Mechanistic Insights into Targeting SARS-CoV-2 Papain-like Protease in the Evolution and Management of COVID-19
Abstract
1. Introduction
2. Implications for Viral Evolution (Target Enzymes and Receptors)
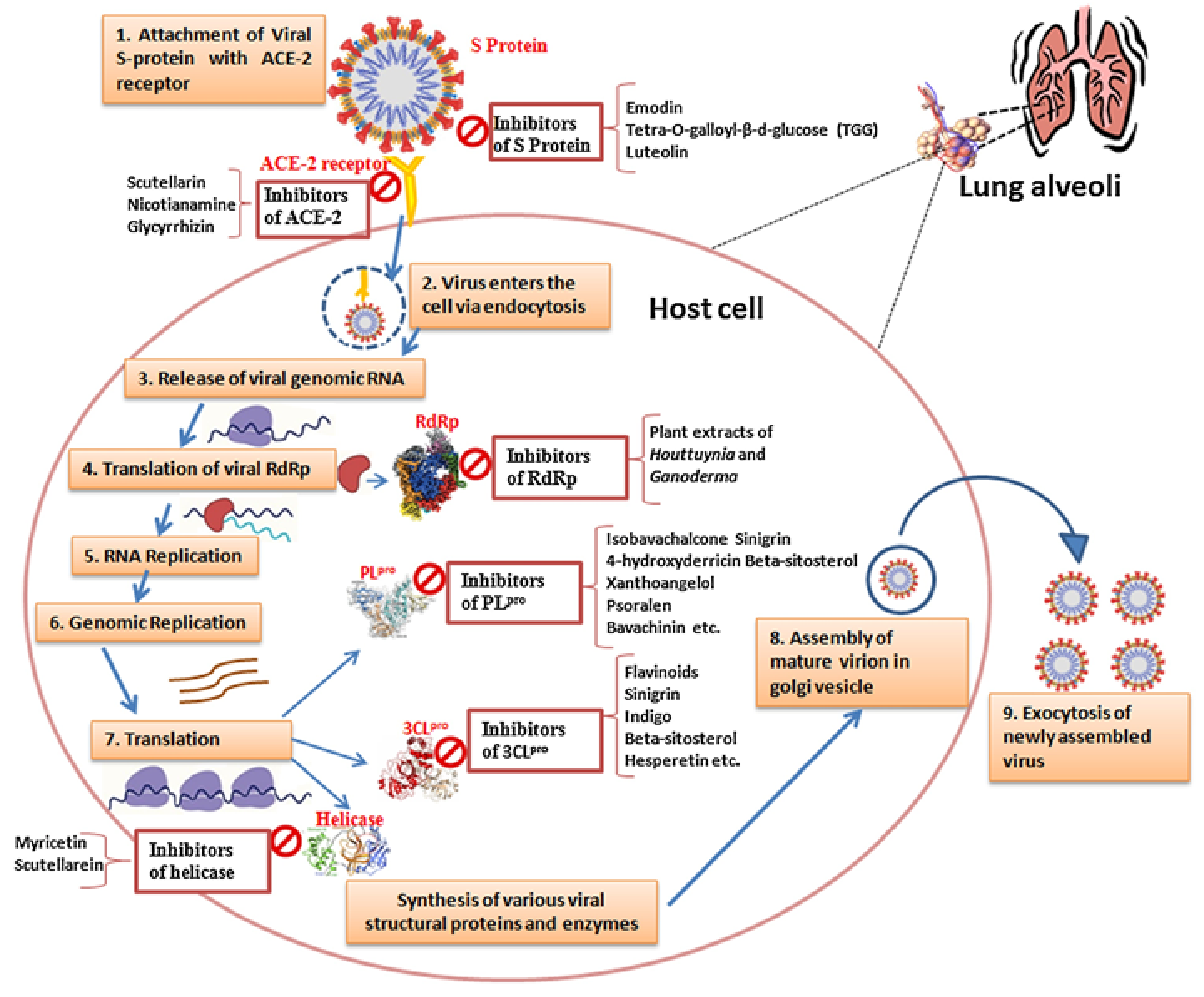
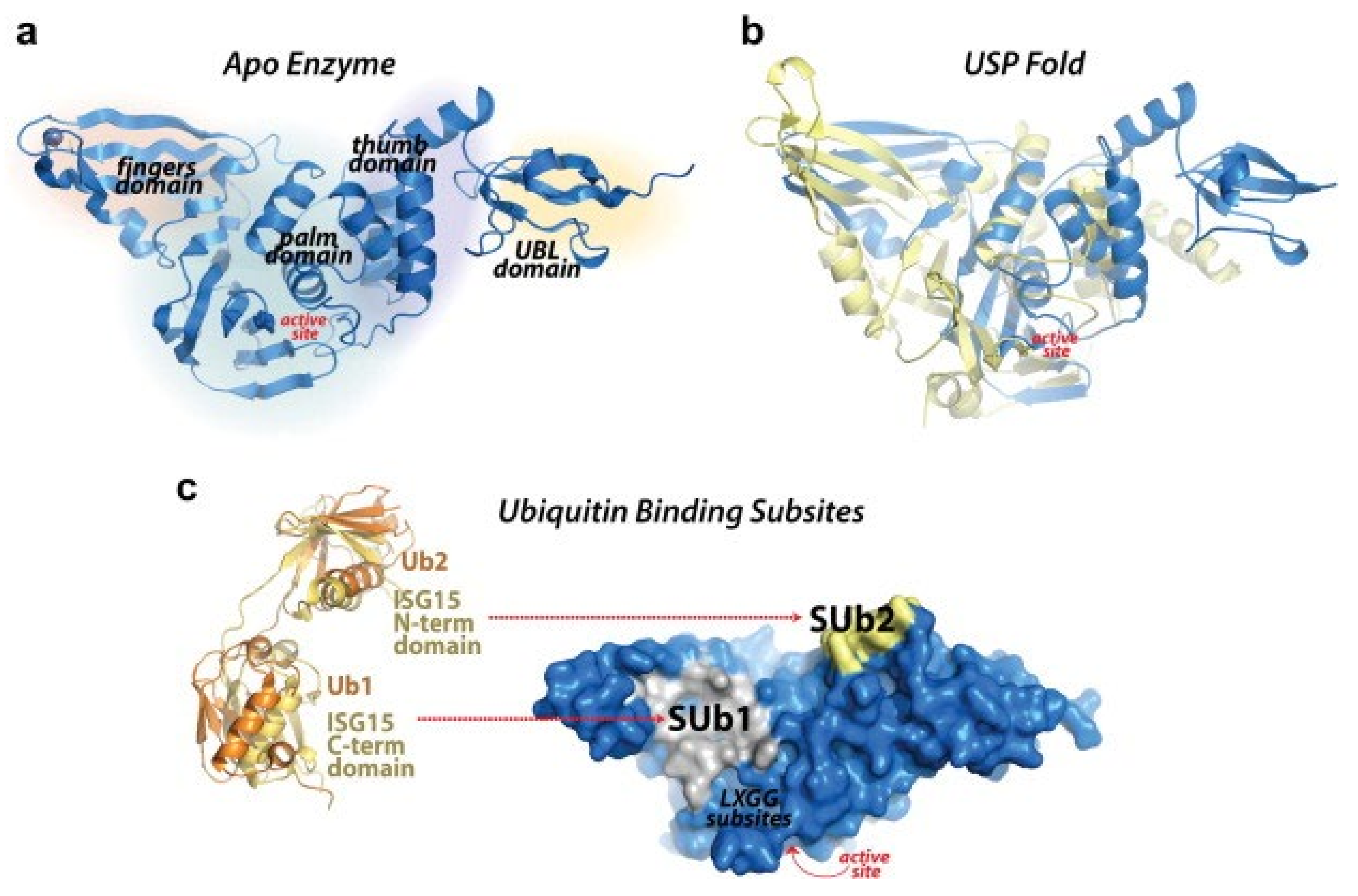
3. Structure and Function of the SARS-CoV-2 PLpro
4. Multifaceted Approach with MD Simulations Targeting SARS-CoV-2 Papain-like Protease (PLpro)
5. Implications of PLpro Targeting beyond COVID-19, Exploration of Its Role in Other Diseases, and Potential as a Target for Broader Antiviral Strategies
6. Clinical and Preclinical Studies: Integrating MD Simulation Insights
7. Challenges and Future Directions: Guided by MD Simulations
8. Conclusions and Author Insights into Targeting SARS-CoV-2 Papain-like Protease
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-specific immune response and the pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef]
- Gadanec, L.K.; McSweeney, K.R.; Qaradakhi, T.; Ali, B.; Zulli, A.; Apostolopoulos, V. Can SARS-CoV-2 virus use multiple receptors to enter host cells? Int. J. Mol. Sci. 2021, 22, 992. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiang, R.; Huo, S.; Zhou, Y.; Jiang, S.; Wang, Q.; Yu, F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target. Ther. 2021, 6, 233. [Google Scholar] [CrossRef]
- Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; Khan, R.B.; Kumalo, H.M. Unravelling Insights into the Evolution and Management of SARS-CoV-2. BioMedInformatics 2024, 4, 385–409. [Google Scholar] [CrossRef]
- Cannalire, R.; Cerchia, C.; Beccari, A.R.; Di Leva, F.S.; Summa, V. Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: State of the art and future opportunities. J. Med. Chem. 2020, 65, 2716–2746. [Google Scholar] [CrossRef]
- Catanzaro, M.; Fagiani, F.; Racchi, M.; Corsini, E.; Govoni, S.; Lanni, C. Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Signal Transduct. Target. Ther. 2020, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Rajarshi, K.; Khan, R.; Singh, M.K.; Ranjan, T.; Ray, S.; Ray, S. Essential functional molecules associated with SARS-CoV-2 infection: Potential therapeutic targets for COVID-19. Gene 2021, 768, 145313. [Google Scholar] [CrossRef]
- Anand, U.; Jakhmola, S.; Indari, O.; Jha, H.C.; Chen, Z.S.; Tripathi, V.; Pérez de la Lastra, J.M. Potential Therapeutic Targets and Vaccine Development for SARS-CoV-2/COVID-19 Pandemic Management: A Review on the Recent Update. Front. Immunol. 2021, 12, 658519. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Davidson, A.M.; Wysocki, J.; Batlle, D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: Therapeutic implications. Hypertension 2020, 76, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Sarker, J.; Das, P.; Sarker, S.; Roy, A.K.; Momen, A.R. A review on expression, pathological roles, and inhibition of TMPRSS2, the serine protease responsible for SARS-CoV-2 spike protein activation. Scientifica 2021, 2021, 2706789. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; Rozano, L.; Falasca, M.; Mancera, R.L. Does the SARS-CoV-2 spike protein receptor binding domain interact effectively with the DPP4 (CD26) receptor? A molecular docking study. Int. J. Mol. Sci. 2021, 22, 7001. [Google Scholar] [CrossRef] [PubMed]
- Mouffouk, C.; Mouffouk, S.; Mouffouk, S.; Hambaba, L.; Haba, H. Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CLpro and PLpro), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2). Eur. J. Pharmacol. 2021, 891, 173759. [Google Scholar] [CrossRef] [PubMed]
- Chitranshi, N.; Gupta, V.K.; Rajput, R.; Godinez, A.; Pushpitha, K.; Shen, T.; Mirzaei, M.; You, Y.; Basavarajappa, D.; Gupta, V. Evolving geographic diversity in SARS-CoV2 and in silico analysis of replicating enzyme 3CL pro targeting repurposed drug candidates. J. Transl. Med. 2020, 18, 278. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Mitra, D.; Paul, M.; Chaudhary, P.; Kamboj, A.; Thatoi, H.; Janmeda, P.; Jain, D.; Panneerselvam, P.; Shrivastav, R. Potential inhibitors of SARS-CoV-2 (COVID 19) proteases PLpro and Mpro/3CLpro: Molecular docking and simulation studies of three pertinent medicinal plant natural components. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100038. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Ariumi, Y. Host cellular RNA helicases regulate SARS-CoV-2 infection. J. Virol. 2022, 96, e00002-22. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, P.; Desikan, R.; Dixit, N.M. Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection. PLoS Comput. Biol. 2020, 16, e1008461. [Google Scholar] [CrossRef]
- Pišlar, A.; Mitrović, A.; Sabotič, J.; Pečar Fonović, U.; Perišić Nanut, M.; Jakoš, T.; Senjor, E.; Kos, J. The role of cysteine peptidases in coronavirus cell entry and replication: The therapeutic potential of cathepsin inhibitors. PLoS Pathog. 2020, 16, e1009013. [Google Scholar] [CrossRef]
- Essalmani, R.; Jain, J.; Susan-Resiga, D.; Andréo, U.; Evagelidis, A.; Derbali, R.M.; Huynh, D.N.; Dallaire, F.; Laporte, M.; Delpal, A. Implications of Spike-glycoprotein processing at S1/S2 by Furin, at S2’by Furin and/or TMPRSS2 and shedding of ACE2: Cell-to-cell fusion, cell entry and infectivity of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Chao, T.-L.; Li, C.-L.; Chiu, M.-F.; Kao, H.-C.; Wang, S.-H.; Pang, Y.-H.; Lin, C.-H.; Tsai, Y.-M.; Lee, W.-H. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020, 33, 108254. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Mushebenge, A.G.; Ugbaja, S.C.; Mtambo, S.E.; Ntombela, T.; Metu, J.I.; Babayemi, O.; Chima, J.I.; Appiah-Kubi, P.; Odugbemi, A.I.; Ntuli, M.L.; et al. Unveiling the Inhibitory Potentials of Peptidomimetic Azanitriles and Pyridyl Esters towards SARS-CoV-2 Main Protease: A Molecular Modelling Investigation. Molecules 2023, 28, 2641. [Google Scholar] [CrossRef] [PubMed]
- Behnam, M.A. Protein structural heterogeneity: A hypothesis for the basis of proteolytic recognition by the main protease of SARS-CoV and SARS-CoV-2. Biochimie 2021, 182, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Osipiuk, J.; Azizi, S.-A.; Dvorkin, S.; Endres, M.; Jedrzejczak, R.; Jones, K.A.; Kang, S.; Kathayat, R.S.; Kim, Y.; Lisnyak, V.G. Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat. Commun. 2021, 12, 743. [Google Scholar] [CrossRef]
- Prajapati, J.; Patel, R.; Rao, P.; Saraf, M.; Rawal, R.; Goswami, D. Perceiving SARS-CoV-2 Mpro and PLpro dual inhibitors from pool of recognized antiviral compounds of endophytic microbes: An in silico simulation study. Struct. Chem. 2022, 33, 1619–1643. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Broad-spectrum prodrugs with anti-SARS-CoV-2 activities: Strategies, benefits, and challenges. J. Med. Virol. 2022, 94, 1373–1390. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Chen, S.; Ouyang, H.; Ren, L. Possible targets of pan-coronavirus antiviral strategies for emerging or re-emerging coronaviruses. Microorganisms 2021, 9, 1479. [Google Scholar] [CrossRef]
- Calleja, D.J.; Lessene, G.; Komander, D. Inhibitors of SARS-CoV-2 PLpro. Front. Chem. 2022, 10, 876212. [Google Scholar] [CrossRef]
- Gao, X.; Qin, B.; Chen, P.; Zhu, K.; Hou, P.; Wojdyla, J.A.; Wang, M.; Cui, S. Crystal structure of SARS-CoV-2 papain-like protease. Acta Pharm. Sin. B 2021, 11, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pradhan, A.; Maurya, V.K.; Kumar, S.; Theengh, A.; Puri, B.; Saxena, S.K. Therapeutic approaches for SARS-CoV-2 infection. Methods 2021, 195, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Dubey, K. SARS-CoV-2: Potential drug targets and its virtual screening. Model. Control Drug Dev. COVID-19 Outbreak Prev. 2022, 366, 203–244. [Google Scholar]
