Abstract
Refractoriness to standard first-line therapy in immune thrombocytopenia (ITP) should foster additional diagnostic work-up to exclude hematological clonal disease, mostly myelodysplatic syndrome (MDS) or clonal cytopenia of unknown significance (CCUS), which may present with isolated thrombocytopenia of immune or non-immune origin. We herein report on a patient who showed a transient leukoerythroblastic reaction (LEB) associated with bone marrow myelofibrosis upon rompilostim treatment, challenging a diagnosis of primary ITP and requiring additional investigations. RUNX-1-mutated myelodysplastic syndrome was eventually diagnosed. Even though LEB and marrow fibrosis have already been rarely reported during romiplostim treatment for ITP, this is the first case to our knowledge in which a background clonal hematopoiesis was diagnosed and deemed potentially involved in the abnormal response to this thrombopoietin receptor agonist (TPO-RA).
1. Introduction
Immune dysregulation has been supposed to mediate thrombocytopenia in a non-negligible percentage of pre-leukemic myeloid clonal disorders [1], where multi-refractoriness to standard primary ITP first-line treatments and suboptimal response to TPO-RAs have also been reported [2]. Therefore, in refractory ITP, hematopoietic clonal disease should be actively investigated through an extended diagnostic work-up, before more aggressive or combinatorial approaches are implemented [3].
TPO-RAs are considered safe [4] but an increase in bone marrow reticulin fibers has been estimated to occur in 5–10% percent of romiplostim-treated trial patients [5], and has also been more recently described with avatrombopag [6]. In this case report, combined refractoriness to romiplostim and the appearance of LEB in a patient with putative primary ITP prompted bone marrow investigations which eventually uncovered clonal hematopoiesis with myelodysplastic changes and fibrosis.
2. Detailed Case Description
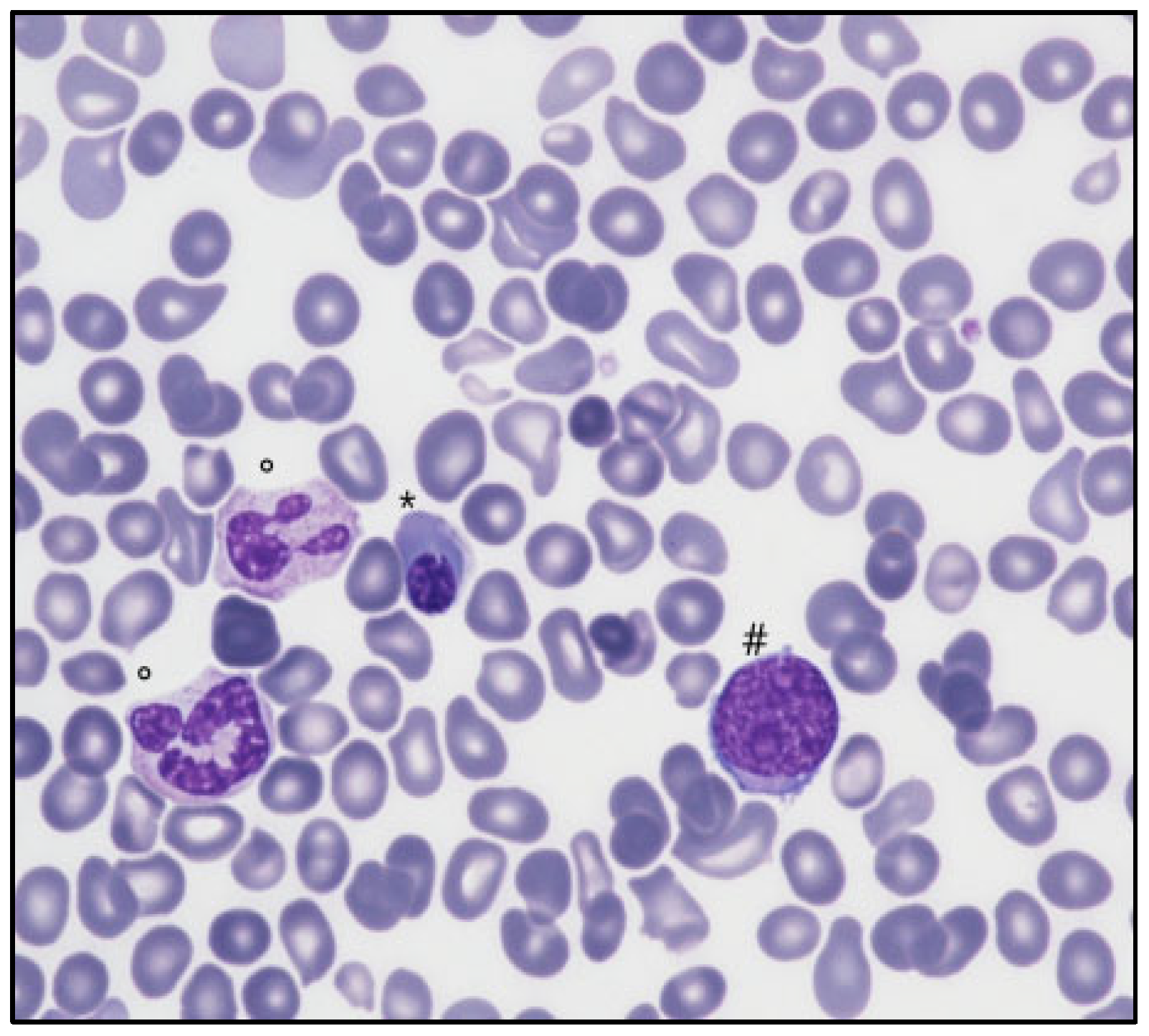
