Inflammatory and Immune Disorders Associated with Myelodysplastic Syndromes
Abstract
:1. Introduction
2. Epidemiology
3. Pathophysiology of MDS-Related Immune Dysregulation Disorders
- -
- Low-risk MDS is characterized by increased apoptosis of hematopoietic progenitors [21,22] secondary to: (i) the production of pro-inflammatory cytokines such as interleukin 6 (IL-6) or tumor necrosis factor alpha (TNF-α) [21,23,24,25,26,27], (ii) the expansion of oligoclonal CD8 T lymphocytes directed against Wilms’ tumor 1 (WT1) antigen or other epitopes overexpressed by the myelodysplastic clone [28,29,30] and (iii) a Th17/Treg imbalance characterized by a quantitative and functional defect of T regulatory lymphocytes (Treg) associated with an excessive Th17 response [31,32,33].
- -
- High-risk MDS is characterized by excess proliferation secondary to: (i) acquisition of resistance to apoptosis by overexpression of the anti-apoptotic TNFR2 receptors of TNF-α [34,35]), (ii) an escapement of the anti-tumor response to the malignant clonal cells [36] by an increase of functional T regulatory lymphocytes [37,38] and (iii) a lack of cytotoxicity of NK cells [34,39] and expansion of myeloid-derived suppressor cells (MDSC) [40,41].
- -
- Th17/Treg imbalance reported during low risk MDS could promote the emergence of dysimmune manifestations through an excess of pro-inflammatory Th17 lymphocytes and a defect of regulatory T lymphocytes at the origin of an immune tolerance breakdown.
- -
- A higher level of interferon regulatory factor 1 (IRF-1), a mediator of type 1 interferon-signaling pathway demonstrated in MDS patients with SIADs compared to MDS patients without SIADs [42].
- -
- During CMML, increased secretion of cytokines such as TNF-α and IL-6 caused by the proliferation of monocytes could promote polyclonal proliferation of B lymphocytes and the production of auto-antibodies. Additionally, a defect in antigen presentation by macrophages could lead to the persistence of deleterious chronic immune activation.
- -
- The quantitative and functional decrease in T-γδ lymphocytes in patients with MDS associated with SIADs could be comparable to that observed in patients with isolated autoimmune diseases [43].
- -
- The inactivating mutations of TET2 and DNMT3A could favor a pro-inflammatory state of monocytic and macrophagic cells [44,45] and/or modify the activity of CD8 T lymphocytes and the polarization of the CD4 T lymphocytes, since TET2 regulates the methylation of the promoter regions of FOXP3 gene [46] and genes of transcription factors TBET, GATA3 and RORgT [47].
- -
4. Characteristics of the Underlying MDS
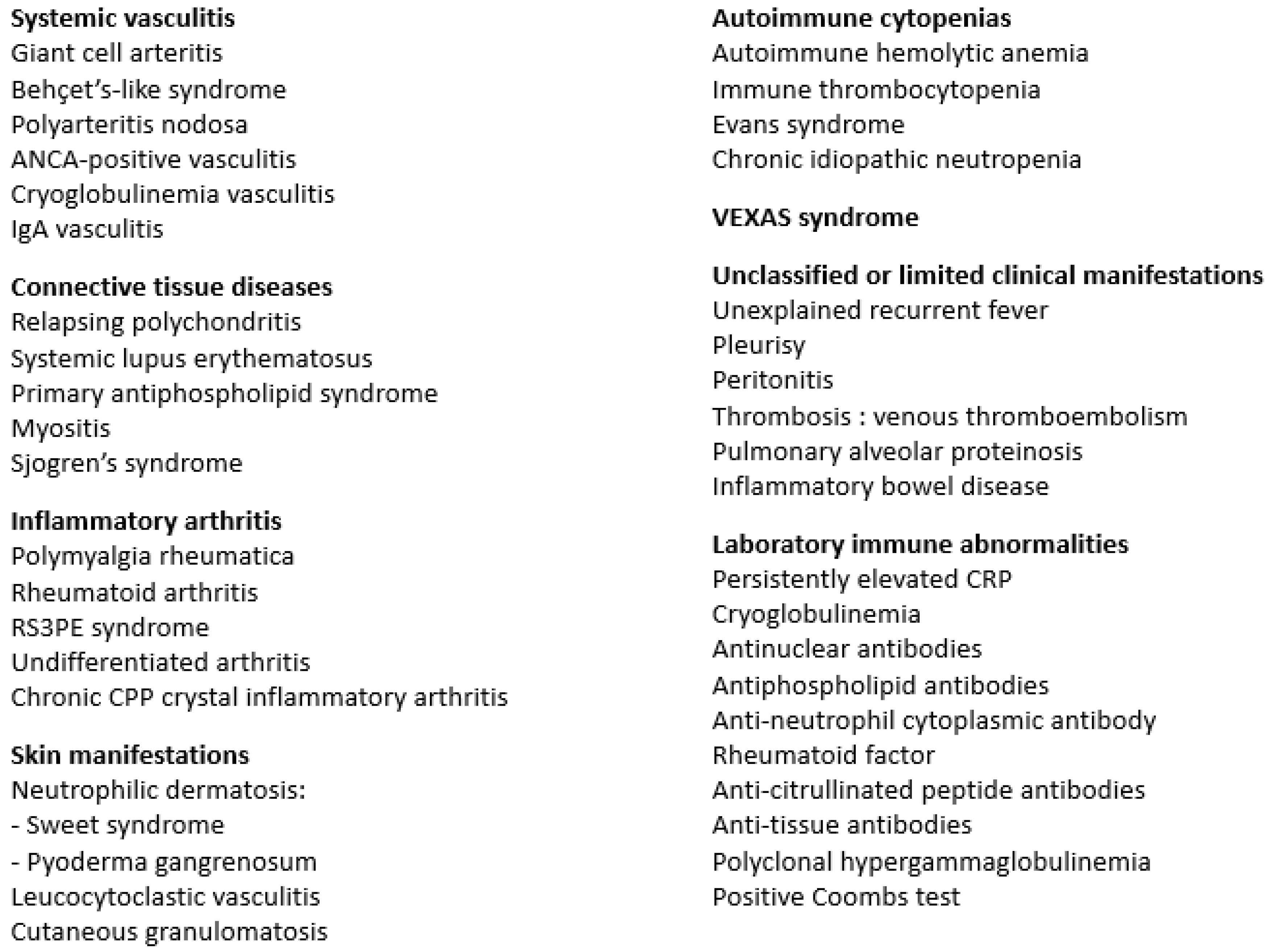
5. Autoimmune Disorder Characteristics
5.1. Systemic Vasculitis
5.2. Connective Tissue Diseases
5.3. Inflammatory Arthritis
5.4. Skin Manifestations
5.5. Autoimmune Cytopenias
5.6. Thrombosis
5.7. VEXAS Syndrome
5.8. Other Manifestations
6. Laboratory Immune Abnormalities in MDS Patients with and without Clinical SIADs
7. Prognosis
8. Treatment of MDS-Related SIADs
8.1. Systemic Glucocorticoids
8.2. Conventional Synthetic and Biological DMARDs
8.3. Other Treatments (IMiDs, Low Dose Interleukin-2, JAK Inhibitors)
8.4. Hypomethylating Agents (HMAs)
8.5. Bone Marrow Engraftment
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adès, L.; Itzykson, R.; Fenaux, P. Myelodysplastic syndromes. Lancet 2014, 383, 2239–2252. [Google Scholar] [CrossRef]
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 2011, 364, 2496–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Qian, Y.; Eksioglu, E.; Epling-Burnette, P.K.; Wei, S. The inflammatory microenvironment in MDS. Cell. Mol. Life Sci. 2015, 72, 1959–1966. [Google Scholar] [CrossRef]
- Shen, J.; Wang, S.; Zhang, Y.-J.; Kappil, M.A.; Chen Wu, H.; Kibriya, M.G.; Wang, Q.; Jasmine, F.; Ahsan, H.; Lee, P.-H.; et al. Genome-wide aberrant DNA methylation of microRNA host genes in hepatocellular carcinoma. Epigenetics 2012, 7, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dunbar, A.; Gondek, L.P.; Mohan, S.; Rataul, M.; O’Keefe, C.; Sekeres, M.; Saunthararajah, Y.; Maciejewski, J.P. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood 2009, 113, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Bacher, U.; Shumilov, E.; Flach, J.; Porret, N.; Joncourt, R.; Wiedemann, G.; Fiedler, M.; Novak, U.; Amstutz, U.; Pabst, T. Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J. 2018, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Mufti, G.J.; Figes, A.; Hamblin, T.J.; Oscier, D.G.; Copplestone, J.A. Immunological abnormalities in myelodysplastic syndromes. I. Serum immunoglobulins and autoantibodies. Br. J. Haematol. 1986, 63, 143–147. [Google Scholar] [CrossRef]
- Saif, M.W.; Hopkins, J.L.; Gore, S.D. Autoimmune phenomena in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk. Lymphoma 2002, 43, 2083–2092. [Google Scholar] [CrossRef]
- Enright, H.; Miller, W. Autoimmune phenomena in patients with myelodysplastic syndromes. Leuk. Lymphoma 1997, 24, 483–489. [Google Scholar] [CrossRef]
- Mekinian, A.; Grignano, E.; Braun, T.; Decaux, O.; Liozon, E.; Costedoat-Chalumeau, N.; Kahn, J.-E.; Hamidou, M.; Park, S.; Puéchal, X.; et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: A French multicentre retrospective study. Rheumatology 2016, 55, 291–300. [Google Scholar] [CrossRef] [Green Version]
- de Hollanda, A.; Beucher, A.; Henrion, D.; Ghali, A.; Lavigne, C.; Lévesque, H.; Hamidou, M.; Subra, J.F.; Ifrah, N.; Belizna, C. Systemic and immune manifestations in myelodysplasia: A multicenter retrospective study. Arthritis Care Res. (Hoboken) 2011, 63, 1188–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marisavljević, D.; Kraguljac, N.; Rolović, Z. Immunologic abnormalities in myelodysplastic syndromes: Clinical features and characteristics of the lymphoid population. Med. Oncol. 2006, 23, 385–391. [Google Scholar] [CrossRef]
