Influence of Implant-Abutment Contact Surfaces and Prosthetic Screw Tightening on the Stress Concentration, Fatigue Life and Microgap Formation: A Finite Element Analysis
Abstract
1. Introduction
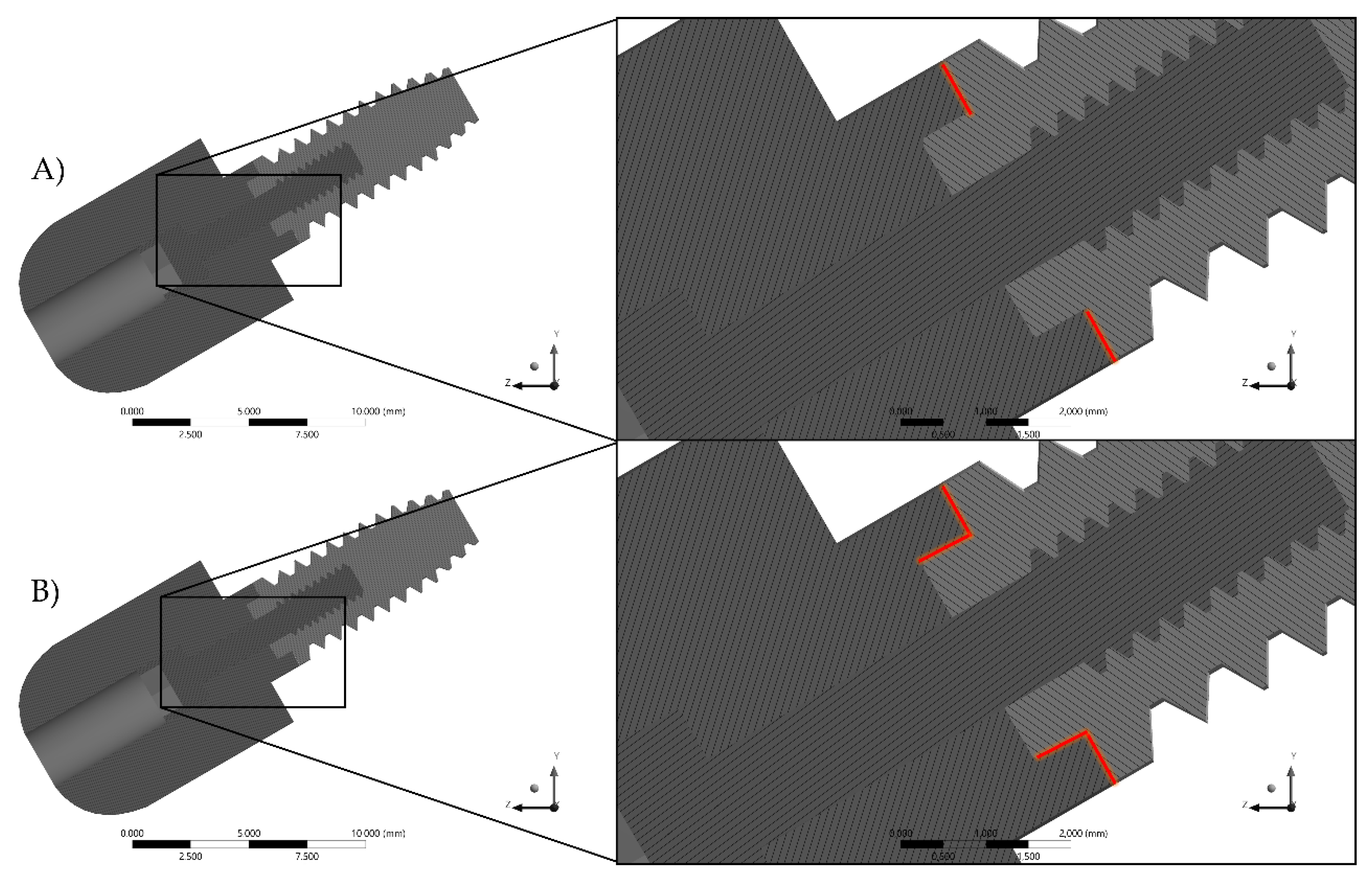
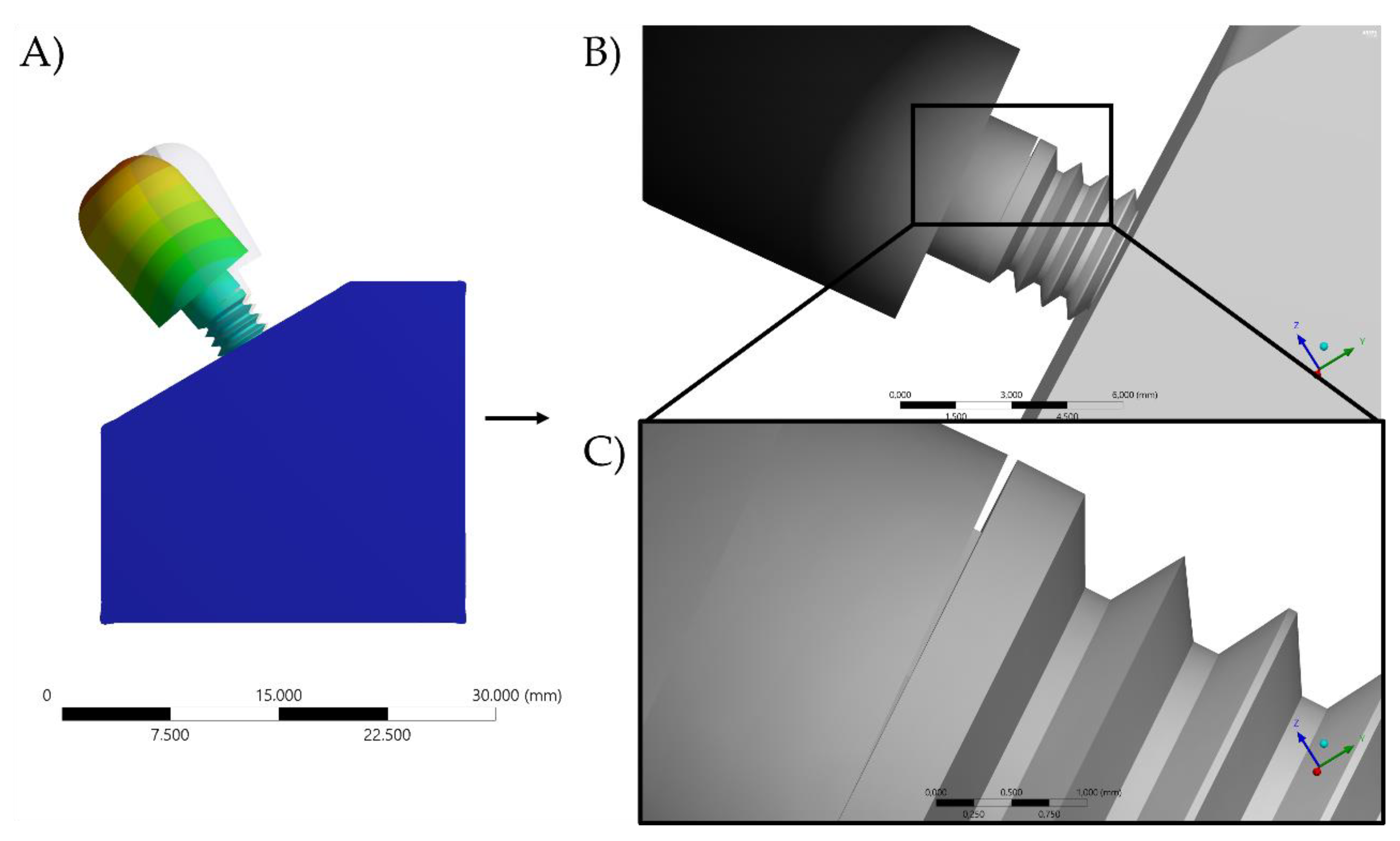
2. Materials and Methods
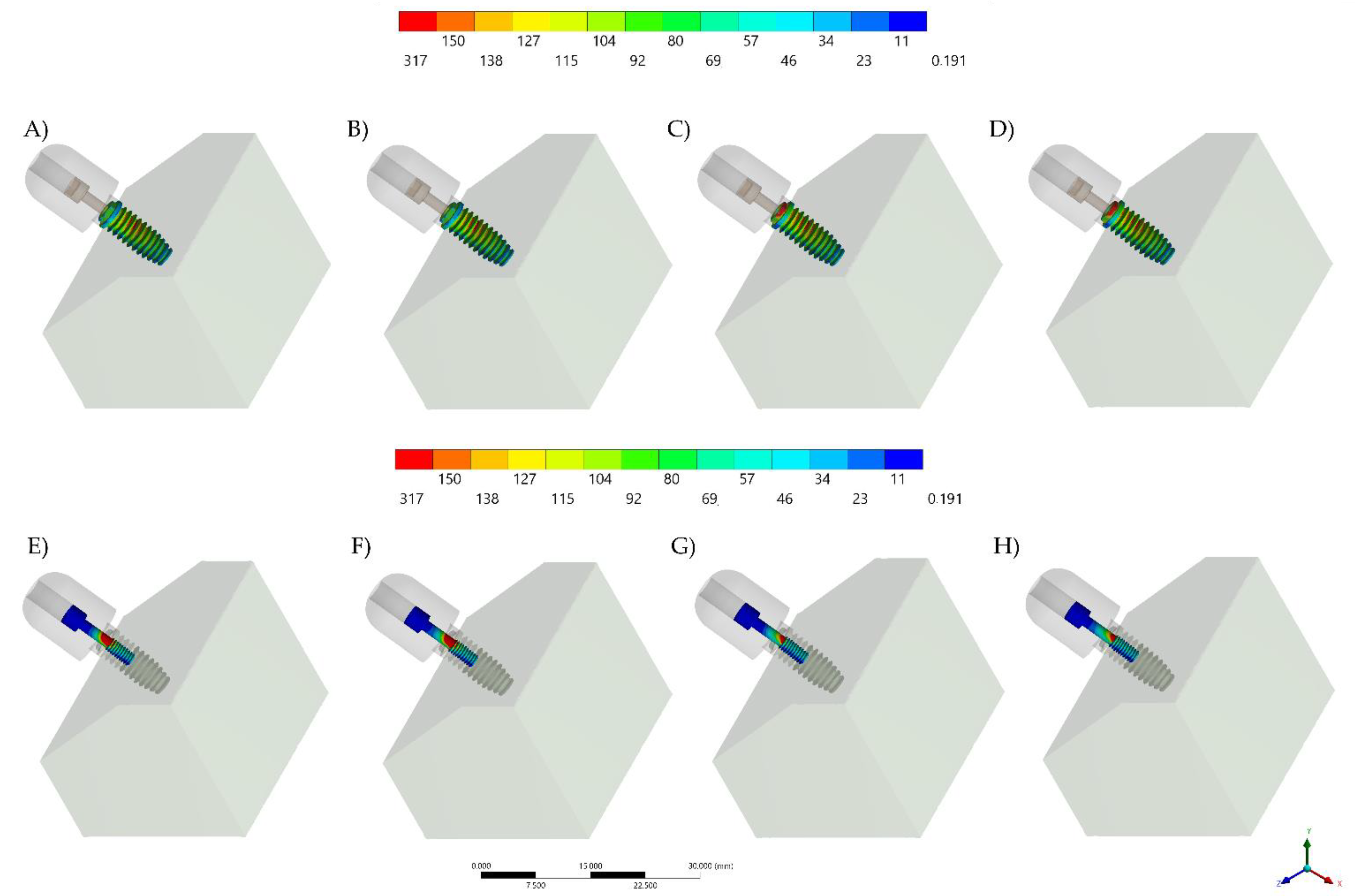
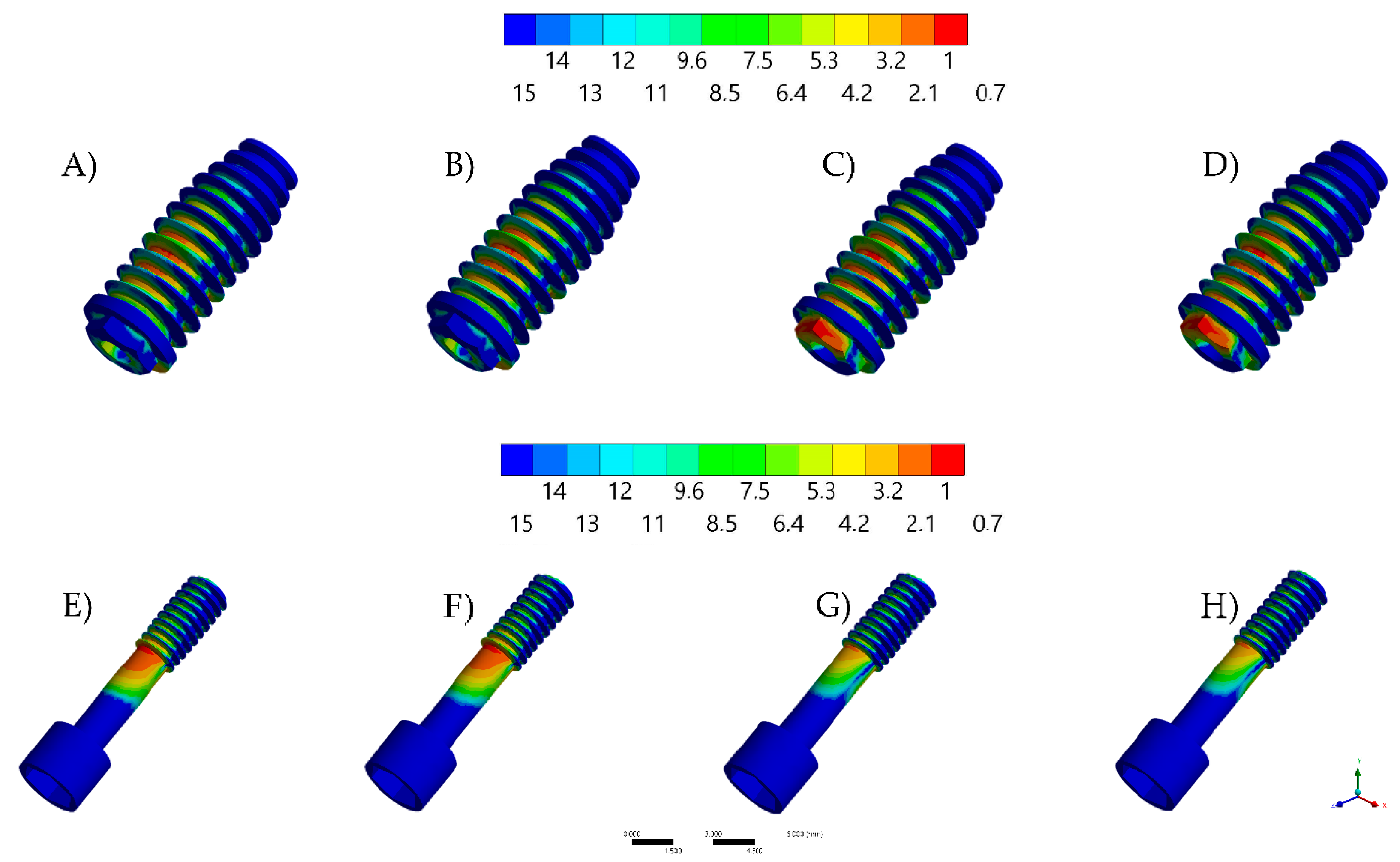
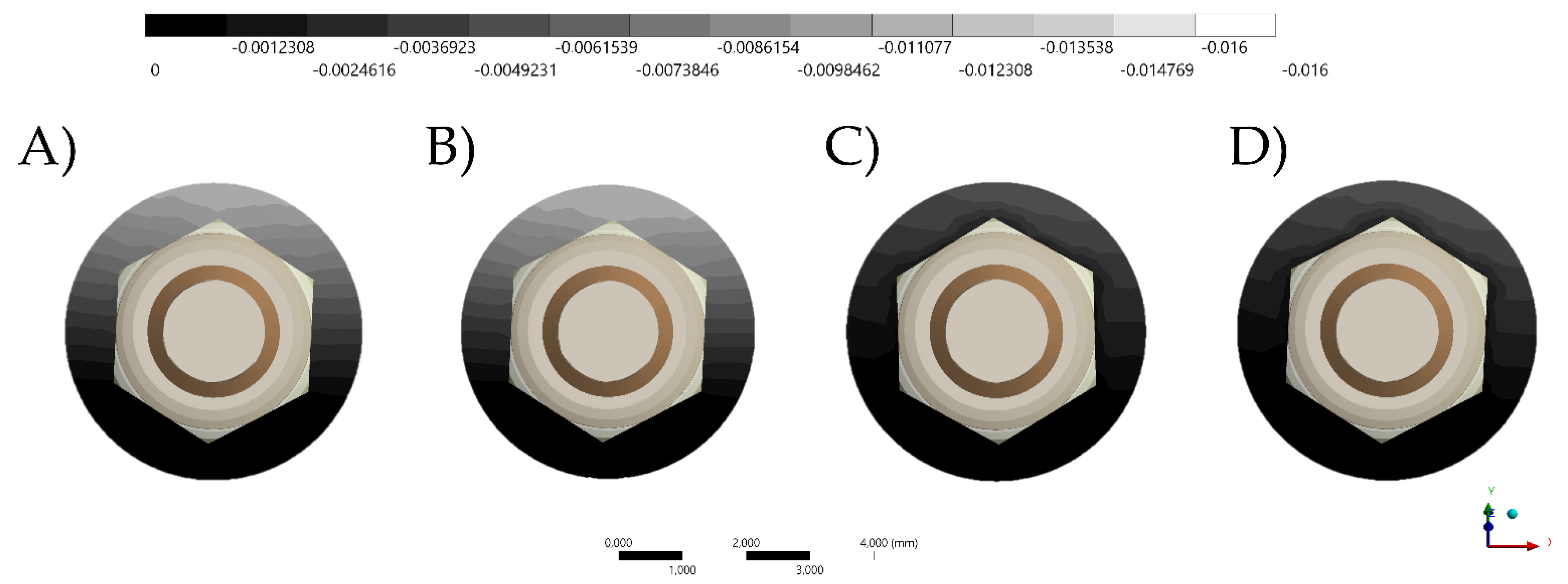
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tricio, J.; Laohapand, P.; van Steenberghe, D.; Quirynen, M.; Naert, I. Mechanical state assessment of the implant-bone continuum: A better understanding of the Periotest method. Int. J. Oral Maxillofac. Implant. 1995, 10, 43–49. [Google Scholar]
- Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials 2020, 13, 1131. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Stähli, A.; Monje, A.; Sculean, A.; Salvi, G.E. Peri-Implantitis: A Clinical Update on Prevalence and Surgical Treatment Outcomes. J. Clin. Med. 2021, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fok, A.; Aparicio, C.; Teng, W. Contact analysis of gap formation at dental implant-abutment interface under oblique loading: A numerical-experimental study. Clin. Implant Dent. Relat. Res. 2019, 21, 741–752. [Google Scholar] [CrossRef]
- Bassi, M.A.; Lopez, M.A.; Confalone, L.; Gaudio, R.M.; Lombardo, L.; Lauritano, D. A prospective evaluation of outcomes of two tapered implant systems. J. Biol. Regul. Homeost. Agents 2016, 30, 1–6. [Google Scholar]