A 76-year-old Caucasian man was evaluated for isolated thrombocytopenia (89 × 109/L). His clinical history was remarkable for ischemic and hypertensive cardiopathy, chronic obstructive lung disease, and a surgically corrected aortic aneurysm. After pseudothrombocytopenia, HVC, HIV, and Helicobacter pylori infections, common variable immunodeficiency, monoclonal gammopathy, and systemic autoimmune diseases were excluded, an operational diagnosis of ITP was provided due to a progressive decrease in platelet counts (nadir 34 × 109/L), lower leg purpura onset, and absence of signs of bone marrow disease (splenomegaly, macrocytosis, and other peripheral blood abnormalities). Since first-line high-dose oral prednisone (1 mg/Kg) provided only partial platelet recovery (50–55 × 109/L), a cycle of high-dose intravenous immunoglobulin was added without any further improvement. A TPO-M, romiplostim (1 µg/Kg), was then introduced and progressively increased (up to 3 µg/Kg) without an effect on the platelet count. Nonetheless, after a month of romiplostim therapy, the appearance of myeloid blasts (1%), early erythroid cells (8%), and hyposegmented hypogranulated neutrophils was noticed on peripheral blood smear examination, and a bone marrow biopsy (BMBx) was scheduled to exclude hematological malignancy. While marrow aspirate smear was not diagnostically reliable due to low and not-well-preserved cellularity, the biopsy reported delayed and hyperplastic maturation of granulopoiesis (myeloid/erythroid ratio 12:1), without excess blasts, and flow cytometry showed absence of hematogones and B-cell precursors, reduced neutrophil SSC, and inhomogeneous CD14 expression on monocytes without increased blast count. Cytogenetic analysis was normal (a molecular study was not performed). Globally, no clear evidence of a myeloid neoplasm was produced, and a diagnosis of primary refractory ITP was made. In the following two months, the dose of romiplostim was progressively increased up to 8 µg/Kg without any improvement in platelet count but with relentlessly worsening of peripheral LEB (myeloid blasts 36%, erythroblasts 59%, myeloid precursors 5%) and appearance of tear-drop poikilocytes (Figure 1).
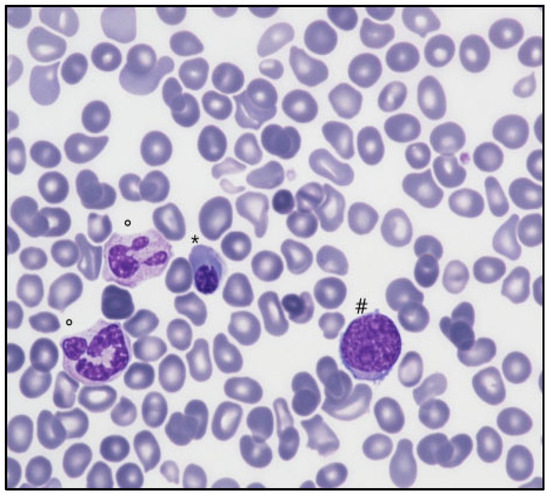
Figure 1.
