinase A) | Endothelial cells, fibroblasts | Tumor cells, endothelial cells | Degrades gelatin, collagen IV, laminin; involved in angiogenesis and metastasis | High tumor grade, increased recurrence, reduced survival | [13] |
| MMP-3 (Stromelysin-1) | Fibroblasts, macrophages | Tumor stroma, macrophages | Degrades ECM components (e.g., proteoglycans, laminin); activates other MMPs | Potential role in tumor invasion and ECM remodeling | [39] |
| MMP-7 (Matrilysin) | Epithelial cells | Tumor epithelial cells | Degrades ECM components (e.g., elastin, fibronectin); involved in tissue repair and inflammation | Increased proliferation, metastasis; poor clinical outcomes | [40] |
| MMP-8 (Collagenase-2) | Neutrophils | Tumor-associated neutrophils | Degrades collagen types I, II, and III; involved in inflammatory processes | Associated with inflammation-related tumor progression | [41] |
| MMP-9 (Gelatinase B) | Neutrophils, macrophages | Tumor cells, endothelial cells, immune cells | Degrades gelatin, collagen IV, elastin; plays a role in cancer metastasis and inflammation | Poor prognosis, therapy resistance, correlation with high grades | [14] |
| MMP-10 (Stromelysin-2) | Fibroblasts, macrophages | Tumor-associated stromal cells, immune cells | Degrades proteoglycans, gelatin; involved in wound healing and tissue remodeling | Linked to tumor invasiveness and tissue remodeling | [42] |
| MMP-11 (Stromelysin-3) | Fibroblasts, tumor cells | Stromal fibroblasts, tumor-associated fibroblasts | Processes ECM components; implicated in cancer cell invasion | Poor prognosis, associated with aggressive tumor behavior | [43] |
| MMP-12 (Macrophage Elastase) | Macrophages | Tumor-infiltrating macrophages | Degrades elastin; involved in tissue remodeling and inflammation | Associated with inflammation-driven tumor progression | [44] |
| MMP-13 (Collagenase-3) | Chondrocytes, fibroblasts | Tumor-associated stromal cells, tumor cells | Degrades type II collagen; important in cartilage remodeling and osteoarthritis | Linked to tumor invasiveness, aggressiveness, poor outcomes | [45] |
| MMP-14 (MT1-MMP) | Fibroblasts, tumor cells | Tumor cell membrane-localized | Degrades collagen types I, II, III; activates MMP-2; involved in cell migration | Inverse correlation with survival, enhanced invasiveness | [46] |
| MMP-15 (MT2-MMP) | Tumor cells | Tumor cell membranes | Involved in ECM remodeling and cancer metastasis | Associated as anti-apoptotic factor in cancer cells | [47] |
| MMP-16 (MT3-MMP) | Tumor cells, fibroblasts | Tumor-associated cells, cell surface | Activates MMP-2; involved in ECM remodeling and angiogenesis | Linked to increased angiogenesis and invasive phenotype | [48] |
| MMP-17 (MT4-MMP) | Macrophages, tumor cells | Tumor cell surfaces, immune cells | ECM remodeling; implicated in cancer progression | Correlated with tumor aggressiveness | [49] |
| MMP-19 | Keratinocytes, fibroblasts | Tumor-associated fibroblasts, stromal cells | Degrades ECM components like collagen IV, laminin; involved in tissue repair | Associated with higher tumor grades and ECM alterations | [50] |
| MMP-20 (Enamelysin) | Odontoblasts | Dental tissue | Specific to enamel development; degrades amelogenin | - | [51] |
| MMP-23 | Tumor cells | Tumor tissue | Implicated in cancer progression; potential regulatory role in ECM | Potential role in tumor progression | [52] |
| MMP-24 (MT5-MMP) | Neurons, tumor cells | Neural tissue, tumor cell membranes | Involved in neural development and ECM remodeling | Elevated in brain tumors, potential prognostic marker | [53] |
| MMP-25 (MT6-MMP) | Leukocytes | Immune cells | ECM remodeling; role in inflammation | Possible role in tumor-associated inflammation | [49] |
| MMP-26 (Endometase) | Endometrial cells, tumor cells | Tumor epithelial cells | Degrades ECM components; implicated in reproductive tissue remodeling and cancer | Linked to aggressive cancer behaviors | [54] |
| MMP-28 (Epilysin) | Keratinocytes, macrophages | Epithelial and immune cells | ECM remodeling; involved in wound healing | Emerging marker for tumor progression | [55] |
3. Roles of MMPs in Glioma Progression
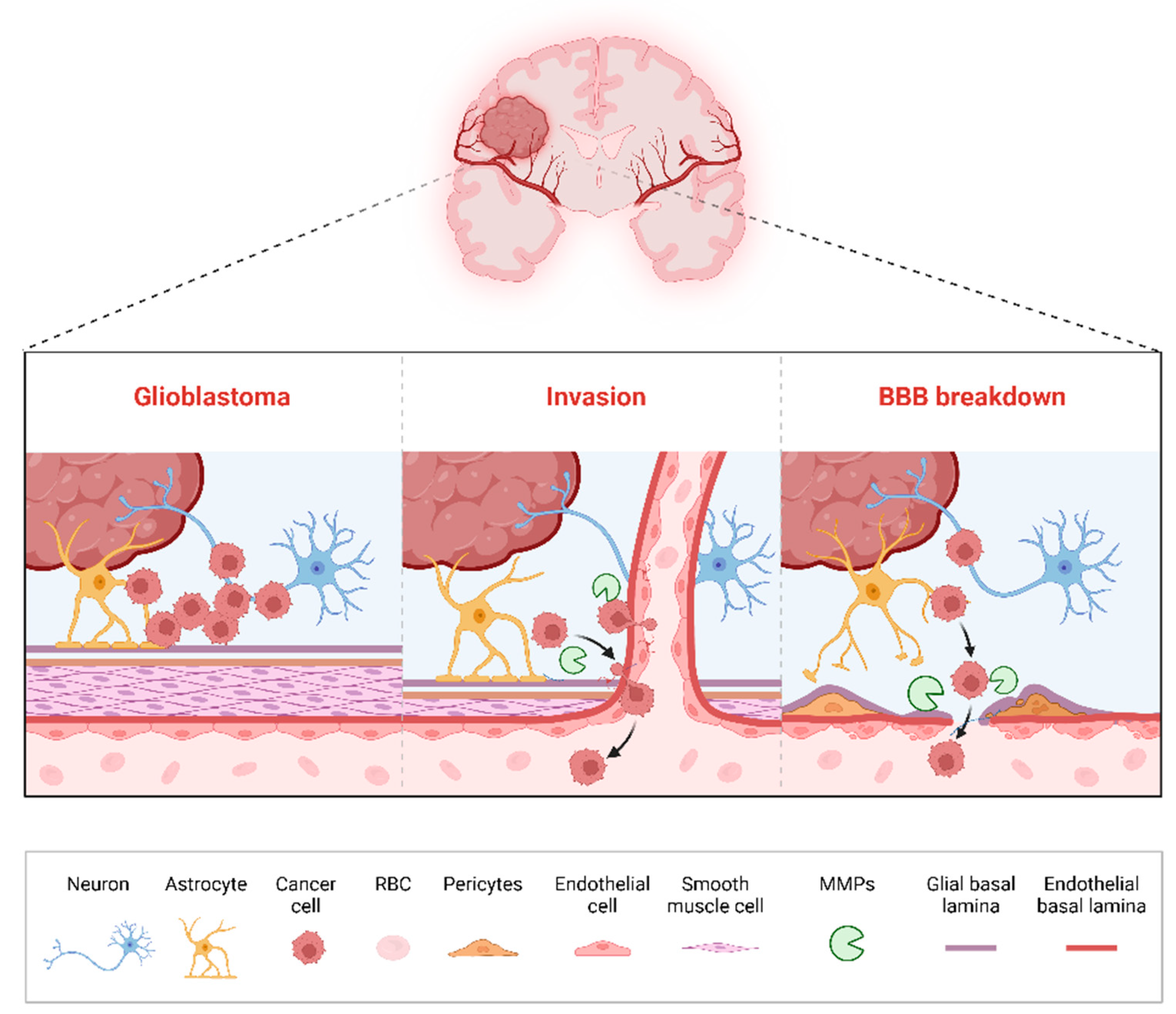
3.1. Tumor Invasion
3.2. Angiogenesis
3.3. Immune Evasion and Modulation
3.4. Microenvironmental Remodeling
3.5. Disruption of the Blood–Brain Barrier (BBB)
4. Prognostic and Diagnostic Relevance of MMPs in Gliomas
5. Therapeutic Targeting of MMPs in Gliomas
5.1. Broad-Spectrum MMP Inhibitors
5.2. Natural Compounds as MMP Inhibitors
