Evaluation of the Performances of a Monolayer Cover with an Elevated Water Table Used for the Reclamation of the Abandoned Aldermac Mine Site (Québec, Canada)
Abstract
:1. Introduction
2. Site Description
- Tailings in the South area were excavated with part of the contaminated soils and confined using a high-density polyethylene (HDPE) geomembrane cover.
- Tailings and contaminated soils along Stream 1 were excavated and deposited in the North area.
- Tailings and contaminated soils from the Intermediate area were excavated and deposited in the North area.
- Two areas separated by a dike (Terrace 1 and 2, see Figure 2) were created in the North area. Tailings in this area were confined with watertight dikes to create ideal conditions for a monolayer-EWT cover composed of a granular material (sand and gravel). The monolayer-EWT cover has a thickness of about 1 m and acts as a protective layer against evaporation.
3. Materials and Methods
3.1. Observation Well Network
3.2. Water Sampling
3.3. Physical and Chemical Analyses
3.4. Statistical Analyses
4. Investigation Results
4.1. Hydrogeological Behavior
4.2. Hydrochemical Behavior
4.2.1. Electrical Conductivity and pH
4.2.2. Correlation between Concentrations of Ca + Mg vs. SO4
4.2.3. Principal Component Analysis
- First campaign
- Tenth campaign
5. Other Results
- Group 1 has a high mineralization and low pH; these samples were collected in the first stage after the mine site reclamation. These waters can be considered contaminated water with existing AMD where the EC ranged between 3020 and 1636 µS/cm and SO4 between 1342 and 1415 mg/L.
- Group 2 has low mineralization and relatively high pH (close to 5 for samples collected during the tenth campaign). These waters represent low metal contamination.
6. Conclusions
- Concentrations of different dissolved elements and EC declined between the first water sampling campaign performed at the end of the site’s reclamation, and the tenth sampling campaign, which was performed three years later.
- Matrix of correlations indicated that mineral associations of the last campaign were different than those obtained during the first campaign.
Author Contributions
Funding
Conflicts of Interest
References
- Aubertin, M.; Bussière, B.; Bernier, L. Environnement et Gestion des Résidus Miniers [CD-ROM]; Les Éditions de l'École Polytechnique de Montréal: Montréal, QC, Canada, 2002. [Google Scholar]
- Ritcey, G.M. Tailings Management, Problems and Solutions in the Mining Industry. In Process Metallurgy 6; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- SRK. Guide Technique Préliminaire sur le Drainage Rocheux Acide; BCAMD Task Force; Centre de Recherches Minérales: Québec, QC, Canada, 1989; Volume 1. [Google Scholar]