- Liu, J.; Cheng, Y.; Zheng, M.; Yuan, B.; Wang, Z.; Li, X.; Yin, J.; Ye, M.; Song, Y. Targeting the ubiquitination/deubiquitination process to regulate immune checkpoint pathways. Signal Transduct. Target. Ther. 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Mio, M.J.; Prince, R.B.; Hughes, T.S.; Moore, J.S. A field guide to foldamers. Chem. Rev. 2001, 101, 3893–4012. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.C.; Pokhrel, S.; Labbe, A.D.; Mathews, I.I.; Cooper, C.J.; Davidson, R.B.; Phillips, G.; Weiss, K.L.; Zhang, Q.; O’Neill, H. Potent and selective covalent inhibition of the papain-like protease from SARS-CoV-2. Nat. Commun. 2023, 14, 1733. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, R.E.; Neto, A.M.; Santos, I.A.; Jardim, A.C.; Corbi, P.P.; Bergamini, F.R. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef]
- Gupta, Y.; Maciorowski, D.; Zak, S.E.; Jones, K.A.; Kathayat, R.S.; Azizi, S.-A.; Mathur, R.; Pearce, C.M.; Ilc, D.J.; Husein, H. Bisindolylmaleimide IX: A novel anti-SARS-CoV2 agent targeting viral main protease 3CLpro demonstrated by virtual screening pipeline and in-vitro validation assays. Methods 2021, 195, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Báez-Santos, Y.M.; St John, S.E.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antivir. Res. 2015, 115, 21–38. [Google Scholar] [CrossRef]
- Amin, S.A.; Banerjee, S.; Ghosh, K.; Gayen, S.; Jha, T. Protease targeted COVID-19 drug discovery and its challenges: Insight into viral main protease (Mpro) and papain-like protease (PLpro) inhibitors. Biorg. Med. Chem. 2021, 29, 115860. [Google Scholar] [CrossRef]
- Mengist, H.M.; Dilnessa, T.; Jin, T. Structural basis of potential inhibitors targeting SARS-CoV-2 main protease. Front. Chem. 2021, 9, 622898. [Google Scholar] [CrossRef] [PubMed]
- Stefani, C.; Fantoni, T.; Bissoli, M.; Thomas, J.; Ruggiero, A. HIV and SARS-CoV-2 Co-Infection: From Population Study Evidence to In Vitro Studies. Life 2022, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Jamal, Q.M.S. Antiviral Potential of Plants against COVID-19 during Outbreaks-An Update. Int. J. Mol. Sci. 2022, 23, 13564. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.S.; Ho, J.; Wills, S.; Kawall, A.; Sharma, A.; Chavada, K.; Ebert, M.C.; Evoli, S.; Singh, A.; Rayalam, S. Aloin isoforms (A and B) selectively inhibits proteolytic and deubiquitinating activity of papain like protease (PLpro) of SARS-CoV-2 In Vitro. Sci. Rep. 2022, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Jha, R.K.; Khan, R.J.; Kumar, A.; Jain, M.; Muthukumaran, J.; Singh, A.K. A computational essential dynamics approach to investigate structural influences of ligand binding on Papain like protease from SARS-CoV-2. Comput. Biol. Chem. 2022, 99, 107721. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.H.; Hussen, N.H.; Shakya, S.; Jamalis, J.; Pratama, M.R.F.; Chander, S.; Kharkwal, H.; Murugesan, S. In silico discovery of multi-targeting inhibitors for the COVID-19 treatment by molecular docking, molecular dynamics simulation studies, and ADMET predictions. Struct. Chem. 2022, 33, 1645–1665. [Google Scholar] [CrossRef]
- Narayanan, A.; Narwal, M.; Majowicz, S.A.; Varricchio, C.; Toner, S.A.; Ballatore, C.; Brancale, A.; Murakami, K.S.; Jose, J. Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. Commun. Biol. 2022, 5, 169. [Google Scholar] [CrossRef] [PubMed]
- Shaheer, M.; Singh, R.; Sobhia, M.E. Protein degradation: A novel computational approach to design protein degrader probes for main protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 10905–10917. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Huang, T.T.; Bekes, M.; Drag, M. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti–COVID-19 drug design. Sci. Adv. 2020, 6, eabd4596. [Google Scholar] [CrossRef]
- Waman, V.P.; Sen, N.; Varadi, M.; Daina, A.; Wodak, S.J.; Zoete, V.; Velankar, S.; Orengo, C. The impact of structural bioinformatics tools and resources on SARS-CoV-2 research and therapeutic strategies. Brief. Bioinform. 2021, 22, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Cui, H.; Gao, Z.; Liu, M.; Lu, S.; Mkandawire, W.; Narykov, O.; Sun, M.; Korkin, D. Structural genomics of SARS-CoV-2 indicates evolutionary conserved functional regions of viral proteins. Viruses 2020, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Heo, L.; Feig, M. Modeling of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins by machine learning and physics-based refinement. BioRxiv 2020. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Mondal, S.K.; Mukhoty, S.; Kundu, H.; Ghosh, S.; Sen, M.K.; Das, S.; Brogi, S. In silico analysis of RNA-dependent RNA polymerase of the SARS-CoV-2 and therapeutic potential of existing antiviral drugs. Comput. Biol. Med. 2021, 135, 104591. [Google Scholar] [CrossRef]
- Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; B. Khan, R.; Kumalo, H.M. Assessing the Potential Contribution of In Silico Studies in Discovering Drug Candidates That Interact with Various SARS-CoV-2 Receptors. Int. J. Mol. Sci. 2023, 24, 15518. [Google Scholar] [CrossRef]
- Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; Khan, R.B.; Kumalo, H.M. A Comprehensive Analysis of Structural and Functional Changes Induced by SARS-CoV-2 Spike Protein Mutations. COVID 2023, 3, 1454–1472. [Google Scholar] [CrossRef]
- Essalmani, R.; Jain, J.; Susan-Resiga, D.; Andréo, U.; Evagelidis, A.; Derbali, R.M.; Huynh, D.N.; Dallaire, F.; Laporte, M.; Delpal, A. Distinctive roles of furin and TMPRSS2 in SARS-CoV-2 infectivity. J. Virol. 2022, 96, e00128-22. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, S.; Shokri, S. Interactions between SARS coronavirus 2 papain-like protease and immune system: A potential drug target for the treatment of COVID-19. Scand. J. Immunol. 2021, 94, e13044. [Google Scholar] [CrossRef] [PubMed]
- Maiti, B.K. Can Papain-like Protease Inhibitors Halt SARS-CoV-2 Replication? ACS Pharmacol. Transl. Sci. 2020, 3, 1017–1019. [Google Scholar] [CrossRef]
- Lanz, J.; Biniaz-Harris, N.; Kuvaldina, M.; Jain, S.; Lewis, K.; Fallon, B.A. Disulfiram: Mechanisms, Applications, and Challenges. Antibiotics 2023, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Kakavandi, S.; Zare, I.; VaezJalali, M.; Dadashi, M.; Azarian, M.; Akbari, A.; Ramezani Farani, M.; Zalpoor, H.; Hajikhani, B. Structural and non-structural proteins in SARS-CoV-2: Potential aspects to COVID-19 treatment or prevention of progression of related diseases. Cell Commun. Signal. 2023, 21, 110. [Google Scholar] [CrossRef]
- Barretto, N.; Jukneliene, D.; Ratia, K.; Chen, Z.; Mesecar, A.D.; Baker, S.C. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 2005, 79, 15189–15198. [Google Scholar] [CrossRef] [PubMed]