- Scarano, A.; Valbonetti, L.; Degidi, M.; Pecci, R.; Piattelli, A.; de Oliveira, P.S.; Perrotti, V. Implant-Abutment Contact Surfaces and Microgap Measurements of Different Implant Connections Under 3-Dimensional X-Ray Microtomography. Implant. Dent. 2016, 25, 656–662. [Google Scholar] [CrossRef]
- Kim, J.H.; Noh, G.; Hong, S.J.; Lee, H. Biomechanical stress and microgap analysis of bone-level and tissue-level implant abutment structure according to the five different directions of occlusal loads. J. Adv. Prosthodont. 2020, 12, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Lim, Y.-J. Axial Displacements and Removal Torque Changes of Five Different Implant-Abutment Connections under Static Vertical Loading. Materials 2020, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; Dal Piva, A.M.D.O.; Lo Giudice, R.; Borges, A.L.S.; Bottino, M.A.; Epifania, E.; Ausiello, P. The Influence of Custom-Milled Framework Design for an Implant-Supported Full-Arch Fixed Dental Prosthesis: 3D-FEA Study. Int. J. Environ. Res. Public Health 2020, 17, 4040. [Google Scholar] [CrossRef] [PubMed]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; López-Jarana, P.; Ríos-Santos, J.V.; Herrero-Climent, M. Review of the Mechanical Behavior of Different Implant–Abutment Connections. Int. J. Environ. Res. Public Health 2020, 17, 8685. [Google Scholar] [CrossRef]
- Bisognin, E.D.C.; Harari, N.D.; Machado, S.J.; da Silva, C.P.; de Almeida Soares, G.D.; Vidigal, G.M., Jr. Evaluation of implant-abutment microgap and bacterial leakage in five external-hex implant systems: An in vitro study. Int. J. Oral Maxillofac. Implant. 2012, 27, 346–351. [Google Scholar]
