BTK Inhibitors and Other Targeted Therapies in Waldenström Macroglobulinemia
Abstract
:1. Introduction
2. Genomic Landscape of WM
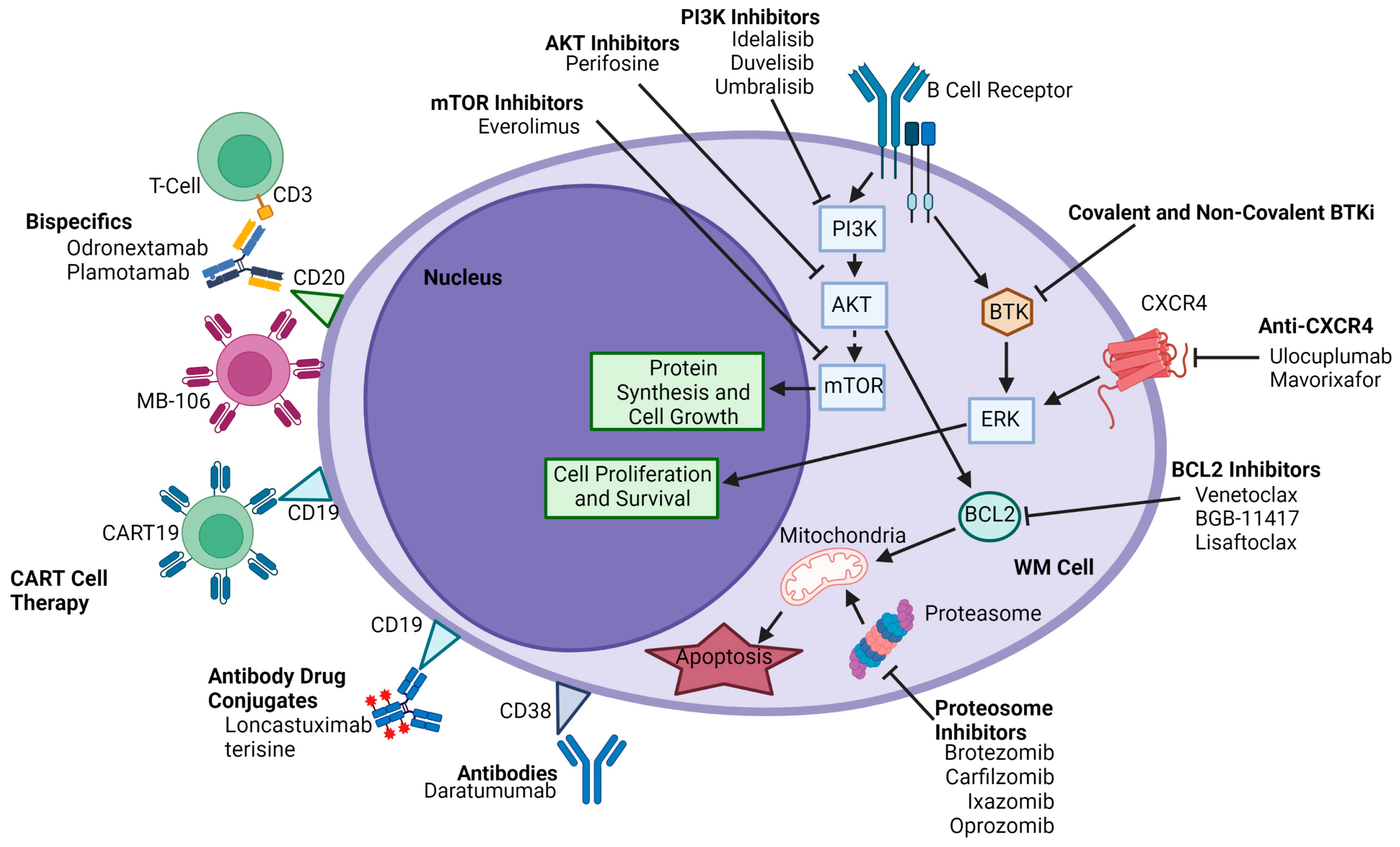
3. Burton Tyrosine Kinase Inhibitors
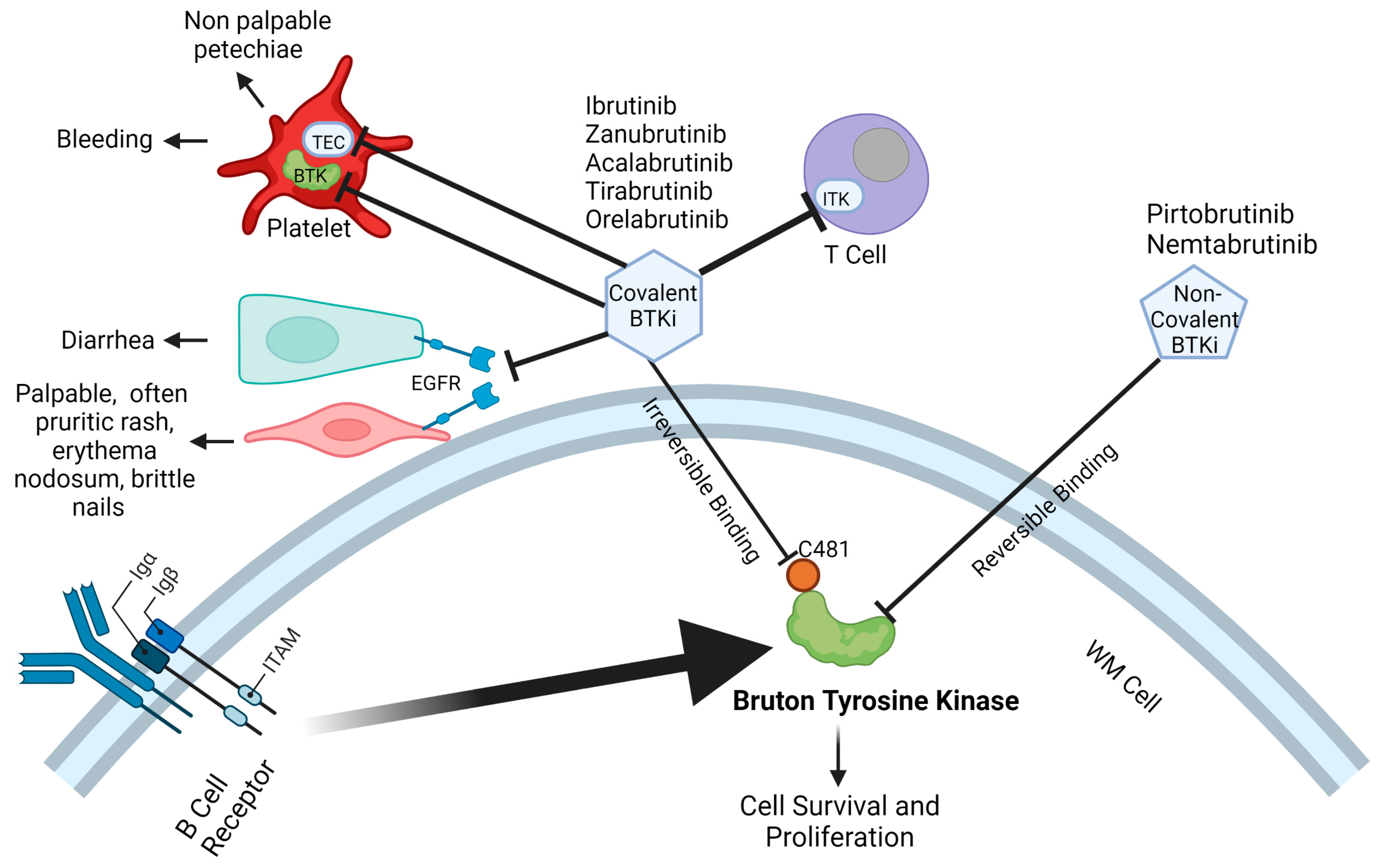
3.1. Covalent Bruton Tyrosine Kinase Inhibitors
3.1.1. Ibrutinib
3.1.2. Zanubrutinib
3.1.3. Acalabrutinib
3.2. Other Emerging Covalent Bruton Tyrosine Kinase Inhibitors
3.3. Non-Covalent Bruton Tyrosine Kinase Inhibitors
3.3.1. Pirtobrutinib
3.3.2. Nemtabrutinib
3.4. Further Considerations with BTKi
4. BCL-2 Inhibitors
5. Proteasome Inhibitors
5.1. Bortezomib
5.2. Carfilzomib
5.3. Ixazomib
5.4. Oprozomib
6. Phosphatidylinositol 3-Kinase Inhibitors
7. CAR-T CD19/CD20
8. Bispecific Agents
9. CLOVER-WaM-Lopfosine
10. Anti-CXCR4 Agents
11. Other Targeted Therapies
12. Challenges of Drug Development and Failed Trials
13. Our Approach in the Era of Targeted Therapies for WM
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gertz, M.A. Waldenstrom macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2021, 96, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Grimont, C.N.; Castillo Almeida, N.E.; Gertz, M.A. Current and Emerging Treatments for Waldenstrom Macroglobulinemia. Acta Haematol. 2021, 144, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Hunter, Z.R.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Manning, R.J.; Tripsas, C.; Patterson, C.J.; Sheehy, P.; et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014, 123, 1637–1646. [Google Scholar] [CrossRef] [Green Version]
- Hunter, Z.R.; Yang, G.; Xu, L.; Liu, X.; Castillo, J.J.; Treon, S.P. Genomics, Signaling, and Treatment of Waldenstrom Macroglobulinemia. J. Clin. Oncol. 2017, 35, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Xu, L.; Hunter, Z. MYD88 Mutations and Response to Ibrutinib in Waldenstrom’s Macroglobulinemia. N. Engl. J. Med. 2015, 373, 584–586. [Google Scholar] [CrossRef]
- Guerrera, M.L.; Tsakmaklis, N.; Xu, L.; Yang, G.; Demos, M.; Kofides, A.; Chan, G.G.; Manning, R.J.; Liu, X.; Chen, J.G.; et al. MYD88 mutated and wild-type Waldenstrom’s Macroglobulinemia: Characterization of chromosome 6q gene losses and their mutual exclusivity with mutations in CXCR4. Haematologica 2018, 103, e408–e411. [Google Scholar] [CrossRef] [Green Version]
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Warren, D.; Varma, G.; Green, R.; Argyropoulos, K.V.; Yang, G.; Cao, Y.; Xu, L.; et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N. Engl. J. Med. 2015, 372, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Harhaj, E.W.; Dixit, V.M. Regulation of NF-kappaB by deubiquitinases. Immunol. Rev. 2012, 246, 107–124. [Google Scholar] [CrossRef] [Green Version]
- García-Sanz, R.; Dogliotti, I.; Zaccaria, G.M.; Ocio, E.M.; Rubio, A.; Murillo, I.; Escalante, F.; Aguilera, C.; García-Mateo, A.; García de Coca, A.; et al. 6q deletion in Waldenstrom macroglobulinaemia negatively affects time to transformation and survival. Br. J. Haematol. 2021, 192, 843–852. [Google Scholar] [CrossRef]
- Ocio, E.M.; Schop, R.F.J.; Gonzalez, B.; Van Wier, S.A.; Hernandez-Rivas, J.M.; Gutierrez, N.C.; Garcia-Sanz, R.; Moro, M.J.; Aguilera, C.; Hernandez, J.; et al. 6q deletion in Waldenstrom macroglobulinemia is associated with features of adverse prognosis. Br. J. Haematol. 2007, 136, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Jurczak, W.; Salem, J.-E.; Dimopoulos, M.A. Managing Waldenström’s macroglobulinemia with BTK inhibitors. Leukemia 2022, 37, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Arcaini, L.; Zibellini, S.; Boveri, E.; Rattotti, S.; Riboni, R.; Corso, A.; Orlandi, E.; Bonfichi, M.; Gotti, M.; et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenstrom’s macroglobulinemia and related lymphoid neoplasms. Blood 2013, 121, 2522–2528. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, C.; Chan, G.G.; Xu, L.; Tsakmaklis, N.; Kofides, A.; Demos, M.G.; Chen, J.; Liu, X.; Munshi, M.; Yang, G.; et al. Genomic evolution of ibrutinib-resistant clones in Waldenstrom macroglobulinaemia. Br. J. Haematol. 2020, 189, 1165–1170. [Google Scholar] [CrossRef]