- MEND. MEND Manual; Report 5.4.2; Secretariat CANMET: Ottawa, ON, Canada, 2001; Volume 4-Prevention and Control. [Google Scholar]
- Fraser, W.W.; Robertson, J.D. Subaqueous Disposal of Reactive Mine Waste: An Overview and Update of Case Studies-MEND/Canada. In Proceedings of the International Land Reclamation and Mine Drainage Conference and 3rd International Conference on the Abatement of Acidic Drainage, Pittsburgh, PA, USA, 24–30 April 1994; pp. 250–259. [Google Scholar]
- Amyot, D.; Vézina, S. Flooding as a Reclamation Solution to an Acidic Tailings Pond-the Solbec case. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; pp. 681–696. [Google Scholar]
- Simms, P.H.; Yanful, E.K.; St-Arnaud, L.; Aubé, B. A laboratory evaluation of metal release and transport in flooded preoxidized mine tailings. Appl. Geochem. 2001, 15, 1245–1263. [Google Scholar] [CrossRef]
- Cabral, A.; Racine, I.; Burnotte, F.; Lefebvre, G. Diffusion of oxygen through a pulp and paper residue barrier. Can. Geotech. J. 2000, 37, 201–217. [Google Scholar] [CrossRef]
- Tassé, N.; Germain, D. Performance of Forestry Wastes for the Treatment of Acid Mine Drainage through a Reactive Barrier. In Proceedings of the 7th International Symposium on Environmental Issues and Waste Management in Energy and Mineral Production (SWEMP 2002), Calgliari, Italy, 7–10 October 2002; pp. 723–773. [Google Scholar]
- Nicholson, R.V.; Gillham, R.W.; Cherry, J.A.; Reardon, E.J. Reduction of acid generation in mine tailings through the use of moisture-retaining cover layers as oxygen barriers. Can. Geotech. J. 1989, 26, 1–8. [Google Scholar] [CrossRef]
- Collin, M.; Rasmuson, A. Mathematical Modelling of Water and Oxygen Transport in Layered Soil Covers for Deposits of Pyritic Mine Tailings, Acid Mine Drainage: Designing for Closure. In Proceedings of the GAC-MAC Annual Meeting, Vancouver, BC, Canada, 16–18 May, 1990; pp. 311–333. [Google Scholar]
- Bussière, B.; Maqsoud, A.; Aubertin, M.; Martschuk, J.; McMullen, J.; Julien, M. Performance of the oxygen limiting cover at the LTA site, Malartic, Québec. Env. Soc. CIM 2006, 99, 1–11. [Google Scholar]
- Bussière, B.; Maqsoud, A.; Aubertin, M.; Martschuk, J.; McMullen, J.; Julien, M. Results from the Monitoring Program at the LTA Site: Hydraulic Behaviour of the Cover. In Proceedings of the Mining Industry Conference, Montréal, QC, Canada, 27–29 May 2003. [Google Scholar]
- Bussière, B.; Maqsoud, A.; Demers, I.; Doumbouya, I. Modélisation Physique de Divers Scénarios de Recouvrement et Étude du Comportement Hydrogéologique: Site Manitou; Report PU-2008-03-371 for MERN; Quebec’s Ministry of Energy and Natural Resources: Rouyn-Noranda, QC, Canada, 2009. [Google Scholar]