- Gigandet, M.; Bigolin, G.; Faoro, F.; Bürgin, W.; Brägger, U. Implants with original and non-original abutment connections. Clin. Implant Dent. Relat. Res. 2014, 16, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Asián, I.; Martínez-González, Á.; Torres-Lagares, D.; Serrera-Figallo, M.-Á.; Gutiérrez-Pérez, J.-L. External Connection versus Internal Connection in Dental Implantology. A Mechanical in vitro Study. Metals 2019, 9, 1106. [Google Scholar] [CrossRef]
- Lee, H.; Jo, M.; Noh, G. Biomechanical effects of dental implant diameter, connection type, and bone density on microgap formation and fatigue failure: A finite element analysis. Comput. Methods Programs Biomed. 2021, 200, 105863. [Google Scholar] [CrossRef]
- Tonin, B.S.H.; He, Y.; Ye, N.; Chew, H.P.; Fok, A. Effects of tightening torque on screw stress and formation of implant-abutment microgaps: A finite element analysis. J. Prosthet. Dent. 2021, 17, S0022–S3913. [Google Scholar]
- Wiest, W.; Rack, A.; Zabler, S.; Schaer, A.; Swain, M.; Nelson, K. Validation of finite-element simulations with synchrotron radiography—A descriptive study of micromechanics in two-piece dental implants. Heliyon 2018, 4, e00524. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D. Fatigue of Dental Implants: Facts and Fallacies. Dent. J. 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Prados-Privado, M.; Ivorra, C.; Martínez-Martínez, C.; Gehrke, S.A.; Calvo-Guirado, J.L.; Prados-Frutos, J.C. A Finite Element Analysis of the Fatigue Behavior and Risk of Failure of Immediate Provisional Implants. Metals 2019, 9, 535. [Google Scholar] [CrossRef]