- Chen, J.G.; Liu, X.; Munshi, M.; Xu, L.; Tsakmaklis, N.; Demos, M.G.; Kofides, A.; Guerrera, M.L.; Chan, G.G.; Patterson, C.J.; et al. BTK(Cys481Ser) drives ibrutinib resistance via ERK1/2 and protects BTK(wild-type) MYD88-mutated cells by a paracrine mechanism. Blood 2018, 131, 2047–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Tsakmaklis, N.; Yang, G.; Chen, J.G.; Liu, X.; Demos, M.; Kofides, A.; Patterson, C.J.; Meid, K.; Gustine, J.; et al. Acquired mutations associated with ibrutinib resistance in Waldenstrom macroglobulinemia. Blood 2017, 129, 2519–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsky, A.; Lamanna, N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 336–345. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Trotman, J.; Tedeschi, A.; Matous, J.V.; Macdonald, D.; Tam, C.; Tournilhac, O.; Ma, S.; Oriol, A.; Heffner, L.T.; et al. Ibrutinib for patients with rituximab-refractory Waldenstrom’s macroglobulinaemia (iNNOVATE): An open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017, 18, 241–250. [Google Scholar] [CrossRef]
- Lewis, K.L.; Cheah, C.Y. Non-Covalent BTK Inhibitors—The New BTKids on the Block for B-Cell Malignancies. J. Pers. Med. 2021, 11, 764. [Google Scholar] [CrossRef]
- Advani, R.H.; Buggy, J.J.; Sharman, J.P.; Smith, S.M.; Boyd, T.E.; Grant, B.; Kolibaba, K.S.; Furman, R.R.; Rodriguez, S.; Chang, B.Y.; et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013, 31, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Treon, S.P.; Meid, K.; Gustine, J.; Yang, G.; Xu, L.; Liu, X.; Patterson, C.J.; Hunter, Z.R.; Branagan, A.R.; Laubach, J.P.; et al. Long-Term Follow-Up of Ibrutinib Monotherapy in Symptomatic, Previously Treated Patients with Waldenstrom Macroglobulinemia. J. Clin. Oncol. 2021, 39, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Gustine, J.; Meid, K.; Yang, G.; Xu, L.; Liu, X.; Demos, M.; Kofides, A.; Tsakmaklis, N.; Chen, J.G.; et al. Ibrutinib Monotherapy in Symptomatic, Treatment-Naive Patients with Waldenstrom Macroglobulinemia. J. Clin. Oncol. 2018, 36, 2755–2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Tedeschi, A.; Trotman, J.; Garcia-Sanz, R.; Macdonald, D.; Leblond, V.; Mahe, B.; Herbaux, C.; Tam, C.; Orsucci, L.; et al. Phase 3 Trial of Ibrutinib plus Rituximab in Waldenstrom’s Macroglobulinemia. N. Engl. J. Med. 2018, 378, 2399–2410. [Google Scholar] [CrossRef]
- Buske, C.; Tedeschi, A.; Trotman, J.; Garcia-Sanz, R.; MacDonald, D.; Leblond, V.; Mahe, B.; Herbaux, C.; Matous, J.V.; Tam, C.S.; et al. Ibrutinib Plus Rituximab Versus Placebo Plus Rituximab for Waldenstrom’s Macroglobulinemia: Final Analysis from the Randomized Phase III iNNOVATE Study. J. Clin. Oncol. 2022, 40, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Vincelette, N.D.; Acharya, U.; Abraham, I. Risk of Atrial Fibrillation and Bleeding Diathesis Associated with Ibrutinib Treatment: A Systematic Review and Pooled Analysis of Four Randomized Controlled Trials. Clin. Lymphoma Myeloma Leuk. 2017, 17, 31–37.e13. [Google Scholar] [CrossRef]
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Sanz, R.G.; et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Sanz, R.G.; Lee, H.P.; Trneny, M.; Varettoni, M.; Opat, S.; D’Sa, S.; Owen, R.G.; Cull, G.; Mulligan, S.; et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenstrom macroglobulinemia: A substudy of the phase 3 ASPEN trial. Blood Adv. 2020, 4, 6009–6018. [Google Scholar] [CrossRef]
- Owen, R.G.; McCarthy, H.; Rule, S.; D’Sa, S.; Thomas, S.K.; Tournilhac, O.; Forconi, F.; Kersten, M.J.; Zinzani, P.L.; Iyengar, S.; et al. Acalabrutinib monotherapy in patients with Waldenstrom macroglobulinemia: A single-arm, multicentre, phase 2 study. Lancet Haematol. 2020, 7, e112–e121. [Google Scholar] [CrossRef]
- Sekiguchi, N.; Rai, S.; Munakata, W.; Suzuki, K.; Handa, H.; Shibayama, H.; Endo, T.; Terui, Y.; Iwaki, N.; Fukuhara, N.; et al. A multicenter, open-label, phase II study of tirabrutinib (ONO/GS-4059) in patients with Waldenstrom’s macroglobulinemia. Cancer Sci. 2020, 111, 3327–3337. [Google Scholar] [CrossRef]
- Palomba, M.L.; Patel, M.R.; Eyre, T.A.; Jurczak, W.; Lewis, D.J.; Gastinne, T.; Ma, S.; Cohen, J.B.; Patel, K.; Brown, J.R.; et al. Efficacy of Pirtobrutinib, a Highly Selective, Non-Covalent (Reversible) BTK Inhibitor in Relapsed / Refractory Waldenström Macroglobulinemia: Results from the Phase 1/2 BRUIN Study. Blood 2022, 140, 557–560. [Google Scholar] [CrossRef]