- Maqsoud, A.; Bussière, B.; Mbonimpa, M.; Aubertin, M.; Chouteau, M. Suction break to control slope-induced effects in covers used as gas barrier. Can. Geotech. J. 2011, 48, 53–71. [Google Scholar] [CrossRef]
- Maqsoud, A.; Bussière, B.; Turcotte, S.; Roy, M. Performance Evaluation of Covers with Capillary Barrier Effects (CCBE) under Deep Groundwater Conditions Using Experimental Cells. In Proceedings of the Conférence Canadienne de Géotechnique, GeoOttawa, Ottawa, ON, Canada, 1–4 October 2017. [Google Scholar]
- Dagenais, A.M. Technique de Contrôle du Drainage Minier Acide Basées sur les Effets de Barrière Capillaire. Ph.D. Thesis, Polytechnique Montréal, Montreal, QC, Canada, 2005. [Google Scholar]
- Ethier, M.P.; Bussière, B.; Aubertin, M.; Maqsoud, A.; Demers, I.; Dionne, J. Field Investigation Results of the Elevated Water Table Technique Combined with a Monolayer Cover on Reactive Tailings. In Proceedings of the Conference Canadienne de Géotechnique, GeoRegina, Regina, SK, Canada, 28 September–1 October 2014 [CD-ROM]. [Google Scholar]
- Ethier, M.P.; Bussière, B.; Aubertin, M.; Maqsoud, A.; Lacroix, R. In Situ Evaluation of the Elevated Water Table Technique Combined with a Monolayer Cover Made with Low Sulphide Tailings: Monitoring Strategy and Preliminary Results. In Proceedings of the 66th Canadian Geotechnical Conference and the 11th Joint CGS/IAH-CNC Groundwater Conference, Montréal, QC, Canada, 29 September–3 October 2013 [CD-ROM]. [Google Scholar]
- Maqsoud, A.; Mbonimpa, M.; Bussière, B.; Dionne, J. Réhabilitation du Site Minier Abandonné Aldermac, Résultats Préliminaires du Suivi de la Nappe Surélevée. In Proceedings of the 66th Canadian Geotechnical Conference and the 11th Joint CGS/IAH-CNC Groundwater Conference, Montréal, QC, Canada, 29 September–3 October 2013. [Google Scholar]
- Maqsoud, A.; Mbonimpa, M.; Bussière, B.; Benzaazoua, M. The Hydrochemical Behaviour of the Aldermac Abandoned Mine Site after its Rehabilitation. In Proceedings of the Conférence Canadienne de Géotechnique, GeoQuébec, Québec, QC, Canada, 20–23 September 2015. [Google Scholar]
- Ouangrawa, M.; Aubertin, M.; Molson, J.W.; Bussière, B.; Zagury, G.J. Preventing acid mine drainage with an elevated water table: Long-term column experiments and parameter analysis. Water Air Soil Pollut. 2010, 213, 437–458. [Google Scholar] [CrossRef]
- Ouangrawa, M.; Molson, J.; Aubertin, M.; Zagury, G.; Bussière, B. The Effect of Water Table Elevation on Acid Mine Drainage from Reactive Tailings: A Laboratory and Numerical Modeling Study. In Proceedings of the 7th International Conference on Acid Rock Drainage (ICARD), St. Louis, MO, USA, 26–30 March 2006; Barnhisel, R.I., Ed.; The American Society of Mining and Reclamation: St. Louis MO, USA, 2006; pp. 1473–1482. [Google Scholar]