5.3. Gene Therapy Approaches Targeting MMPs
5.4. Emerging Delivery Systems: Nanoparticles and TIMP Mimetics
5.5. Challenges and Future Directions
6. Emerging Research and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nabors, L.B.; Ammirati, M.; Bierman, P.J.; Brem, H.; Butowski, N.; Chamberlain, M.C.; DeAngelis, L.M.; Fenstermaker, R.A.; Friedman, A.; Gilbert, M.R.; et al. Central nervous system cancers. J. Natl. Compr. Cancer Netw. 2013, 11, 1114–1151. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, K.; Adhikary, K.; Acharjee, A.; Acharjee, P.; Trigun, S.K.; Mutlaq, A.S.; Ashique, S.; Yasmin, S.; Alshahrani, A.M.; Ansari, M.Y. Biological significance and pathophysiological role of Matrix Metalloproteinases in the Central Nervous System. Int. J. Biol. Macromol. 2024, 280, 135967. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A. Potential of MMP-2 and MMP-9 Gelatinase Blockade as a Therapeutic Strategy in Fibrosarcoma Treatment: A Decadal Review. Targets 2024, 2, 104–125. [Google Scholar] [CrossRef]
- Radisky, E.S. Extracellular proteolysis in cancer: Proteases, substrates, and mechanisms in tumor progression and metastasis. J. Biol. Chem. 2024, 300, 107347. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Tan, X.J.; Li, Z.X.; Wang, H.Y. Immunomodulatory role of metalloproteases in cancers: Current progress and future trends. Front. Immunol. 2022, 13, 1064033. [Google Scholar] [CrossRef]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.; Anacker, J.; Ernestus, R.I.; Vince, G.H. A complete compilation of matrix metalloproteinase expression in human malignant gliomas. World J. Clin. Oncol. 2012, 3, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Sinceviciute, R.; Vaitkiene, P.; Urbanaviciute, R.; Steponaitis, G.; Tamasauskas, A.; Skiriute, D. MMP2 is associated with glioma malignancy and patient outcome. Int. J. Clin. Exp. Pathol. 2018, 11, 3010–3018. [Google Scholar]
- Dobra, G.; Gyukity-Sebestyen, E.; Bukva, M.; Harmati, M.; Nagy, V.; Szabo, Z.; Pankotai, T.; Klekner, A.; Buzas, K. MMP-9 as Prognostic Marker for Brain Tumours: A Comparative Study on Serum-Derived Small Extracellular Vesicles. Cancers 2023, 15, 712. [Google Scholar] [CrossRef]
- Oldak, L.; Chludzinska-Kasperuk, S.; Milewska, P.; Grubczak, K.; Reszec, J.; Gorodkiewicz, E. MMP-1, UCH-L1, and 20S Proteasome as Potential Biomarkers Supporting the Diagnosis of Brain Glioma. Biomolecules 2022, 12, 1477. [Google Scholar] [CrossRef]
- Dimitrova, I.; Tacheva, T.; Mindov, I.; Petrov, B.; Aleksandrova, E.; Valkanov, S.; Gulubova, M.; Vlaykova, T. Serum levels of MMP-7 in primary brain cancers and brain metastases. Biotechnol. Biotechnol. Equip. 2019, 33, 881–885. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Corrigendum to “Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer” [Crit. Rev. Oncol. Hematol. 137, May (2019) 57–83]. Crit. Rev. Oncol./Hematol. 2019, 138, 172. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.K.; Tonn, J.C.; Rao, J.S. Matrix metalloproteinases and their biological function in human gliomas. Int. J. Dev. Neurosci. 1999, 17, 495–502. [Google Scholar] [CrossRef]
- Fingleton, B. MMPs as therapeutic targets—Still a viable option? Semin. Cell Dev. Biol. 2008, 19, 61–68. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Shoari, A.; Khalili-Tanha, G.; Coban, M.A.; Radisky, E.S. Structure and computation-guided yeast surface display for the evolution of TIMP-based matrix metalloproteinase inhibitors. Front. Mol. Biosci. 2023, 10, 1321956. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Farina, A.R.; Mackay, A.R. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers 2014, 6, 240–296. [Google Scholar] [CrossRef]
- Van Hove, I.; Lemmens, K.; Van de Velde, S.; Verslegers, M.; Moons, L. Matrix metalloproteinase-3 in the central nervous system: A look on the bright side. J. Neurochem. 2012, 123, 203–216. [Google Scholar] [CrossRef]
- Turunen, S.P.; Tatti-Bugaeva, O.; Lehti, K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 1974–1988. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Papaharalambus, C.A.; Griendling, K.K. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc. Med. 2007, 17, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Pullen, N.A.; Anand, M.; Cooper, P.S.; Fillmore, H.L. Matrix metalloproteinase-1 expression enhances tumorigenicity as well as tumor-related angiogenesis and is inversely associated with TIMP-4 expression in a model of glioblastoma. J. Neurooncol 2012, 106, 461–471. [Google Scholar] [CrossRef]