- Armentia, M.; Abasolo, M.; Coria, I.; Albizuri, J. Fatigue Design of Dental Implant Assemblies: A Nominal Stress Approach. Metals 2020, 10, 744. [Google Scholar] [CrossRef]
- UNE-EN ISO 14801:2016. Dentistry—Implants—Dynamic Loading Test for Endosseous Dental Implants; International Organozation for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Tribst, J.P.M.; Piva, A.M.D.O.D.; Anami, L.C.; Borges, A.L.S.; Bottino, M. A Influence of implant connection on the stress distribution in restorations performed with hybrid abutments. J. Osseointegration 2019, 11, 507–512. [Google Scholar]
- Sahoo, P.; Das, S.K.; Davim, J.P. Tribology of materials for biomedical applications. Mech. Behav. Biomater. 2019, 1–45. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Janeček, M.; Nový, F.; Harcuba, P.; Stráský, J.; Trško, L.; Mhaede, M.; Wagner, L. The Very High Cycle Fatigue Behavior of Ti-6Al-4V Alloy. Acta Phys. Pol. A 2015, 128, 497–502. [Google Scholar] [CrossRef]
- Jörn, D.; Kohorst, P.; Besdo, S.; Borchers, L.; Stiesch, M. Three-Dimensional Nonlinear Finite Element Analysis and Microcomputed Tomography Evaluation of Microgap Formation in a Dental Implant under Oblique Loading. Int. J. Oral Maxillofac. Implant. 2016, 31, 32–42. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Wang, K.S.; Woo, J.C. Fatigue life and reliability evaluation for dental implants based on computer simulation and limited test data. J. Mech. Eng. Sci. 2013, 227, 554–564. [Google Scholar] [CrossRef]