- Komrokji, R.S.; Kulasekararaj, A.; Al Ali, N.H.; Kordasti, S.; Bart-Smith, E.; Craig, B.M.; Padron, E.; Zhang, L.; Lancet, J.E.; Pinilla-Ibarz, J.; et al. Autoimmune diseases and myelodysplastic syndromes. Am. J. Hematol. 2016, 91, E280–E283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguier, J.; Gelsi-Boyer, V.; Ebbo, M.; Hamidou, Z.; Charbonnier, A.; Bernit, E.; Durand, J.-M.; Harlé, J.-R.; Vey, N.; Schleinitz, N. Autoimmune diseases in myelodysplastic syndrome favors patients survival: A case control study and literature review. Autoimmun. Rev. 2019, 18, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, S.; Voulgarelis, M.; Zintzaras, E.; Tzioufas, A.G.; Moutsopoulos, H.M. Autoimmune phenomena in myelodysplastic syndromes: A 4-yr prospective study. Rheumatology (Oxford) 2004, 43, 626–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthier, S.; Magy, N.; Gil, H.; Schneider, M.B.; Vuitton, D.A.; Dupond, J.L. Myelodysplasias and systemic diseases. A non-fortuitous association. Rev. Med. Interne 2001, 22, 428–432. [Google Scholar] [CrossRef]
- Hamidou, M.A.; Derenne, S.; Audrain, M.A.; Berthelot, J.M.; Boumalassa, A.; Grolleau, J.Y. Prevalence of rheumatic manifestations and antineutrophil cytoplasmic antibodies in haematological malignancies. A prospective study. Rheumatology (Oxford) 2000, 39, 417–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.A.; Pfeiffer, R.M.; Landgren, O.; Gadalla, S.; Berndt, S.I.; Engels, E.A. Risks of myeloid malignancies in patients with autoimmune conditions. Br. J. Cancer 2009, 100, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Kristinsson, S.Y.; Björkholm, M.; Hultcrantz, M.; Derolf, Å.R.; Landgren, O.; Goldin, L.R. Chronic Immune Stimulation Might Act As a Trigger for the Development of Acute Myeloid Leukemia or Myelodysplastic Syndromes. J. Clin. Oncol. 2011, 29, 2897–2903. [Google Scholar] [CrossRef] [Green Version]
- Dalamaga, M.; Petridou, E.; Cook, F.E.; Trichopoulos, D. Risk factors for myelodysplastic syndromes: A case-control study in Greece. Cancer Causes Control 2002, 13, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Fenaux, P. Myelodysplastic Syndromes (MDS) and autoimmune disorders (AD): Cause or consequence? Best Pract. Res. Clin. Haematol. 2013, 26, 327–336. [Google Scholar] [CrossRef]
- Parker, J.E.; Mufti, G.J. Excessive apoptosis in low risk myelodysplastic syndromes (MDS). Leuk. Lymphoma 2000, 40, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Miyazato, A.; Chen, G.; Kajigaya, S.; Young, N.S.; Maciejewski, J.P. Interferon-gamma-induced gene expression in CD34 cells: Identification of pathologic cytokine-specific signature profiles. Blood 2006, 107, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, M.; Saito, I.; Kuwata, T.; Yoshida, S.; Yamaguchi, S.; Takahashi, M.; Tanizawa, T.; Kamiyama, R.; Hirokawa, K. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia 1997, 11, 2049–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gañán-Gómez, I.; Wei, Y.; Starczynowski, D.T.; Colla, S.; Yang, H.; Cabrero-Calvo, M.; Bohannan, Z.S.; Verma, A.; Steidl, U.; Garcia-Manero, G. Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia 2015, 29, 1458–1469. [Google Scholar] [CrossRef]
- Claessens, Y.-E.; Bouscary, D.; Dupont, J.-M.; Picard, F.; Melle, J.; Gisselbrecht, S.; Lacombe, C.; Dreyfus, F.; Mayeux, P.; Fontenay-Roupie, M. In vitro proliferation and differentiation of erythroid progenitors from patients with myelodysplastic syndromes: Evidence for Fas-dependent apoptosis. Blood 2002, 99, 1594–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewski, J.P.; Selleri, C.; Sato, T.; Anderson, S.; Young, N.S. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br. J. Haematol. 1995, 91, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Baumann, I.; Scheid, C.; Koref, M.S.; Swindell, R.; Stern, P.; Testa, N.G. Autologous lymphocytes inhibit hemopoiesis in long-term culture in patients with myelodysplastic syndrome. Exp. Hematol. 2002, 30, 1405–1411. [Google Scholar] [CrossRef]
- Zheng, Z.; Qianqiao, Z.; Qi, H.; Feng, X.; Chunkang, C.; Xiao, L. In vitro deprivation of CD8(+)CD57(+)T cells promotes the malignant growth of bone marrow colony cells in patients with lower-risk myelodysplastic syndrome. Exp. Hematol. 2010, 38, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Sloand, E.M.; Melenhorst, J.J.; Tucker, Z.C.G.; Pfannes, L.; Brenchley, J.M.; Yong, A.; Visconte, V.; Wu, C.; Gostick, E.; Scheinberg, P.; et al. T-cell immune responses to Wilms tumor 1 protein in myelodysplasia responsive to immunosuppressive therapy. Blood 2011, 117, 2691–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordasti, S.Y.; Ingram, W.; Hayden, J.; Darling, D.; Barber, L.; Afzali, B.; Lombardi, G.; Wlodarski, M.W.; Maciejewski, J.P.; Farzaneh, F.; et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 2007, 110, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Kordasti, S.Y.; Afzali, B.; Lim, Z.; Ingram, W.; Hayden, J.; Barber, L.; Matthews, K.; Chelliah, R.; Guinn, B.; Lombardi, G.; et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br. J. Haematol. 2009, 145, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, W.; Ogawara, H.; Handa, H.; Tsukamoto, N.; Nojima, Y.; Murakami, H. Clinical significance of regulatory T cells in patients with myelodysplastic syndrome. Eur. J. Haematol. 2009, 82, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Swerdlow, S.H.; TenEyck, S.P.; Boyiadzis, M.; Felgar, R.E. Natural killer cell (NK) subsets and NK-like T-cell populations in acute myeloid leukemias and myelodysplastic syndromes. Cytom. B Clin. Cytom. 2016, 90, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Sawanobori, M.; Yamaguchi, S.; Hasegawa, M.; Inoue, M.; Suzuki, K.; Kamiyama, R.; Hirokawa, K.; Kitagawa, M. Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leuk. Res. 2003, 27, 583–591. [Google Scholar] [CrossRef]