- Kim, E.M.; Hwang, O. Role of matrix metalloproteinase-3 in neurodegeneration. J. Neurochem. 2011, 116, 22–32. [Google Scholar] [CrossRef]
- Rome, C.; Arsaut, J.; Taris, C.; Couillaud, F.; Loiseau, H. MMP-7 (matrilysin) expression in human brain tumors. Mol. Carcinog. 2007, 46, 446–452. [Google Scholar] [CrossRef]
- Lee, E.J.; Han, J.E.; Woo, M.S.; Shin, J.A.; Park, E.M.; Kang, J.L.; Moon, P.G.; Baek, M.C.; Son, W.S.; Ko, Y.T.; et al. Matrix metalloproteinase-8 plays a pivotal role in neuroinflammation by modulating TNF-alpha activation. J. Immunol. 2014, 193, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Miyake, M.; Lawton, A.; Goodison, S.; Rosser, C.J. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 2014, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Stojic, J.; Hagemann, C.; Haas, S.; Herbold, C.; Kuhnel, S.; Gerngras, S.; Roggendorf, W.; Roosen, K.; Vince, G.H. Expression of matrix metalloproteinases MMP-1, MMP-11 and MMP-19 is correlated with the WHO-grading of human malignant gliomas. Neurosci. Res. 2008, 60, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Li, G.S.; Tang, Y.X.; Zhang, W.; Li, J.D.; Huang, H.Q.; Liu, J.; Fu, Z.W.; He, R.Q.; Kong, J.L.; Zhou, H.F.; et al. MMP12 is a Potential Predictive and Prognostic Biomarker of Various Cancers Including Lung Adenocarcinoma. Cancer Control 2024, 31, 10732748241235468. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Wang, J.; Li, C.; Yu, K.; Wang, Q. Increased expression of matrix metalloproteinase-13 in glioma is associated with poor overall survival of patients. Med. Oncol. 2012, 29, 2432–2437. [Google Scholar] [CrossRef]
- Ulasov, I.; Yi, R.; Guo, D.; Sarvaiya, P.; Cobbs, C. The emerging role of MMP14 in brain tumorigenesis and future therapeutics. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2014, 1846, 113–120. [Google Scholar] [CrossRef]
- Abraham, R.; Schafer, J.; Rothe, M.; Bange, J.; Knyazev, P.; Ullrich, A. Identification of MMP-15 as an anti-apoptotic factor in cancer cells. J. Biol. Chem. 2005, 280, 34123–34132. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, J.; Dong, C.; Wei, W.; Li, J.; Li, X. Membranous type matrix metalloproteinase 16 induces human prostate cancer metastasis. Oncol. Lett. 2017, 14, 3096–3102. [Google Scholar] [CrossRef]
- Sohail, A.; Sun, Q.; Zhao, H.; Bernardo, M.M.; Cho, J.A.; Fridman, R. MT4-(MMP17) and MT6-MMP (MMP25), A unique set of membrane-anchored matrix metalloproteinases: Properties and expression in cancer. Cancer Metastasis Rev. 2008, 27, 289–302. [Google Scholar] [CrossRef]
- Lettau, I.; Hattermann, K.; Held-Feindt, J.; Brauer, R.; Sedlacek, R.; Mentlein, R. Matrix metalloproteinase-19 is highly expressed in astroglial tumors and promotes invasion of glioma cells. J. Neuropathol. Exp. Neurol. 2010, 69, 215–223. [Google Scholar] [CrossRef]
- Turk, B.E.; Lee, D.H.; Yamakoshi, Y.; Klingenhoff, A.; Reichenberger, E.; Wright, J.T.; Simmer, J.P.; Komisarof, J.A.; Cantley, L.C.; Bartlett, J.D. MMP-20 is predominately a tooth-specific enzyme with a deep catalytic pocket that hydrolyzes type V collagen. Biochemistry 2006, 45, 3863–3874. [Google Scholar] [CrossRef] [PubMed]
- Galea, C.A.; Nguyen, H.M.; George Chandy, K.; Smith, B.J.; Norton, R.S. Domain structure and function of matrix metalloprotease 23 (MMP23): Role in potassium channel trafficking. Cell. Mol. Life Sci. 2014, 71, 1191–1210. [Google Scholar] [CrossRef]
- Llano, E.; Pendas, A.M.; Freije, J.P.; Nakano, A.; Knauper, V.; Murphy, G.; Lopez-Otin, C. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase a overexpressed in brain tumors. Cancer Res. 1999, 59, 2570–2576. [Google Scholar] [PubMed]
- Guo, J.G.; Guo, C.C.; He, Z.Q.; Cai, X.Y.; Mou, Y.G. High MMP-26 expression in glioma is correlated with poor clinical outcome of patients. Oncol. Lett. 2018, 16, 2237–2242. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Sun, L.; Bi, X.; He, H.; Chen, L.; Pang, J. The function of MMP-28/TGF-beta induced cell apoptosis in human glioma cells. Exp. Ther. Med. 2018, 16, 2867–2874. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Pullen, N.A.; Pickford, A.R.; Perry, M.M.; Jaworski, D.M.; Loveson, K.F.; Arthur, D.J.; Holliday, J.R.; Van Meter, T.E.; Peckham, R.; Younas, W. Current insights into matrix metalloproteinases and glioma progression: Transcending the degradation boundary. Met. Med. 2018, 5, 13–30. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Hannocks, M.J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019, 75–76, 102–113. [Google Scholar] [CrossRef]
