Abstract
Peri-implantitis is marked by ongoing bone loss and soft tissue inflammation surrounding dental implants. Due to its ability to intensify tissue healing and its compatibility with the body, hyaluronic acid (HA) has appeared as a viable biomaterial for managing this oral condition. The goal of this scoping review is to examine current trends and future directions for refining HA-based interventions as an adjuvant peri-implant therapy over the past decade. Using the PRISMA-ScR guidelines, a review of the literature for the past 10 years was conducted. Articles related to HA’s anti-inflammatory, antimicrobial, regenerative mechanisms were taken into consideration to underline both achievements and research related to peri-implantitis treatment management. A total of 105 articles were found, and finally, five clinical studies were included. Despite encouraging results that showed good efficacy of HA use as an adjuvant and/or preventive therapeutic agent for peri-implantitis treatment, a larger number of randomized controlled trials and standardized protocols are needed to confirm HA’s therapeutic efficacy and establish its place in routine clinical practice.
1. Introduction
Peri-implantitis has been defined differently over the years [1,2,3,4,5,6,7,8]. As such, it was concluded that peri-implantitis is considered to be a progressive, multifactorial inflammatory disease that affects both the soft and hard tissues surrounding osseointegrated dental implants [6,8]. If left untreated, it can cause irreversible bone loss and implant failure. The etiology of peri-implantitis is complex, involving microbial biofilm accumulation [3], a dysregulated host immune response [5], and individual risk factors such as smoking, poor oral hygiene, systemic diseases (e.g., diabetes), a history of periodontitis, genetic predispositions, oral bisphosphonate treatment, and occlusal overload [9,10,11,12,13,14,15]. Generally, it has been reported that its prevalence within the population ranges roughly from 10% to 50%, depending on the diagnostic criteria, follow-up duration, and patient populations studied [4,15,16].
As a prevention method [4], for patients with dental implants covered by fixed or removable overdentures, the recommended follow-up for implant maintenance is quarterly appointments [17]. Thus, the most indicated and frequent procedures are periodontal probing and sequential radiographies [8], or even CBCTs [18].
In case of any inflammation, dental plaque or biofilm formation detection, the dental practitioners are recommended to follow clinically updated guidelines [4]. Thus, the traditional surgical therapies, with or without additional non-surgical methods, aim to reduce bleeding on probing (BOP) values and maintain good local homeostasis at the site of the dental implants [7,8]. Therefore, based on the clinical guidelines, the following conditions need to be taken into consideration for peri-implantitis as a diagnostic criterion: (a) BOP—bleeding on probing; (b) PPD—pocket probing depth (>6 mm); and (c) BS—bone loss (>3 mm) [19].
In some cases where peri-implant inflammation, mucositis, and/or bone loss occur, conventional treatment strategies include mechanical debridement, antiseptic irrigation (e.g., Chlorhexidine), systemic or local antibiotics, and surgical approaches—either resective or regenerative [19,20].
However, these methods often yield unpredictable results, especially in advanced lesions, and are limited by incomplete decontamination or compromise of implant surfaces, poor tissue regeneration, and recurrence of infection [21,22,23,24].
As such, the focus has shifted to adjuvant therapies that promote host modulation, improve local soft tissue healing, and regenerate lost tissues [25,26]. Given the data, adjuvant therapies may play an essential role in using less aggressive methods at the implant level compared with traditional surgical interventions.
One such agent is hyaluronic acid (HA), a genuine linear glycosaminoglycan that is present within the extracellular matrix of connective, epithelial, and neural tissues [25,26,27]. It has an essential role in tissue repair [28,29,30], inflammation control [29,30], and antimicrobial [30,31,32,33] and cellular transmission [31,32,33,34,35].
Generally, HA shows multiple biological functions by interacting with specific cell-surface receptors, such as CD44 and RHAMM [33,35], thereby influencing cell proliferation, migration, and angiogenesis [34,35]. It also aids in matrix stabilization, hydration, and tissue viscoelasticity [31,34,35]. Moreover, HA has intrinsic anti-inflammatory [36,37,38] and bacteriostatic properties [39], making it particularly useful for treating biofilm-related infections [40,41,42], such as peri-implantitis [43,44] or other oral tissue defects [45,46,47].
Depending on its molecular weight, which ranges from 10 kDa to over 1000 kDa, hyaluronic acid (HA) can significantly influence its biological behavior [27]. This includes the following: (a) Non-modified linear HA [36] is characterized by two main variants—high-molecular-weight HA (HMW-HA, >1000 kDa) and low-molecular-weight HA (LMW-HA, <500 kDa). HMW-HA has been shown to exert anti-inflammatory and immunomodulatory effects by suppressing cytokines such as IL-1β and TNF-α, while stabilizing the extracellular matrix, thereby forming a protective barrier against oxidative stress and microbial invasion. As a result, it encourages fibroblast proliferation and collagen synthesis, promoting soft tissue regeneration [27,36]. Conversely, LMW-HA may act as a proinflammatory molecule, interacting with Toll-like receptors (TLR 2 and TLR 4), and stimulating immune responses with the production of IL-6 and IL-8 [36]. Consequently, it exhibits high tissue permeability and a strong ability to support soft tissue healing. It also makes microbial biofilms more vulnerable to mechanical debridement, increasing the permeability of microbial membranes [27]. (b) Modified linear HA (cross-linked hyaluronic acid—CLHA or xHA) undergoes chemical modifications to extend bioavailability, resulting in a stable gel that enhances hydration and promotes tissue regeneration [36]. Some studies [46,48] have shown that it reduces probing depths and improves bone fill. Additionally, various studies involving animals and humans have demonstrated that it stimulates fibroblast and osteoblast differentiation, aiding tissue and bone regeneration [42,43,46,48,49,50]. (c) Bioengineered HA-enriched systems include antibiotics, growth factors, and scaffolds [36]. Rapid advances in biomaterials and tissue engineering have led to the development of HA-enriched systems such as microspheres, microtubes [36], injectable hydrogels [48], scaffold coatings [51,52,53,54], nanoengineered surfaces [52,53,54], and HA- based drug delivery structures [53]. These biostructures aim to improve biocompatibility, regulate local drug release, and support osseointegration and soft tissue healing [53].
New therapeutic approaches in bio-tissue engineering [54,55,56] have shown the potential use of HA either alone or combined with various molecules or bone graft materials [57,58], or as a therapeutic agent in a preventive strategy [59,60,61,62,63] for patients with clinically healthy signs of peri-implant tissues.
Nevertheless, to our knowledge, the literature lacks studies specifically related to the efficacy of HA or its modified variants for peri-implantitis treatment.