- Cao, X.X.; Jin, J.; Fu, C.C.; Yi, S.H.; Zhao, W.L.; Sun, Z.M.; Yang, W.; Li, D.J.; Cui, G.H.; Hu, J.D.; et al. Evaluation of orelabrutinib monotherapy in patients with relapsed or refractory Waldenstrom’s macroglobulinemia in a single-arm, multicenter, open-label, phase 2 study. EClinicalMedicine 2022, 52, 101682. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Allan, J.N.; Siddiqi, T.; Advani, R.H.; Meid, K.; Leventoff, C.; White, T.P.; Flynn, C.A.; Sarosiek, S.; Branagan, A.R.; et al. Venetoclax in Previously Treated Waldenstrom Macroglobulinemia. J. Clin. Oncol. 2022, 40, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Anagnostopoulos, A.; Kyrtsonis, M.C.; Castritis, E.; Bitsaktsis, A.; Pangalis, G.A. Treatment of relapsed or refractory Waldenstrom’s macroglobulinemia with bortezomib. Haematologica 2005, 90, 1655–1658. [Google Scholar]
- Chen, C.I.; Kouroukis, C.T.; White, D.; Voralia, M.; Stadtmauer, E.; Stewart, A.K.; Wright, J.J.; Powers, J.; Walsh, W.; Eisenhauer, E.; et al. Bortezomib is active in patients with untreated or relapsed Waldenstrom’s macroglobulinemia: A phase II study of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Kanan, S.; Sheehy, P.; Chuma, S.; Xu, L.; Cao, Y.; Yang, G.; Liu, X.; et al. Carfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenstrom’s macroglobulinemia. Blood 2014, 124, 503–510. [Google Scholar] [CrossRef]
- Castillo, J.J.; Meid, K.; Flynn, C.A.; Chen, J.; Demos, M.G.; Guerrera, M.L.; Kofides, A.; Liu, X.; Munshi, M.; Tsakmaklis, N.; et al. Ixazomib, dexamethasone, and rituximab in treatment-naive patients with Waldenstrom macroglobulinemia: Long-term follow-up. Blood Adv. 2020, 4, 3952–3959. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Vij, R.; Siegel, D.; Badros, A.; Kaufman, J.; Raje, N.; Jakubowiak, A.; Savona, M.R.; Obreja, M.; Berdeja, J.G. A Phase Ib/II Study of Oprozomib in Patients with Advanced Multiple Myeloma and Waldenström Macroglobulinemia. Clin. Cancer Res. 2019, 25, 4907–4916. [Google Scholar] [CrossRef]
- Tomowiak, C.; Poulain, S.; Herbaux, C.; Perrot, A.; Mahe, B.; Morel, P.; Aurran, T.; Tournilhac, O.; Lepretre, S.; Assaad, S.; et al. Obinutuzumab and idelalisib in symptomatic patients with relapsed/refractory Waldenstrom macroglobulinemia. Blood Adv. 2021, 5, 2438–2446. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Tsui, S.T.; Liu, D. Second-generation inhibitors of Bruton tyrosine kinase. J. Hematol. Oncol. 2016, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Garcia-Sanz, R.; et al. P1161: Aspen: Long-term follow-up results of a phase 3 randomized trial of zanubrutinib (ZANU) vs ibrutinib (IBR) in patients (PTS) with waldenström macroglobulinemia (WM). HemaSphere 2022, 6, 1048–1049. [Google Scholar] [CrossRef]
- Tam, C.S.L.; Garcia-Sanz, R.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.-P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; et al. ASPEN: Long-term follow-up results of a phase 3 randomized trial of zanubrutinib (ZANU) versus ibrutinib (IBR) in patients with Waldenström macroglobulinemia (WM). J. Clin. Oncol. 2022, 40, 7521. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illes, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Aslan, B.; Kismali, G.; Iles, L.R.; Manyam, G.C.; Ayres, M.L.; Chen, L.S.; Gagea, M.; Bertilaccio, M.T.S.; Wierda, W.G.; Gandhi, V. Pirtobrutinib inhibits wild-type and mutant Bruton’s tyrosine kinase-mediated signaling in chronic lymphocytic leukemia. Blood Cancer J. 2022, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Woyach, J.A.; Flinn, I.W.; Awan, F.T.; Eradat, H.; Brander, D.; Tees, M.; Parikh, S.A.; Phillips, T.J.; Ghori, R.; Reddy, N.M.; et al. Efficacy and Safety of Nemtabrutinib, a Wild-Type and C481S-Mutated Bruton Tyrosine Kinase Inhibitor for B-Cell Malignancies: Updated Analysis of the Open-Label Phase 1/2 Dose-Expansion Bellwave-001 Study. Blood 2022, 140, 7004–7006. [Google Scholar] [CrossRef]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Fotiou, D.; Dimopoulos, M.A. Current and novel BTK inhibitors in Waldenstrom’s macroglobulinemia. Ther. Adv. Hematol. 2021, 12, 2040620721989586. [Google Scholar] [CrossRef]
- Gustine, J.N.; Meid, K.; Dubeau, T.; Severns, P.; Hunter, Z.R.; Guang, Y.; Xu, L.; Treon, S.P.; Castillo, J.J. Ibrutinib discontinuation in Waldenstrom macroglobulinemia: Etiologies, outcomes, and IgM rebound. Am. J. Hematol. 2018, 93, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Shadman, M.; Flinn, I.W.; Levy, M.Y.; Porter, R.; Burke, J.M.; Cultrera, J.L.; Misleh, J.; Zafar, S.F.; Freeman, B.; Rao, S.S.; et al. Phase 2 Study of Zanubrutinib in BTK Inhibitor-Intolerant Patients (Pts) with Relapsed/Refractory B-Cell Malignancies. Blood 2021, 138, 1410. [Google Scholar] [CrossRef]