- Kandwal, S.; Fayne, D. Genetic conservation across SARS-CoV-2 non-structural proteins–Insights into possible targets for treatment of future viral outbreaks. Virology 2023, 581, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.; Lorenzo-Leal, A.C.; Hernández, L.R.; Bach, H. Targeting SARS-coV-2 non-structural proteins. Int. J. Mol. Sci. 2023, 24, 13002. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Wang, M.; Liu, J.; Ma, P.; Pang, S.; Liu, W.; Liu, A. Diagnostics and analysis of SARS-CoV-2: Current status, recent advances, challenges and perspectives. Chem. Sci. 2023, 14, 6149–6206. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Cohen, S.M. Rhenium (V) complexes as cysteine-targeting coordinate covalent warheads. J. Med. Chem. 2023, 66, 3088–3105. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, V.J.; De Silva, A.; Mark, B.L.; Kikkert, M. Viral deubiquitinating proteases and the promising strategies of their inhibition. Virus Res. 2024, 344, 199368. [Google Scholar] [CrossRef]
- Zmudzinski, M.; Rut, W.; Olech, K.; Granda, J.; Giurg, M.; Burda-Grabowska, M.; Kaleta, R.; Zgarbova, M.; Kasprzyk, R.; Zhang, L. Ebselen derivatives inhibit SARS-CoV-2 replication by inhibition of its essential proteins: PLpro and Mpro proteases, and nsp14 guanine N7-methyltransferase. Sci. Rep. 2023, 13, 9161. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.S.; Wang, C.-K.; Rao, P.; Mancini, F.; Clemens, R.A.; Wirakartakusumah, A.; Chiu, H.-F.; Yen, C.-H.; Porretta, S.; Mathai, I. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID. NPJ Sci. Food 2024, 8, 19. [Google Scholar] [CrossRef]
- Darvishi, M.; Hajilou, F. Review on Virology of Coronaviridae. Clinic 2024, 1, 520–2644. [Google Scholar]
- Van Huizen, M.; Bloeme-ter Horst, J.R.; de Gruyter, H.L.; Geurink, P.P.; van der Heden van Noort, G.J.; Knaap, R.C.; Nelemans, T.; Ogando, N.S.; Leijs, A.A.; Urakova, N. Deubiquitinating activity of SARS-CoV-2 papain-like protease does not influence virus replication or innate immune responses in vivo. PLoS Pathog. 2024, 20, e1012100. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wu, G. Potential 3-chymotrypsin-like cysteine protease cleavage sites in the coronavirus polyproteins pp1a and pp1ab and their possible relevance to COVID-19 vaccine and drug development. FASEB J. 2021, 35, e21573. [Google Scholar] [CrossRef] [PubMed]
- Ziebuhr, J.; Schelle, B.; Karl, N.; Minskaia, E.; Bayer, S.; Siddell, S.G.; Gorbalenya, A.E.; Thiel, V. Human coronavirus 229E papain-like proteases have overlapping specificities but distinct functions in viral replication. J. Virol. 2007, 81, 3922–3932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choudhary, S.; Nehul, S.; Singh, A.; Panda, P.K.; Kumar, P.; Sharma, G.K.; Tomar, S. Unraveling antiviral efficacy of multifunctional immunomodulatory triterpenoids against SARS-COV-2 targeting main protease and papain-like protease. IUBMB Life 2024, 76, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Dai, R.; Su, R. Computer-aided drug design for the pain-like protease (PLpro) inhibitors against SARS-CoV-2. Biomed. Pharmacother. 2023, 159, 114247. [Google Scholar] [CrossRef] [PubMed]
- Omage, F.B.; Madabeni, A.; Tucci, A.R.; Nogara, P.A.; Bortoli, M.; Rosa, A.D.S.; Neuza Dos Santos Ferreira, V.; Teixeira Rocha, J.B.; Miranda, M.D.; Orian, L. Diphenyl diselenide and SARS-CoV-2: In silico exploration of the mechanisms of inhibition of main protease (Mpro) and papain-like protease (PLpro). J. Chem. Inf. Model. 2023, 63, 2226–2239. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Zhang, X.; Ansari, A.; Jadhav, P.; Tan, H.; Li, K.; Chopra, A.; Ford, A.; Chi, X.; Ruiz, F.X. Design of a SARS-CoV-2 papain-like protease inhibitor with antiviral efficacy in a mouse model. Science 2024, 383, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Alhadhrami, A. Nano-Crystallites of Ruthenium (III) Violurate Complex: Synthesis, Characterization, PXRD and DFT Structural Analysis. DNA/HSA-Binding, Antiviral Activity Against COVID-19 and Molecular Docking Study. New J. Chem. 2024, 48, 9718–9737. [Google Scholar] [CrossRef]
- Arduino, I.; Francese, R.; Civra, A.; Feyles, E.; Argenziano, M.; Volante, M.; Cavalli, R.; Mougharbel, A.M.; Kortz, U.; Donalisio, M. Polyoxometalate exerts broad-spectrum activity against human respiratory viruses hampering viral entry. Antivir. Res. 2024, 226, 105897. [Google Scholar] [CrossRef]
- Mehrotra, R.; Shukla, S.N.; Gaur, P. Metallo-antiviral aspirants: Answer to the upcoming virus outbreak. Eur. J. Med. Chem. Rep. 2023, 8, 100104. [Google Scholar] [CrossRef]
- Gil-Moles, M.; O’Beirne, C.; Esarev, I.V.; Lippmann, P.; Tacke, M.; Cinatl, J.; Bojkova, D.; Ott, I. Silver N-heterocyclic carbene complexes are potent uncompetitive inhibitors of the papain-like protease with antiviral activity against SARS-CoV-2. RSC Med. Chem. 2023, 14, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Balsera-Manzanero, M.; Soengas, R.G.; Carretero-Ledesma, M.; Ratia, C.; Iglesias, M.J.; Pachón, J.; López-Ortiz, F.; Cordero, E.; Soto, S.M.; Sánchez-Céspedes, J. Heteroleptic (S^ C)-cyclometallated gold (III) complexes as novel antiviral agents. Heliyon 2024, 10, e27601. [Google Scholar] [CrossRef] [PubMed]
- Mufassirin, M.M.; Newton, M.H.; Sattar, A. Artificial intelligence for template-free protein structure prediction: A comprehensive review. Artif. Intell. Rev. 2023, 56, 7665–7732. [Google Scholar] [CrossRef]
- Dhingra, S.; Sowdhamini, R.; Cadet, F.; Offmann, B. A glance into the evolution of template-free protein structure prediction methodologies. Biochimie 2020, 175, 85–92. [Google Scholar] [CrossRef]
- Vincenzi, M.; Mercurio, F.A.; Leone, M. Looking for SARS-CoV-2 Therapeutics Through Computational Approaches. Curr. Med. Chem. 2023, 30, 3158–3214. [Google Scholar] [CrossRef]
- Mbunge, E.; Akinnuwesi, B.; Fashoto, S.G.; Metfula, A.S.; Mashwama, P. A critical review of emerging technologies for tackling COVID-19 pandemic. Hum. Behav. Emerg. Technol. 2021, 3, 25–39. [Google Scholar] [CrossRef]
- Selvaraj, C.; Dinesh, D.C.; Panwar, U.; Abhirami, R.; Boura, E.; Singh, S.K. Structure-based virtual screening and molecular dynamics simulation of SARS-CoV-2 Guanine-N7 methyltransferase (nsp14) for identifying antiviral inhibitors against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 4582–4593. [Google Scholar] [CrossRef] [PubMed]
- Frances-Monerris, A.; Hognon, C.; Miclot, T.; Garcia-Iriepa, C.; Iriepa, I.; Terenzi, A.; Grandemange, S.; Barone, G.; Marazzi, M.; Monari, A. Molecular basis of SARS-CoV-2 infection and rational design of potential antiviral agents: Modeling and simulation approaches. J. Proteome Res. 2020, 19, 4291–4315. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.W.; Hasannuzaman, M.; Cuce, E.; Cuce, P.M. Global technological advancement and challenges of glazed window, facade system and vertical greenery-based energy savings in buildings: A comprehensive review. Energy Built Environ. 2023, 4, 206–226. [Google Scholar] [CrossRef]
- Andreini, C.; Arnesano, F.; Rosato, A. The zinc proteome of SARS-CoV-2. Metallomics 2022, 14, mfac047. [Google Scholar] [CrossRef]
- Debnath, S.K.; Debnath, M.; Srivastava, R.; Omri, A. Drugs repurposing for SARS-CoV-2: New insight of COVID-19 druggability. Expert. Rev. Anti Infect. Ther. 2022, 20, 1187–1204. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M.; Shahabi, D.; Chapman, M.E.; Mesecar, A.D. Drug development and medicinal chemistry efforts toward SARS-coronavirus and COVID-19 therapeutics. ChemMedChem 2020, 15, 907–932. [Google Scholar] [CrossRef] [PubMed]