- Wang, M.; Wang, T.; Liu, S.; Yoshida, D.; Teramoto, A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003, 20, 65–72. [Google Scholar] [CrossRef]
- Yu, C.F.; Chen, F.H.; Lu, M.H.; Hong, J.H.; Chiang, C.S. Dual roles of tumour cells-derived matrix metalloproteinase 2 on brain tumour growth and invasion. Br. J. Cancer 2017, 117, 1828–1836. [Google Scholar] [CrossRef]
- Ricci, S.; Guadagno, E.; Bruzzese, D.; Del Basso De Caro, M.; Peca, C.; Sgulo, F.G.; Maiuri, F.; Di Carlo, A. Evaluation of matrix metalloproteinase type IV-collagenases in serum of patients with tumors of the central nervous system. J. Neuro-Oncol. 2017, 131, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Dibdiakova, K.; Majercikova, Z.; Galanda, T.; Richterova, R.; Kolarovszki, B.; Racay, P.; Hatok, J. Relationship between the Expression of Matrix Metalloproteinases and Their Tissue Inhibitors in Patients with Brain Tumors. Int. J. Mol. Sci. 2024, 25, 2858. [Google Scholar] [CrossRef] [PubMed]
- Hummel, V.; Kallmann, B.A.; Wagner, S.; Fuller, T.; Bayas, A.; Tonn, J.C.; Benveniste, E.N.; Toyka, K.V.; Rieckmann, P. Production of MMPs in human cerebral endothelial cells and their role in shedding adhesion molecules. J. Neuropathol. Exp. Neurol. 2001, 60, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Nakada, M.; Nakamura, H.; Ikeda, E.; Fujimoto, N.; Yamashita, J.; Sato, H.; Seiki, M.; Okada, Y. Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am. J. Pathol. 1999, 154, 417–428. [Google Scholar] [CrossRef]
- Kim, W.Y.; Lee, H.Y. Brain angiogenesis in developmental and pathological processes: Mechanism and therapeutic intervention in brain tumors. FEBS J. 2009, 276, 4653–4664. [Google Scholar] [CrossRef]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar] [CrossRef]
- Komatsu, K.; Nakanishi, Y.; Nemoto, N.; Hori, T.; Sawada, T.; Kobayashi, M. Expression and quantitative analysis of matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor Pathol. 2004, 21, 105–112. [Google Scholar] [CrossRef]
- Bodey, B.; Bodey, B., Jr.; Siegel, S.E.; Kaiser, H.E. Matrix metalloproteinase expression in childhood astrocytomas. Anticancer Res. 2000, 20, 3287–3292. [Google Scholar]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Gearing, A.J.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.; Davidson, A.H.; Drummond, A.H.; Galloway, W.A.; Gilbert, R.; Gordon, J.L.; et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature 1994, 370, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Abbes, A.; Zayani, Y.; Zidi, W.; Hammami, M.B.; Mebazaa, A.; El Euch, D.; Ben Ammar, A.; Sanhaji, H.; El May, M.V.; Mokni, M.; et al. Matrix metalloproteinase-7 could be a predictor for acute inflammation in psoriatic patients. Cytokine 2020, 134, 155195. [Google Scholar] [CrossRef]
- Andersen, R.S.; Anand, A.; Harwood, D.S.L.; Kristensen, B.W. Tumor-Associated Microglia and Macrophages in the Glioblastoma Microenvironment and Their Implications for Therapy. Cancers 2021, 13, 4255. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neuro-Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Evans, K.; Xiao, C.; DeVito, N.; Theivanthiran, B.; Holtzhausen, A.; Siska, P.J.; Blobe, G.C.; Hanks, B.A. Stromal Fibroblasts Mediate Anti-PD-1 Resistance via MMP-9 and Dictate TGFbeta Inhibitor Sequencing in Melanoma. Cancer Immunol. Res. 2018, 6, 1459–1471. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Yuan, Z.N.; Li, Y.P.; Zhang, S.F.; Wang, X.Y.; Dou, H.; Yu, X.; Zhang, Z.R.; Yang, S.S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Toborek, M. Blood-brain Barrier Remodeling during Brain Metastasis Formation. Mol. Med. 2016, 22, 32–40. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Feng, S.; Cen, J.; Huang, Y.; Shen, H.; Yao, L.; Wang, Y.; Chen, Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS ONE 2011, 6, e20599. [Google Scholar] [CrossRef]
- Liu, X.; Su, P.; Meng, S.; Aschner, M.; Cao, Y.; Luo, W.; Zheng, G.; Liu, M. Role of matrix metalloproteinase-2/9 (MMP2/9) in lead-induced changes in an in vitro blood-brain barrier model. Int. J. Biol. Sci. 2017, 13, 1351–1360. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Ruda, R. Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Mohanty, S.; Chen, Z.; Li, K.; Morais, G.R.; Klockow, J.; Yerneni, K.; Pisani, L.; Chin, F.T.; Mitra, S.; Cheshier, S.; et al. A Novel Theranostic Strategy for MMP-14-Expressing Glioblastomas Impacts Survival. Mol. Cancer Ther. 2017, 16, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Steeg, P.S.; Stetler-Stevenson, W.G. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell 1991, 64, 327–336. [Google Scholar] [CrossRef]