- Epling-Burnette, P.K.; Painter, J.S.; Rollison, D.E.; Ku, E.; Vendron, D.; Widen, R.; Boulware, D.; Zou, J.X.; Bai, F.; List, A.F. Prevalence and clinical association of clonal T-cell expansions in Myelodysplastic Syndrome. Leukemia 2007, 21, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotsianidis, I.; Bouchliou, I.; Nakou, E.; Spanoudakis, E.; Margaritis, D.; Christophoridou, A.V.; Anastasiades, A.; Tsigalou, C.; Bourikas, G.; Karadimitris, A.; et al. Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia 2009, 23, 510–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailloux, A.W.; Sugimori, C.; Komrokji, R.S.; Yang, L.; Maciejewski, J.P.; Sekeres, M.A.; Paquette, R.; Loughran, T.P.; List, A.F.; Epling-Burnette, P.K. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J. Immunol. 2012, 189, 3198–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejazi, M.; Manser, A.R.; Fröbel, J.; Kündgen, A.; Zhao, X.; Schönberg, K.; Germing, U.; Haas, R.; Gattermann, N.; Uhrberg, M. Impaired cytotoxicity associated with defective natural killer cell differentiation in myelodysplastic syndromes. Haematologica 2015, 100, 643–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Fu, R.; Wang, H.; Li, L.; Qu, W.; Liang, Y.; Wang, G.; Wang, X.; Wu, Y.-H.; Liu, H.; et al. Increased circulating of myeloid-derived suppressor cells in myelodysplastic syndrome. Chin. Med. J. (Engl.) 2013, 126, 2582–2584. [Google Scholar] [PubMed]
- Chen, X.; Eksioglu, E.A.; Zhou, J.; Zhang, L.; Djeu, J.; Fortenbery, N.; Epling-Burnette, P.; Van Bijnen, S.; Dolstra, H.; Cannon, J.; et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J. Clin. Investig. 2013, 123, 4595–4611. [Google Scholar] [CrossRef]
- Giannouli, S.; Tzoanopoulos, D.; Ritis, K.; Kartalis, G.; Moutsopoulos, H.M.; Voulgarelis, M. Autoimmune manifestations in human myelodysplasia: A positive correlation with interferon regulatory factor-1 (IRF-1) expression. Ann. Rheum. Dis. 2004, 63, 578–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiladjian, J.-J.; Visentin, G.; Viey, E.; Chevret, S.; Eclache, V.; Stirnemann, J.; Bourhis, J.H.; Chouaib, S.; Fenaux, P.; Caignard, A. Activation of cytotoxic T-cell receptor gammadelta T lymphocytes in response to specific stimulation in myelodysplastic syndromes. Haematologica 2008, 93, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.-L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Zhao, K.; Shen, Q.; Han, Y.; Gu, Y.; Li, X.; Zhao, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 2015, 525, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Trifari, S.; Äijö, T.; Tsagaratou, A.; Pastor, W.A.; Zepeda-Martínez, J.A.; Lio, C.-W.J.; Li, X.; Huang, Y.; Vijayanand, P.; et al. Control of Foxp3 stability through modulation of TET activity. J. Exp. Med. 2016, 213, 377–397. [Google Scholar] [CrossRef] [Green Version]
- Ichiyama, K.; Chen, T.; Wang, X.; Yan, X.; Kim, B.-S.; Tanaka, S.; Ndiaye-Lobry, D.; Deng, Y.; Zou, Y.; Zheng, P.; et al. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity 2015, 42, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Mundle, S.D.; Venugopal, P.; Cartlidge, J.D.; Pandav, D.V.; Broady-Robinson, L.; Gezer, S.; Robin, E.L.; Rifkin, S.R.; Klein, M.; Alston, D.E.; et al. Indication of an involvement of interleukin-1 beta converting enzyme-like protease in intramedullary apoptotic cell death in the bone marrow of patients with myelodysplastic syndromes. Blood 1996, 88, 2640–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, T.; Yamashita, K.; Ohno, T.; Izumi, T.; Takaori-Kondo, A.; Sasada, M.; Uchiyama, T. Familial Mediterranean fever gene as a possible modifier of Sweet syndrome with chronic myelogenous leukemia. Acta Haematol. 2008, 120, 57–62. [Google Scholar] [CrossRef]
- Jo, T.; Horio, K.; Migita, K. Sweet’s syndrome in patients with MDS and MEFV mutations. N. Engl. J. Med. 2015, 372, 686–688. [Google Scholar] [CrossRef]
- Wolach, O.; Stone, R. Autoimmunity and Inflammation in Myelodysplastic Syndromes. Acta Haematol. 2016, 136, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; Kacar, M.; Bragazzi, N.L.; Zhou, Q.; Jassam, M.; Taylor, J.; Roman, E.; Smith, A.; Jones, R.A.; Amital, H.; et al. Somatic Mutations and the Risk of Undifferentiated Autoinflammatory Disease in MDS: An Under-Recognized but Prognostically Important Complication. Front. Immunol. 2021, 12, 610019. [Google Scholar] [CrossRef]
- Hamidou, M.A.; Boumalassa, A.; Larroche, C.; El Kouri, D.; Blétry, O.; Grolleau, J.Y. Systemic medium-sized vessel vasculitis associated with chronic myelomonocytic leukemia. Semin. Arthritis Rheum. 2001, 31, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Grignano, E.; Mekinian, A.; Braun, T.; Liozon, E.; Hamidou, M.; Decaux, O.; Puéchal, X.; Kahn, J.E.; Schoindre, Y.; Rossignol, J.; et al. Autoimmune and inflammatory diseases associated with chronic myelomonocytic leukemia: A series of 26 cases and literature review. Leuk. Res. 2016, 47, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wesner, N.; Drevon, L.; Guedon, A.; Fraison, J.B.; Terrier, B.; Trad, S.; Kahn, J.E.; Aouba, A.; Gillard, J.; Ponsoye, M.; et al. Gastrointestinal Behcet’s-like disease with myelodysplastic neoplasms with trisomy 8: A French case series and literature review. Leuk. Lymphoma 2019, 60, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, D.; Hamada, T.; Yamasaki, O.; Sando, Y.; Saeki, K.; Fujii, N.; Kawakami, Y.; Iwatsuki, K. Successful use of etoposide for pyoderma gangrenosum associated with myelodysplastic syndrome and trisomy 8: Cytokine profiles during treatment. Eur. J. Dermatol. 2017, 27, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Hoshida, S.; Yoneda, S. Sweet’s syndrome associated with recurrent fever in a patient with trisomy 8 myelodysplastic syndrome. Int. J. Hematol. 2003, 77, 383–386. [Google Scholar] [CrossRef]