- Demers, I.; Bussière, B.; Rousselle, M.; Aubertin, M.; Pabst, T.; Lacroix, R. Laboratory Evaluation of Reclamation Scenarios for the Spillage Areas of the Abandoned Manitou Mine Site Using Goldex Tailings. In Proceedings of the World Mining Congress, Montréal, QC, Canada, 11–15 August 2013. [Google Scholar]
- Pabst, T.; Aubertin, M.; Bussière, B.; Molson, J. Column tests to characterize the hydrogeochemical response of pre-oxidised acid-generating tailings with a monolayer cover. Water Air Soil Pollut. 2014, 225, 1841. [Google Scholar] [CrossRef]
- Maqsoud, A.; Bussère, B.; Mbonimpa, M. Low Saturated Hydraulic Conductivity Covers. In Hard Rock Mine Reclamation: From Prediction to Management of Acid Mine Drainage; Bussière, B., Guittonny, M., Eds.; CRC Press: New York, NY, USA, 2021. [Google Scholar]
- SNC-Lavalin. SNC-Lavalin. Restauration du Site Minier Aldermac, Rapport tel que construit des travaux effectués en 2008-2009, Ministère des Ressources naturelles et de la Faune Rouyn-Noranda, Québec, 2010, SNC-Lavalin, Mines et Métallurgie. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fmern.gouv.qc.ca%2Fwp-content%2Fuploads%2FDocument_17-01-033-N.pdf&clen=17914532&chunk=true (accessed on 1 February 2022).
- Cyr, J.; Maurice, R.; Isabelle, D. Restauration du Site Minier Aldermac. In Proceedings of the Symposium Rouyn-Noranda: Mines and the Environment, Rouyn-Noranda, Québec, QC, Canada, 6–19 November 2011 [CD-ROM]. [Google Scholar]
- Elberling, B.; Nicholson, R.V. Field determination of sulphide oxidation rates in mines tailings. Water Resour. Res. 1996, 32, 1773–1784. [Google Scholar] [CrossRef]
- Tibble, P.A.; Nicholson, R.V. Oxygen consumption on sulphide tailings and covers: Measured rates and applications. In Proceedings of the 4th International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997; pp. 647–661. [Google Scholar]
- Elberling, B.; Damgaard, L.R. Microscale measurement of oxygen diffusion and consumption in subaqueous sulphide tailings. Geochem. Cosmochim. Acta 2001, 65, 1897–1905. [Google Scholar] [CrossRef]
- Hollings, P.; Hendry, M.J.; Nicholson, R.V.; Kirkland, R.A. Quantification of oxygen consumption and sulphate release rates for waste rock piles using kinetic cells: Cluff Lake uranium mine, northern Saskatchewan, Canada. Appl. Geochem. 2001, 16, 1215–1230. [Google Scholar] [CrossRef]
- Mbonimpa, M.; Aubertin, M.; Aachib, M.; Bussière, B. Diffusion and consumption of oxygen in unsaturated cover materials. Can. Geotech. J. 2003, 40, 916–932. [Google Scholar] [CrossRef]