- Velasco, G.; Cal, S.; Merlos-Suarez, A.; Ferrando, A.A.; Alvarez, S.; Nakano, A.; Arribas, J.; Lopez-Otin, C. Human MT6-matrix metalloproteinase: Identification, progelatinase A activation, and expression in brain tumors. Cancer Res. 2000, 60, 877–882. [Google Scholar] [PubMed]
- Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013–2023). Molecules 2023, 28, 5567. [Google Scholar] [CrossRef]
- Jelski, W.; Mroczko, B. Molecular and Circulating Biomarkers of Brain Tumors. Int. J. Mol. Sci. 2021, 22, 7039. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 12, 28. [Google Scholar] [CrossRef]
- Du, R.; Petritsch, C.; Lu, K.; Liu, P.; Haller, A.; Ganss, R.; Song, H.; Vandenberg, S.; Bergers, G. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro-Oncol. 2008, 10, 254–264. [Google Scholar] [CrossRef]
- Karimi, N.; Kheiri, H.; Zarrinpour, V.; Forghanifard, M.M. Bioinformatic analysis of MMP family members in GBM. Inform. Med. Unlocked 2023, 39, 101240. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Wang, H.; Xu, L.; Yu, Y. MMP-2 expression and correlation with pathology and MRI of glioma. Oncol. Lett. 2019, 17, 1826–1832. [Google Scholar] [CrossRef]
- Choe, G.; Park, J.K.; Jouben-Steele, L.; Kremen, T.J.; Liau, L.M.; Vinters, H.V.; Cloughesy, T.F.; Mischel, P.S. Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin. Cancer Res. 2002, 8, 2894–2901. [Google Scholar] [PubMed]
- Ramachandran, R.K.; Sorensen, M.D.; Aaberg-Jessen, C.; Hermansen, S.K.; Kristensen, B.W. Expression and prognostic impact of matrix metalloproteinase-2 (MMP-2) in astrocytomas. PLoS ONE 2017, 12, e0172234. [Google Scholar] [CrossRef]
- Wong, E.T.; Alsop, D.; Lee, D.; Tam, A.; Barron, L.; Bloom, J.; Gautam, S.; Wu, J.K. Cerebrospinal fluid matrix metalloproteinase-9 increases during treatment of recurrent malignant gliomas. Cerebrospinal Fluid Res. 2008, 5, 1. [Google Scholar] [CrossRef]
- Li, Q.; Chen, B.; Cai, J.; Sun, Y.; Wang, G.; Li, Y.; Li, R.; Feng, Y.; Han, B.; Li, J.; et al. Comparative Analysis of Matrix Metalloproteinase Family Members Reveals That MMP9 Predicts Survival and Response to Temozolomide in Patients with Primary Glioblastoma. PLoS ONE 2016, 11, e0151815. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kato, Y.; Erzinger, S.A.; Kiriakova, G.M.; Qian, Y.; Palmieri, D.; Steeg, P.S.; Price, J.E. The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer 2012, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, A.; Miekus, N.; Stefanowicz, J.; Adamkiewicz-Drozynska, E. Selected Matrix Metalloproteinases (MMP-2, MMP-7) and Their Inhibitor (TIMP-2) in Adult and Pediatric Cancer. Diagnostics 2020, 10, 547. [Google Scholar] [CrossRef]
- Houson, H.; Kasten, B.; Jiang, K.; Rao, J.H.; Warram, J. MMP-14 as a noninvasive marker for PET and NIRF imaging of glioblastoma multiforme. J. Nucl. Med. 2019, 60, 1033. [Google Scholar]
- Kan, L.K.; Drummond, K.; Hunn, M.; Williams, D.; O’Brien, T.J.; Monif, M. Potential biomarkers and challenges in glioma diagnosis, therapy and prognosis. BMJ Neurol. Open 2020, 2, e000069. [Google Scholar] [CrossRef]
- Hormigo, A.; Gu, B.; Karimi, S.; Riedel, E.; Panageas, K.S.; Edgar, M.A.; Tanwar, M.K.; Rao, J.S.; Fleisher, M.; DeAngelis, L.M.; et al. YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin. Cancer Res. 2006, 12, 5698–5704. [Google Scholar] [CrossRef]
- Manicone, A.M.; McGuire, J.K. Matrix metalloproteinases as modulators of inflammation. Semin. Cell Dev. Biol. 2008, 19, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Lv, S.; Zong, Z.; Wu, L.; Tang, X.; Kuang, W.; Zhang, P.; Li, X.; Fu, J.; Xiao, M.; et al. Cerebrospinal fluid biomarkers for brain tumor detection: Clinical roles and current progress. Am. J. Transl. Res. 2020, 12, 1379–1396. [Google Scholar]
- Papadimitrakis, D.; Perdikakis, M.; Gargalionis, A.N.; Papavassiliou, A.G. Biomarkers in Cerebrospinal Fluid for the Diagnosis and Monitoring of Gliomas. Biomolecules 2024, 14, 801. [Google Scholar] [CrossRef]
- Smith, E.R.; Zurakowski, D.; Saad, A.; Scott, R.M.; Moses, M.A. Urinary biomarkers predict brain tumor presence and response to therapy. Clin. Cancer Res. 2008, 14, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Tepper, D.; Leonard, A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.J.; Asselin, M.C.; Hinz, R.; Parkes, L.M.; Allan, S.; Schiessl, I.; Boutin, H.; Dickie, B. In vivo methods for imaging blood-brain barrier function and dysfunction. Eur. J. Nucl. Med. Mol. I 2023, 50, 1051–1083. [Google Scholar] [CrossRef]