- Shadman, M.; Flinn, I.W.; Kingsley, E.C.; Freeman, B.; Levy, M.Y.; Holmes, H.; Farber, C.M.; Chaudhry, A.; Crescenzo, R.; Idoine, A.; et al. Zanubrutinib in Acalabrutinib-Intolerant Patients (Pts) with B-Cell Malignancies. Blood 2022, 140, 3655–3657. [Google Scholar] [CrossRef]
- Sarosiek, S.; Gustine, J.N.; Flynn, C.A.; Leventoff, C.; Little, M.; White, T.; Meid, K.; Treon, S.P.; Castillo, J.J. Dose reductions in patients with Waldenström macroglobulinaemia treated with ibrutinib. Br. J. Haematol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Chng, W.J.; Schop, R.F.; Price-Troska, T.; Ghobrial, I.; Kay, N.; Jelinek, D.F.; Gertz, M.A.; Dispenzieri, A.; Lacy, M.; Kyle, R.A.; et al. Gene-expression profiling of Waldenstrom macroglobulinemia reveals a phenotype more similar to chronic lymphocytic leukemia than multiple myeloma. Blood 2006, 108, 2755–2763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, M.A.; Geyer, S.M.; Badros, A.; Kahl, B.S.; Erlichman, C. Early results of a phase I trial of oblimersen sodium for relapsed or refractory Waldenstrom’s macroglobulinemia. Clin. Lymphoma 2005, 5, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, G.; Hunter, Z.R.; Liu, X.; Xu, L.; Chen, J.; Tsakmaklis, N.; Hatjiharissi, E.; Kanan, S.; Davids, M.S.; et al. The BCL2 antagonist ABT-199 triggers apoptosis, and augments ibrutinib and idelalisib mediated cytotoxicity in CXCR4 Wild-type and CXCR4 WHIM mutated Waldenstrom macroglobulinaemia cells. Br. J. Haematol. 2015, 170, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I First-in-Human Study of Venetoclax in Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, J.J.; Sarosiek, S.; Branagan, A.R.; Sermer, D.J.; Flynn, C.A.; Leventoff, C.; Little, M.; White, T.P.; Meid, K.; Canning, A.; et al. Ibrutinib and Venetoclax in Previously Untreated Waldenström Macroglobulinemia. Blood 2022, 140, 564–565. [Google Scholar] [CrossRef]
- Hu, N.; Guo, Y.; Xue, H.; Liu, Y.; Guo, Y.; Wang, F.; Song, X.; Guo, Y.; Chen, S.; Xu, H.; et al. Abstract 3077: Preclinical characterization of BGB-11417, a potent and selective Bcl-2 inhibitor with superior antitumor activities in haematological tumor models. Cancer Res. 2020, 80, 3077. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Lasica, M.; Opat, S.; Cheah, C.Y.; Chan, H.; Verner, E.; González Barca, E.; Tedeschi, A.; Hilger, J.; Fang, Y.; et al. A Phase 1 Study with the Novel B-Cell Lymphoma 2 (Bcl-2) Inhibitor Bgb-11417 As Monotherapy or in Combination with Zanubrutinib (ZANU) in Patients (Pts) with Non-Hodgkin Lymphoma (NHL) or Waldenström Macroglobulinemia (WM): Preliminary Data. Blood 2022, 140, 9325–9327. [Google Scholar] [CrossRef]
- Deng, J.; Paulus, A.; Fang, D.D.; Manna, A.; Wang, G.; Wang, H.; Zhu, S.; Chen, J.; Min, P.; Yin, Y.; et al. Lisaftoclax (APG-2575) Is a Novel BCL-2 Inhibitor with Robust Antitumor Activity in Preclinical Models of Hematologic Malignancy. Clin. Cancer Res. 2022, 28, 5455–5468. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Chanan-Khan, A.A.A.; Chen, Z.; Huang, B.; Konopleva, M.; Brander, D.M.; Rizzieri, D.; Lasica, M.; Tam, C.S.L.; Yannakou, C.K.; et al. First-in-human study of lisaftoclax (APG-2575), a novel BCL-2 inhibitor (BCL-2i), in patients (pts) with relapsed/refractory (R/R) CLL and other hematologic malignancies (HMs). J. Clin. Oncol. 2021, 39, 7502. [Google Scholar] [CrossRef]
- Alencar, A.J.; Roeker, L.E.; Hoffmann, M.; Guru Murthy, G.S.; Patel, V.; Ku, N.C.; Pauff, J.M.; Eyre, T.A.; Jurczak, W.; Le Gouill, S. A First-in-Human Phase 1 Study of Oral LOXO-338, a Selective BCL2 Inhibitor, in Patients with Advanced Hematologic Malignancies (Trial in Progress). Blood 2021, 138, 2424. [Google Scholar] [CrossRef]
- Hungria, V.T.M.; Crusoe, E.Q.; Bittencourt, R.I.; Maiolino, A.; Magalhaes, R.J.P.; Sobrinho, J.D.N.; Pinto, J.V.; Fortes, R.C.; Moreira, E.S.; Tanaka, P.Y. New proteasome inhibitors in the treatment of multiple myeloma. Hematol. Transfus. Cell. Ther. 2019, 41, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treon, S.P.; Ioakimidis, L.; Soumerai, J.D.; Patterson, C.J.; Sheehy, P.; Nelson, M.; Willen, M.; Matous, J.; Mattern, J., 2nd; Diener, J.G.; et al. Primary therapy of Waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J. Clin. Oncol. 