- Mbonimpa, M.; Aubertin, M.; Bussière, B. Oxygen consumption test to evaluate the diffusive flux into reactive tailings: Interpretation and numerical assessment. Can. Geotech. J. 2011, 48, 878–890. [Google Scholar] [CrossRef]
- Maqsoud, A.; Bussière, B.; Bouzahzah, H. Physical modelling of different reclamation scenarios for the abandoned Principale mine site (Quebec, Canada). In Proceedings of the Tailing and mine waste, Vancouver, BC, Canada, 19–23 November 2019. [Google Scholar]
- Maqsoud, A.; Diaby, S.; Mbonimpa, M. Performance evaluation of Ity mine site reclamation (Ivory Coast) using physical models based on clay materials. J. Afr. Earth Sci. 2021, 176, 104110. [Google Scholar] [CrossRef]
- Puls, R.W.; Barcelona, M.J. Low-Flow (Minimal Grawdown) Groundwater Sampling Procedures in Ground Water Issues, US; EPA, EPA/540/540/S-95/504; EPA Environmental Research Center: Ada, OK, USA, 1996. [Google Scholar]
- Puls, R.W.; Cynthia, J.P. Low-Flow Purging and Sampling of Ground Water Monitoring Wells with Dedicated Systems. Groundw. Monit. Remediat. 1995, 15, 116–123. [Google Scholar] [CrossRef]
- Curran, C.A.; Eng, K.; Konrad, C.P. Commission Analysis of Low Flows and Selected Methods for Estimating Low-Flow Characteristics at Partial-Record and Ungagged Stream Sites in Western Washington; Report 2012–5078; Survey Scientific Investigations; U.S. Geological Survey: Reston, VA, USA, 2012. Available online: http://pubs.usgs.gov/sir/2012/5078/pdf/sir20125078.pdf (accessed on 31 January 2022).
- Maqsoud, A.; Neculita, C.; Bussière, B.; Benzaazoua, M. Hydrochemical evolution of the Abandoned Manitou Mine site during and after tailings deposition. Environ. Sci. Pollut. Res. 2016, 23, 9054–9072. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Chemometric and intelligent. Lab. Syst. 1987, 2, 27–52. [Google Scholar]
- Farnham, I.M.; Johannesson, K.H.; Singh, A.K.; Hodge, V.F.; Stetzenbach, K.J. Factor analytical approaches for evaluating groundwater trace elementchemistry data. Anal. Chim. Data 2003, 490, 123–138. [Google Scholar] [CrossRef]
- Joliffe, I. Principal Component Analysis. In Encyclopedia of Statistics in Behavioral Science; John Wiley & Sons, Ltd: Hoboken, NJ, USA, Print; ISBN 9780470860809.
- Lghoul, M.; Maqsoud, A.; Hakkou, R.; Kchikach, A. Hydrochemical study on the contamination of groundwater in an abandoned mine area, Kettra (Morocco). J. Geochem. Explor. 2013, 144, 456–467. [Google Scholar] [CrossRef]
- Bakalowicz, M. Water geochemistry: Water quality and dynamics. Groundw. Ecol. 1994, 4, 97–127. [Google Scholar]