- Lopez-Avila, V.; Spencer, J.V. Methods for detection of matrix metalloproteinases as biomarkers in cardiovascular disease. Clin. Med. Cardiol. 2008, 2, CMC-S484. [Google Scholar] [CrossRef]
- Irmer, B.; Chandrabalan, S.; Maas, L.; Bleckmann, A.; Menck, K. Extracellular Vesicles in Liquid Biopsies as Biomarkers for Solid Tumors. Cancers 2023, 15, 1307. [Google Scholar] [CrossRef]
- Lawrence, S.R.; Shah, K.M. Prospects and Current Challenges of Extracellular Vesicle-Based Biomarkers in Cancer. Biology 2024, 13, 694. [Google Scholar] [CrossRef]
- Batool, S.M.; Hsia, T.; Khanna, S.K.; Gamblin, A.S.; Rosenfeld, Y.; You, D.G.; Carter, B.S.; Balaj, L. Decoding vesicle-based precision oncology in gliomas. Neuro-Oncol. Adv. 2022, 4, ii53–ii60. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, T.G.; Salave, S.; Shinde, T.; Srikanth, I.; Gyanani, V.; Haley, J.C.; Jain, A. Understanding the role of endothelial cells in brain tumor formation and metastasis: A proposition to be explored for better therapy. J. Natl. Cancer Cent. 2023, 3, 222–235. [Google Scholar] [CrossRef]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef]
- Ganji, M.; Bakhshi, S.; Shoari, A.; Cohan, R.A. Discovery of potential FGFR3 inhibitors via QSAR, pharmacophore modeling, virtual screening and molecular docking studies against bladder cancer. J. Transl. Med. 2023, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, X.; Wan, Q. Combined machine learning models, docking analysis, ADMET studies and molecular dynamics simulations for the design of novel FAK inhibitors against glioblastoma. BMC Chem. 2024, 18, 203. [Google Scholar] [CrossRef]
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Takeuchi, T.; Komatsu, K.; Miyazaki, K.; Sato, M.; Higashi, S. Structural basis for matrix metalloproteinase-2 (MMP-2)-selective inhibitory action of beta-amyloid precursor protein-derived inhibitor. J. Biol. Chem. 2011, 286, 33236–33243. [Google Scholar] [CrossRef]
- Shoari, A.; Rasaee, M.J.; Kanavi, M.R.; Daraei, B. Functional mimetic peptide discovery isolated by phage display interacts selectively to fibronectin domain and inhibits gelatinase. J. Cell Biochem. 2019, 120, 19699–19711. [Google Scholar] [CrossRef]
- Lee, H.; Youn, I.; Demissie, R.; Vaid, T.M.; Che, C.T.; Azar, D.T.; Han, K.Y. Identification of small molecule inhibitors against MMP-14 via High-Throughput screening. Bioorg. Med. Chem. 2023, 85, 117289. [Google Scholar] [CrossRef]
- Uhm, J.H.; Dooley, N.P.; Villemure, J.G.; Yong, V.W. Glioma invasion in vitro: Regulation by matrix metalloprotease-2 and protein kinase C. Clin. Exp. Metastasis 1996, 14, 421–433. [Google Scholar] [CrossRef]
- Li, X.Y.; Huang, G.H.; Liu, Q.K.; Yang, X.T.; Wang, K.; Luo, W.Z.; Liang, T.S.; Yuan, S.P.; Zhen, Y.W.; Yan, D.M. Porf-2 Inhibits Tumor Cell Migration Through the MMP-2/9 Signaling Pathway in Neuroblastoma and Glioma. Front. Oncol. 2020, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Kuang, X.; Xie, Z.; Liang, L.; Zhang, Z.; Zhang, Y.; Ma, F.; Gao, Q.; Chang, R.; Lee, H.H.; et al. Small-molecule MMP2/MMP9 inhibitor SB-3CT modulates tumor immune surveillance by regulating PD-L1. Genome Med. 2020, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Hadass, O.; Tomlinson, B.N.; Gooyit, M.; Chen, S.Y.; Purdy, J.J.; Walker, J.M.; Zhang, C.Y.; Giritharan, A.B.; Purnell, W.; Robinson, C.R.; et al. Selective Inhibition of Matrix Metalloproteinase-9 Attenuates Secondary Damage Resulting from Severe Traumatic Brain Injury. PLoS ONE 2013, 8, e76904. [Google Scholar] [CrossRef] [PubMed]
- Thanh, H.D.; Lee, S.; Nguyen, T.T.; Huu, T.N.; Ahn, E.J.; Cho, S.H.; Kim, M.S.; Moon, K.S.; Jung, C. Temozolomide promotes matrix metalloproteinase 9 expression through p38 MAPK and JNK pathways in glioblastoma cells. Sci. Rep. 2024, 14, 14341. [Google Scholar] [CrossRef]
- Zhong, J.; Shan, W.; Zuo, Z. Norepinephrine inhibits migration and invasion of human glioblastoma cell cultures possibly via MMP-11 inhibition. Brain Res. 2021, 1756, 147280. [Google Scholar] [CrossRef]
- Guan, N.; Huo, X.; Zhang, Z.; Zhang, S.; Luo, J.; Guo, W. Ginsenoside Rh2 inhibits metastasis of glioblastoma multiforme through Akt-regulated MMP13. Tumour Biol. 2015, 36, 6789–6795. [Google Scholar] [CrossRef]
- Takino, T.; Nakada, M.; Li, Z.; Yoshimoto, T.; Domoto, T.; Sato, H. Tip60 regulates MT1-MMP transcription and invasion of glioblastoma cells through NF-kappaB pathway. Clin. Exp. Metastasis 2016, 33, 45–52. [Google Scholar] [CrossRef]