2009, 27, 3830–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Garcia-Sanz, R.; Gavriatopoulou, M.; Morel, P.; Kyrtsonis, M.C.; Michalis, E.; Kartasis, Z.; Leleu, X.; Palladini, G.; Tedeschi, A.; et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): Long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood 2013, 122, 3276–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leblebjian, H.; Noonan, K.; Paba-Prada, C.; Treon, S.P.; Castillo, J.J.; Ghobrial, I.M. Cyclophosphamide, bortezomib, and dexamethasone combination in waldenstrom macroglobulinemia. Am. J. Hematol. 2015, 90, E122–E123. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Dimopoulos, M.A.; Grunenberg, A.; Kastritis, E.; Tomowiak, C.; Mahé, B.; Troussard, X.; Hajek, R.; Viardot, A.; Tournilhac, O.; et al. Bortezomib in Combination with Dexamethasone, Rituximab and Cyclophosphamide (B-DRC) As First-Line Treatment of Waldenstrom’s Macroglobulinemia: Results of a Prospectively Randomized Multicenter European Phase II Trial. Blood 2020, 136, 26. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Chen, Q.; Voorhees, P.M.; Strader, J.S.; Shenk, K.D.; Sun, C.M.; Demo, S.D.; Bennett, M.K.; van Leeuwen, F.W.; Chanan-Khan, A.A.; et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 2007, 110, 3281–3290. [Google Scholar] [CrossRef] [Green Version]
- Demo, S.D.; Kirk, C.J.; Aujay, M.A.; Buchholz, T.J.; Dajee, M.; Ho, M.N.; Jiang, J.; Laidig, G.J.; Lewis, E.R.; Parlati, F.; et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007, 67, 6383–6391. [Google Scholar] [CrossRef] [Green Version]
- Groen, K.; van de Donk, N.; Stege, C.; Zweegman, S.; Nijhof, I.S. Carfilzomib for relapsed and refractory multiple myeloma. Cancer Manag. Res. 2019, 11, 2663–2675. [Google Scholar] [CrossRef] [Green Version]
- Vesole, D.H.; Richter, J.; Biran, N.; McBride, L.; Anand, P.; Huang, M.; Kumeli, A.Z.; Klippel, Z.; Iskander, K.; Siegel, D.S. Carfilzomib as salvage therapy in Waldenstrom macroglobulinemia: A case series. Leuk. Lymphoma 2018, 59, 259–261. [Google Scholar] [CrossRef]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Gertz, M.A.; Laplant, B.; Malave, G.C.; Wolfe, E.; Muchtar, E.; Siddiqui, M.A.; Gonsalves, W.I.; Emanuel, A.R.; Kourelis, T.; et al. Phase 2 Trial of Daratumumab, Ixazomib, Lenalidomide and Modified Dose Dexamethasone in Patients with Newly Diagnosed Multiple Myeloma. Blood 2019, 134, 864. [Google Scholar] [CrossRef]
- Castillo, J.J.; Meid, K.; Gustine, J.N.; Dubeau, T.; Severns, P.; Hunter, Z.R.; Yang, G.; Xu, L.; Treon, S.P. Prospective Clinical Trial of Ixazomib, Dexamethasone, and Rituximab as Primary Therapy in Waldenstrom Macroglobulinemia. Clin. Cancer Res. 2018, 24, 3247–3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersten, M.J.; Amaador, K.; Minnema, M.C.; Vos, J.M.I.; Nasserinejad, K.; Kap, M.; Kastritis, E.; Gavriatopoulou, M.; Kraan, W.; Chamuleau, M.E.D.; et al. Combining Ixazomib with Subcutaneous Rituximab and Dexamethasone in Relapsed or Refractory Waldenström’s Macroglobulinemia: Final Analysis of the Phase I/II HOVON124/ECWM-R2 Study. J. Clin. Oncol. 2021, 40, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Parrondo, R.D.; Laplant, B.; Alegria, V.R.; Elliott, J.B.; Zimmerman, A.; Heslop, K.; Chapin, D.; Sher, T.; Roy, V.; et al. Phase II Study of Ibrutinib in Combination with Ixazomib in Patients with Waldenström Macroglobulinemia (WM). Blood 2022, 140, 9331–9332. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Sacco, A.; Husu, E.N.; Pitsillides, C.; Vesole, S.; Azab, A.K.; Azab, F.; Melhem, M.; Ngo, H.T.; Quang, P.; et al. Dual targeting of the PI3K/Akt/mTOR pathway as an antitumor strategy in Waldenstrom macroglobulinemia. Blood 2010, 115, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Liu, X.; Zhou, Y.; Xu, L.; Cao, Y.; Manning, R.; Patterson, C.; Tripsas, C.K.; Hunter, Z.; Buhrlage, S.; et al. PI3K/AKT Pathway Is Activated by MYD88 L265P and Use Of PI3K-Delta Inhibitors Induces Robust Tumor Cell Killing in Waldenstrom’s Macroglobulinemia. Blood 2013, 122, 4255. [Google Scholar] [CrossRef]
- Pene, F.; Claessens, Y.E.; Muller, O.; Viguie, F.; Mayeux, P.; Dreyfus, F.; Lacombe, C.; Bouscary, D. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene 2002, 21, 6587–6597. [Google Scholar] [CrossRef] [Green Version]
- Younes, H.; Leleu, X.; Hatjiharissi, E.; Moreau, A.-S.; Hideshima, T.; Richardson, P.; Anderson, K.C.; Ghobrial, I.M. Targeting the Phosphatidylinositol 3-Kinase Pathway in Multiple Myeloma. Clin. Cancer Res. 2007, 13, 3771–3775. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.J.; Gustine, J.N.; Meid, K.; Dubeau, T.; Yang, G.; Xu, L.; Hunter, Z.R.; Treon, S.P. Idelalisib in Waldenström macroglobulinemia: High incidence of hepatotoxicity. Leuk. Lymphoma 2017, 58, 1002–1004. [Google Scholar] [CrossRef]