- Owji, H.; Negahdaripour, M.; Hajighahramani, N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020, 88, 106924. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zheng, Y.; Zeng, X.; He, B.; Cheng, W. Structural biology of SARS-CoV-2: Open the door for novel therapies. Signal Transduct. Target. Ther. 2022, 7, 26. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, P.; Zhang, J. Potential inhibitors targeting papain-like protease of SARS-CoV-2: Two birds with one stone. Front. Chem. 2022, 10, 822785. [Google Scholar] [CrossRef]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 2020, 369, m1849. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Gil, C.; Ginex, T.; Maestro, I.; Nozal, V.; Barrado-Gil, L.; Cuesta-Geijo, M.Á.; Urquiza, J.; Ramírez, D.; Alonso, C.; Campillo, N.E. COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020, 63, 12359–12386. [Google Scholar] [CrossRef]
- Martinotti, C.; Ruiz-Perez, L.; Deplazes, E.; Mancera, R.L. Molecular dynamics simulation of small molecules interacting with biological membranes. Chemphyschem 2020, 21, 1486–1514. [Google Scholar] [CrossRef]
- Parmar, P.; Rao, P.; Sharma, A.; Shukla, A.; Rawal, R.M.; Saraf, M.; Patel, B.V.; Goswami, D. Meticulous assessment of natural compounds from NPASS database for identifying analogue of GRL0617, the only known inhibitor for SARS-CoV2 papain-like protease (PLpro) using rigorous computational workflow. Mol. Divers. 2022, 26, 389–407. [Google Scholar] [CrossRef]
- Sanachai, K.; Mahalapbutr, P.; Sanghiran Lee, V.; Rungrotmongkol, T.; Hannongbua, S. In silico elucidation of potent inhibitors and rational drug design against SARS-CoV-2 papain-like protease. J. Phys. Chem. B 2021, 125, 13644–13656. [Google Scholar] [CrossRef]
- Naidoo, D.; Kar, P.; Roy, A.; Mutanda, T.; Bwapwa, J.; Sen, A.; Anandraj, A. Structural insight into the binding of cyanovirin-n with the spike glycoprotein, Mpro and PLpro of SARS-CoV-2: Protein–protein interactions, dynamics simulations and free energy calculations. Molecules 2021, 26, 5114. [Google Scholar] [CrossRef]
- Selvaraj, V.; Rathinavel, T.; Ammashi, S.; Nasir Iqbal, M. Polyphenolic phytochemicals exhibit promising SARS-CoV-2 papain like protease (PLpro) inhibition validated through a computational approach. Polycycl. Aromat. Compd. 2023, 43, 5545–5566. [Google Scholar] [CrossRef]
- Muralidharan, N.; Sakthivel, R.; Velmurugan, D.; Gromiha, M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 protease against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 2673–2678. [Google Scholar] [CrossRef]
- Bera, K.; Reeda, V.J.; Babila, P.; Dinesh, D.C.; Hritz, J.; Karthick, T. An in silico molecular dynamics simulation study on the inhibitors of SARS-CoV-2 proteases (3CLpro and PLpro) to combat COVID-19. Mol. Simul. 2021, 47, 1168–1184. [Google Scholar] [CrossRef]
- Yan, F.; Gao, F. An overview of potential inhibitors targeting non-structural proteins 3 (PLpro and Mac1) and 5 (3CLpro/Mpro) of SARS-CoV-2. Comput. Struct. Biotechnol. J. 2021, 19, 4868–4883. [Google Scholar] [CrossRef]
- Xu, L.; Tong, J.; Wu, Y.; Zhao, S.; Lin, B.-L. A computational evaluation of targeted oxidation strategy (TOS) for potential inhibition of SARS-CoV-2 by disulfiram and analogues. Biophys. Chem. 2021, 276, 106610. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hu, Y.; Townsend, J.A.; Lagarias, P.I.; Marty, M.T.; Kolocouris, A.; Wang, J. Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharmacol. Transl. Sci. 2020, 3, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L.; Zhao, X.-E. Co-crystallization and structure determination: An effective direction for anti-SARS-CoV-2 drug discovery. Comput. Struct. Biotechnol. J. 2021, 19, 4684–4701. [Google Scholar] [CrossRef]
- Talevi, A.; Bellera, C.L. Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics. Expert. Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef]
- Pang, J.; Gao, S.; Sun, Z.; Yang, G. Discovery of small molecule PLpro inhibitor against COVID-19 using structure-based virtual screening, molecular dynamics simulation, and molecular mechanics/Generalized Born surface area (MM/GBSA) calculation. Struct. Chem. 2021, 32, 879–886. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, Z.; Zhang, J.; Wu, D.; Li, H.; Geng, F. Molecular dynamics simulation of the interaction of food proteins with small molecules. Food Chem. 2023, 405, 134824. [Google Scholar] [CrossRef]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA 2020, 323, 844–853. [Google Scholar] [CrossRef]
- Zrieq, R.; Ahmad, I.; Snoussi, M.; Noumi, E.; Iriti, M.; Algahtani, F.D.; Patel, H.; Saeed, M.; Tasleem, M.; Sulaiman, S. Tomatidine and patchouli alcohol as inhibitors of SARS-CoV-2 enzymes (3CLpro, PLpro and NSP15) by molecular docking and molecular dynamics simulations. Int. J. Mol. Sci. 2021, 22, 10693. [Google Scholar] [CrossRef]
- Thangavel, N.; Albratty, M. Benchmarked molecular docking integrated molecular dynamics stability analysis for prediction of SARS-CoV-2 papain-like protease inhibition by olive secoiridoids. J. King Saud. Univ. Sci. 2023, 35, 102402. [Google Scholar] [CrossRef]
- Patel, D.; Athar, M.; Jha, P.C. Computational investigation of binding of chloroquinone and hydroxychloroquinone against PLPro of SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 3071–3081. [Google Scholar] [CrossRef]
- Kandeel, M.; Abdelrahman, A.; Oh-Hashi, K.; Ibrahim, A.; Venugopala, K.; Morsy, M.; Ibrahim, M. Repurposing of FDA-approved antivirals, antibiotics, anthelmintics, antioxidants, and cell protectives against SARS-CoV-2 papain-like protease. J. Biomol. Struct. Dyn. 2020, 39, 5129–5136. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Joshi, M.; Degani, M.S. Tackling SARS-CoV-2: Proposed targets and repurposed drugs. Future Med. Chem. 2020, 12, 1579–1601. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-W.; Xie, Y.; Tang, L.-S.; Pu, D.; Zhu, Y.-J.; Liu, J.-Y.; Ma, X.-L. Therapeutic targets and interventional strategies in COVID-19: Mechanisms and clinical studies. Signal Transduct. Target. Ther. 2021, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.K.; Faheem; Sekhar, K.; Ojha, R.; Prajapati, V.K.; Pai, A.; Murugesan, S. Pharmacophore based virtual screening, molecular docking, molecular dynamics and MM-GBSA approach for identification of prospective SARS-CoV-2 inhibitor from natural product databases. J. Biomol. Struct. Dyn. 2022, 40, 1363–1386. [Google Scholar] [CrossRef] [PubMed]
- Jupudi, S.; Rajagopal, K.; Murugesan, S.; Kumar, B.K.; Raman, K.; Byran, G.; Chennaiah, J.; pillai Muthiah, V.; Sankaran, S. Identification of Papain-Like Protease inhibitors of SARS CoV-2 through HTVS, Molecular docking, MMGBSA and Molecular dynamics approach. S. Afr. J. Bot. 2022, 151, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, S.; Sahoo, S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luan, J.; Zhang, L. Molecular docking of potential SARS-CoV-2 papain-like protease inhibitors. Biochem. Biophys. Res. Commun. 2021, 538, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Hu, Y.; Jadhav, P.; Tan, B.; Wang, J. Progress and challenges in targeting the SARS-CoV-2 papain-like protease. J. Med. Chem. 2022, 65, 7561–7580. [Google Scholar] [CrossRef] [PubMed]
- Klemm, T.; Ebert, G.; Calleja, D.J.; Allison, C.C.; Richardson, L.W.; Bernardini, J.P.; Lu, B.G.; Kuchel, N.W.; Grohmann, C.; Shibata, Y. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020, 39, e106275. [Google Scholar] [CrossRef]
- Su, C.-M.; Du, Y.; Rowland, R.R.; Wang, Q.; Yoo, D. Reprogramming viral immune evasion for a rational design of next-generation vaccines for RNA viruses. Front. Immunol. 2023, 14, 1172000. [Google Scholar] [CrossRef]