Peripheral blood film (May Grunwald-Giemsa, 200×) showing nucleated red cells (*), myeloid blast cells (#), dysplastic neutrophils (°), and rare dacryocytes.
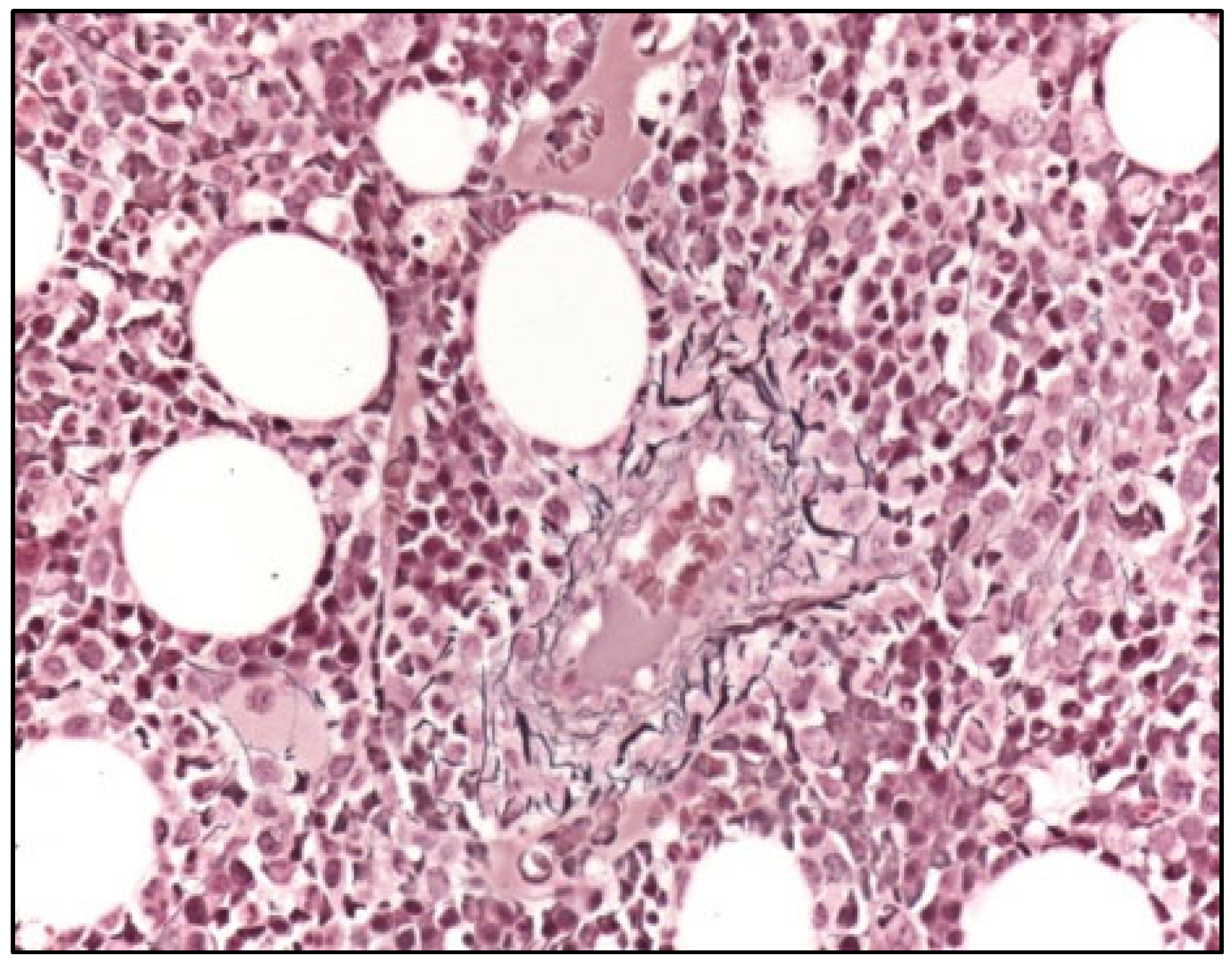
A second BMBx was thus performed: the aspirate was again suboptimal for diagnostic purposes; flow cytometry revealed the presence of immunophenotypically myeloid blasts (21% of total cellularity), mild abnormalities in erythroid maturation (reduced CD71), and myeloid maturation (reduced SSC) and the absence of lymphoid B precursors and hematogones. The BMBx showed dysplastic megakaryocytes, hyperplastic and dysplastic granulopoieisis without excess blasts (<5%), erythroid hypoplasia, and reticulin marrow fibrosis (MF2 on WHO scale) (Figure 2) on Gomori staining, suggesting myelofibrosis secondary to myelodysplastic syndrome (unclassifiable); cytogenetic analysis was normal but a RUNX1 mutation was detected on next-generation sequencing with a variant allele frequency (VAF) of 38%, which further confirmed an underlying myelodysplastic syndrome.