2. Materials and Methods
This scoping review was conducted according to Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines [63,64,65]. A Patient–Concept/Exposure–Context/Outcome (PCC/PEO) modified version of the PICO (Population, Intervention, Comparison, Outcome) model was applied for this review to formulate focused questions and to determine and assess the outcomes of this oral health setting [66,67]. The PCC model applied for this review followed three main elements: Population—patients with peri-implantitis/peri-implant disease/dental implant failure; Concept—HA used as treatment strategy in peri-implantitis management; Context/Outcome—no limitation was applied for any type of specific cultural setting.
2.1. Focused Objectives
- To assess the outcomes of hyaluronic acid as a non-surgical treatment strategy for patients with peri-implantitis.
- To assess the HA’s effect as an adjuvant therapeutic agent on the clinical parameter criteria involved in peri-implantitis.
2.2. Search Strategy and Data Sources
To reduce bias and maintain consistency, two researchers (BB and AO) independently conducted the research, assessing articles published between January 2015 and January 2025 from the following databases: Medline/PubMed, ScienceDirect, Scopus, Web of Science (WOS), and Grey literature (www.worldcat.org; www.greynet.org).
For the four databases, the electronic search was accomplished by using the medical subject headings (MeSH terms), specific keywords and terms that are not indexed; words such as “peri-implantitis*,” “hyaluronic acid,” “implant failure”, “antimicrobial agents,” “bone regeneration”, “regenerative therapy”, and “biomaterials” were inserted within the electronic search along with Boolean operators (AND/OR). Additionally, we applied a manual search for Grey literature consultation. The electronic research was supplemented by a manual application of the filters (such as time range (2015–2025), article type, studies on humans, and English language) to focus the findings based on the inclusion criteria. Thus, a central core of research strategy was applied for all four databases: (“Hyaluronic Acid” OR crosslinked) AND (hyaluronic) AND (acid OR hyaluronan) AND (“dental implant failure” OR “peri-implantitis” OR “peri-implant defect”) AND dental implants) as either medical subject headings (MeSH) or keywords. A detailed electronic search strategy is listed in the Supplementary Materials Section, Table S1.
2.3. Eligible Criteria
Along with the research strategy, we used some inclusion and exclusion criteria as shown below (Table 1). Only clinical trials, prospective case series, or any type of clinical study related to the subject, published in English, were included. Studies were screened by title and abstract; full texts were reviewed when relevant.

Table 1.
Inclusion and exclusion criteria used for the search strategy.
2.4. Extraction and Method of Data Assessment
To organize the articles, we utilized Rayyan.ai, a web-based application for systematic reviews [68] with the Blind on function, and then we used the PRISMA flowchart [64] (Figure 1). After the resolution of the conflicts within the Rayyan application between the two researchers, we were able to collect the results based on bibliographic details (authors, title, publication year, and journal), methodology, study design, sample size, key findings, and outcomes. At the end of the data extraction, a third researcher (RSC) checked the completeness, integrity, and accuracy of the initial data extraction.
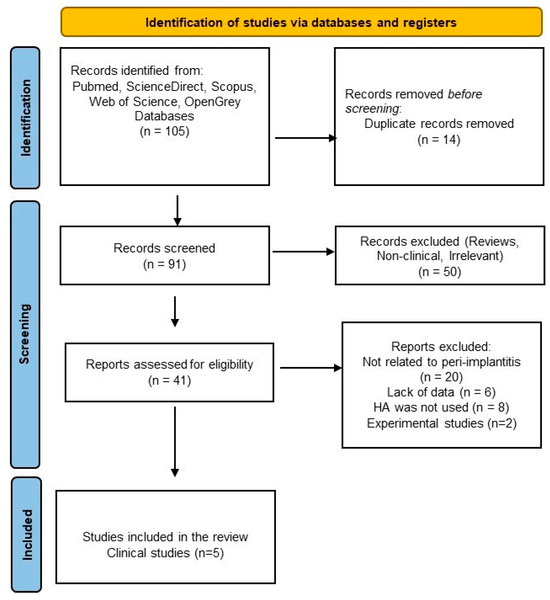
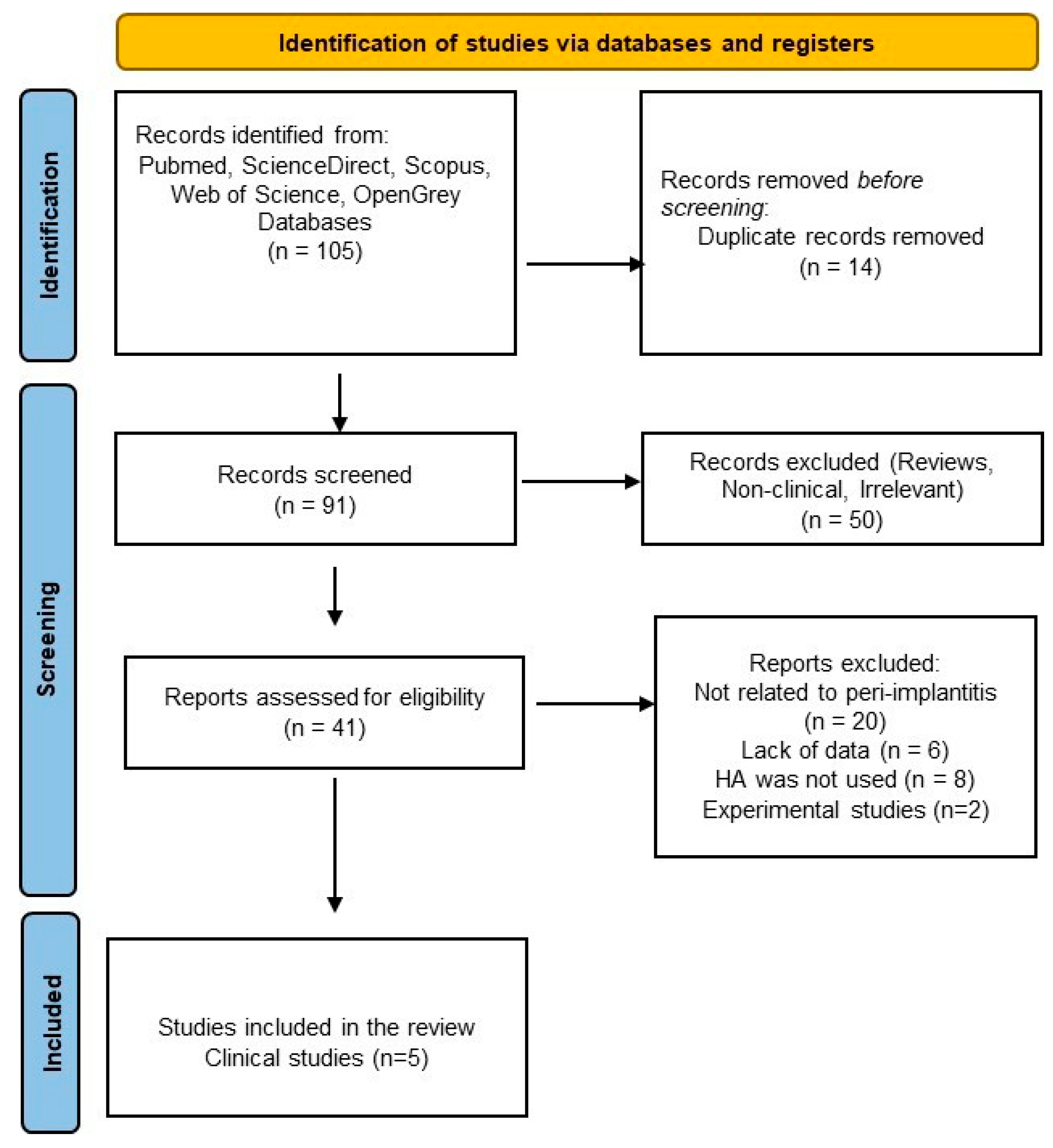
Figure 1.