- Flinn, I.W.; O’Brien, S.; Kahl, B.; Patel, M.; Oki, Y.; Foss, F.F.; Porcu, P.; Jones, J.; Burger, J.A.; Jain, N.; et al. Duvelisib, a novel oral dual inhibitor of PI3K-delta,gamma, is clinically active in advanced hematologic malignancies. Blood 2018, 131, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: The phase 2 ELARA trial. Nat. Med. 2022, 28, 325–332. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jager, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Vidriales, M.B.; Ocio, E.; Mateo, G.; Sanchez-Guijo, F.; Sanchez, M.L.; Escribano, L.; Barez, A.; Moro, M.J.; Hernandez, J.; et al. Immunophenotypic analysis of Waldenstrom’s macroglobulinemia. Semin. Oncol. 2003, 30, 187–195. [Google Scholar] [CrossRef]
- Palomba, M.L.; Qualls, D.; Monette, S.; Sethi, S.; Dogan, A.; Roshal, M.; Senechal, B.; Wang, X.; Riviere, I.; Sadelain, M.; et al. CD19-directed chimeric antigen receptor T cell therapy in Waldenstrom macroglobulinemia: A preclinical model and initial clinical experience. J. Immunother. Cancer 2022, 10, e004128. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.; Redman, M.W.; Lee, S.Y.; Lee, D.H.; Ramachandran, A.; Ra, S.; Marzbani, E.A.; Graf, S.A.; Warren, E.H.; et al. Third Generation CD20 Targeted CAR T-Cell Therapy (MB-106) for Treatment of Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Blood 2020, 136, 38–39. [Google Scholar] [CrossRef]
- Salvaris, R.; Ong, J.; Gregory, G.P. Bispecific Antibodies: A Review of Development, Clinical Efficacy and Toxicity in B-Cell Lymphomas. J. Pers. Med. 2021, 11, 355. [Google Scholar] [CrossRef]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martinez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef]
- Bannerji, R.; Arnason, J.E.; Advani, R.H.; Brown, J.R.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; O’Brien, S.M.; Chavez, J.C.; Duell, J.; et al. Odronextamab, a human CD20xCD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): Results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022, 9, e327–e339. [Google Scholar] [CrossRef]
- Parrondo, R.D.; Paulus, A.; Alegria, V.; Liebowitz, D.; Johnson, C.; Clynes, R.; Roy, V.; Menke, D.M.; Jiang, L.; Chanan-Khan, A.A.; et al. Plamotamab (XmAb((R))13676) for Ibrutinib-refractory CXCR4-mutated extramedullary Waldenstrom macroglobulinemia. Leuk. Lymphoma 2022, 63, 738–742. [Google Scholar] [CrossRef]
- Longcor, J.; Callander, N.; Oliver, K.; Chanan-Khan, A.; Ailawadhi, S. Iopofosine I-131 treatment in late-line patients with relapsed/refractory multiple myeloma post anti-BCMA immunotherapy. Blood Cancer J. 2022, 12, 130. [Google Scholar] [CrossRef]
- Lubner, S.J.; Mullvain, J.; Perlman, S.; Pishvaian, M.; Mortimer, J.; Oliver, K.; Heideman, J.; Hall, L.; Weichert, J.; Liu, G. A Phase 1, Multi-Center, Open-Label, Dose-Escalation Study of 131I-CLR1404 in Subjects with Relapsed or Refractory Advanced Solid Malignancies. Cancer Investig. 2015, 33, 483–489. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Stiff, P.; Ibrahim, E.; Green, D.J.; Lipe, B.; Cull, E.H.; Callander, N.S.; Friend, J.; Longcor, J.; Oliver, K. CLR 131 (Iopofosine I-131) Treatment in Triple Class Refractory and Beyond Multiple Myeloma Patients: Preliminary Efficacy and Safety Results from the Phase 2 Clover-1 Trial. Blood 2021, 138, 1652. [Google Scholar] [CrossRef]
- Treon, S.P.; Meid, K.; Hunter, Z.R.; Flynn, C.A.; Sarosiek, S.R.; Leventoff, C.R.; White, T.P.; Cao, Y.; Roccaro, A.M.; Sacco, A.; et al. Phase 1 study of ibrutinib and the CXCR4 antagonist ulocuplumab in CXCR4-mutated Waldenström macroglobulinemia. Blood 2021, 138, 1535–1539. [Google Scholar] [CrossRef]
- Treon, S.P.; Buske, C.; Thomas, S.K.; Castillo, J.J.; Branagan, A.R.; Dimopoulos, M.A.; Gavriatopoulou, M.; Cadavid, D.; Garzon, F.T.; Tang, W.; et al. Preliminary Clinical Response Data from a Phase 1b Study of Mavorixafor in Combination with Ibrutinib in Patients with Waldenström’s Macroglobulinemia with MYD88 and CXCR4 Mutations. Blood 2021, 138, 1362. [Google Scholar] [CrossRef]