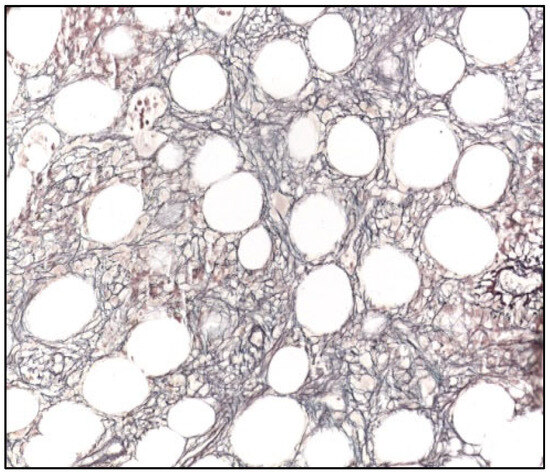
Figure 2.
Bone marrow biopsy (Gomori, 400×), showing diffuse increase in reticulin fibers (WHO MF2).
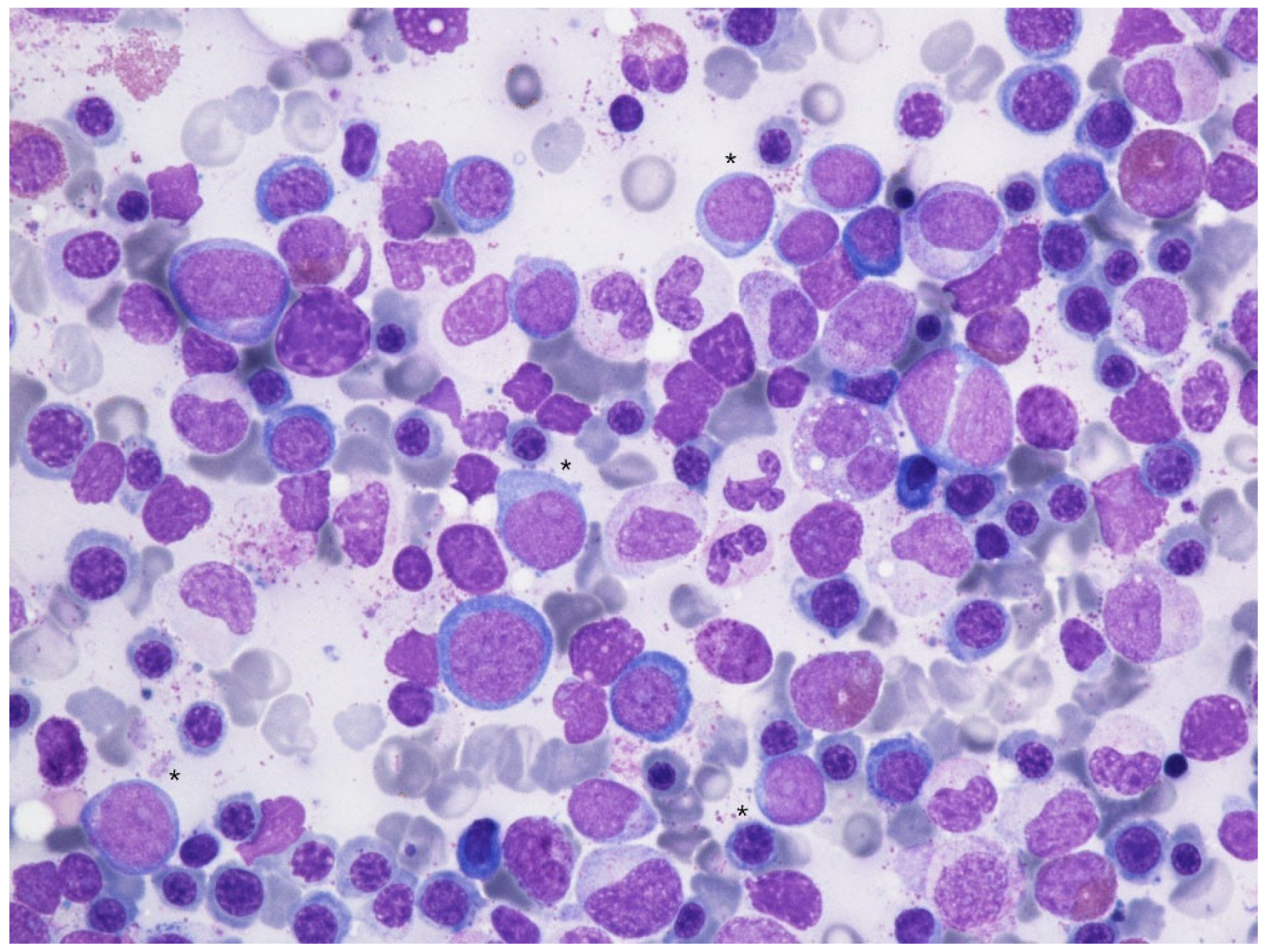
However, given the discrepancy in blast counts, a cautious wait-and-watch approach was undertaken while withdrawing romiplostim treatment and introducing danazole. In the following two months, the peripheral LEB disappeared, while the platelet count did not improve, leaving the patient platelet transfusion-dependent. Disappearance of tear-drop