PRISMA flowchart for the literature selection process.
Based on the modified PCC model adopted for this review, which maps the current trends of hyaluronic application as a treatment strategy in humans, the quality assessment was not accomplished, despite the individual studies and clinical outcomes of the analyzed treatment. This agrees with the PRISMA-ScR guidelines, which state that the risk of bias analysis among the articles should not be performed in the case of a scoping review [64,65].
3. Results of the Literature Research
Initially, a total of 530 results were obtained from a rough search of each database (PubMed, ScienceDirect, Scopus, Web of Science, and Grey literature) [see Supplementary Table S1]. After the applied filters were inserted and a thorough manual checking, a total of 105 studies were inserted in the Rayyan application (18 studies for PubMed, 10 studies for Scopus, 64 for ScienceDirect, and 13 studies for WOS).
After screening titles and abstracts from the total number of studies, 14 were removed due to duplication; 50 articles were excluded because they were irrelevant or lacked clinical data. The full texts of forty-one potentially eligible articles were reviewed, and ultimately, only five clinical trials were selected for further final assessment: two randomized clinical trials (RCTs), two pilot RCTs, and one prospective case series [69,70,71,72,73]. The literature selection process is outlined in the PRISMA flowchart (Figure 1).
The results were summarized to provide a structured understanding of the impact of hyaluronic acid in peri-implantitis therapy, based on the analyzed studies. Below are revised tables (Table 2 and Table 3), followed by a detailed interpretation of the clinical and biomaterial data.

Table 2.
Summary of clinical trials involving the use of hyaluronic acid in peri-implantitis.

Table 3.
Treatment strategies of hyaluronic acid (HA) found based on the assessed studies.
Overall, 157 participants were enrolled in the clinical studies (Table 2). Two of the studies [68,70] divided patients into two control groups (n = 21). As they mentioned [69], both studies were conducted by researchers from the same research group.
Nearly 250 dental implants were analyzed within the clinical studies (Table 2). Notably, two studies investigated the anti-inflammatory and antimicrobial effects of HA [69,70], one used HA in reconstructive surgery [71], one study used HA as adjuvant therapy [72], and one study analyzed the effects of a combination of HA mixed with ribose-collagen matrix and reconstructive surgical intervention [73].
4. Discussion
The detailed use of hyaluronic acid in periodontal therapy and bone regeneration has been recently and widely discussed [28,29,37,38,41,46,48,50]. However, in the past decade, several recent experimental and animal studies [49,74,75,76,77], a meta-analysis [34], and five clinical studies [69,70,71,72,73] have been published on hyaluronic acid used as a therapeutic or adjuvant biomaterial related to peri-implantitis treatment management.
This scoping review compiles current clinical trends and biomaterial-based evidence on the therapeutic role of hyaluronic acid (HA) in managing peri-implantitis. Five clinical trials were assessed for HA’s capacity to reduce inflammation, promote tissue regeneration, and improve clinical parameters related to peri-implant healing outcomes.
Considering the limited number of randomized clinical trials and clinical case studies conducted to date on the effects and benefits of HA in peri-implantitis diseases, we have decided to provide our analysis of the literature, focusing primarily on the four therapeutic concepts (Table 3): (A) anti-inflammatory and antibacterial effect; (B) reconstructive regenerative therapy; (C) HA-mixed derivatives and reconstructive therapy; (D) adjuvant therapy.
4.1. Treatment Strategies of Hyaluronic Acid for Peri-Implantitis
- (A)
- Anti-inflammatory and antibacterial effects
It is well-known that hyaluronic acid has a bacteriostatic potential [39]; however, recently, most studies have focused their research on the anti-inflammatory and antibacterial ability of HA towards the periodontal microbiome [25,30,35]. But the newly developed research technologies based on tissue engineering have emphasized the importance of linear HA for different dental research areas [26,27].
In their clinical research, Lucarini et al. [33] investigated some markers of inflammation related to peri-implantitis; thus, they showed that the mucosal tissues affected by peri-implantitis had a high concentration of vascular endothelial growth factor (VEGF), micro-vascular densities (MVDs), and also CD-44. But in a systematic review conducted by Duarte et al. [74], it was shown that there was a high concentration of proinflammatory cytokines (interleukin [IL]-1β, IL-6, IL-12, IL-17) and tumor necrosis factor [TNF]-α in the crevicular fluid in the case of peri-implantitis compared with healthy areas. Moreover, in a 5-year follow-up study, a significantly high concentration of tumor necrosis factor [TNF]-α and the presence of salivary biomarkers (IL-1β, IL-10, RANK, OPG, MMP-2, TGF-β) were observed when no peri-implant maintenance therapy was applied [75].
Recently, a systematic review [76] highlighted the importance of considering alternative treatment options for peri-implant diseases when the oral pathogens persist despite the use of conventional therapies.
One of the alternative therapies is proposed in their clinical trial by Soriano-Lerma et al. [70]. They treated patients with a 0.8% subgingival HA gel, which resulted in a significant decrease in stratum 2 pathogens (Streptococcus, Veillonella, Rothia, and Granulicatella), and a similar significant decrease in stratum 3 pathogens (Prevotella and Campylobacter) (p < 0.05) after using the tested HA gel.
An in vitro study demonstrated the high efficiency of HA over P. gingivalis, acting at the gene level (fimA, mfa1, hagA, rgpA, and kgp), compared to Azithromycin and Chlorhexidine [77].
Peri-implantitis is a condition defined as being activated by inflammation of the implant-surrounding mucosal tissue and bone loss [5,78]. But as it has been shown by other studies [35], HA can reduce inflammation by drastically decreasing the immune markers.