- Nishida, A.; Miyamoto, A.; Yamamaoto, H.; Uchida, N.; Izutsu, K.; Wake, A.; Ohta, Y.; Fujii, T.; Araoka, H.; Taniguchi, S.; et al. Possible association of trisomy 8 with secondary pulmonary alveolar proteinosis in myelodysplastic syndrome. Am. J. Respir. Crit. Care Med. 2011, 184, 279–280. [Google Scholar] [CrossRef]
- Wesner, N.; Drevon, L.; Guedon, A.; Fraison, J.B.; Trad, S.; Kahn, J.E.; Aouba, A.; Gillard, J.; Ponsoye, M.; Hanslik, T.; et al. Inflammatory disorders associated with trisomy 8-myelodysplastic syndromes: French retrospective case-control study. Eur. J. Haematol. 2019, 102, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.-P.; Boy, M.; Azoulay, C.; Clappier, E.; Sébert, M.; Amable, L.; Klibi, J.; Benlagha, K.; Espéli, M.; Balabanian, K.; et al. Genomic landscape of MDS/CMML associated with systemic inflammatory and autoimmune disease. Leukemia 2021. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Shin, D.-Y.; Hwang, S.M.; Kim, S.-M.; Im, K.; Park, H.S.; Kim, J.-A.; Song, Y.W.; Márquez, A.; Martín, J.; et al. Mutation of ten-eleven translocation-2 is associated with increased risk of autoimmune disease in patients with myelodysplastic syndrome. Korean J. Intern. Med. 2020, 35, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Roupie, A.L.; Guedon, A.; Terrier, B.; Lahuna, C.; Jachiet, V.; Regent, A.; de Boysson, H.; Carrat, F.; Seguier, J.; Terriou, L.; et al. Vasculitis associated with myelodysplastic syndrome and chronic myelomonocytic leukemia: French multicenter case-control study. Semin. Arthritis Rheum. 2020, 50, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Roupie, A.L.; de Boysson, H.; Thietart, S.; Carrat, F.; Seguier, J.; Terriou, L.; Versini, M.; Queyrel, V.; Groh, M.; Benhamou, Y.; et al. Giant-cell arteritis associated with myelodysplastic syndrome: French multicenter case control study and literature review. Autoimmun. Rev. 2020, 19, 102446. [Google Scholar] [CrossRef] [PubMed]
- Dion, J.; Costedoat-Chalumeau, N.; Sène, D.; Cohen-Bittan, J.; Leroux, G.; Dion, C.; Francès, C.; Piette, J.-C. Relapsing Polychondritis Can Be Characterized by Three Different Clinical Phenotypes: Analysis of a Recent Series of 142 Patients. Arthritis Rheumatol. 2016, 68, 2992–3001. [Google Scholar] [CrossRef] [PubMed]
- Francès, C.; el Rassi, R.; Laporte, J.L.; Rybojad, M.; Papo, T.; Piette, J.C. Dermatologic manifestations of relapsing polychondritis. A study of 200 cases at a single center. Medicine (Baltimore) 2001, 80, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Gould, J.; Dolan, G. Relapsing polychondritis and myelodysplasia: A report of two cases and review of the current literature. Clin. Lab. Haematol. 2000, 22, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Piette, J.C.; Papo, T.; Chavanon, P.; Huong, D.L.; Frances, C.; Godeau, P. Myelodysplasia and relapsing polychondritis. J. Rheumatol. 1995, 22, 1208–1209. [Google Scholar] [PubMed]
- Lu, M.; Bernatsky, S.; Ramsey-Goldman, R.; Petri, M.; Manzi, S.; Urowitz, M.B.; Gladman, D.; Fortin, P.R.; Ginzler, E.M.; Yelin, E.; et al. Non-lymphoma hematological malignancies in systemic lupus erythematosus. Oncology 2013, 85, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Voulgarelis, M.; Giannouli, S.; Tasidou, A.; Anagnostou, D.; Ziakas, P.D.; Tzioufas, A.G. Bone marrow histological findings in systemic lupus erythematosus with hematologic abnormalities: A clinicopathological study. Am. J. Hematol. 2006, 81, 590–597. [Google Scholar] [CrossRef]
- Chalayer, E.; Costedoat-Chalumeau, N.; Beyne-Rauzy, O.; Ninet, J.; Durupt, S.; Tebib, J.; Asli, B.; Lambotte, O.; Ffrench, M.; Vasselon, C.; et al. Bone marrow involvement in systemic lupus erythematosus. QJM 2017, 110, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonetta, F.; Posa, M.; Villard, J.; Marceau-Renaut, A.; Preudhomme, C.; Samii, K.; Chizzolini, C. Restoration of hematopoiesis in a case of myelodysplastic syndrome associated with systemic lupus erythematosus treated with rituximab. Ann. Hematol. 2015, 94, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Kameoka, J.; Hirabayashi, Y.; Takahashi, R.; Ishii, T.; Sasaki, T.; Harigae, H. Reversible bone marrow dysplasia in patients with systemic lupus erythematosus. Intern. Med. 2008, 47, 737–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricard, L.; Hirsch, P.; Largeaud, L.; Deswarte, C.; Jachiet, V.; Mohty, M.; Rivière, S.; Malard, F.; Tenon, M.; de Vassoigne, F.; et al. Clonal haematopoiesis is increased in early onset in systemic sclerosis. Rheumatology (Oxford) 2020, 59, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Mekinian, A.; Braun, T.; Decaux, O.; Falgarone, G.; Toussirot, E.; Raffray, L.; Omouri, M.; Gombert, B.; De Wazieres, B.; Buchdaul, A.-L.; et al. Inflammatory Arthritis in Patients with Myelodysplastic Syndromes: A Multicenter Retrospective Study and Literature Review of 68 Cases. Medicine 2014, 93, 1–10. [Google Scholar] [CrossRef]