poikilocytes was evident, but refractory progressive neutropenia appeared and was complicated by septic cholangiopathy, requiring hospitalization and surgery. After resolution of sepsis, with the patient still being on danazol (200 mg/d), a follow-up BMBx (9 months after the previous one) was performed that showed complete resolution of fibrosis (Figure 3) with persistent myelodysplastic changes and a blast count of 8% on bone marrow biopsy and 3–6% on aspirate smear (Figure 4). RUNX1 mutation was confirmed (VAF 19%).
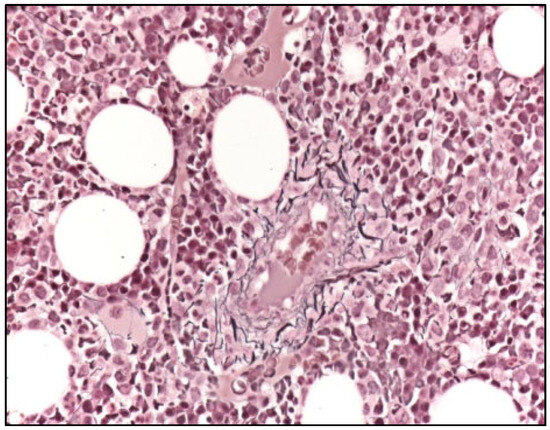
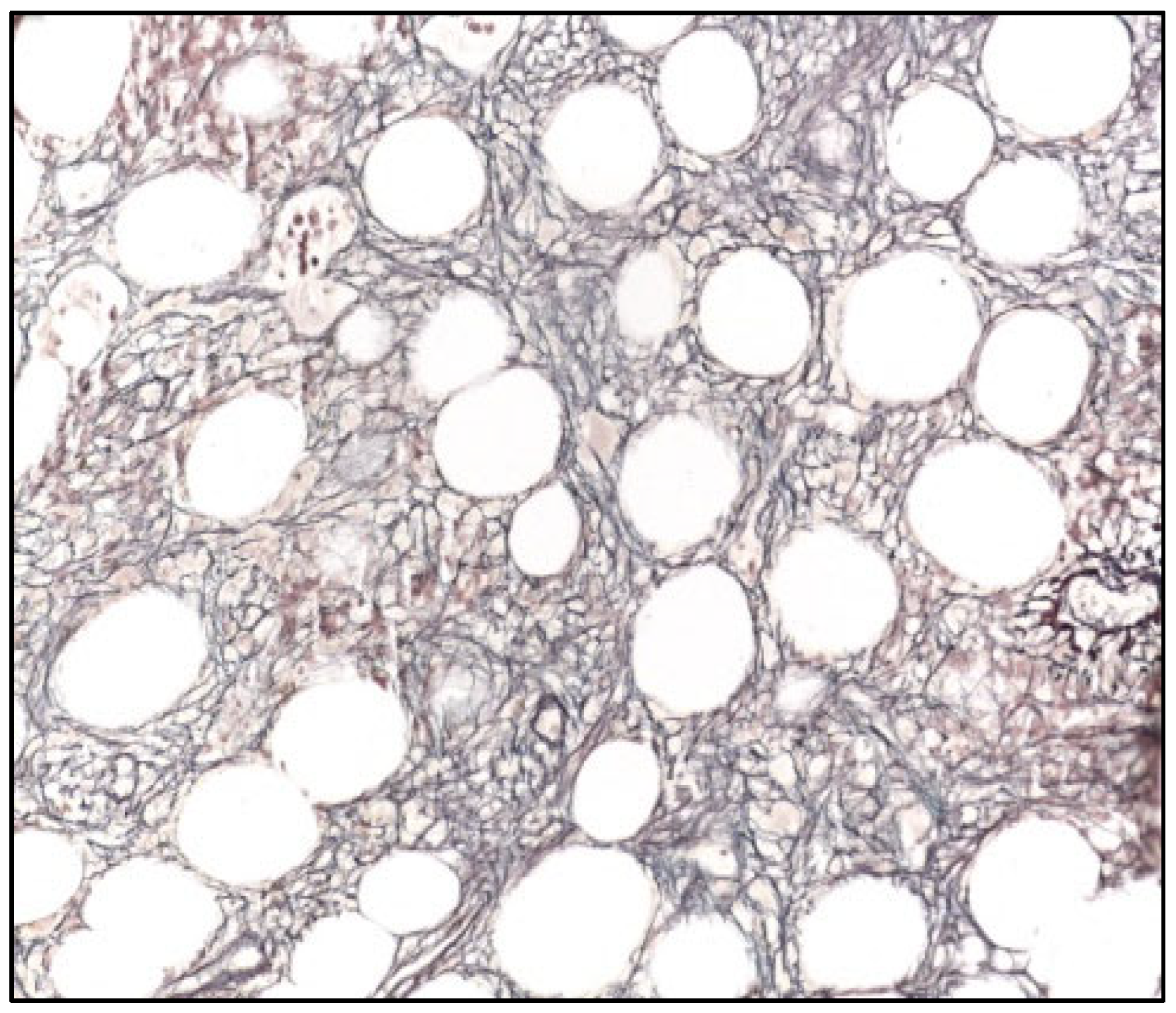
Figure 3.
Bone marrow biopsy (Gomori. 200×) showing normal reticulin network (WHO MF0) and reference staining of the Tunica externa of a small vessel.
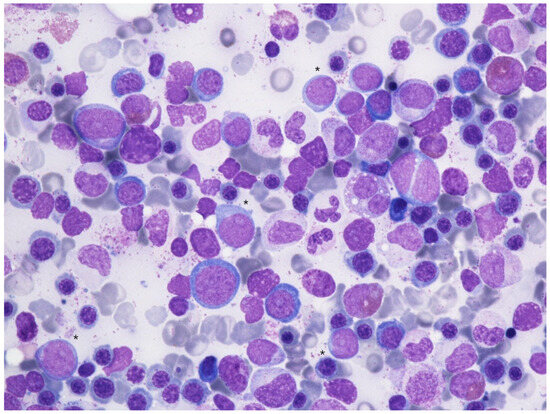
Figure 4.
Bone marrow aspirate smear (May-Grünwald Giemsa, 600×) showing dysplastic changes in erythropoiesis (megaloblastic changes, binuclear erythroblasts), granulopoiesis (hypogranular and hyposegmented neutrophils) with an excess of myeloid blasts (*).