Thus, Sánchez-Fernández et al. [69] showed significant reductions in the proinflammatory cytokine IL-1β and a notable decrease in probing depth, indicating effective modulation of inflammation and clinical improvement in patients with peri-implantitis.
- (B)
- Reconstructive Regenerative Therapy
An important component to sustain a healthy integrated dental implant is the bone site where it is inserted. It was shown that bone loss and various bone defects are caused by peri-implantitis conditions [3,22]. One of the traditional ways considered a “golden standard” in treating such a condition is the surgical reconstructive method [4,23,24]. Given the high complexity of peri-implantitis, it has been observed over time that the sole implementation of surgical interventions does not always yield a positive outcome in cases of peri-implant diseases [24]. Incorporating additional anti-inflammatory and antibacterial non-surgical methods could potentially enhance the outcomes [69,70,76].
Considering that another property of HA is its ability to induce cell differentiation and bone regeneration [42], regenerative therapy may be an area where it can be effectively used [37].
Periodontal therapy and oral surgery have become increasingly associated with the use of HA [37,38,46,48]; thus, many studies have reported its beneficial properties in repairing periodontal bone and soft tissue defects [57,79].
Focusing on regenerative outcomes by combining HA with a bovine bone graft (BBG), Rakasević et al. [71] reported a significant enhancement of bone fill and improved soft tissue healing in peri-implant defects; also, a decrease in BOP was reported.
Recently, one meta-analysis [45] and an experimental animal study [80] reported a beneficial use of HA in bone defect reconstruction during surgical and periodontal procedures. This might be due to the use of HA gel, which has a flowable viscosity and therefore did not succeed in maintaining the surgical periodontal site [45]. This constructive interaction between HA and grafting materials supports its role in regenerative strategies.
- (C)
- HA-mixed derivatives and Regenerative Reconstructive therapy
A combination of cross-linked HA + ribose-crosslinked collagen (RCLC) matrix was used by Friedmann et al. [73] for reconstructive surgical treatment in peri-implantitis bone defects. The authors reported closure of the pocket defect within six weeks after surgical treatment, reduced bleeding sites and 62.8% of bone fill and improvement of radiographic bone defects after 12 months of follow-up. These results are in agreement with the findings reported by other authors [38,48]. This could be explained by the ability of HA to act at the cell level as an anti-inflammatory agent. Nevertheless, this is not in agreement with Rakasević et al. [71] who reported a complete reduction after six months postoperatively. Moreover, Sánchez-Fernández et al. [69] and Lopez et al. [72] reported a decrease in the BOP indicator. Still, this information must be considered with precaution and awareness, knowing that in the case of peri-implantitis, an inflammatory condition may exist even when the POB indicator has decreased clinical values [78].
Recently, there have been significant advancements in bioengineered variants of HA mixed with different antibacterial molecules/particles [81], molecule-carriers to induce osteogenesis [82], or 3D printed scaffolds for bone regeneration and repair [83]. All of these showed a favorable behavior of HA, but more clinical studies are necessary to fill the knowledge gap.
- (D)
- Adjuvant therapy
Adjuvant treatment in case of peri-implantitis refers to the local application of Chlorhexidine or antibiotics with or without additional conventional surgical interventions [83,84].
From a therapeutic standpoint, HA offers clear advantages over conventional antiseptics, such as Chlorhexidine or other substances [84,85], which, although effective, are associated with cytotoxicity and delayed tissue healing [27,45,53,57]. In contrast, HA promotes hydration, angiogenesis, and matrix remodeling, facilitating faster and safer recovery [27,53,59].
Since 2017, Lopez et al. [72] have attempted to utilize an adjuvant approach to nebulized HA application and analyze it in a non-surgical context. The authors showed the ability of sprayed HA to improve clinical signs of inflammation, particularly probing depth and bleeding scores, within just 8 weeks. These findings are partially in agreement with the other clinical studies [69,70]. The results reported by Lopez et al. [72] (reductions in probing depths and bleeding indices after nebulized HA use), align with Pilloni et al. [37] and Vela et al. [38] and also with the findings described by Iorio-Siciliano et al. [46] and Pilloni et al. [79].
- (E)
- Preventive therapy
During the assessment of the present review, we observed a tendency of the researchers to use HA and bioengineered HA-enriched systems in a more preventive way. Although this concept of therapy was not included in our search, we will discuss below the findings of some of the clinically related articles [60,62,63] to address the broader second question of this paper and in conjunction with the fourth therapeutic strategy (“(D) Adjuvant Therapy”).
We understand “preventive therapy” by using HA alone or mixed (1) applied at the moment when the implant is inserted into the bone [60,62,63] and (2) applied on the dental implant’s surface employing tissue engineering methods [56,61,86,87].
A few years ago, in a clinical study, Kaya et al. [63] used a combination of HA with a xenograft and collagen membrane applied directly on implants inserted in Class I buccal dehiscence bone type (small and medium-sized). They reported a significant increase in vertical bone level for medium-sized bony defects in 6 months, and a statistical overall increase in vertical bone height for both bone type defects within the groups where they used HA mixed with xenograft and collagen matrix. Other groups of researchers also found similar findings: Pilloni et al. [37,79], Iorio-Siciliano et al. [46], and Vela et al. [38].
In the same period, other researchers considered using hyaluronic acid at the biomolecular level. Thus, a biomolecular coating of a “covalently linked nanolayer of hyaluronan” industrially placed on the surface of Ti implants was tested by Lupi et al. in a crossover clinical trial [62] in comparison with SLA-based Ti implants at a 36-month follow-up. In this randomized crossover clinical investigation, clinical success was reported for HA-coated Ti implants compared with the control group of acid-etched surface Ti implants. These findings are sustained by recent data that implied the use of tissue bioengineering technology [61,86,87].
Recently, new preventive approaches were used in an RCT study by Bawankar et al. [60], who combined the microneedling technique with HA injectable gel application as a preventive method. The authors reported a successful reduction in inflammation and an increase in the keratinized tissue around the papilla. These results agree with similar studies that tested HA as an anti-inflammatory agent [69,70,72].
Biomaterial-focused studies expand the role of HA into the domain of multifunctional tissue engineering platforms. Zhou et al. [81] developed a hydrogel matrix incorporating HA with chitosan and dexamethasone, resulting in a system with dual functionality: anti-inflammatory and antimicrobial properties. This hydrogel demonstrated effective epithelial healing and suppression of common peri-implant pathogens, such as P. gingivalis, making it a strong candidate for managing infected sites. This finding is consistent with those described by Alharbi et al. [77], who reported a reduction in the cellular level of P. gingivalis, particularly for specific virulence genes (fimA, mfa1, hagA, and rgp).