- Paulus, A.; Manna, A.; Akhtar, S.; Paulus, S.M.; Sharma, M.; Coignet, M.V.; Jiang, L.; Roy, V.; Witzig, T.E.; Ansell, S.M.; et al. Targeting CD38 with daratumumab is lethal to Waldenstrom macroglobulinaemia cells. Br. J. Haematol. 2018, 183, 196–211. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.J.; Libby, E.N.; Ansell, S.M.; Palomba, M.L.; Meid, K.; Flynn, C.A.; Leventoff, C.; Hergott, C.B.; Sewastianik, T.; Morgan, E.A.; et al. Multicenter phase 2 study of daratumumab monotherapy in patients with previously treated Waldenstrom macroglobulinemia. Blood Adv. 2020, 4, 5089–5092. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Gertz, M.; Laplant, B.; Camoriano, J.; Hayman, S.; Lacy, M.; Chuma, S.; Harris, B.; Leduc, R.; Rourke, M.; et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenstrom macroglobulinemia. J. Clin. Oncol. 2010, 28, 1408–1414. [Google Scholar] [CrossRef] [Green Version]
- Treon, S.P.; Meid, K.; Tripsas, C.; Heffner, L.T.; Eradat, H.; Badros, A.Z.; Xu, L.; Hunter, Z.R.; Yang, G.; Patterson, C.J.; et al. Prospective, Multicenter Clinical Trial of Everolimus as Primary Therapy in Waldenstrom Macroglobulinemia (WMCTG 09-214). Clin. Cancer Res. 2017, 23, 2400–2404. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Siegel, D.; Gutierrez, M.; Jacoby, M.; Hofmeister, C.C.; Gabrail, N.; Baz, R.; Mau-Sorensen, M.; Berdeja, J.G.; Savona, M.; et al. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood 2018, 131, 855–863. [Google Scholar] [CrossRef]
- Hamadani, M.; Radford, J.; Carlo-Stella, C.; Caimi, P.F.; Reid, E.; O’Connor, O.A.; Feingold, J.M.; Ardeshna, K.M.; Townsend, W.; Solh, M.; et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 2021, 137, 2634–2645. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Roccaro, A.; Hong, F.; Weller, E.; Rubin, N.; Leduc, R.; Rourke, M.; Chuma, S.; Sacco, A.; Jia, X.; et al. Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom’s macroglobulinemia. Clin. Cancer Res. 2010, 16, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Bishton, M.; Spencer, A.; Dickinson, M.; Ritchie, D. A single-arm, phase II study of the anti-Blys monoclonal antibody belimumab in symptomatic Waldenstrom macroglobulinemia. Clin. Lymphoma Myeloma Leuk. 2013, 13, 575–578. [Google Scholar] [CrossRef]
- Kothari, J.; Eyre, T.A.; Rismani, A.; McCarthy, H.; Collins, A.; Lewis, D.J.; Arulogun, S.O.; Wilson, W.; Clifton-Hadley, L.; Edwards, D.; et al. Pembrowm: Results of a Multi-Centre Phase II Trial Investigating the Safety and Efficacy of Rituximab and Pembrolizumab in Relapsed/Refractory Waldenström’s Macroglobulinaemia. Blood 2022, 140, 3624–3626. [Google Scholar] [CrossRef]
- Bourhill, T.; Narendran, A.; Johnston, R.N. Enzastaurin: A lesson in drug development. Crit. Rev. Oncol. Hematol. 2017, 112, 72–79. [Google Scholar] [CrossRef]
- Thomas, S.K.; Harb, W.A.; Beck, J.T.; Nashat, G.; Palomba, M.L.; Ansell, S.M.; Eradat, H.; Libby, E.N., III; Advani, R.H.; Hajdenberg, J.; et al. Preliminary Results from a Phase 1/2, Open-Label, Dose-Escalation Clinical Trial of IMO-8400 in Patients with Relapsed or Refractory Waldenstrom’s Macroglobulinemia. Blood 2015, 126, 1540. [Google Scholar] [CrossRef]
- Treon, S.P.; Soumerai, J.D.; Hunter, Z.R.; Patterson, C.J.; Ioakimidis, L.; Kahl, B.; Boxer, M. Long-term follow-up of symptomatic patients with lymphoplasmacytic lymphoma/Waldenström macroglobulinemia treated with the anti-CD52 monoclonal antibody alemtuzumab. Blood 2011, 118, 276–281. [Google Scholar] [CrossRef] [Green Version]
| Class | Agent (Ref) | No of WM Pts | Response | Overall Survival | Progression-Free Survival | Notable Adverse Events * | Comments |
|---|---|---|---|---|---|---|---|
| BTK Inhibitors | Ibrutinib [7] | 63 R/R | ORR: 91% MRR: 73% | 2-year OS: 95% | 2-year PFS: 69% | Atrial fibrillation: 5% (2%) Bleeding: 2% (0) Neutropenia: 22% (15%) Thrombocytopenia: 14% (13%) | Response rate higher in MYD88L265PCXCR4WT vs. MYD88WTCXCR4WT or MYD88WT CXCR4WHIM |
| Ibrutinib [22] | 30 TN MYD88MUT | ORR: 100% MRR: 83% | 18-mo OS: 100% | 18-mo PFS: 92% | Atrial fibrillation 7% (0) Hypertension: 13% (7%) Bruising: 7% (0) Neutropenia: 7% (0) | No patients with MYD88WT status were enrolled. | |
| Ibrutinib/rituximab vs. placebo/rituximab (23) | 150 | ORR: Ibr/R 92% vs. Pcb/R 47% MRR: Ibr/R 72% vs. Pcb/R 32% | 30-mo OS: Ibr/R 94% vs. Pcb/R 92% | 30-mo PFS: Ibr/R 82% vs. Pcb/R 28% | Atrial fibrillation (grade 3 or higher): 12% Ibr/R vs. 1% Pcb/R Hypertension (grade 3 or higher): 13% Ibr/R vs. 4% Pcb/R Major hemorrhage (all): 4% both arms | Patients in the control arm could cross over to the IR arm upon progression. Reduced rates of rituximab related infusion reactions were noted with concomitant ibrutinib administration. | |