- Prados-Privado, M.; Prados-Frutos, J.C.; Manchón, Á.; Rojo, R.; Felice, P.; Bea, J.A. Dental implants fatigue as a possible failure of implantologic treatment: The importance of randomness in fatigue behaviour. BioMed Res. Int. 2015, 2015, 825402. [Google Scholar] [CrossRef]
- Prabhudesai, A.; Dhatrak, P.N.; Padmanabhan, D. Fatigue Life Prediction of Dental Implants. Int. J. Eng. Technol. Comput. Res. 2017, 5, 153–160. [Google Scholar]
- Tribst, J.P.M.; Dal Piva, A.M.O.; Borges, A.L.S.; Anami, L.C.; Kleverlaan, C.J.; Bottino, M.A. Survival Probability, Weibull Characteristics, Stress Distribution, and Fractographic Analysis of Polymer-Infiltrated Ceramic Network Restorations Cemented on a Chairside Titanium Base: An In Vitro and In Silico Study. Materials 2020, 13, 1879. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.; Blasón-González, S.; García-García, I.; Lamela-Rey, M.J.; Fernández-Canteli, A.; Álvarez-Arenal, Á. Optimized Planning and Evaluation of Dental Implant Fatigue Testing: A Specific Software Application. Biology 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; Dal Piva, A.M.D.O.; Borges, A.L.S.; Bottino, M.A. Different combinations of CAD/CAM materials on the biomechanical behavior of a two-piece prosthetic solution. Int. J. Comput. Dent. 2019, 22, 171–176. [Google Scholar] [PubMed]