- Blavoux, B.; Mudry, J. Utilisation de l’analyse en composantes principales pour l’étude du fonctionnement hydrocinématique de trois aquifères karstiques de la France. Hydrogéologie 1985, 1, 53–59. [Google Scholar]
- Helena, B.; Pardo, R.; Vega, M.; Barrado, E.; Fernandez, J.M.; Fernandez, L. Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res. 2000, 34, 807–816. [Google Scholar] [CrossRef]
- Maqsoud, A.; Veillette, J.; Bakalowicz, M. Hydrochimie de l’esker Saint Mathieu-Lac Berry. In Proceedings of the 57th Canadian Geotechnical Conference, Québec, QC, Canada, 25–27 October 2004; pp. 28–35. [Google Scholar]
- Singh, E.J.K.; Gupta, A.; Singh, N.R. Groundwater quality in Imphal West District, Manipur, India, with multivariate statistical analysis of data. Environ. Sci. Pollut. Res. 2013, 20, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, Z.; Urieta, J.S.; Lanaja, F.J.; Pardo, A.; Rodriguez-Casals, C. Hydrochemistry dynamics in remote mountain lakes and its relation to catchment and atmospheric features: The case study of Sabocos Tarn, Pyrenees. Environ. Sci. Pollut. Res. 2015, 22, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Güler, C.; Thyne, G.-D.; McCray, J.-E.; Turner, A.-K. Evaluation of graphical and multivariante statistical methods for classification of water chemistry data. Hydrogeol. J. 2002, 10, 455–474. [Google Scholar] [CrossRef]













| Thickness (m) | Sand and Gravel | Tailings | Top Soil | Clay | Glacial Till | Rock |
|---|---|---|---|---|---|---|
| T7 | 1.07 | 2.89 | 1.68 | 10.87 | 1.83 | |
| T8 | 1.52 | 2.59 | 1.52 | 17.15 | 1.57 | |
| T9 | 4.93 | |||||
| T10 | 1.10 | 4.29 | ||||
| T11 | 1.52 | 3.35 | 0.76 | 1.09 | ||
| T12 | 1.10 | 3.76 | 2.05 | |||
| CP1 | 4.24 | |||||
| CP2 | 4.12 | 1.24 |
| Observation Well | Minimum (m) | Maximum (m) | Average (m) |
|---|---|---|---|
| T1 | 0.75 | 0.88 | 0.79 |
| T2 | 0.85 | 0.99 | 0.93 |
| T3 | 0.59 | 0.73 | 0.66 |
| T4 | 0.83 | 0.98 | 0.91 |
| T5 | 0.59 | 0.97 | 0.80 |
| T6 | 0.34 | 0.48 | 0.40 |
| Factor F1 (87.5%) | Factor F2 (6.4%) | Factor F3 (5%) | |
|---|---|---|---|
| SO4 | 0.97 | −0.19 | −0.10 |
| Pb | 0.61 | 0.41 | 0.67 |
| Ni | 0.99 | 0.04 | −0.07 |
| Mn | 0.89 | 0.30 | −0.31 |
| Mg | 0.98 | 0.06 | −0.15 |
| Fe | 0.88 | −0.30 | −0.14 |
| Cu | 0.95 | −0.21 | 0.09 |
| Co | 0.96 | 0.16 | −0.07 |
| Cr | 0.93 | 0.22 | −0.18 |
| Ca | 0.98 | −0.10 | −0.14 |
| Cd | 0.96 | 0.12 | 0.26 |
| Ba | −0.20 | 0.82 | −0.28 |
| Al | 0.99 | 0.01 | 0.03 |
| Ti | 0.96 | 0.20 | 0.14 |
| Zn | 0.95 | −0.04 | 0.27 |
| EC | 0.98 | −0.11 | −0.17 |
| pH | −0.92 | 0.28 | −0.02 |
| Variables | SO4 | Pb | Ni | Mn | Mg | Fe | Cu | Co | Cr | Ca | Cd | Ba | Al | Ti | Zn | Ec | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO4 | 1 | ||||||||||||||||