Flow cytometry reported the same dysplastic changes previously reported but a reduced blast count (2%). A final diagnosis of MDS with excess blasts (ICC 2022)/MDS with increased blasts—IB1 (WHO 2022) was made. After azacytidine treatment, the patient remained clinically stable and transfusion-independent, and a follow-up BMBx after 16 cycles demonstrated complete remission with partial hematological recovery as per IWG 2023 response criteria for higher-risk myelodysplastic syndrome [7].
3. Discussion
Our case is unique in that an LEB with reversible bone marrow fibrosis uncovering MDS has not been reported before, at least to our knowledge, in patients treated with romiplostim for presumed ITP.
Romiplostim treatment for ITP has been associated with bone marrow fibrosis in a variable percentage of cases. After being first reported in clinical trials [8,9], a dedicated safety study, performed by Kuter et al. [5] to track the incidence and evolution of reticulin fibrosis, suggested that a significant proportion of patients (5–10%) show a mild non-progressive increase in reticulin fiber formation, which is likely to be dose-related and reversible. In the following decade, other authors [10,11] investigated the long-term consequences of marrow fibrotic changes, showing that a moderate increase in reticulin fibrosis (MF-2) was observed in 18% at a median time of treatment of 2.5 years [10]. Severe grades of reticulin fibrosis (MF-3 and/or collagen fibrosis) were extremely rare in both studies. In general, it did not seem that TPO-RAs induced substantial fibrosis or changes in the number or morphology of peripheral blood cells. Both reticulin and collagen fibrosis regressed in most patients after discontinuation of TPO-RA.
While formal indications for bone marrow monitoring in romiplostim-treated patients have not been established, clinical studies and case reports mandate active vigilance in ITP patients on long-term TPO-RAs, especially when changes in peripheral blood counts and morphology are detected. Besides these subtle fibrotic changes of apparently benign significance, the onset of higher-grade myelofibrosis with peripheral leukoerythroblastosis has been seldom reported in the literature [12,13], maybe reflecting an idiosyncratic mechanism related to genetic individual susceptibility or other unknown factors.
The appearance of LEB, in combination with romiplostim refractoriness, led us to perform serial bone marrow investigations until a diagnosis of myelodysplastic syndrome with RUNX1 mutation was established, adding a new layer of complexity and suggesting a new potential pathophysiological explanation. In fact, even though a cytogenetic clonality marker was not detected at first bone marrow work-up (unfortunately, a molecular study was not available at our center at that time), preventing us from establishing an earlier definitive diagnosis of MDS or CCUS, the morphological and flow cytometric analysis of peripheral blood and bone marrow had displayed mild signs of multilineage dysplasia, favoring this hypothesis. It seems thus likely the refractoriness to standard ITP treatment and the leukoerythroblastic crisis with myelofibrosis were related to undetected RUNX1-driven clonal hematopoiesis with abnormal sensitivity to TPO receptor stimulation [14]. For obvious ethical reasons, such a hypothesis could not be proven by re-administration of romiplostim. Whether, in this patient, the mechanism of thrombocytopenia was, at least in part, immune-mediated could not be evaluated since no validated laboratory and clinical criteria are available to disentangle this conundrum.
In conclusion, even though the latest guidelines state that bone marrow examination in patients with isolated thrombocytopenia is not necessary irrespective of age, this case report confirms previous experience by Lapis et al., 2023 [15], i.e., that a myeloid neoplasm should not be overlooked in patients presenting with isolated thrombocytopenia, especially those of older age (>60), male sex, and with mild thrombocytopenia (e.g., >40.000/mcL), in which cases a bone marrow investigations could be anticipated at the earliest stages of diagnostic work-up.