- Tribst, J.P.M.; Dal Piva, A.M.O.; Shibli, J.A.; Borges, A.L.S.; Tango, R.N. Influence of implantoplasty on stress distribution of exposed implants at different bone insertion levels. Braz. Oral Res. 2017, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Datte, C.E.; Tribst, J.P.; Dal Piva, A.O.; Nishioka, R.S.; Bottino, M.A.; Evangelhista, A.M.; Monteiro, F.M.M.; Borges, A.L. Influence of different restorative materials on the stress distribution in dental implants. J. Clin. Exp. Dent. 2018, 10, 439–444. [Google Scholar] [CrossRef]
- Adolfi, D.; Mendes Tribst, J.P.; Souto Borges, A.L.; Bottino, M.A. Torque Maintenance Capacity, Vertical Misfit, Load to Failure, and Stress Concentration of Zirconia Restorations Cemented or Notched to Titanium Bases. Int. J. Oral Maxillofac. Implant. 2020, 35, 357–365. [Google Scholar] [CrossRef]
- Melo Filho, A.B.D.; Tribst, J.P.M.; Ramos, N.D.C.; Luz, J.N.; Jardini, M.A.N.; Borges, A.L.S.; Santamaria, M.P.; Melo, R.M.D. Failure probability, stress distribution and fracture analysis of experimental screw for micro conical abutment. Braz. Dent. J. 2019, 30, 157–163. [Google Scholar] [CrossRef]
- Torres-Alemany, A.; Fernández-Estevan, L.; Agustín-Panadero, R.; Montiel-Company, J.M.; Labaig-Rueda, C.; Mañes-Ferrer, J.F. Clinical Behavior of Short Dental Implants: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 3271. [Google Scholar] [CrossRef]
- Ausiello, P.; Dal Piva, A.M.d.O.; Borges, A.L.S.; Lanzotti, A.; Zamparini, F.; Epifania, E.; Mendes Tribst, J.P. Effect of Shrinking and No Shrinking Dentine and Enamel Replacing Materials in Posterior Restoration: A 3D-FEA Study. Appl. Sci. 2021, 11, 2215. [Google Scholar] [CrossRef]