- Castro, M.; Conn, D.L.; Su, W.P.; Garton, J.P. Rheumatic manifestations in myelodysplastic syndromes. J. Rheumatol. 1991, 18, 721–727. [Google Scholar]
- Espinosa, G.; Font, J.; Muñoz-Rodríguez, F.J.; Cervera, R.; Ingelmo, M. Myelodysplastic and myeloproliferative syndromes associated with giant cell arteritis and polymyalgia rheumatica: A coincidental coexistence or a causal relationship? Clin. Rheumatol. 2002, 21, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Boggio, E.; Sola, D.; Gibbin, A.; Gualerzi, A.; Favretto, S.; Guaschino, G.; Bonometti, R.; Pedrazzoli, R.; Pirisi, M.; et al. Association between rheumatic diseases and cancer: Results from a clinical practice cohort study. Intern. Emerg. Med. 2017, 12, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Paira, S.; Graf, C.; Roverano, S.; Rossini, J. Remitting seronegative symmetrical synovitis with pitting oedema: A study of 12 cases. Clin. Rheumatol. 2002, 21, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.K.; Stone, R.M.; Helfgott, S.M. Calcium Pyrophosphate Crystal Inflammatory Arthritis (Pseudogout) with Myelodysplastic Syndrome: A New Paraneoplastic Syndrome? J. Rheumatol. 2017, 44, 1101–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, C.; Bulai Livideanu, C.; Jegu, J.; Paul, C.; Viraben, R.; Lamant, L.; Delavigne, K.; Adoue, D.; Laurent, G.; Beyne Rauzy, O. Prevalence and prognostic value of cutaneous manifestations in patients with myelodysplastic syndrome. J. Eur. Acad Dermatol. Venereol. 2010, 24, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Yoneta, K.; Fujimoto, N.; Teramura, K.; Takayama, S.; Tanaka, T. Disseminated granulomatous skin lesions associated with myelodysplastic syndrome treated successfully with tranilast: A case report and review of the literature. Eur. J. Dermatol. 2016, 26, 398–400. [Google Scholar] [CrossRef]
- Balin, S.J.; Wetter, D.A.; Kurtin, P.J.; Letendre, L.; Pittelkow, M.R. Myelodysplastic syndrome presenting as generalized granulomatous dermatitis. Arch. Dermatol. 2011, 147, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Lepelletier, C.; Bouaziz, J.-D.; Rybojad, M.; Bagot, M.; Georgin-Lavialle, S.; Vignon-Pennamen, M.-D. Neutrophilic Dermatoses Associated with Myeloid Malignancies. Am. J. Clin. Dermatol. 2019, 20, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, D.; Battistella, M.; Bouaziz, J.-D. Neutrophilic dermatosis: Disease mechanism and treatment. Curr. Opin. Hematol. 2015, 22, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Sweet’s syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J. Rare Dis. 2007, 2, 34. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ai, M.; Yang, W.; Li, X. Vital organ involvement in Sweet’s syndrome with myelodysplastic syndrome: A case report and literature review. Int. J. Dermatol. 2015, 54, 1303–1308. [Google Scholar] [CrossRef]
- Requena, L.; Kutzner, H.; Palmedo, G.; Pascual, M.; Fernández-Herrera, J.; Fraga, J.; García-Díez, A.; Yus, E.S. Histiocytoid Sweet syndrome: A dermal infiltration of immature neutrophilic granulocytes. Arch. Dermatol. 2005, 141, 834–842. [Google Scholar] [CrossRef]
- Chavan, R.N.; Cappel, M.A.; Ketterling, R.P.; Wada, D.A.; Rochet, N.M.; Knudson, R.; Gibson, L.E. Histiocytoid Sweet syndrome may indicate leukemia cutis: A novel application of fluorescence in situ hybridization. J. Am. Acad Dermatol. 2014, 70, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Alegría-Landa, V.; Rodríguez-Pinilla, S.M.; Santos-Briz, A.; Rodríguez-Peralto, J.L.; Alegre, V.; Cerroni, L.; Kutzner, H.; Requena, L. Clinicopathologic, Immunohistochemical, and Molecular Features of Histiocytoid Sweet Syndrome. JAMA Dermatol. 2017, 153, 651–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoufi, L.; Ortonne, N.; Ingen-Housz-Oro, S.; Barhoumi, W.; Begon, E.; Haioun, C.; Pautas, C.; Beckerich, F.; Robin, C.; Wolkenstein, P.; et al. Histiocytoid Sweet Syndrome Is More Frequently Associated With Myelodysplastic Syndromes Than the Classical Neutrophilic Variant: A Comparative Series of 62 Patients. Medicine (Baltimore) 2016, 95, e3033. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Noe, M.H.; McMahon, C.M.; Gowda, A.; Wu, B.; Ashchyan, H.J.; Perl, A.E.; James, W.D.; Micheletti, R.G.; Rosenbach, M. Sweet syndrome in patients with and without malignancy: A retrospective analysis of 83 patients from a tertiary academic referral center. J. Am. Acad Dermatol. 2018, 78, 303–309.e4. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, K.; Gill, R.M.; McMahon, P.; Chigurupati, R.; Siddiqi, I.; Fox, L.; Damon, L.; McCalmont, T.H.; Jordan, R.; Wolf, J. 20q-clonality in a case of oral sweet syndrome and myelodysplasia. Am. J. Clin. Pathol. 2012, 137, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sujobert, P.; Cuccuini, W.; Vignon-Pennamen, D.; Martin-Garcia, N.; Albertini, A.F.; Uzunov, M.; Redjoul, R.; Dombret, H.; Raffoux, E. Evidence of differentiation in myeloid malignancies associated neutrophilic dermatosis: A fluorescent in situ hybridization study of 14 patients. J. Investig. Dermatol. 2013, 133, 1111–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magro, C.M.; Kiani, B.; Li, J.; Crowson, A.N. Clonality in the setting of Sweet’s syndrome and pyoderma gangrenosum is not limited to underlying myeloproliferative disease. J. Cutan. Pathol. 2007, 34, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Wang, X.; Wang, Y.; Li, Y.; Zhang, R. Clonal neutrophil infiltrates in concurrent Sweet’s syndrome and acute myeloid leukemia: A case report and literature review. Cancer Genet. 2018, 226–227, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Passet, M.; Lepelletier, C.; Vignon-Pennamen, M.-D.; Chasset, F.; Hirsch, P.; Battistella, M.; Duriez, P.; Sicre de Fontbrune, F.; Boissel, N.; Legrand, O.; et al. Next-Generation Sequencing in Myeloid Neoplasm-Associated Sweet’s Syndrome Demonstrates Clonal Relation between Malignant Cells and Skin-Infiltrating Neutrophils. J. Investig. Dermatol. 2020, 140, 1873–1876.e5. [Google Scholar] [CrossRef] [PubMed]