Various groups of researchers, such as Chua et al. [56], Li et al. [86], and Wang et al. [87], have studied different types of surface bioengineered coatings for titanium implants using HA combined derivatives (polyelectrolytic layer with HA/chitosan, microRNA-21-loaded nanoparticles, and a HBPL (hyperbranched polylysine) antimicrobial layer), and thus, the integration of HA with gene-modulatory agents suggesting a frontier for regenerative therapies. Furthermore, this system promoted gingival fibroblast adhesion and proliferation. These studies demonstrate that HA-based coatings enhance osteoconductivity and cellular integration on implant surfaces, which expands their functionality to prevent infection and promote long-term implant success.
Oral hygiene for patients with dental implants is very important and is strongly linked to soft tissue inflammation and bone loss [5,7]. In a retrospective cohort study [88] that followed 475 participants and 1991 implants over 10 years, they found that signs of peri-implantitis usually do not appear before year five, with a peak around year seven. Implementing preventive methods, such as HA alone or bioengineered HA-enriched systems, immediately or within the first 12 months seems to be a relevant treatment strategy to prevent the progression of mucosal inflammation and bone loss.
Our assessment of the literature in this scoping review aimed to highlight the current trend of using hyaluronic acid as a therapeutic approach for peri-implantitis. We identified four key therapy concepts: anti-inflammatory and antimicrobial treatments; reconstructive regenerative therapy; HA-mixed collagen matrix combined with reconstructive regenerative therapy; and adjuvant treatment. Because clinical signs of peri-implantitis tend to appear later after the implant is placed in the bone tissue [88], a preventive method was also considered for discussion. Thus, HA’s biocompatibility and unique viscoelastic properties make it a versatile biomaterial and an effective adjunctive agent for regenerative procedures and infection control, especially in cases of peri-implant diseases.
4.2. Limitations of the Study and Future Research
The assessed clinical investigations vary in design, sample size, HA type, application frequency, and follow-up duration. To underscore the importance of the inconsistencies mentioned above, we must note that studies by Lucarini et al. [33] and other researchers [9,10,11,12,13,14,15] have observed variability in HA’s effectiveness based on patient factors, such as smoking and various systemic health.
The inconsistency in applying clinical protocols is a major problem for conducting clinical investigations. Furthermore, multiple studies [89,90,91] have called for standardized protocols and more extended follow-up periods for peri-implant disease treatments and therapies.
While some studies applied HA once during surgery [71,73], others performed multiple subgingival applications over several weeks [69,70,72]. Only a few studies measured long-term outcomes, such as implant survival or radiographic bone levels [88].
Despite encouraging outcomes, several limitations were noticed in the current body of research:
- -
- All the studies had small sample sizes, limiting the generalizability of results.
- -
- There was substantial heterogeneity in HA formulations used, including variations in molecular weight, cross-linking, and concentration.
- -
- Application protocols varied, with inconsistencies in treatment duration, dosage frequency, and delivery methods.
- -
- Follow-up durations were often short (≤24 months), restricting conclusions about long-term implant stability.
- -
- Patient-related factors such as smoking, systemic diseases, or prior periodontitis were often not controlled for or stratified.
Future studies should include patient-reported outcome measures (PROMs), microbiological profiles, and biomarker analyses [30,75] to gain a more comprehensive understanding of HA treatment effectiveness. Additionally, the use of Artificial Intelligence for predicting implant failure and/or peri-implantitis could be considered for future clinical applications [92,93,94].
The versatility of HA, based on its physicochemical properties and molecular interactions [30], makes it highly adaptable for designing next-generation therapies.
5. Conclusions
Given the limitations of the study, the following conclusions emerged:
- HA may be associated with improvements in clinical indicators of peri-implantitis (including inflammatory markers, probing depth, bleeding indices, and the healing of bone and soft tissue).
- HA may be effectively used as a potential additional treatment agent for bone loss or bone defects in peri-surgical regenerative dentistry.
- Overall, HA may be considered a safe, multifunctional adjunctive agent in peri-implantitis management.
- HA showed favorable potential in preventive treatment for patients with dental implants.
- However, clinical heterogeneity continues to be a concern; further clinical studies are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oral5030068/s1, Table S1: Summary Table of the Research Strategy.
Author Contributions
Conceptualization, B.B. and A.O.; methodology, B.B.; software, B.B.; validation, B.B., A.O. and R.S.C.; investigation, B.B. and A.O.; resources, A.O.; data curation, B.B. and A.O.; writing—original draft preparation, B.B.; writing—review and editing, A.O.; visualization, A.O.; supervision, R.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lindhe, J.; Meyle, J.; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T.; Working Group 4 of the Seventh European Workshop on P. Periimplant diseases: Where are we now?—Consensus of the seventh European workshop on periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 178–181. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Meyle, J. Peri-implant tissue destruction. The third EAO consensus conference 2012. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 108–110. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and treatment of peri-implant diseases-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 4–76. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Aaboe, M.; Araujo, M.; Carrión, J.B.; Cavalcanti, R.; Cionca, N.; Cochran, D.; Darby, I.; Funakoshi, E.; Gierthmuehlen, P.C.; et al. Group 4 ITI Consensus Report: Risks and Biologic Complications Associated with Implant Dentistry. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 351–358. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L. Peri-implant mucositis and peri-implantitis: Key features and differences. Br. Dent. J. 2024, 236, 791–794. [Google Scholar] [CrossRef]