- Martorelli, M.; Ausiello, P. A novel approach for a complete 3D tooth reconstruction using only 3D crown data. Int. J. Interact. Des. Manuf. 2013, 7, 125–133. [Google Scholar] [CrossRef]
- Prati, C.; Tribst, J.P.M.; Dal Piva, A.M.d.O.; Borges, A.L.S.; Ventre, M.; Zamparini, F.; Ausiello, P. 3D Finite Element Analysis of Rotary Instruments in Root Canal Dentine with Different Elastic Moduli. Appl. Sci. 2021, 11, 2547. [Google Scholar] [CrossRef]




| Material | Elastic Modulus (GPa) | Poisson Ratio | Ultimate Tensile Strength (MPa) | Yield Strength (MPa) |
|---|---|---|---|---|
| Fixation base | 3.6 | 0.3 | - | - |
| Titanium | 110 | 0.3 | 930 | 860 |
| Torque of Screw | Contacting Surfaces | Stress (MPa) | |
|---|---|---|---|
| Implant | Screw | ||
| 20 Ncm | Axials and surrounding | 279 | 283 |
| Surrounding only | 256 | 374 | |
| 30 Ncm | Axials and surrounding | 268 | 287 |
| Surrounding only | 253 | 419 | |
| Torque of Screw | Contacting Surfaces | Safety Factor | |
|---|---|---|---|
| Implant | Screw | ||
| 20 Ncm | Axials and surrounding | 1.60 | 1.51 |
| Surrounding only | 1.49 | 0.76 | |
| 30 Ncm | Axials and surrounding | 1.61 | 1.44 |
| Surrounding only | 1.50 | 0.72 | |
| Torque of Screw | Contacting Surfaces | Life (Cycles) | |
|---|---|---|---|
| Implant | Screw | ||
| 20 Ncm | Axials and surrounding | 1 × 1010 | 1 × 1010 |
| Surrounding only | 1 × 1010 | 63,567 | |
| 30 Ncm | Axials and surrounding | 1 × 1010 | 1 × 1010 |
| Surrounding only | 1 × 1010 | 57,389 | |
| Torque of Screw | Contacting Surfaces | Microgap (mm) |
|---|---|---|
| Interface Implant-Abutment | ||