- Osio, A.; Battistella, M.; Feugeas, J.-P.; Cuccuini, W.; Noguera, M.-E.; Petrella, T.; Raffoux, E.; Janin, A.; Pennamen, V. Myelodysplasia Cutis Versus Leukemia Cutis. J. Investig. Dermatol. 2015, 135, 2321–2324. [Google Scholar] [CrossRef] [Green Version]
- Avivi, I.; Rosenbaum, H.; Levy, Y.; Rowe, J. Myelodysplastic syndrome and associated skin lesions: A review of the literature. Leuk. Res. 1999, 23, 323–330. [Google Scholar] [CrossRef]
- Ahronowitz, I.; Harp, J.; Shinkai, K. Etiology and management of pyoderma gangrenosum: A comprehensive review. Am. J. Clin. Dermatol. 2012, 13, 191–211. [Google Scholar] [CrossRef]
- Maverakis, E.; Ma, C.; Shinkai, K.; Fiorentino, D.; Callen, J.P.; Wollina, U.; Marzano, A.V.; Wallach, D.; Kim, K.; Schadt, C.; et al. Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum: A Delphi Consensus of International Experts. JAMA Dermatol. 2018, 154, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Celada, A.; Farquet, J.J.; Muller, A.F. Refractory sideroblastic anemia secondary to autoimmune hemolytic anemia. Acta Haematol. 1977, 58, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Ustwani, O.A.; Ford, L.A.; Sait, S.J.N.; Block, A.M.W.; Barcos, M.; Vigil, C.E.; Griffiths, E.A.; Thompson, J.E.; Wang, E.S.; Ambrus, J.; et al. Myelodysplastic syndromes and autoimmune diseases—Case series and review of literature. Leuk. Res. 2013, 37, 894–899. [Google Scholar] [CrossRef] [Green Version]
- Van Rhee, F.; Abela, M. Coombs negative haemolytic anaemia responding to intravenous immunoglobulins in a patient with myelodysplastic syndrome. Clin. Lab. Haematol. 1991, 13, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Novaretti, M.C.; Sopelete, C.R.; Velloso, E.R.; Rosa, M.F.; Dorlhiac-Llacer, P.E.; Chamone, D.A. Immunohematological findings in myelodysplastic syndrome. Acta Haematol. 2001, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jachiet, V.; Moulis, G.; Hadjadj, J.; Seguier, J.; Laribi, K.; Schleinitz, N.; Vey, N.; Sacre, K.; Godeau, B.; Beyne-Rauzy, O.; et al. Clinical spectrum, outcome and management of immune thrombocytopenia associated with myelodysplastic syndromes and chronic myelomonocytic leukemia. Haematologica 2021, 106, 1414. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Malcovati, L.; Gallì, A.; Travaglino, E.; Ambaglio, I.; Rizzo, E.; Molteni, E.; Elena, C.; Ferretti, V.V.; Catricalà, S.; Bono, E.; et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood 2017, 129, 3371–3378. [Google Scholar] [CrossRef]
- Barcellini, W.; Fattizzo, B.; Zaninoni, A.; Valli, V.; Ferri, V.; Gianelli, U.; Cortelezzi, A. Clinical evolution of autoimmune cytopenias to idiopathic cytopenias/dysplasias of uncertain significance (ICUS/IDUS) and bone marrow failure syndromes. Am. J. Hematol. 2017, 92, E26–E29. [Google Scholar] [CrossRef]
- Tabata, R.; Tabata, C.; Omori, K.; Nagai, T. Disappearing myelodysplastic syndrome-associated hemolytic anemia in leukemic transformation. Int Arch. Allergy Immunol. 2010, 152, 407–412. [Google Scholar] [CrossRef]
- Beck, D.B.; Ferrada, M.A.; Sikora, K.A.; Ombrello, A.K.; Collins, J.C.; Pei, W.; Balanda, N.; Ross, D.L.; Ospina Cardona, D.; Wu, Z.; et al. Somatic Mutations in UBA1 and Severe Adult-Onset Autoinflammatory Disease. N. Engl. J. Med. 2020, 383, 2628–2638. [Google Scholar] [CrossRef]
- Obiorah, I.E.; Beck, D.B.; Wang, W.; Ombrello, A.; Ferrada, M.A.; Wu, Z.; Sikora, K.A.; Trick, M.A.; Dulau-Florea, A.; Patel, B.A.; et al. Myelodysplasia and Bone Marrow Manifestations of Somatic UBA1 Mutated Autoinflammatory Disease. Blood 2020, 136, 20–21. [Google Scholar] [CrossRef]
- Oganesyan, A.; Jachiet, V.; Chasset, F.; Hirsch, P.; Hage-Sleiman, M.; Fabiani, B.; Duriez, P.; Georgin-Lavialle, S.; Delhommeau, F.; Hakobyan, Y.; et al. VEXAS syndrome: Still expanding the clinical phenotype. Rheumatology (Oxford) 2021. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Tazawa, R.; Kaneko, C.; Saraya, T.; Inoue, Y.; Hamano, E.; Kogure, Y.; Tomii, K.; Terada, M.; Takada, T.; et al. Clinical features of secondary pulmonary alveolar proteinosis: Pre-mortem cases in Japan. Eur. Respir. J. 2011, 37, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Y.; Liu, Y. Concurrent inflammatory bowel disease and myelodysplastic syndrome: Report of nine new cases and a review of the literature. Dig. Dis. Sci. 2008, 53, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- Fraison, J.-B.; Grignano, E.; Braun, T.; Adès, L.; Chollet-Martin, S.; Roland-Nicaise, P.; Fenaux, P.; Fain, O.; Mekinian, A. Autoantibodies in myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk. Lymphoma 2019, 60, 2594–2596. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Okada, M.; Mori, A.; Saheki, K.; Takatsuka, H.; Wada, H.; Tamura, A.; Fujimori, Y.; Takemoto, Y.; Kanamaru, A.; et al. Correlation between immunological abnormalities and prognosis in myelodysplastic syndrome patients. Int. J. Hematol. 1997, 66, 345–351. [Google Scholar] [CrossRef]
- Zhao, S.; Mao, H.; Wang, H.; Yu, J. The relationship between myelodysplastic syndromes and autoimmune disorders. Zhonghua Xue Ye Xue Za Zhi 2002, 23, 311–313. [Google Scholar]
- Lee, S.J.; Park, J.K.; Lee, E.Y.; Joo, S.H.; Jung, K.C.; Lee, E.B.; Song, Y.W.; Yoon, S.-S. Certain Autoimmune Manifestations Are Associated With Distinctive Karyotypes and Outcomes in Patients With Myelodysplastic Syndrome: A Retrospective Cohort Study. Medicine 2016, 95, e3091. [Google Scholar] [CrossRef]