- Misch, C.E.; Suzuki, J.B.; Misch-Dietsh, F.M.; Bidez, M.W. A positive correlation between occlusal trauma and peri-implant bone loss: Literature support. Implant. Dent. 2005, 14, 108–116. [Google Scholar] [CrossRef]
- Guney, Z.; Altingoz, S.; Has, H.; Serdar, M.A.; Kurgan, S. The impact of electronic cigarettes on peri-implant health: A systematic review and meta-analysis. J. Dent. 2024, 143, 104883. [Google Scholar] [CrossRef]
- Giovannoli, J.-L.; Roccuzzo, M.; Albouy, J.-P.; Duffau, F.; Lin, G.-H.; Serino, G. Local risk indicators—Consensus report of working group 2. Int. Dent. J. 2019, 69 (Suppl. S2), 7–11. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pimentel, S.P.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z. Host response and peri-implantitis. Braz. Oral Res. 2019, 33 (Suppl. S1), e066. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: A systematic literature review. J. Periodontol. 2009, 80, 1719–1730. [Google Scholar] [CrossRef]
- Yip, J.K.; Borrell, L.N.; Cho, S.; Francisco, H.; Tarnow, D.P. Association between oral bisphosphonate use and dental implant failure among middle-aged women. J. Clin. Periodontol. 2012, 39, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Salcetti, J.M.; Moriarty, J.D.; Cooper, L.F.; Smith, F.W.; Collins, J.G.; Socransky, S.S.; Offenbacher, S. The clinical, microbial, and host response characteristics of the failing implant. Int. J. Oral Maxillofac. Implant. 1997, 12, 32–42. [Google Scholar]
- Ting, M.; Suzuki, J.B. Peri-Implantitis. Dent. J. 2024, 12, 251. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.M.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhu, Y.; Fan, L.F.; Zhang, X.; Jiang, Y.H.; Gu, Y.X. Intra-and inter-observer agreements in detecting peri-implant bone defects between periapical radiography and cone beam computed tomography: A clinical study. J. Dent. Sci. 2021, 16, 948956. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Schwarz, F. Current Concepts for the Treatment of Peri-implant Disease. Int. J. Prosthodont. 2024, 37, 124–134. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Mitrea, M.; Bozomitu, L.I.; Mihaela, M.; Tepordei, R.T.; Gurzu, I.L.; Walid, E.A.H.; Niculescu, S.; Tecuceanu, A.; Ciurcanu, O.E.; Moraru, M.C.; et al. Our Experience Regarding Peri-Implantitis. Rom. J. Oral Rehabil. 2023, 15, 379–385. [Google Scholar]
- Serino, G.; Turri, A.; Lang, N.P. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin. Oral Implant. Res. 2013, 24, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.; Claffey, N. Surgical therapy for peri-implantitis. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 84–94. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Bhati, A.; Fageeh, H.; Ibraheem, W.; Fageeh, H.; Chopra, H.; Panda, S. Role of Hyaluronic Acid in Periodontal Therapy (Review). Biomed. Rep. 2022, 17, 91. [Google Scholar] [CrossRef]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.D.; Trilli, I.; Ferrante, L.; Casamassima, L.; Nardelli, P.; Inchingolo, F.; Palermo, A.; Severino, M.; Inchingolo, A.M.; et al. Recent Use of Hyaluronic Acid in Dental Medicine. Materials 2025, 18, 1863. [Google Scholar] [CrossRef]
- Mostafa, D.; Alzahrani, M.; Alatawi, J.A.; Alsirhani, S.F.; Alshehri, A.; Mazyed Almutiri, A. Effect of Hyaluronic Acid Gel on Healing of Simple Dental Extraction Sockets: A Pilot Study. Open Access Maced. J. Med. Sci. 2021, 9, 190–195. [Google Scholar] [CrossRef]
- Jentsch, H.; Pomowski, R.; Kundt, G.; Göcke, R. Treatment of Gingivitis with Hyaluronan. J. Clin. Periodontol. 2003, 30, 159–164. [Google Scholar] [CrossRef]
- Pardo, A.; Magnani, V.; Montagna, P.; Ala, A.; Brancato, G.; Melloni, F.; Lombardo, G.; De Santis, D. Clinical, Microbiological, and Biochemical Outcomes of Hyaluronic Acid in Non-Surgical Periodontal Therapy: A Systematic Review and Meta-Analysis. Appl. Sci. 2025, 15, 5975. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Valachová, K.; Hassan, M.E.; Šoltés, L. Hyaluronan: Sources, Structure, Features and Applications. Molecules 2024, 29, 739. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, G.; Zizzi, A.; Rubini, C.; Ciolino, F.; Aspriello, S.D. VEGF, Microvessel Density, and CD44 as Inflammation Markers in Peri-Implant Healthy Mucosa, Peri-Implant Mucositis, and Peri-Implantitis: Impact of Age, Smoking, PPD, and Obesity. Inflammation 2019, 42, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valverde, N.; López-Valverde, A.; Blanco Rueda, J.A. Role of Hyaluronic Acid in the Treatment of Peri-Implant Diseases: Results of a Meta-Analysis. Front. Oral Health 2025, 6, 1564599. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, R.; Prpic, J. Hyaluronic Acid: The Reason for Its Variety of Physiological and Biochemical Functional Properties. Appl. Clin. Res. Clin. Trials Regul. Aff. Heitz 2019, 6, 112–159. [Google Scholar] [CrossRef]
- Pilloni, A.; Rojas, M.A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of Intrabony Defects Following Regenerative Surgery by Means of Single-Flap Approach in Conjunction with Either Hyaluronic Acid or an Enamel Matrix Derivative: A 24-Month Randomized Controlled Clinical Trial. Clin. Oral Investig. 2021, 25, 5095–5107. [Google Scholar] [CrossRef]
- Vela, O.C.; Boariu, M.; Rusu, D.; Iorio-Siciliano, V.; Ramaglia, L.; Boia, S.; Radulescu, V.; Ilyes, I.; Stratul, S.-I. Healing of Periodontal Suprabony Defects Following Treatment with Open Flap Debridement with or without Hyaluronic Acid (HA) Application. Medicina 2024, 60, 829. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic Effects of Hyaluronic Acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef]