- Laronha, H.; Carpinteiro, I.; Portugal, J.; Azul, A.; Polido, M.; Petrova, K.T.; Salema-Oom, M.; Caldeira, J. Challenges in Matrix Metalloproteinases Inhibition. Biomolecules 2020, 10, 717. [Google Scholar] [CrossRef]
- Taheri, E.; Raeeszadeh-Sarmazdeh, M. Effect of TIMPs and their minimally engineered variants in blocking invasion and migration of brain cancer cells. Oncotarget 2025, 16, 118–130. [Google Scholar] [CrossRef]
- Kalantar, M.; Kalanther, I.; Kumar, S.; Buxton, E.K.; Raeeszadeh-Sarmazdeh, M. Determining key residues of engineered scFv antibody variants with improved MMP-9 binding using deep sequencing and machine learning. Comput. Struct. Biotechnol. J. 2024, 23, 3759–3770. [Google Scholar] [CrossRef]
- Kalantar, M.; Hilpert, G.A.; Mosca, E.R.; Raeeszadeh-Sarmazdeh, M. Engineering metalloproteinase inhibitors: Tissue inhibitors of metalloproteinases or antibodies, that is the question. Curr. Opin. Biotechnol. 2024, 86, 103094. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, H.L.; Liang, Y.; Worthington, K.; Luo, C.; Gianneschi, N.C.; Christman, K.L. Enzyme-Responsive Nanoparticles for the Targeted Delivery of an MMP Inhibitor to Acute Myocardial Infarction. Biomacromolecules 2023, 24, 4695–4704. [Google Scholar] [CrossRef]
- Pourmasoumi, P.; Banihashemian, S.A.; Zamani, F.; Rasouli-Nia, A.; Mehrabani, D.; Karimi-Busheri, F. Nanoparticle-Based Approaches in the Diagnosis and Treatment of Brain Tumors. J. Clin. Med. 2024, 13, 7449. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Nicolazzo, J.; Scott, A.M.; Gan, H.K. Antibody Drug Conjugates in Glioblastoma—Is There a Future for Them? Front. Oncol. 2021, 11, 718590. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Suzuki, T.; Ohishi, T.; Isemura, M.; Nakamura, Y.; Unno, K. Effects of Epigallocatechin-3-Gallate on Matrix Metalloproteinases in Terms of Its Anticancer Activity. Molecules 2023, 28, 525. [Google Scholar] [CrossRef]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Glowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Asuthkar, S.; Velpula, K.K.; Chetty, C.; Gorantla, B.; Rao, J.S. Epigenetic Regulation of miRNA-211 by MMP-9 Governs Glioma Cell Apoptosis, Chemosensitivity and Radiosensitivity. Oncotarget 2012, 3, 1439–1454. [Google Scholar] [CrossRef]
- Veeravalli, K.K.; Rao, J.S. MMP-9 and uPAR regulated glioma cell migration. Cell Adhes. Migr. 2012, 6, 509–512. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, F.; Yuan, J.; Li, Z.; Zhang, W. Sevoflurane inhibits migration and invasion of glioma cells via regulating miR-34a-5p/MMP-2 axis. Life Sci. 2020, 256, 117897. [Google Scholar] [CrossRef]
- Thakur, V.; Thakur, V.S.; Aguila, B.; Slepak, T.I.; Wang, M.; Song, W.; Konai, M.; Mobashery, S.; Chang, M.; Rana, A.B.; et al. Targeting extracellular matrix remodeling sensitizes glioblastoma to ionizing radiation. Neuro-Oncol. Adv. 2022, 4, vdac147. [Google Scholar] [CrossRef]
- Banerjee, K.; Núñez, F.J.; Haase, S.; McClellan, B.L.; Faisal, S.M.; Carney, S.V.; Yu, J.; Alghamri, M.S.; Asad, A.S.; Candia, A.J.N.; et al. Current Approaches for Glioma Gene Therapy and Virotherapy. Front. Mol. Neurosci. 2021, 14, 621831. [Google Scholar] [CrossRef] [PubMed]
- Lara-Velazquez, M.; Alkharboosh, R.; Norton, E.S.; Ramirez-Loera, C.; Freeman, W.D.; Guerrero-Cazares, H.; Forte, A.J.; Quiñones-Hinojosa, A.; Sarabia-Estrada, R. Chitosan-Based Non-viral Gene and Drug Delivery Systems for Brain Cancer. Front. Neurol. 2020, 11, 740. [Google Scholar] [CrossRef]
- Chao, C.N.; Yang, Y.H.; Wu, M.S.; Chou, M.C.; Fang, C.Y.; Lin, M.C.; Tai, C.K.; Shen, C.H.; Chen, P.L.; Chang, D.C.; et al. Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Sci. Rep. 2018, 8, 2213. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Yee, G.T.; Khang, D. Nanoparticle-Based Combinational Strategies for Overcoming the Blood-Brain Barrier and Blood-Tumor Barrier. Int. J. Nanomed. 2024, 19, 2529–2552. [Google Scholar] [CrossRef]
- Justo, B.L.; Jasiulionis, M.G. Characteristics of TIMP1, CD63, and beta1-Integrin and the Functional Impact of Their Interaction in Cancer. Int. J. Mol. Sci. 2021, 22, 9319. [Google Scholar] [CrossRef]
- Su, C.W.; Lin, C.W.; Yang, W.E.; Yang, S.F. TIMP-3 as a therapeutic target for cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919864247. [Google Scholar] [CrossRef]
- Hernandez-Barrantes, S.; Shimura, Y.; Soloway, P.D.; Sang, Q.A.; Fridman, R. Differential roles of TIMP-4 and TIMP-2 in pro-MMP-2 activation by MT1-MMP. Biochem. Biophys. Res. Commun. 2001, 281, 126–130. [Google Scholar] [CrossRef]