- Montoro, J.; Gallur, L.; Merchán, B.; Molero, A.; Roldán, E.; Martínez-Valle, F.; Villacampa, G.; Navarrete, M.; Ortega, M.; Castellví, J.; et al. Autoimmune disorders are common in myelodysplastic syndrome patients and confer an adverse impact on outcomes. Ann. Hematol. 2018, 97, 1349–1356. [Google Scholar] [CrossRef]
- Ertz-Archambault, N.; Kosiorek, H.; Taylor, G.E.; Kelemen, K.; Dueck, A.; Castro, J.; Marino, R.; Gauthier, S.; Finn, L.; Sproat, L.Z.; et al. Association of Therapy for Autoimmune Disease With Myelodysplastic Syndromes and Acute Myeloid Leukemia. JAMA Oncol. 2017, 3, 936–943. [Google Scholar] [CrossRef]
- Mekinian, A.; Dervin, G.; Lapidus, N.; Kahn, J.-E.; Terriou, L.; Liozon, E.; Grignano, E.; Piette, J.-C.; Rauzy, O.B.; Grobost, V.; et al. Biologics in myelodysplastic syndrome-related systemic inflammatory and autoimmune diseases: French multicenter retrospective study of 29 patients. Autoimmun. Rev. 2017, 16, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thieu, K.P.; Rosenbach, M.; Xu, X.; Kist, J.M. Neutrophilic dermatosis complicating lenalidomide therapy. J. Am. Acad Dermatol. 2009, 61, 709–710. [Google Scholar] [CrossRef]
- Tageja, N.; Giorgadze, T.; Zonder, J. Dermatological complications following initiation of lenalidomide in a patient with chronic lymphocytic leukaemia. Intern. Med. J. 2011, 41, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Hoverson, A.R.; Davis, M.D.P.; Weenig, R.H.; Wolanskyj, A.P. Neutrophilic dermatosis (Sweet syndrome) of the hands associated with lenalidomide. Arch. Dermatol. 2006, 142, 1070–1071. [Google Scholar] [CrossRef]
- Saadoun, D.; Rosenzwajg, M.; Joly, F.; Six, A.; Carrat, F.; Thibault, V.; Sene, D.; Cacoub, P.; Klatzmann, D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 2011, 365, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Rosenzwajg, M.; Lorenzon, R.; Cacoub, P.; Pham, H.P.; Pitoiset, F.; El Soufi, K.; RIbet, C.; Bernard, C.; Aractingi, S.; Banneville, B.; et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 2019, 78, 209–217. [Google Scholar] [CrossRef]
- Corfmat, M.; Willekens, C.; Vinit, J.; Bussone, G.; Fenaux, P.; Fain, O.; Klatzmann, D.; Mekinian, A.; Comont, T. Low dose IL-2 in patients with steroid-dependent dysimmune manifestations associated with myelodysplastic syndromes: A three-case report. Rheumatology (Oxford) 2020. [Google Scholar] [CrossRef]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Bourbon, E.; Heiblig, M.; Gerfaud-Valentin, M.; Barba, T.; Durel, C.A.; Lega, J.-C.; Barraco, F.; Seve, P.; Jamilloux, Y.; Sujobert, P. Therapeutic options in Vexas syndrome: Insights from a retrospective series. Blood 2021. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.F.; Platzbecker, U.; Mey, U. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.; Ho, A.; Creamer, J.D.; du Vivier, A.W.P.; Salisbury, J.R.; Mufti, G.J. Complete response of deep neutrophilic dermatosis associated with myelodysplastic syndrome to 5-azacytidine. Br. J. Dermatol. 2007, 156, 1039–1041. [Google Scholar] [CrossRef]
- Al Ustwani, O.; Francis, J.; Wallace, P.K.; Ambrus, J.; Wetzler, M. Treating myelodysplastic syndrome improves an accompanying autoimmune disease along with a reduction in regulatory T-cells. Leuk. Res. 2011, 35, e35–e36. [Google Scholar] [CrossRef] [Green Version]
- Frietsch, J.J.; Dornaus, S.; Neumann, T.; Scholl, S.; Schmidt, V.; Kunert, C.; Sayer, H.G.; Hochhaus, A.; La Rosée, P. Paraneoplastic inflammation in myelodysplastic syndrome or bone marrow failure: Case series with focus on 5-azacytidine and literature review. Eur. J. Haematol. 2014, 93, 247–259. [Google Scholar] [CrossRef]
- Pilorge, S.; Doleris, L.M.; Dreyfus, F.; Park, S. The autoimmune manifestations associated with myelodysplastic syndrome respond to 5-azacytidine: A report on three cases. Br. J. Haematol. 2011, 153, 664–665. [Google Scholar] [CrossRef] [PubMed]
- Kudo, D.; Shimizu, M.; Kuroda, A.; Suyama, T.; Shinagawa, A.; Ito, S. Myelodysplastic syndrome with neutrophilic dermatosis successfully treated with azacitidine. Rinsho Ketsueki 2017, 58, 607–612. [Google Scholar]
- Kono, M.; Komeda, Y.; Sakurai, T.; Okamoto, A.; Minaga, K.; Kamata, K.; Hagiwara, S.; Inoue, H.; Enoki, E.; Matsumura, I.; et al. Induction of Complete Remission by Azacitidine in a Patient with Myelodysplastic Syndrome-Associated Inflammatory Bowel Disease. J. Crohns Colitis 2018, 12, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Erden, A.; Bilgin, E.; Kılıç, L.; Sarı, A.; Armağan, B.; Büyükaşık, Y.; Kalyoncu, U. Remission of relapsing polychondritis after successful treatment of myelodysplastic syndrome with azacitidine: A case and review of the literature. Drug Metab. Pers. Ther. 2018, 33, 105–108. [Google Scholar] [CrossRef]
- Costantini, B.; Kordasti, S.Y.; Kulasekararaj, A.G.; Jiang, J.; Seidl, T.; Abellan, P.P.; Mohamedali, A.; Thomas, N.S.B.; Farzaneh, F.; Mufti, G.J. The effects of 5-azacytidine on the function and number of regulatory T cells and T-effectors in myelodysplastic syndrome. Haematologica 2013, 98, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Bontkes, H.J.; Ruben, J.M.; Alhan, C.; Westers, T.M.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Azacitidine differentially affects CD4pos T-cell polarization in vitro and in vivo in high risk myelodysplastic syndromes. Leuk. Res. 2012, 36, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Goodyear, O.C.; Dennis, M.; Jilani, N.Y.; Loke, J.; Siddique, S.; Ryan, G.; Nunnick, J.; Khanum, R.; Raghavan, M.; Cook, M.; et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 2012, 119, 3361–3369. [Google Scholar] [CrossRef]