- Kalimeri, E.; Roccuzzo, A.; Stähli, A.; Oikonomou, I.; Berchtold, A.; Sculean, A.; Kloukos, D. Adjunctive Use of Hyaluronic Acid In The Treatment Of Gingival Recessions: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2024, 28, 329. [Google Scholar] [CrossRef]
- Shukla, K.; Pebbili, K.K. Role of Hyaluronic Acid during Periodontal Therapy and Post-Periodontal Surgeries. Arch. Dent. Res. 2023, 12, 89–96. [Google Scholar] [CrossRef]
- Zhu, X.; von Werdt, L.; Zappalà, G.; Sculean, A.; Eick, S.; Stähli, A. In Vitro Activity of Hyaluronic Acid and Human Serum on Periodontal Biofilm and Periodontal Ligament Fibroblasts. Clin. Oral Investig. 2023, 27, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Soanca, A.; Mastica, V.; Pall, E.; Roman, A.; Ciurea, A.; Onet, D.; Micu, L.C.; Rusu, D. A brief overview on the applications of hyaluronic acid in periodontal therapy. Rom. J. Stomatol. 2022, 68, 188–192. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Eliezer, M.; Imber, J.-C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic Acid as Adjunctive to Non-Surgical and Surgical Periodontal Therapy: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Blasi, A.; Mauriello, L.; Salvi, G.E.; Ramaglia, L.; Sculean, A. Non-Surgical Treatment of Moderate Periodontal Intrabony Defects with Adjunctive Cross-Linked Hyaluronic Acid: A Single-Blinded Randomized Controlled Clinical Trial. J. Clin. Periodon. 2024, 52, 310–322. [Google Scholar] [CrossRef]
- Bertl, K.; Bruckmann, C.; Isberg, P.-E.; Klinge, B.; Gotfredsen, K.; Stavropoulos, A. Hyaluronan in Non-Surgical and Surgical Periodontal Therapy: A Systematic Review. J. Clin. Periodontol. 2015, 42, 236–246. [Google Scholar] [CrossRef]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef]
- Kono, S.; Sasaki, S.; Matsuda, S.; Takeda, K.; Iwata, T.; Ouhara, K.; Kajiya, M.; Kurihara, H.; Mizuno, N. Brain-Derived Neurotrophic Factor Promotes Bone Regeneration in a Canine Model of Peri-Implantitis. Int. J. Implant. Dent. 2024, 10, 59. [Google Scholar] [CrossRef]
- Pilloni, A.; Marini, L.; Gagliano, N.; Canciani, E.; Dellavia, C.; Cornaghi, L.; Costa, E.; Rojas, M. Clinical, Histological, Immunohistochemical, and Biomolecular Analysis of Hyaluronic Acid in Early Wound Healing of Human Gingival Tissues: A Randomized, Split-Mouth Trial. J. Periodont. 2023, 94, 868–881. [Google Scholar] [CrossRef]
- Takeda, K.; Sakai, N.; Shiba, H.; Nagahara, T.; Fujita, T.; Kajiya, M.; Iwata, T.; Matsuda, S.; Kawahara, K.; Kawaguchi, H.; et al. Characteristics of High-Molecular-Weight Hyaluronic Acid as a Brain-Derived Neurotrophic Factor Scaffold in Periodontal Tissue Regeneration. Tissue Eng. Part A 2011, 17, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Liu, S.; Chen, M.; Zhang, H. Surface Bio-Functionalization of Anti-Bacterial Titanium Implants: A Review. Coatings 2022, 12, 1125. [Google Scholar] [CrossRef]
- Alkabli, J. Recent Advances in the Development of Chitosan/Hyaluronic Acid-Based Hybrid Materials for Skin Protection, Regeneration, and Healing: A Review. Int. J. Biol. Macromol. 2024, 279, 135357. [Google Scholar] [CrossRef] [PubMed]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging Roles of Hyaluronic Acid Bioscaffolds in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface Functionalization of Titanium with Hyaluronic Acid/Chitosan Polyelectrolyte Multilayers and RGD for Promoting Osteoblast Functions and Inhibiting Bacterial Adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Machiulskiene, V.; Dvyliene, U.M.; Eliezer, M.; Sculean, A. Clinical Evaluation of a Novel Combination of Sodium Hypochlorite/Amino Acid and Cross-linked Hyaluronic Acid Adjunctive to Non-surgical Periodontal Treatment: A Case Series. Oral Health Prev. Dent. 2023, 21, 279–284. [Google Scholar] [CrossRef]
- Papuc, A.; Bran, S.; Moldovan, M.; Lucaciu, O.; Armencea, G.; Baciut, G.; Dinu, C.; Onișor, F.; Kretschmer, W.; Baciut, M. How Is Bone Regeneration Influenced by Polymer Membranes? Insight into the Histological and Radiological Point of View in the Literature. Membranes 2024, 14, 193. [Google Scholar] [CrossRef]
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’Amico, C.; Oteri, G.; Cicciù, M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. Int. J. Environ. Res. Public Health 2021, 18, 4670. [Google Scholar] [CrossRef]
- Bawankar, P.V.; Tuli, P.; Kolte, A.P.; Kolte, R.A. Evaluation of the efficacy of microneedling alone and in combination with injectable hyaluronic acid in augmentation of peri-implant soft tissues: A randomized controlled trial. J. Indian Soc. Periodontol. 2024, 28, 643–650. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, Q.; Hong, G.; Bai, Z.; Bian, J.; Xie, H.; Chen, C. A chlorogenic acid-chitosan complex bifunctional coating for improving osteogenesis differentiation and bactericidal properties of zirconia implants. Colloids Surf. B Biointerfaces 2023, 230, 113484. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.M.; Rodriguez y Baena, A.; Cassinelli, C.; Iviglia, G.; Tallarico, M.; Morra, M.; Rodriguez Y Baena, R. Covalently-Linked Hyaluronan versus Acid Etched Titanium Dental Implants: A Crossover RCT in Humans. Int. J. Mol. Sci. 2019, 20, 763. [Google Scholar] [CrossRef] [PubMed]
- Kaya, O.A.; Muglali, M.; Torul, D.; Kaya, I. Peri-implant bone defects: A 1-year follow-up comparative study of use of hyaluronic acid and xenografts. Niger. J. Clin. Pract. 2019, 22, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Munn, Z.; Stern, C.; Aromataris, E.; Lockwood, C.; Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med. Res. Methodol. 2018, 18, 5. [Google Scholar] [CrossRef]
- Khan, K.S.; Kunz, R.; Kleijnen, J.; Antes, G. Systematic Reviews to Support Evidence-based Medicine: How to Review and Apply Findings of Healthcare Research, 1st ed.; Khan, K.S., Kunz, R., Kleijnen, J., Antes, G., Eds.; Hodder Education Publishers: London, UK, 2003; p. 148. [Google Scholar]
- Rayyan—Intelligent Systematic Review. Available online: https://www.rayyan.ai (accessed on 1 February 2025).