- Moustaqil, M.; Ollivier, E.; Chiu, H.-P.; Van Tol, S.; Rudolffi-Soto, P.; Stevens, C.; Bhumkar, A.; Hunter, D.J.; Freiberg, A.N.; Jacques, D. SARS-CoV-2 proteases PLpro and 3CLpro cleave IRF3 and critical modulators of inflammatory pathways (NLRP12 and TAB1): Implications for disease presentation across species. Emerg. Microbes Infect. 2021, 10, 178–195. [Google Scholar] [CrossRef]
- Wong, N.A.; Saier, M.H., Jr. The SARS-coronavirus infection cycle: A survey of viral membrane proteins, their functional interactions and pathogenesis. Int. J. Mol. Sci. 2021, 22, 1308. [Google Scholar] [CrossRef] [PubMed]
- Yapasert, R.; Khaw-On, P.; Banjerdpongchai, R. Coronavirus infection-associated cell death signaling and potential therapeutic targets. Molecules 2021, 26, 7459. [Google Scholar] [CrossRef] [PubMed]
- Cavasotto, C.N.; Lamas, M.S.; Maggini, J. Functional and druggability analysis of the SARS-CoV-2 proteome. Eur. J. Pharmacol. 2021, 890, 173705. [Google Scholar] [CrossRef]
- Durairajan, S.S.K.; Singh, A.K.; Saravanan, U.B.; Namachivayam, M.; Radhakrishnan, M.; Huang, J.D.; Dhodapkar, R.; Zhang, H. Gastrointestinal Manifestations of SARS-CoV-2: Transmission, Pathogenesis, Immunomodulation, Microflora Dysbiosis, and Clinical Implications. Viruses 2023, 15, 1231. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Mei, H.; Chen, Y.; Griffin, J.D.; Liu, Q.; Weisberg, E.; Yang, J. Repurposing clinically available drugs and therapies for pathogenic targets to combat SARS-CoV-2. MedComm 2023, 4, e254. [Google Scholar] [CrossRef] [PubMed]
- Clemente, V.; D’arcy, P.; Bazzaro, M. Deubiquitinating enzymes in coronaviruses and possible therapeutic opportunities for COVID-19. Int. J. Mol. Sci. 2020, 21, 3492. [Google Scholar] [CrossRef]
- Seyoum Tola, F. The role of ubiquitin-proteasome system in the pathogenesis of severe acute respiratory syndrome coronavirus-2 disease. Int. J. Inflamm. 2023, 2023, 6698069. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, H.; Zhu, J.; Dong, Q.; Wang, J.; Fan, H.; Chen, Y.; Zhang, X.; Han, X.; Li, Q. Ubiquitin-modified proteome of SARS-CoV-2-infected host cells reveals insights into virus–host interaction and pathogenesis. J. Proteome Res. 2021, 20, 2224–2239. [Google Scholar] [CrossRef]
- Chourasia, M.; Koppula, P.R.; Battu, A.; Ouseph, M.M.; Singh, A.K. EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules 2021, 26, 1200. [Google Scholar] [CrossRef]
- Tretter, F.; Wolkenhauer, O.; Meyer-Hermann, M.; Dietrich, J.W.; Green, S.; Marcum, J.; Weckwerth, W. The quest for system-theoretical medicine in the COVID-19 era. Front. Med. 2021, 8, 640974. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Lin, X. A review on applications of computational methods in drug screening and design. Molecules 2020, 25, 1375. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.B.; Gebhardt, T.; Golebiewski, M.; Karaderi, T.; Hillemanns, M.; Khan, F.M.; Salehzadeh-Yazdi, A.; Kirschner, M.; Krobitsch, S.; Consortium, E.-S.P. Computational models for clinical applications in personalized medicine—Guidelines and recommendations for data integration and model validation. J. Pers. Med. 2022, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Muratov, E.N.; Amaro, R.; Andrade, C.H.; Brown, N.; Ekins, S.; Fourches, D.; Isayev, O.; Kozakov, D.; Medina-Franco, J.L.; Merz, K.M. A critical overview of computational approaches employed for COVID-19 drug discovery. Chem. Soc. Rev. 2021, 50, 9121–9151. [Google Scholar] [CrossRef] [PubMed]
- Rahmandad, H.; Xu, R.; Ghaffarzadegan, N. Enhancing long-term forecasting: Learning from COVID-19 models. PLOS Comput. Biol. 2022, 18, e1010100. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Rong, H.; Qi, D.; Valencia-Cabrera, L.; Zhang, G.; Pérez-Jiménez, M.J. A Review of Membrane Computing Models for Complex Ecosystems and a Case Study on a Complex Giant Panda System. Complexity 2020, 2020, 1312824. [Google Scholar] [CrossRef]
- Maharao, N.; Antontsev, V.; Wright, M.; Varshney, J. Entering the era of computationally driven drug development. Drug Metab. Rev. 2020, 52, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Wang, R.; Chen, J.; Cheng, L.; Frishcosy, J.; Huzumi, Y.; Qiu, Y.; Schluckbier, T.; Wei, X.; Wei, G.-W. Methodology-centered review of molecular modeling, simulation, and prediction of SARS-CoV-2. Chem. Rev. 2022, 122, 11287–11368. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Luccioni, A.; Pham, K.H.; Lam, C.S.N.; Luengo-Oroz, M. Mapping the landscape of artificial intelligence applications against COVID-19. J. Artif. Intell. Res. 2020, 69, 807–845. [Google Scholar] [CrossRef]
- Çalışkaner, Z.O. Determination of Binding Potential of HCV Protease Inhibitors Against to SARS-CoV-2 Papain-like Protease wtih Computational Docking. Lett. Drug Des. Discov. 2021, 18, 949–960. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, B.; Tang, J.; Liu, S.; Liu, M.; Ye, Y.; Liu, Z.; Xiong, Y.; Zhu, W.; Cao, D. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat. Commun. 2021, 12, 488. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Sharun, K.; Tiwari, R.; Dadar, M.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W. COVID-19, an emerging coronavirus infection: Advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin. Immunother. 2020, 16, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Farah, M.A.; Al-Anazi, K.M. An updated review of computer-aided drug design and its application to COVID-19. BioMed Res. Int. 2021, 2021, 8853056. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Jannat, T.; Brishty, S.R.; Roy, U.; Mitra, S.; Rafi, M.O.; Islam, M.R.; Nesa, M.L.; Islam, M.A.; Emran, T.B. Clinical efficacy and safety of antiviral drugs in the extended use against COVID-19: What we know so Far. Biologics 2021, 1, 252–284. [Google Scholar] [CrossRef]
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Mei, K.; Zhang, J. The Kinetic parameters, Thermodynamic parameters, and Mechanism of PLpro from SARS-CoV and SARS-CoV-2. In Proceedings of the 2023 4th International Symposium on Artificial Intelligence for Medicine Science, Chengdu, China, 27–29 October 2023; pp. 1218–1222. [Google Scholar]
- Singh, U.; Gandhi, H.A.; Nikita; Bhattacharya, J.; Tandon, R.; Tiwari, G.; Tandon, R. Cyanometabolites: Molecules with immense antiviral potential. Arch. Microbiol. 2023, 205, 164. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Vanet, A.; Francesconi, V.; Tagliazucchi, L.; Tassone, G.; Venturelli, A.; Spyrakis, F.; Mazzorana, M.; Costi, M.P.; Tonelli, M. Antitarget, anti-SARS-CoV-2 leads, drugs, and the drug discovery–genetics alliance perspective. J. Med. Chem. 2023, 66, 3664–3702. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Ingale, K. In silico approaches for drug repurposing for SARS-CoV-2 Infection. RSC 2022. [Google Scholar] [CrossRef]
- Sharma, P.; Joshi, T.; Mathpal, S.; Tamta, S.; Chandra, S. Computational approaches for drug discovery against COVID-19. In Omics Approaches and Technologies in COVID-19; Elsevier: Amsterdam, The Netherlands, 2023; pp. 321–337. [Google Scholar]
- Jade, D.; Ayyamperumal, S.; Tallapaneni, V.; Nanjan, C.M.J.; Barge, S.; Mohan, S.; Nanjan, M.J. Virtual high throughput screening: Potential inhibitors for SARS-CoV-2 PLPRO and 3CLPRO proteases. Eur. J. Pharmacol. 2021, 901, 174082. [Google Scholar] [CrossRef]