| Ibrutinib vs. Zanubrutinib [26] | 164 R/R, 37 TN (Zanu: 102 Ibr: 99) | ORR: Zanu 94% vs. Ibr 93% MRR: Zanu 77% vs. Ibr 78% | 18-mo OS: Zanu 97% vs. Ibr 93% | 18-mo PFS: Zanu 85% vs. Ibr 84% | Atrial fibrillation (grade 3 or higher): 0% Zanu vs. 4% Ibr Hypertension (grade 3 or higher): 6% Zanu vs. 11% Ibr Neutropenia (grade 3 or higher): 20% Zanu vs. 8% Ibr | VGPR rates were numerically higher with zanubrutinib. | |
| Zanubrutinib [27] | 23 R/R 5 TN | ORR: 81% MRR: 50% | 18-mo OS: 88% | 18-mo PFS: 68% | All hemorrhage: 39% (7%) Hypertension: 11% (11%) Neutropenia: 18% (11%) Atrial fibrillation: 4% (0%) | All pts MYD88WT | |
| Acalabrutinib [28] | 14 TN, 92 R/R | ORR: 93% TN, 92% R/R MRR: 79% TN, 78% R/R | 24-mo OS: 92% TN, 89% R/R | 24-mo PFS: 90% TN, 82% R/R | Bleeding: 58% (3%) Atrial fibrillation: 5% (1%) Pneumonia: 9% (7%) Hypertension: 5% (3%) | Response rates were similar in both TN and R/R patients | |
| Tirabrutinib [29] | 18 TN, 9 R/R | ORR: 96% MRR: 89% | NR | NR | Neutropenia: 30% (11%) Lymphopenia 11% (11%) Rash: 44% (NA) | Short follow-up duration but therapy efficacy appears comparable in TN and R/R patients. Rash is a major toxicity. | |
| Pirtobrutinib [30] | 80 R/R | ORR: 84% MRR: 73% | 19 mo in prior BTKi patients | NR in prior BTKi patients | Bruising: 24% (0%) Neutropenia 24% (20%) Atrial fibrillation/flutter 3% (1%) Hypertension 9% (2%) | High rates of response seen in patients with previous BTKi treatment | |
| Orelabrutinib [31] | 66 R/R | ORR: 89% MRR: 81% | 12-mo OS: 94% | 12-mo PFS: 89% | Neutropenia: 19% (11%) Thrombocytopenia: 28% (6%) Pneumonia 4% (4%) Hepatitis B reactivation: 2% (2%) | Hepatitis B reactivation is a major safety concern needing further exploration. | |
| BCL2 Inhibitors | Venetoclax [32] | 32 R/R | ORR: 84% MRR: 81% | 30-mo OS: 100% | 24-mo PFS: 80% | Neutropenia 53% (45%) Anemia: 25% (3%) Tumor lysis syndrome: 3% (3%) | Progression noted in 10 patients within a year of completion of 24-mo fixed duration treatment |
| PI | Bortezomib [33] | 10 R/R | ORR: 80% PR: 60% | NR | NR | Thrombocytopenia: 40% (20%) Fatigue: 70% (20%) Peripheral neuropathy: 30% (20%) | Peripheral neuropathy was a major concern limiting applicability |
| Bortezomib [34] | 12 TN, 15 R/R | ORR: 26% MRR: 26% | OS: NA | PFS: 16.3 mos | Thrombocytopenia: 70% (30%) Neutropenia: 67% (19%) Peripheral Neuropathy: 74% (19%) | Peripheral neuropathy was a major concern limiting applicability | |
| Carfilzomib-rituximab-dexamethasone (CaRD) [35] | 33 TN | ORR: 87% MRR: 68% | 15.4 median follow-up: OS 100% | 15.4 median follow-up: PFS 65%, | Infusion reaction: 23% (0%) Rash: 29% (0%) Thrombocytopenia: 3% (0%) Hyperlipasemia: 56% (16%) | Cardiopulmonary toxicity remains a major concern with carfilzomib-based regimens. | |
| Ixazomib-dexamethasone-rituximab (IDR) [36] | 26 TN | ORR: 96% MRR: 77% | OS 100% at 52-month follow-up | Median PFS: 40 months | Insomnia: 27% (8%) Rash: 27% (8%) Infusion reaction: 39% (19%) | Grade 3 neuropathy was noted in only 1 patient | |
| Oprozomib [37] | 71 WM and MM | ORR: 71% 2/7 schedule 50% 5/14 schedule MRR: 50% 2/7 schedule, 29% 5/14 schedule | OS: NA | Median PFS: 6.1 mos 2/7 schedule, 3.7 mos 5/14 schedule | Phase II Events for WM- Diarrhea: 2/7 schedule 93% (27%), 5/14 schedule 71% (6%) Sepsis: 2/7 schedule 13% (13%), 5/14 schedule 0% Decreased Platelets: 2/7 schedule 40% (7%), 5/14 18% (0%) | Therapy limited by grade 3 or greater gastrointestinal and hematologic events | |
| PI3Ki | Idelalisib and Obinutuzumab [38] | 48 R/R | ORR: 71% MRR: 65% | 12-mo OS: 90% | 12-mo PFS: 55% | Neutropenia: 19% (16%) Diarrhea: 9% (5%) Hepatic Toxicity: 9% (7%) | Hepatic toxicity limited clinical applicability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chohan, K.L.; Kapoor, P. BTK Inhibitors and Other Targeted Therapies in Waldenström Macroglobulinemia. Hemato 2023, 4, 135-157. https://doi.org/10.3390/hemato4020012
Chohan KL, Kapoor P. BTK Inhibitors and Other Targeted Therapies in Waldenström Macroglobulinemia. Hemato. 2023; 4(2):135-157. https://doi.org/10.3390/hemato4020012
Chicago/Turabian StyleChohan, Karan L., and Prashant Kapoor. 2023. "BTK Inhibitors and Other Targeted Therapies in Waldenström Macroglobulinemia" Hemato 4, no. 2: 135-157. https://doi.org/10.3390/hemato4020012
APA StyleChohan, K. L., & Kapoor, P. (2023). BTK Inhibitors and Other Targeted Therapies in Waldenström Macroglobulinemia. Hemato, 4(2), 135-157. https://doi.org/10.3390/hemato4020012