- Sohlberg, E.; Pfefferle, A.; Andersson, S.; Baumann, B.C.; Hellström-Lindberg, E.; Malmberg, K.-J. Imprint of 5-azacytidine on the natural killer cell repertoire during systemic treatment for high-risk myelodysplastic syndrome. Oncotarget 2015, 6, 34178–34190. [Google Scholar] [CrossRef] [Green Version]
- Gang, A.O.; Frøsig, T.M.; Brimnes, M.K.; Lyngaa, R.; Treppendahl, M.B.; Grønbæk, K.; Dufva, I.H.; Straten, P.T.; Hadrup, S.R. 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J. 2014, 4, e197. [Google Scholar] [CrossRef]
- Kopp, L.M.; Ray, A.; Denman, C.J.; Senyukov, V.S.; Somanchi, S.S.; Zhu, S.; Lee, D.A. Decitabine has a biphasic effect on natural killer cell viability, phenotype, and function under proliferative conditions. Mol. Immunol. 2013, 54, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Frikeche, J.; Clavert, A.; Delaunay, J.; Brissot, E.; Grégoire, M.; Gaugler, B.; Mohty, M. Impact of the hypomethylating agent 5-azacytidine on dendritic cells function. Exp. Hematol. 2011, 39, 1056–1063. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, Y.; Shen, Q.; Li, G.; Hu, L.; Zhang, X. Demethylating agent decitabine disrupts tumor-induced immune tolerance by depleting myeloid-derived suppressor cells. J. Cancer Res. Clin. Oncol. 2017, 143, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Poplutz, M.K.; Wessels, I.; Rink, L.; Uciechowski, P. Regulation of the Interleukin-6 gene expression during monocytic differentiation of HL-60 cells by chromatin remodeling and methylation. Immunobiology 2014, 219, 619–626. [Google Scholar] [CrossRef]
- Fraison, J.-B.; Mekinian, A.; Grignano, E.; Kahn, J.-E.; Arlet, J.-B.; Decaux, O.; Denis, G.; Buchdahl, A.-L.; Omouri, M.; Maigne, G.; et al. Efficacy of Azacitidine in autoimmune and inflammatory disorders associated with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk. Res. 2016, 43, 13–17. [Google Scholar] [CrossRef]
- Yamato, K. Successful cord blood stem cell transplantation for myelodysplastic syndrome with Behçet disease. Int. J. Hematol. 2003, 77, 82–85. [Google Scholar] [CrossRef]
- Tomonari, A.; Tojo, A.; Takahashi, T.; Iseki, T.; Ooi, J.; Takahashi, S.; Nagamura, F.; Uchimaru, K.; Asano, S. Resolution of Behçet’s disease after HLA-mismatched unrelated cord blood transplantation for myelodysplastic syndrome. Ann. Hematol. 2004, 83, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Nonami, A.; Takenaka, K.; Sumida, C.; Aizawa, K.; Kamezaki, K.; Miyamoto, T.; Harada, N.; Nagafuji, K.; Teshima, T.; Harada, M. Successful treatment of myelodysplastic syndrome (MDS)-related intestinal Behçet’s disease by up-front cord blood transplantation. Intern. Med. 2007, 46, 1753–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomomatsu, J.; Hamano, Y.; Ando, J.; Komatsu, N.; Sugimoto, K. Non-myeloablative allogenic BMT for myelodysplastic syndrome successfully controlled accompanying relapsing polychondritis. Bone Marrow Transplant. 2012, 47, 742–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kook, M.-H.; Yhim, H.-Y.; Lee, N.-R.; Song, E.-K.; Kim, H.S.; Yim, C.-Y.; Kwak, J.-Y. Successful treatment of myelodysplastic syndrome and Behcet colitis after allogeneic hematopoietic stem cell transplantation. Korean J. Intern. Med. 2014, 29, 123–125. [Google Scholar] [CrossRef]
- Soysal, T.; Salihoğlu, A.; Esatoğlu, S.N.; Gültürk, E.; Eşkazan, A.E.; Hatemi, G.; Hatemi, I.; Öngören Aydın, Ş.; Erzin, Y.Z.; Başlar, Z.; et al. Bone marrow transplantation for Behçet’s disease: A case report and systematic review of the literature. Rheumatology (Oxford) 2014, 53, 1136–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-S.; Ahn, J.-S.; Yun, S.J.; Park, D.-J. Successful treatment of a patient with myelodysplastic syndrome accompanied by pyoderma gangrenosum and Behçet’s disease using allogeneic stem cell transplantation. Blood Res. 2017, 52, 319–321. [Google Scholar] [CrossRef] [Green Version]
- Daikeler, T.; Hügle, T.; Farge, D.; Andolina, M.; Gualandi, F.; Baldomero, H.; Bocelli-Tyndall, C.; Brune, M.; Dalle, J.H.; Urban, C.; et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transplant. 2009, 44, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Snowden, J.A.; Badoglio, M.; Labopin, M.; Giebel, S.; McGrath, E.; Marjanovic, Z.; Burman, J.; Moore, J.; Rovira, M.; Wulffraat, N.M.; et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017, 1, 2742–2755. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jachiet, V.; Fenaux, P.; Sevoyan, A.; Hakobyan, Y.; Ades, L.; Fain, O.; Mekinian, A.; on behalf of the MINHEMON and GFM. Inflammatory and Immune Disorders Associated with Myelodysplastic Syndromes. Hemato 2021, 2, 329-346. https://doi.org/10.3390/hemato2020019
Jachiet V, Fenaux P, Sevoyan A, Hakobyan Y, Ades L, Fain O, Mekinian A, on behalf of the MINHEMON and GFM. Inflammatory and Immune Disorders Associated with Myelodysplastic Syndromes. Hemato. 2021; 2(2):329-346. https://doi.org/10.3390/hemato2020019
Chicago/Turabian StyleJachiet, Vincent, Pierre Fenaux, Anna Sevoyan, Yervand Hakobyan, Lionel Ades, Olivier Fain, Arsène Mekinian, and on behalf of the MINHEMON and GFM. 2021. "Inflammatory and Immune Disorders Associated with Myelodysplastic Syndromes" Hemato 2, no. 2: 329-346. https://doi.org/10.3390/hemato2020019
APA StyleJachiet, V., Fenaux, P., Sevoyan, A., Hakobyan, Y., Ades, L., Fain, O., Mekinian, A., & on behalf of the MINHEMON and GFM. (2021). Inflammatory and Immune Disorders Associated with Myelodysplastic Syndromes. Hemato, 2(2), 329-346. https://doi.org/10.3390/hemato2020019