- Khaledi, M.; Sameni, F.; Yahyazade, S.; Radandish, M.; Owlia, P.; Bagheri, N.; Afkhami, H.; Mahjoor, M.; Esmaelpour, Z.; Kohansal, M. COVID-19 and the potential of Janus family kinase (JAK) pathway inhibition: A novel treatment strategy. Front. Med. 2022, 9, 961027. [Google Scholar] [CrossRef]
- Schake, P.; Dishnica, K.; Kaiser, F.; Leberecht, C.; Haupt, V.J.; Schroeder, M. An interaction-based drug discovery screen explains known SARS-CoV-2 inhibitors and predicts new compound scaffolds. Sci. Rep. 2023, 13, 9204. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guan, Y. COVID-19 drug repurposing: A review of computational screening methods, clinical trials, and protein interaction assays. Med. Res. Rev. 2021, 41, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Tshinanu, F.M. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Agoramoorthy, G.; Lee, S.-S. The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: Lessons learned from major clinical studies. Front. Pharmacol. 2021, 12, 704205. [Google Scholar] [CrossRef] [PubMed]
- McCradden, M.D.; Anderson, J.A.; A. Stephenson, E.; Drysdale, E.; Erdman, L.; Goldenberg, A.; Zlotnik Shaul, R. A research ethics framework for the clinical translation of healthcare machine learning. Am. J. Bioeth. 2022, 22, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Fu, W.; Jiang, D.; Sun, H.; Wang, J.; Zhang, X.; Weng, G.; Liu, H.; Tao, P.; Hou, T. VAD-MM/GBSA: A Variable Atomic Dielectric MM/GBSA Model for Improved Accuracy in Protein–Ligand Binding Free Energy Calculations. J. Chem. Inf. Model. 2021, 61, 2844–2856. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Consortium, W.S.T. Repurposed antiviral drugs for COVID-19—Interim WHO solidarity trial results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Stockand, J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020, 83, 104327. [Google Scholar] [CrossRef]
- Hamdy, R.; Fayed, B.; Mostafa, A.; Shama, N.M.A.; Mahmoud, S.H.; Mehta, C.H.; Nayak, Y.; M. Soliman, S.S. Iterated virtual screening-assisted antiviral and enzyme inhibition assays reveal the discovery of novel promising anti-SARS-CoV-2 with dual activity. Int. J. Mol. Sci. 2021, 22, 9057. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, A.; Gosavi, S.; Faccioli, P.; Wintrode, P.L. Successes and challenges in simulating the folding of large proteins. J. Biol. Chem. 2020, 295, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Bhati, A.P.; Hoti, A.; Potterton, A.; Bieniek, M.K.; Coveney, P.V. Long Time Scale Ensemble Methods in Molecular Dynamics: Ligand-Protein Interactions and Allostery in SARS-CoV-2 Targets. J. Chem. Theory Comput. 2023, 19, 3359–3378. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.W.; Gilmer, J.B.; Matsumoto, R.A.; Quach, C.D.; Shamaprasad, P.; Yang, A.H.; Iacovella, C.R.; McCabe, C.; Cummings, P.T. Towards molecular simulations that are transparent, reproducible, usable by others, and extensible (TRUE). Mol. Phys. 2020, 118, e1742938. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.; Ouldridge, T.E.; Henrich, O.; Rovigatti, L.; Šulc, P. A primer on the oxDNA model of DNA: When to use it, how to simulate it and how to interpret the results. Front. Mol. Biosci. 2021, 8, 693710. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O.; Iwaloye, O.; Josiah, S.S.; Lawal, A.O.; Akinjiyan, M.O.; Ariyo, E.O. Molecular docking studies, molecular dynamics and ADME/tox reveal therapeutic potentials of STOCK1N-69160 against papain-like protease of SARS-CoV-2. Mol. Divers. 2021, 25, 1761–1773. [Google Scholar] [CrossRef]
- Citarella, A.; Scala, A.; Piperno, A.; Micale, N. SARS-CoV-2 Mpro: A potential target for peptidomimetics and small-molecule inhibitors. Biomolecules 2021, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Patamia, V.; Scala, A.; Sciortino, M.T.; Piperno, A.; Rescifina, A. Putative inhibitors of SARS-CoV-2 main protease from a library of marine natural products: A virtual screening and molecular modeling study. Mar. Drugs 2020, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Minetti, C.A.; Remeta, D.P. Forces driving a magic bullet to its target: Revisiting the role of thermodynamics in drug design, development, and optimization. Life 2022, 12, 1438. [Google Scholar] [CrossRef]
- Badavath, V.N.; Kumar, A.; Samanta, P.K.; Maji, S.; Das, A.; Blum, G.; Jha, A.; Sen, A. Determination of potential inhibitors based on isatin derivatives against SARS-CoV-2 main protease (mpro): A molecular docking, molecular dynamics and structure-activity relationship studies. J. Biomol. Struct. Dyn. 2022, 40, 3110–3128. [Google Scholar] [CrossRef] [PubMed]
- Boike, L.; Henning, N.J.; Nomura, D.K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 2022, 21, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Al-Karmalawy, A.A.; El-Gamil, D.S.; El-Shesheny, R.; Sharaky, M.; Alnajjar, R.; Kutkat, O.; Moatasim, Y.; Elagawany, M.; Al-Rashood, S.T.; Binjubair, F.A. Design and statistical optimisation of emulsomal nanoparticles for improved anti-SARS-CoV-2 activity of N-(5-nitrothiazol-2-yl)-carboxamido candidates: In vitro and in silico studies. J. Enzym. Inhib. Med. Chem. 2023, 38, 2202357. [Google Scholar] [CrossRef]
- De Souza Neto, L.R.; Moreira-Filho, J.T.; Neves, B.J.; Maidana, R.L.B.R.; Guimarães, A.C.R.; Furnham, N.; Andrade, C.H.; Silva, F.P., Jr. In silico strategies to support fragment-to-lead optimization in drug discovery. Front. Chem. 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Trisciuzzi, D.; Villoutreix, B.O.; Siragusa, L.; Baroni, M.; Cruciani, G.; Nicolotti, O. Targeting protein-protein interactions with low molecular weight and short peptide modulators: Insights on disease pathways and starting points for drug discovery. Expert. Opin. Drug Discov. 2023, 18, 737–752. [Google Scholar] [CrossRef]
- Kumar, H.M.S.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Poongavanam, V.; Ramaswamy, V. Computational Drug Discovery: Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Mouvet, F.; Villard, J.; Bolnykh, V.; Rothlisberger, U. Recent advances in first-principles based molecular dynamics. Acc. Chem. Res. 2022, 55, 221–230. [Google Scholar] [CrossRef]
- Li, H.; Komori, A.; Li, M.; Chen, X.; Yang, A.W.H.; Sun, X.; Liu, Y.; Hung, A.; Zhao, X.; Zhou, L. Multi-ligand molecular docking, simulation, free energy calculations and wavelet analysis of the synergistic effects between natural compounds baicalein and cubebin for the inhibition of the main protease of SARS-CoV-2. J. Mol. Liq. 2023, 374, 121253. [Google Scholar] [CrossRef]
- Yuda, G.P.W.C.; Hanif, N.; Hermawan, A. Computational Screening Using a Combination of Ligand-Based Machine Learning and Molecular Docking Methods for the Repurposing of Antivirals Targeting the SARS-CoV-2 Main Protease. DARU J. Pharm. Sci. 2023, 32, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Ullah, S.; Halim, S.A.; Rehman, N.U.; Ali, A.; Jan, A.; Muhsinah, A.B.; Khan, A.; Al-Harrasi, A. Targeting papain-like protease by natural products as novel therapeutic potential SARS-CoV-2. Int. J. Biol. Macromol. 2024, 258, 128812. [Google Scholar] [CrossRef]
- Prieto-Martínez, F.D.; Galván-Ciprés, Y. Free Energy Estimation for Drug Discovery: Background and Perspectives. Appl. Comput. Aided Drug Des. Models Methods 2023, 310–345. Available online: https://books.google.co.za/books?hl=en&lr=&id=t7DpEAAAQBAJ&oi=fnd&pg=PA310&dq=Prieto-Mart%C3%ADnez,+F.D.%3B+Galv%C3%A1n-Cipr%C3%A9s,+Y.+Free+Energy+Estimation+for+Drug+Discovery:+Background+and+Perspectives.+Appl.+Comput.+Aided+Drug+Des.+Models+Methods+2 (accessed on 25 May 2024).