Author Contributions
Conceptualization, G.M.; methodology, P.D. and A.C.; investigation P.D.; resources, A.E., P.D., A.R. and S.P.; data curation, G.M., C.P., R.D.R. and A.E.; writing—original draft preparation, G.M.; writing—review and editing, P.D. and A.C.; supervision, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from the subject.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rodeghiero, F. Immune thrombocytopenia in myeloid and lymphoid clonal disorders: An intriguing association. Haematologica 2021, 106, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Jachiet, V.; Moulis, G.; Hadjadj, J.; Seguier, J.; Laribi, K.; Schleinitz, N.; Vey, N.; Sacre, K.; Godeau, B.; Beyne-Rauzy, O.; et al. Clinical spectrum, outcome and management of immune thrombocytopenia associated with myelodysplastic syndromes and chronic myelomonocytic leukemia. Haematologica 2021, 106, 1414–1422. [Google Scholar] [CrossRef]
- Miltiadous, O.; Hou, M.; Bussel, J.B. Identifying and treating refractory ITP: Difficulty in diagnosis and role of combination treatment. Blood 2020, 135, 472–490. [Google Scholar] [CrossRef]
- Provan, D. Real-world evidence confirms thrombopoietin receptor agonists are safe and effective for all stages of immune thrombocytopenia. Am. J. Hematol. 2024, 99, 4–5. [Google Scholar] [CrossRef]
- Kuter, D.J.; Mufti, G.J.; Bain, B.J.; Hasserjian, R.P.; Davis, W.; Rutstein, M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood 2009, 114, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.; Bagot, C.; Leach, M.; Bain, B.J. Thrombopoietin mimetic-induced bone marrow fibrosis. Am. J. Hematol. 2024, 99, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Platzbecker, U.; Bewersdorf, J.P.; Stahl, M.; Adès, L.; Borate, U.; Bowen, D.T.; Buckstein, R.J.; Brunner, A.M.; E Carraway, H.; et al. Consensus proposal for revised International Working Group 2023 response criteria for higher-risk myelodysplastic syndromes. Blood 2023, 141, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Kuter, D.J.; Pullarkat, V.; Lyons, R.M.; Guo, M.; Nichol, J.L. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009, 113, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Bussel, J.B.; Lyons, R.M.; Pullarkat, V.; Gernsheimer, T.B.; Senecal, F.M.; Aledort, L.M.; George, J.N.; Kessler, C.M.; Sanz, M.A.; et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: A double-blind randomised controlled trial. Lancet 2008, 371, 395–403. [Google Scholar] [CrossRef]
- Ghanima, W.; Geyer, J.T.; Lee, C.S.; Boiocchi, L.; Imahiyerobo, A.A.; Orazi, A.; Bussel, J.B. Bone marrow fibrosis in 66 Immune Thrombocytopenia patients treated with thrombopoietin receptor agonists: A single center long-term follow-up. Haematologica 2014, 99, 937–944. [Google Scholar] [CrossRef]
- Janssens, A.; Rodeghiero, F.; Anderson, D.; Chong, B.H.; Boda, Z.; Pabinger, I.; Červinek, L.; Terrell, D.R.; Wang, X.; Franklin, J. Changes in bone marrow morphology in adults receiving romiplostim for the treatment of thrombocytopenia associated with primary immune thrombocytopenia. Ann. Hematol. 2016, 95, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Park, S.W.; Kim, J.H.; Kang, J.H.; Lee, W.S.; Song, H.N. Romiplostim-related myelofibrosis in refractory primary immune thrombocytopenia: A Case report. Medicine 2019, 98, e15882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leung, T.; Lokan, J.; Turner, P.; Smith, C. Reversible bone marrow reticulin fibrosis as a side effect of romiplostim therapy for the treatment of chronic refractory immune thrombocytopenia. Pathology 2011, 43, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Matsumura, I.; Tanaka, H.; Ezoe, S.; Fukushima, K.; Tokunaga, M.; Yasumi, M.; Shibayama, H.; Mizuki, M.; Era, T.; et al. AML1/RUNX1 works as a negative regulator of c-Mpl in hematopoietic stem cells. J. Biol. Chem. 2008, 283, 30045–30056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liapis, K.; Papadopoulos, V.; Pontikoglou, C.; Vrachiolias, G.; Stavroulaki, E.; Kourakli, A.; Lazaris, V.; Galanopoulos, A.G.; Papoutselis, M.; Papageorgiou, S.G.; et al. Myelodysplastic neoplasm with isolated thrombocytopenia and immune thrombocytopenic purpura in adults: Insights from a comparison of two national registries. Leukemia 2023, 37, 708–711. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).