- Sánchez-Fernández, E.; Magán-Fernández, A.; O’Valle, F.; Bravo, M.; Mesa, F. Hyaluronic acid reduces inflammation and crevicular fluid IL-1β concentrations in peri-implantitis: A randomized controlled clinical trial. J. Periodontal. Implant. Sci. 2021, 51, 63–74. [Google Scholar] [CrossRef]
- Soriano-Lerma, A.; Magán-Fernández, A.; Gijón, J.; Sánchez-Fernández, E.; Soriano, M.; García-Salcedo, J.A.; Mesa, F. Short-term effects of hyaluronic acid on the subgingival microbiome in peri-implantitis: A randomized controlled clinical trial. J. Periodontol. 2020, 91, 734–745. [Google Scholar] [CrossRef]
- Rakasević, D.; Šćepanović, M.; Mijailović, I.; Mišić, T.; Janjić, B.; Soldatović, I.; Marković, A. Reconstructive peri-implantitis therapy using bovine bone substitute with or without hyaluronic acid: A randomized clinical pilot study. J. Funct. Biomater. 2023, 14, 149. [Google Scholar] [CrossRef]
- Lopez, M.A.; Manzulli, N.; D’Angelo, A.; Lauritano, D.; Papalia, R.; Candotto, V. The use of hyaluronic acid as an adjuvant in peri-implantitis management. J. Biol. Regul. Homeost. Agents 2017, 31, 123–127. [Google Scholar]
- Friedmann, A.; Jung, R.; Bilhan, H.; Ghawi-Begovic, H.A.; Kauffmann, F.; Diehl, D. Reconstructive Surgical Therapy of Peri-Implant Defects with Ribose Cross-Linked Collagen Matrix and Crosslinked Hyaluronic Acid—A Prospective Case Series. Clin. Oral Investig. 2024, 28, 536. [Google Scholar] [CrossRef]
- Duarte, P.M.; Serrão, C.R.; Miranda, T.S.; Zanatta, L.C.; Bastos, M.F.; Faveri, M.; Figueiredo, L.C.; Feres, M. Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J. Periodontal Res. 2016, 51, 689–698. [Google Scholar] [CrossRef]
- Gomes, A.M.; Douglas-de-Oliveira, D.W.; Ferreira, S.D.; Silva, T.A.D.; Cota, L.O.M.; Costa, F.O. Periodontal disease, peri-implant disease and levels of salivary biomarkers IL-1β, IL-10, RANK, OPG, MMP-2, TGF-β and TNF-α: Follow-up over 5 years. J. Appl. Oral Sci. 2019, 27, e20180316. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pisano, M.; Di Palo, M.P.; Salzano, F.; Rupe, A.; Fiorino, A.; Rengo, C. Potential Impact of Microbial Variations After Peri-Implantitis Treatment on Peri-Implant Clinical, Radiographic, and Crevicular Parameters: A Systematic Review. Dent. J. 2024, 12, 414. [Google Scholar] [CrossRef]
- Alharbi, M.S.; Alshehri, F.A. High Molecular Weight Hyaluronic Acid Reduces the Expression of Virulence Genes fimA, mfa1, hagA, rgpA, and kgp in the Oral Pathogen Porphyromonas gingivalis. Pharmaceutics 2022, 14, 1628. [Google Scholar] [CrossRef]
- Yu, X.; Lin, X.; Wang, F.; Wu, Y. Long-term predictive value of bleeding on probing in peri-implantitis diagnosis: A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2024, 24, 102034. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2019, 23, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: A preclinical study in dogs. Quintessence Int. 2021, 52, 308–316. [Google Scholar] [PubMed]
- Zhou, Z.; Zhang, Q.; Wang, Y. Preparation and characterization of antibacterial and anti-inflammatory hyaluronic acid-chitosan-dexamethasone hydrogels for peri-implantitis repair. J. Biomater. Appl. 2022, 36, 1141–1150. [Google Scholar] [CrossRef]
- Pan, H.; Han, J.J.; Park, Y.D.; Cho, T.H.; Hwang, S.J. Effect of sustained release of rhBMP-2 from dried and wet hyaluronic acid hydrogel carriers compared with direct dip coating of rhBMP-2 on peri-implant osteogenesis of dental implants in canine mandibles. J. Cranio-Maxillofac. Surg. 2016, 44, 116–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, M.; Zhu, J.; Li, T.; Li, N.; Su, B.; Sun, G.-D.; Li, L.; Zhou, C. Exosome-loaded hyaluronic acid hydrogel composite with oxygen-producing 3D printed polylactic acid scaffolds for bone tissue repair and regeneration. Int. J. Biol. Macromol. 2024, 274, 132970, Erratum in: Int. J. Biol. Macromol. 2025, 308, 142032. https://doi.org/10.1016/j.ijbiomac.2025.142032. [Google Scholar] [CrossRef]
- de Araújo Nobre, M.; Cintra, N.; Maló, P. Peri-Implant Maintenance of Immediate Function Implants: A Pilot Study Comparing Hyaluronic Acid and Chlorhexidine. Int. J. Dent. Hyg. 2007, 5, 87–94. [Google Scholar] [CrossRef]
- Benyei, L.; Friedmann, A.; Ostermann, T.; Kotsakis, D. Non-surgical treatment of residual periodontal pockets using sodium hypochlorite/amino acid gel and cross-linked hyaluronic acid-a 9-month pilot randomized controlled clinical trial. Clin. Oral Investig. 2024, 28, 513. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Yu, H.; Zhang, S.; Liang, H. Construction of an HBPL antibacterial coating on a phase-transition lysozyme-modified titanium surface. Front. Oral Health 2025, 6, 1615280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, G.; Yang, Z.; Li, X.; Feng, Z.; Zhao, Y. Chitosan/Hyaluronic Acid/MicroRNA-21 Nanoparticle-Coated Smooth Titanium Surfaces Promote the Functionality of Human Gingival Fibroblasts. Int. J. Nanomed. 2022, 17, 3793–3807. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, A.; Rinaldo, F.; Pasqualotto, D.; Sorrentino, F.; La Torre, G.; Guerra, F. A retrospective cohort study on peri-implant complications in implants up to 10 years of functional loading in periodontally compromised patients. J. Periodontol. 2020, 91, 995–1002. [Google Scholar] [CrossRef]
- Morton, D.; Wismeijer, D.; Chen, S.; Hamilton, A.; Wittneben, J.; Casentini, P.; Gonzaga, L.; Lazarin, R.; Martin, W.; Molinero-Mourelle, P.; et al. Group 5 ITI Consensus Report: Implant Placement and Loading Protocols. Clin. Oral Implant. Res. 2023, 34, 349–356. [Google Scholar] [CrossRef]
- Hart, I.; Wells, C.; Tsigarida, A.; Bezerra, B. Effectiveness of mechanical and chemical decontamination methods for the treatment of dental implant surfaces affected by peri-implantitis: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2024, 10, e839. [Google Scholar] [CrossRef]
- De Santis, D.; Graziani, P.; Castellani, R.; Zanotti, G.; Gelpi, F.; Marconcini, S.; Bertossi, D.; Nocini, P.F. A New Radiologic Protocol and a New Occlusal Radiographic Index for Computer-Guided Implant Surgery. J. Craniofac. Surg. 2016, 27, e506–e510. [Google Scholar] [CrossRef]
- Rekawek, P.; Herbst, E.A.; Suri, A.; Ford, B.P.; Rajapakse, C.S.; Panchal, N. Machine Learning and Artificial Intelligence: A Web-Based Implant Failure and Peri-Implantitis Prediction Model for Clinicians. Int. J. Oral Maxillofac. Implant. 2023, 38, 576–582b. [Google Scholar] [CrossRef]
- Mugri, M.H. Accuracy of Artificial Intelligence Models in Detecting Peri-Implant Bone Loss: A Systematic Review. Diagnostics 2025, 15, 655. [Google Scholar] [CrossRef]
- Baima, G.; Citterio, F.; Romandini, M.; Romano, F.; Mariani, G.M.; Buduneli, N.; Aimetti, M. Surface Decontamination Protocols for Surgical Treatment of Peri-Implantitis: A Systematic Review with Meta-Analysis. Clin. Oral Implant. Res. 2022, 33, 1069–1086. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).