- Chen, L.; Wu, Y.; Wu, C.; Silveira, A.; Sherman, W.; Xu, H.; Gallicchio, E. Performance and Analysis of the Alchemical Transfer Method for Binding-Free-Energy Predictions of Diverse Ligands. J. Chem. Inf. Model. 2023, 64, 250–264. [Google Scholar] [CrossRef]
- Parveen, S.; Shehzadi, S.; Shafiq, N.; Rashid, M.; Naz, S.; Mehmood, T.; Riaz, R.; Almaary, K.S.; Nafidi, H.-A.; Bourhia, M. A discovery of potent kaempferol derivatives as multi-target medicines against diabetes as well as bacterial infections: An in silico approach. J. Biomol. Struct. Dyn. 2024, 1–23. [Google Scholar] [CrossRef]
- Kurisaki, I.; Suzuki, M. Simulation toolkits at the molecular scale for trans-scale thermal signaling. Comput. Struct. Biotechnol. J. 2023, 21, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Schlick, T.; Portillo-Ledesma, S. Biomolecular modeling thrives in the age of technology. Nat. Comput. Sci. 2021, 1, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Nochebuena, J.; Naseem-Khan, S.; Cisneros, G.A. Development and Application of QM/MM Methods with Advanced Polarizable Potentials. arXiv 2020, arXiv:2010.14723. [Google Scholar] [CrossRef]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. In silico methods for drug design and discovery. Front. Media SA 2020, 8, 612. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Pantaleão, S.Q.; Fernandes, P.O.; Gonçalves, J.E.; Maltarollo, V.G.; Honorio, K.M. Recent advances in the prediction of pharmacokinetics properties in drug design studies: A review. ChemMedChem 2022, 17, e202100542. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, X.; Shu, Y.; Guo, M.; Zhang, H.; Tao, W. Insights from nanotechnology in COVID-19 treatment. Nano today 2021, 36, 101019. [Google Scholar] [CrossRef]
- Abdel-Basset, M.; Hawash, H.; Elhoseny, M.; Chakrabortty, R.K.; Ryan, M. DeepH-DTA: Deep learning for predicting drug-target interactions: A case study of COVID-19 drug repurposing. Ieee Access 2020, 8, 170433–170451. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Wang, J.; Cao, Y.; Kato, N. When machine learning meets privacy in 6G: A survey. IEEE Commun. Surv. Tutor. 2020, 22, 2694–2724. [Google Scholar] [CrossRef]
- Sabe, V.T.; Ntombela, T.; Jhamba, L.A.; Maguire, G.E.; Govender, T.; Naicker, T.; Kruger, H.G. Current trends in computer aided drug design and a highlight of drugs discovered via computational techniques: A review. Eur. J. Med. Chem. 2021, 224, 113705. [Google Scholar] [CrossRef]
- Zhan, P.; Pannecouque, C.; De Clercq, E.; Liu, X. Anti-HIV drug discovery and development: Current innovations and future trends: Miniperspective. J. Med. Chem. 2016, 59, 2849–2878. [Google Scholar] [CrossRef] [PubMed]
- Rakib, A.; Nain, Z.; Sami, S.A.; Mahmud, S.; Islam, A.; Ahmed, S.; Siddiqui, A.B.F.; Babu, S.O.F.; Hossain, P.; Shahriar, A. A molecular modelling approach for identifying antiviral selenium-containing heterocyclic compounds that inhibit the main protease of SARS-CoV-2: An in silico investigation. Brief. Bioinform. 2021, 22, 1476–1498. [Google Scholar] [CrossRef] [PubMed]
- Carracedo-Reboredo, P.; Liñares-Blanco, J.; Rodríguez-Fernández, N.; Cedrón, F.; Novoa, F.J.; Carballal, A.; Maojo, V.; Pazos, A.; Fernandez-Lozano, C. A review on machine learning approaches and trends in drug discovery. Comput. Struct. Biotechnol. J. 2021, 19, 4538–4558. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.; Cornell, W.; Luan, B. In silico Exploration of Inhibitors for SARS-CoV-2’s Papain-Like Protease. Front. Chem. 2020, 8, 624163. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors–an in silico docking and molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2021, 39, 4362–4374. [Google Scholar] [CrossRef]
- Wang, J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. 2020, 60, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Chekroud, A.M.; Bondar, J.; Delgadillo, J.; Doherty, G.; Wasil, A.; Fokkema, M.; Cohen, Z.; Belgrave, D.; DeRubeis, R.; Iniesta, R. The promise of machine learning in predicting treatment outcomes in psychiatry. World Psychiatry 2021, 20, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. A review of open source ventilators for COVID-19 and future pandemics. F1000Research 2020, 9, 218. [Google Scholar] [CrossRef]
- Di Pietro, G.; Biagi, F.; Costa, P.; Karpiński, Z.; Mazza, J. The Likely Impact of COVID-19 on Education: Reflections Based on the Existing Literature and Recent International Datasets; Publications Office of the European Union Luxembourg: Luxembourg, 2020; Volume 30275. [Google Scholar]
- Sohraby, F.; Aryapour, H. Unraveling the unbinding pathways of SARS-CoV-2 Papain-like proteinase known inhibitors by Supervised Molecular Dynamics simulation. PLoS ONE 2021, 16, e0251910. [Google Scholar] [CrossRef]
- Alanine, A.; Nettekoven, M.; Roberts, E.; Thomas, A.W. Lead generation-enhancing the success of drug discovery by investing in the hit to lead process. Comb. Chem. High. Throughput Screen. 2003, 6, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Aftab, S.O.; Ghouri, M.Z.; Masood, M.U.; Haider, Z.; Khan, Z.; Ahmad, A.; Munawar, N. Analysis of SARS-CoV-2 RNA-dependent RNA polymerase as a potential therapeutic drug target using a computational approach. J. Transl. Med. 2020, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Chen, J.; Zhang, H. An investigation of testing capacity for evaluating and modeling the spread of coronavirus disease. Inf. Sci. 2021, 561, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Shen, Z.; Ratia, K.; Cooper, L.; Kong, D.; Lee, H.; Kwon, Y.; Li, Y.; Alqarni, S.; Huang, F.; Dubrovskyi, O. Design of SARS-CoV-2 PLpro inhibitors for COVID-19 antiviral therapy leveraging binding cooperativity. J. Med. Chem. 2021, 65, 2940–2955. [Google Scholar] [CrossRef]

| Enzyme/Receptor (Crystal Structure) | Function/Role | Targets for Therapeutic Development | Virus–Host Interaction | Implications for Viral Evolution and SARS-CoV-2 Management | References |
|---|---|---|---|---|---|
ACE2 | Viral entry, lung protection | Therapeutic target | Facilitates viral entry and crosstalk with host cells | Dual role in COVID-19 infections; potential protection from acute lung injury and ARDS; increased susceptibility due to high ACE2 expression | [10,11] |
TMPRSS2 | Viral entry, priming | Potential inhibitor | Mediates viral entry and spike protein cleavage | Important for viral entry; inhibitors may prevent infection and reduce viral spread | [12] |
DPP4 | Possible receptor | Role uncertain | Possible binding to SARS-CoV-2 | Role in virus–host interaction needs further investigation; may be involved in viral entry | [13] |
PLpro (Protease) | Viral replication, polyprotein cleavage | Drug target for inhibition | Essential for viral replication | Critical for cleaving polyproteins; potential drug target for inhibiting viral replication | [14] |
3CLpro (Mpro)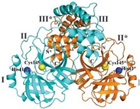 | Polyprotein cleavage, replication | Drug target for inhibition | Essential for viral replication | Key for cleaving polyproteins; promising target for antiviral strategies | [15,16] |
RdRp (RNA Polymerase) | RNA synthesis, replication | Potential drug target | Crucial for viral genome replication | Required for replication; promising drug target for antiviral strategies | [17] |
Helicase (NSP13)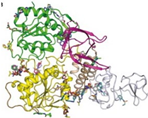 | RNA unwinding, replication | Potential target | Facilitates RNA unwinding and replication | Important for viral genome replication; potential therapeutic target for anti-COVID-19 strategies | [18] |
Cathepsin B/L | Viral entry | Target for inhibiting entry | Involved in viral entry | Blocking cathepsin activity can prevent viral entry | [19,20,58] |
Furin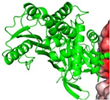 | S protein cleavage | Potential drug target | Cleavage of S protein and virus entry | Promising target for inhibiting viral entry and spread | [22,58] |
| Inhibitor/Drug Candidate and Chemical Structure | Simulation Method | Key/Type of Interaction | Simulation Length | Force Field Used | Binding Free Energy | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|---|
GRL-0617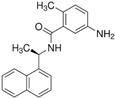 | Molecular dynamics simulation | Non-covalent binding | 100 ns | AMBER | −21.5 kcal/mol | Noncovalent inhibition of PLpro | [103] |
| VIR250 and VIR251 | Molecular dynamics simulation | Irreversible binding | 50 ns | OPLS-AA | Not available | Irreversible inhibitors of PLpro | [104,105] |
Neobavaisoflavone | Molecular dynamics simulation | Low-energy binding | 75 ns | CHARMM36 | Not available | Binding to the catalytic triad of PLpro | [106] |
Ritonavir | Molecular dynamics simulation | Binding analysis | 50 ns | GROMOS | −8.2 kcal/mol | Investigated potential PLpro inhibition | [107] |
Dasabuvir (A17) | Molecular dynamics simulation | Stable binding | 100 ns | CHARMM27 | −11.7 kcal/mol | Stable binding with PLpro | [108] |
Methisazone (A34)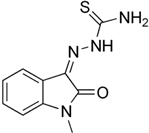 | Molecular dynamics simulation | Stable binding | 75 ns | AMBER | −12.3 kcal/mol | Exhibits stable dynamic behavior in a complex | [108] |
Vaniprevir (A53)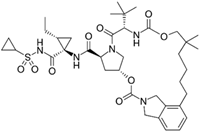 | Molecular dynamics simulation | High binding affinity | 100 ns | OPLS-AA | Not available | Shows high binding affinity for PLpro | [108] |
Baicalein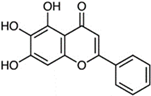 | Molecular dynamics simulation | Binding to the active site | 50 ns | CHARMM36 | −12.8 kcal/mol | Binds to the active site of PLpro | [109] |
Disulfiram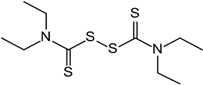 | Molecular dynamics simulation | Inhibition analysis | 75 ns | GROMOS | Not available | Repurposed for potential PLpro inhibition | [110] |
Carmofur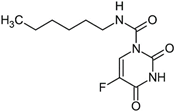 | Molecular dynamics simulation | Binding to PLpro | 100 ns | AMBER | −10.5 kcal/mol | Demonstrates binding to PLpro. | [111] |
Ebselen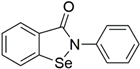 | Molecular dynamics simulation | Antiviral activity | 75 ns | CHARMM27 | Not available | Investigated for its antiviral activity | [69] |
Tideglusib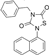 | Molecular dynamics simulation | Potential inhibitor | 50 ns | CHARMM36 | Not available | Explored for its potential as an inhibitor | [111] |
Shikonin | Molecular dynamics simulation | Active site binding | 100 ns | AMBER | −15.6 kcal/mol | Binds to the active site of PLpro | [112] |
PX-12 (Belinostat) | Molecular dynamics simulation | Inhibition potential | 75 ns | GROMOS | Not available | Investigated for its inhibition potential | [101] |