- Han, J.; Jing, Y.; Han, F.; Sun, P. Comprehensive analysis of expression, prognosis and immune infiltration for TIMPs in glioblastoma. BMC Neurol. 2021, 21, 447. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Groft, L.L.; Muzik, H.; Rewcastle, N.B.; Johnston, R.N.; Knäuper, V.; Lafleur, M.A.; Forsyth, P.A.; Edwards, D.R. Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br. J. Cancer 2001, 85, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Waller, V.; Pruschy, M. Combined Radiochemotherapy: Metalloproteinases Revisited. Front. Oncol. 2021, 11, 676583. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Fields, G.B. Mechanisms of Action of Novel Drugs Targeting Angiogenesis-Promoting Matrix Metalloproteinases. Front. Immunol. 2019, 10, 1278. [Google Scholar] [CrossRef]
- Melssen, M.M.; Sheybani, N.D.; Leick, K.M.; Slingluff, C., Jr. Barriers to immune cell infiltration in tumors. J. Immunother. Cancer 2023, 11, e006401. [Google Scholar] [CrossRef]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.; Thaci, B.; Sarvaiya, P.; Yi, R.; Guo, D.; Auffinger, B.; Pytel, P.; Zhang, L.; Kim, C.K.; Borovjagin, A.; et al. Inhibition of MMP14 potentiates the therapeutic effect of temozolomide and radiation in gliomas. Cancer Med. 2013, 2, 457–467. [Google Scholar] [CrossRef]
- Gusev, Y.; Bhuvaneshwar, K.; Song, L.; Zenklusen, J.C.; Fine, H.; Madhavan, S. The REMBRANDT study, a large collection of genomic data from brain cancer patients. Sci. Data 2018, 5, 180158. [Google Scholar] [CrossRef]
- Duan, H.; Ren, J.L.; Wei, S.Y.; Yang, Z.Y.; Li, C.; Wang, Z.N.; Li, M.C.; Wei, Z.; Liu, Y.; Wang, X.Q.; et al. Integrated analyses of multi-omic data derived from paired primary lung cancer and brain metastasis reveal the metabolic vulnerability as a novel therapeutic target. Genome Med. 2024, 16, 138. [Google Scholar] [CrossRef]
- Shi, Y.X.; Zhang, Q.L.; Mei, J.; Liu, J.H. Editorial: Multi-omics analysis in tumor microenvironment and tumor heterogeneity. Front. Genet. 2023, 14, 1271295. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chen, R.; Wa, Y.; Ding, S.K.; Yang, Y.J.; Liao, J.B.; Tong, L.; Xiao, G.L. Tumor Immune Microenvironment and Immunotherapy in Brain Metastasis from Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 829451. [Google Scholar] [CrossRef] [PubMed]
- Czajka-Francuz, P.; Prendes, M.J.; Mankan, A.; Quintana, A.; Pabla, S.; Ramkissoon, S.; Jensen, T.J.; Peiró, S.; Severson, E.A.; Achyut, B.R.; et al. Mechanisms of immune modulation in the tumor microenvironment and implications for targeted therapy. Front. Oncol. 2023, 13, 1200646. [Google Scholar] [CrossRef]
- Tang, L.; Feng, Y.C.; Gao, S.; Mu, Q.C.; Liu, C.Y. Nanotherapeutics Overcoming the Blood-Brain Barrier for Glioblastoma Treatment. Front. Pharmacol. 2021, 12, 786700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Cui, H.J.; Gan, N.; Ma, X.H.; Ren, W.Z.; Wu, A.G. Recent advances in matrix metalloproteinases-responsive nanoprobes for cancer diagnosis and therapy. Rev. Anal. Chem. 2022, 41, 198–216. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Chekhonin, V.P. Extracellular vesicles shed by glioma cells: Pathogenic role and clinical value. Tumor Biol. 2014, 35, 8425–8438. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Coban, M.; Mahajan, S.; Hockla, A.; Sankaran, B.; Downey, G.P.; Radisky, D.C.; Radisky, E.S. Engineering of tissue inhibitor of metalloproteinases TIMP-1 for fine discrimination between closely related stromelysins MMP-3 and MMP-10. J. Biol. Chem. 2022, 298, 101654. [Google Scholar] [CrossRef]
- Ashja Ardalan, A.; Khalili-Tanha, G.; Shoari, A. Shaping the Landscape of Lung Cancer: The Role and Therapeutic Potential of Matrix Metalloproteinases. Int. J. Transl. Med. 2024, 4, 661–679. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitchison, E.E.; Dimesa, A.M.; Shoari, A. Matrix Metalloproteinases in Glioma: Drivers of Invasion and Therapeutic Targets. BioTech 2025, 14, 28. https://doi.org/10.3390/biotech14020028
Aitchison EE, Dimesa AM, Shoari A. Matrix Metalloproteinases in Glioma: Drivers of Invasion and Therapeutic Targets. BioTech. 2025; 14(2):28. https://doi.org/10.3390/biotech14020028
Chicago/Turabian StyleAitchison, Ella E., Alexandra M. Dimesa, and Alireza Shoari. 2025. "Matrix Metalloproteinases in Glioma: Drivers of Invasion and Therapeutic Targets" BioTech 14, no. 2: 28. https://doi.org/10.3390/biotech14020028
APA StyleAitchison, E. E., Dimesa, A. M., & Shoari, A. (2025). Matrix Metalloproteinases in Glioma: Drivers of Invasion and Therapeutic Targets. BioTech, 14(2), 28. https://doi.org/10.3390/biotech14020028