| 20 Ncm | Axials and surrounding | 0.000861 |
| Surrounding only | 0.001817 | |
| 30 Ncm | Axials and surrounding | 0.000820 |
| Surrounding only | 0.001751 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tribst, J.P.M.; Dal Piva, A.M.d.O.; da Silva-Concílio, L.R.; Ausiello, P.; Kalman, L. Influence of Implant-Abutment Contact Surfaces and Prosthetic Screw Tightening on the Stress Concentration, Fatigue Life and Microgap Formation: A Finite Element Analysis. Oral 2021, 1, 88-101. https://doi.org/10.3390/oral1020009
Tribst JPM, Dal Piva AMdO, da Silva-Concílio LR, Ausiello P, Kalman L. Influence of Implant-Abutment Contact Surfaces and Prosthetic Screw Tightening on the Stress Concentration, Fatigue Life and Microgap Formation: A Finite Element Analysis. Oral. 2021; 1(2):88-101. https://doi.org/10.3390/oral1020009
Chicago/Turabian StyleTribst, João Paulo Mendes, Amanda Maria de Oliveira Dal Piva, Laís Regiane da Silva-Concílio, Pietro Ausiello, and Les Kalman. 2021. "Influence of Implant-Abutment Contact Surfaces and Prosthetic Screw Tightening on the Stress Concentration, Fatigue Life and Microgap Formation: A Finite Element Analysis" Oral 1, no. 2: 88-101. https://doi.org/10.3390/oral1020009
APA StyleTribst, J. P. M., Dal Piva, A. M. d. O., da Silva-Concílio, L. R., Ausiello, P., & Kalman, L. (2021). Influence of Implant-Abutment Contact Surfaces and Prosthetic Screw Tightening on the Stress Concentration, Fatigue Life and Microgap Formation: A Finite Element Analysis. Oral, 1(2), 88-101. https://doi.org/10.3390/oral1020009