| Computational Technique | Applicability of the Technique to SARS-CoV-2 | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Molecular Dynamics (MD) | For an atomistic analysis of the dynamic interactions between inhibitors and PLpro or other enzymes in SARS-CoV-2, MD simulations are a good choice. They could record conformational changes over time, offering insights into the kinetics and binding mechanisms of the creation of protein–ligand complexes. MD simulations provide useful information regarding the flexibility and stability of the binding site, thereby making structure-based drug design easier. | By thoroughly examining binding events, MD simulations help scientists understand the intricate relationships between inhibitors and target enzymes. They offer an atomistic-level understanding of the dynamic behavior of the protein–ligand complex, which helps to rationally design new inhibitors with a higher selectivity and affinity. Furthermore, MD simulations can capture the impacts of ions and solvent molecules on the binding process, thereby improving the precision of binding free energy estimates. | MD simulations are computationally demanding despite their high level of realism, especially when extended periods are required to observe uncommon binding events. The selection of force field parameters and simulation techniques, which may induce bias or inaccuracies, also affects the accuracy of MD simulations. Furthermore, the predictive power of MD simulations may be limited by their inability to adequately mimic specific features of protein dynamics, such as extensive conformational changes or allosteric effects. | [189,190,191] |
| Molecular Docking | One popular technique for estimating the binding affinities and mechanisms of small-molecule inhibitors to PLpro or other SARS-CoV-2 enzymes is molecular docking. Large compound libraries can be virtually screened to identify possible inhibitors and rank lead compounds for additional experimental confirmation. By offering insightful information about the interactions between inhibitors and target enzymes, molecular docking helps optimize lead compounds for structure-based drug design strategies. | High-throughput screening capabilities provided by molecular docking enable researchers to quickly assess how well various possible inhibitors bind to PLpro or other enzymes. This makes it easier to rationally design new inhibitors with an improved potency and specificity by precisely predicting the binding modes and poses of ligands within the active site of the enzyme. Moreover, researchers with limited computer resources can access molecular docking because it is comparatively less expensive to compute than other approaches. | The precision with which molecular docking techniques can capture protein flexibility may be limited, which could result in estimates of the ligand binding affinity that are either falsely positive or falsely negative. They depend on stiff receptor architectures, which may not accurately capture the dynamic character of the interaction between a protein and a ligand. The consideration of solvent effects and conformational changes in the binding site may be limited in molecular docking predictions of binding free energies. Furthermore, the quality of the protein structure and the scoring function employed affect the accuracy of molecular docking results, which could add uncertainty to the predictions. | [76,192,193,194] |
| Free Energy Perturbation (FEP) | FEP calculations provide a quantitative assessment of the binding free energies between inhibitors and PLpro or other enzymes in SARS-CoV-2. By shedding light on the thermodynamic stability of the protein–ligand complex, it is possible to rank lead compounds according to how well they are projected to bind. FEP calculations can capture minor energy differences between ligands, guiding sensible tuning of inhibitor potency and selectivity. | By providing researchers with a thorough and quantitative evaluation of binding affinities, FEP calculations enable them to rank potential inhibitors for additional experimental validation and compare the effectiveness of various inhibitors. By taking solvent effects, entropy fluctuations, and molecule flexibility into consideration, they provide insightful information about the energetics of ligand binding. Furthermore, by highlighting important interactions between inhibitors and target enzymes, FEP calculations can direct structure-based drug design efforts and help rationalize the development of innovative treatments with increased efficacy. | FEP computations are computationally and time intensive because they require meticulous equilibration and substantial sampling to obtain accurate findings. To guarantee the validity of the forecasts, they depend on precise force field parameters and simulation techniques, which, if improperly calibrated, may introduce uncertainties or inaccuracies. Furthermore, some features of protein–ligand interactions, such as conformational changes or solvent effects, may be difficult for FEP calculations to adequately simulate, which could result in inaccurate binding free energy predictions. Furthermore, the computing expense of FEP computations can restrict their suitability for intricate biological systems or sizable compound libraries, necessitating cautious evaluation of available resources. | [190,195,196] |
| MM/PBSA and MM/GBSA | When determining the binding free energies between inhibitors and PLpro or other enzymes in SARS-CoV-2, the MM/PBSA and MM/GBSA approaches work well, especially in large systems with many flexible regions or binding sites. They provide a computationally affordable substitute for explicit solvent simulations, facilitating the expeditious identification of putative inhibitors and the order of importance of lead compounds. The energetics of protein–ligand interactions are better understood using the MM/PBSA and MM/GBSA techniques, which help in the logical adjustment of inhibitor potency and selectivity. | Because the MM/PBSA and MM/GBSA techniques are less computationally expensive than explicit solvent simulations, even researchers with limited computing resources can use them. They provide a useful and effective method for calculating binding free energies in large systems, making it possible to quickly screen for possible inhibitors and identify lead compounds for additional experimental confirmation. Furthermore, the contributions of certain residues to ligand binding can be captured using the MM/PBSA and MM/GBSA techniques, offering important insights into the major interactions influencing inhibitor efficacy and selectivity. | The MM/PBSA and MM/GBSA techniques depend on implicit solvent representations and simplified energy models, which may not be sufficiently accurate to capture intricate protein–ligand interactions. They are susceptible to the selection of force field parameters, and if not calibrated correctly, may yield erratic results. Furthermore, the projected binding free energies may contain errors or uncertainties due to the inability of the MM/PBSA and MM/GBSA methodologies to consider solvent effects and conformational changes in the binding site. Furthermore, in systems with high protein flexibility or allosteric effects, the accuracy of MM/PBSA and MM/GBSA calculations may be impaired, necessitating rigorous validation and interpretation of the results. | [76,190,197] |
| Quantum Mechanics/Molecular Mechanics (QM/MM) | Enzyme–substrate interactions in PLpro or other SARS-CoV-2 enzymes can be studied with a high accuracy using QM/MM simulations, which consider the chemical reactivity and electronic effects during binding. They offer thorough insights into the mechanisms underlying inhibitor binding and enzyme catalysis, which help to rationally develop innovative medicines with increased selectivity and efficacy. QM/MM simulations can capture the effects of substrate changes or active site mutations on the enzyme activity and inhibitor binding, making them an excellent tool for investigating complex biological systems. | A thorough understanding of enzyme–substrate interactions at the quantum mechanical level is possible with QM/MM simulations, which also provide important insights into the underlying chemical mechanisms of inhibitor binding and enzyme catalysis. They enable the logical optimization of inhibitor potency and selectivity by enabling researchers to investigate the energetics of bond creation and breaking in enzyme processes. Furthermore, by highlighting important interactions between inhibitors and target enzymes, QM/MM simulations can direct structure-based drug design efforts and help develop innovative therapies with improved specificity and efficacy. | The usefulness of QM/MM simulations is limited to tiny systems or short timelines because of their high processing costs and requirements. To guarantee the veracity of the forecasts, they depend on precise force field parameters and quantum mechanical techniques, which, if improperly calibrated, may introduce uncertainties or inaccuracies. Furthermore, the effects of solvent molecules and protein flexibility on the interactions between the enzyme and substrate may be difficult to simulate in QM/MM simulations, which could result in inaccurate binding free energy predictions. In addition, the intricacy of quantum mechanical computations and the requirement for specific knowledge in computational chemistry may make it difficult to interpret the outcomes of QM/MM simulations. | [198,199,200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magwaza, N.N.; Mushebenge, A.G.-A.; Ugbaja, S.C.; Mbatha, N.A.; Khan, R.B.; Kumalo, H.M. Mechanistic Insights into Targeting SARS-CoV-2 Papain-like Protease in the Evolution and Management of COVID-19. BioChem 2024, 4, 268-299. https://doi.org/10.3390/biochem4030014
Magwaza NN, Mushebenge AG-A, Ugbaja SC, Mbatha NA, Khan RB, Kumalo HM. Mechanistic Insights into Targeting SARS-CoV-2 Papain-like Protease in the Evolution and Management of COVID-19. BioChem. 2024; 4(3):268-299. https://doi.org/10.3390/biochem4030014
Chicago/Turabian StyleMagwaza, Nonjabulo Ntombikhona, Aganze Gloire-Aimé Mushebenge, Samuel Chima Ugbaja, Nonkululeko Avril Mbatha, Rene B. Khan, and Hezekiel M. Kumalo. 2024. "Mechanistic Insights into Targeting SARS-CoV-2 Papain-like Protease in the Evolution and Management of COVID-19" BioChem 4, no. 3: 268-299. https://doi.org/10.3390/biochem4030014
APA StyleMagwaza, N. N., Mushebenge, A. G.-A., Ugbaja, S. C., Mbatha, N. A., Khan, R. B., & Kumalo, H. M. (2024). Mechanistic Insights into Targeting SARS-CoV-2 Papain-like Protease in the Evolution and Management of COVID-19. BioChem, 4(3), 268-299. https://doi.org/10.3390/biochem4030014