| Pb | 0.46 | 1 | |||||||||||||||
| Ni | 0.95 | 0.57 | 1 | ||||||||||||||
| Mn | 0.83 | 0.46 | 0.93 | 1 | |||||||||||||
| Mg | 0.95 | 0.52 | 0.99 | 0.95 | 1 | ||||||||||||
| Fe | 0.96 | 0.34 | 0.83 | 0.68 | 0.84 | 1 | |||||||||||
| Cu | 0.97 | 0.57 | 0.90 | 0.73 | 0.89 | 0.96 | 1 | ||||||||||
| Co | 0.89 | 0.60 | 0.99 | 0.95 | 0.98 | 0.72 | 0.82 | 1 | |||||||||
| Cr | 0.85 | 0.53 | 0.96 | 0.98 | 0.97 | 0.68 | 0.76 | 0.99 | 1 | ||||||||
| Ca | 0.99 | 0.47 | 0.97 | 0.89 | 0.98 | 0.92 | 0.95 | 0.92 | 0.91 | 1 | |||||||
| Cd | 0.88 | 0.81 | 0.94 | 0.81 | 0.91 | 0.76 | 0.90 | 0.92 | 0.87 | 0.88 | 1 | ||||||
| Ba | −0.28 | −0.04 | −0.19 | 0.08 | −0.15 | −0.23 | −0.29 | −0.13 | −0.06 | −0.23 | −0.17 | 1 | |||||
| Al | 0.96 | 0.63 | 0.99 | 0.89 | 0.98 | 0.85 | 0.93 | 0.97 | 0.93 | 0.96 | 0.97 | −0.21 | 1 | ||||
| Ti | 0.88 | 0.76 | 0.97 | 0.89 | 0.95 | 0.73 | 0.86 | 0.97 | 0.94 | 0.90 | 0.98 | −0.12 | 0.93 | 1 | |||
| Zn | 0.92 | 0.76 | 0.90 | 0.73 | 0.88 | 0.86 | 0.97 | 0.85 | 0.78 | 0.90 | 0.97 | −0.22 | 0.95 | 0.93 | 1 | ||
| EC | 0.99 | 0.45 | 0.97 | 0.88 | 0.98 | 0.94 | 0.95 | 0.91 | 0.89 | 0.99 | 0.88 | −0.21 | 0.96 | 0.89 | 0.90 | 1 | |
| pH | −0.95 | −0.46 | −0.89 | −0.69 | −0.87 | −0.92 | −0.94 | −0.82 | −0.75 | −0.89 | −0.86 | 0.35 | −0.92 | −0.90 | −0.90 | 0.92 | 1 |
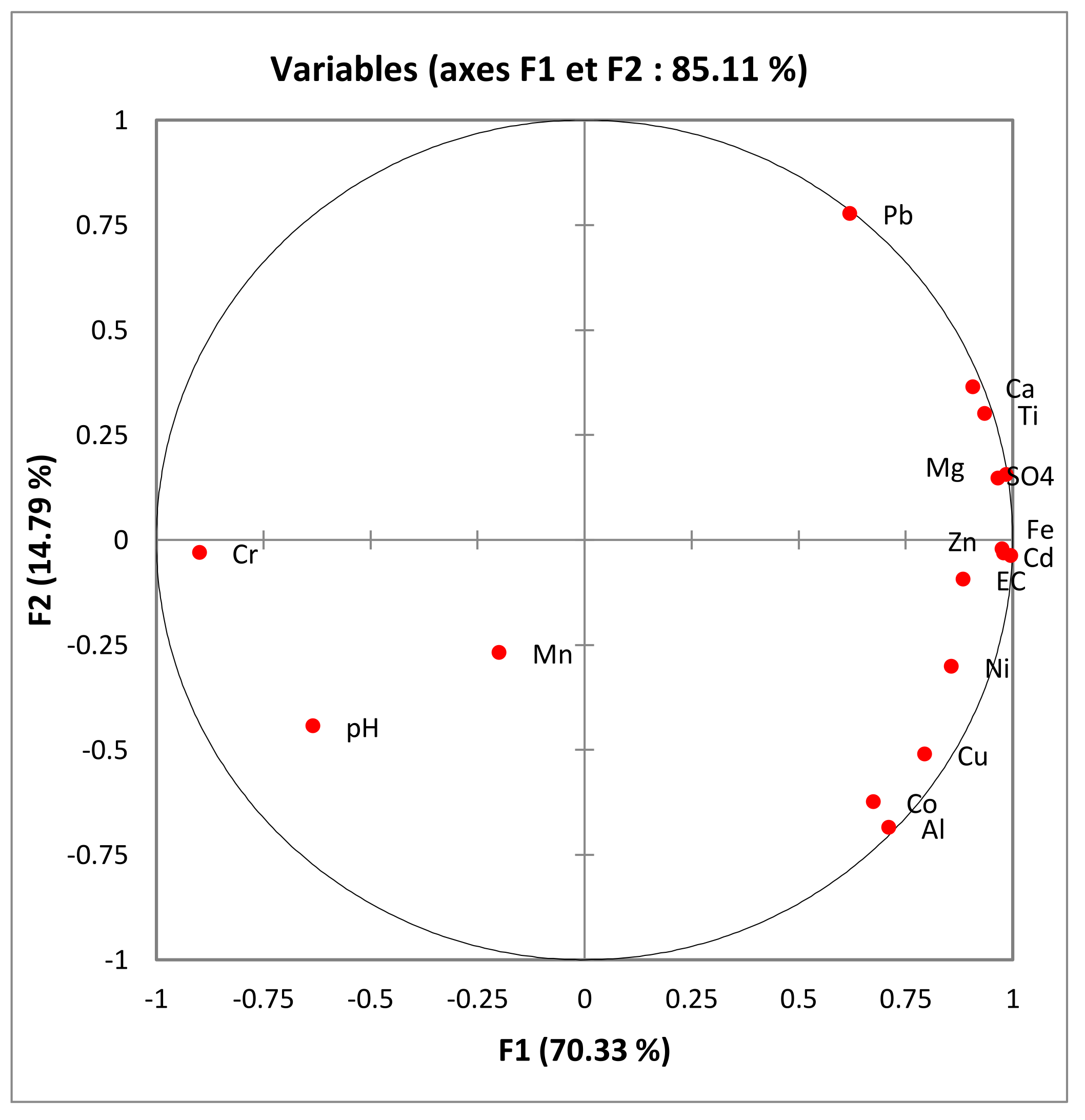
| Factor F1 (70.3%) | Factor F2 (14.8%) | Factor F3 (9%) | |
|---|---|---|---|
| SO4 | 0.99 | 0.16 | 0.03 |
| Pb | 0.62 | 0.78 | −0.05 |
| Ni | 0.86 | −0.30 | 0.22 |
| Mn | −0.20 | −0.27 | 0.86 |
| Mg | 0.97 | 0.15 | 0.21 |
| Fe | 0.98 | −0.02 | −0.15 |
| Cu | 0.79 | −0.51 | −0.25 |
| Co | 0.68 | −0.62 | 0.16 |
| Cr | −0.90 | −0.03 | 0.43 |
| Ca | 0.91 | 0.36 | 0.12 |
| Cd | 0.99 | −0.04 | −0.03 |
| Al | 0.71 | −0.69 | −0.13 |
| Ti | 0.94 | 0.30 | 0.04 |
| Zn | 0.98 | −0.03 | 0.06 |
| EC | 0.89 | −0.09 | 0.13 |
| pH | −0.63 | −0.44 | −0.34 |
| Variables | SO4 | Pb | Ni | Mn | Mg | Fe | Cu | Co | Cr | Ca | Cd | Al | Ti | Zn | Ec | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO4 | 1 | |||||||||||||||
| Pb | 0.74 | 1 | ||||||||||||||
| Ni | 0.79 | 0.25 | 1 | |||||||||||||
| Mn | −0.19 | −0.34 | −0.03 | 1 | ||||||||||||
| Mg | 0.98 | 0.70 | 0.82 | −0.05 | 1 | |||||||||||
| Fe | 0.96 | 0.61 | 0.76 | −0.26 | 0.91 | 1 | ||||||||||
| Cu | 0.71 | 0.13 | 0.71 | −0.16 | 0.64 | 0.86 | 1 | |||||||||
| Co | 0.55 | −0.11 | 0.93 | 0.04 | 0.59 | 0.60 | 0.74 | 1 | ||||||||
| Cr | −0.88 | −0.61 | −0.66 | 0.55 | −0.79 | −0.94 | −0.81 | −0.51 | 1 | |||||||
| Ca | 0.96 | 0.85 | 0.65 | −0.12 | 0.96 | 0.87 | 0.52 | 0.36 | −0.78 | 1 | ||||||
| Cd | 0.97 | 0.59 | 0.88 | −0.24 | 0.95 | 0.97 | 0.81 | 0.71 | −0.91 | 0.87 | 1 | |||||
| Al | 0.59 | −0.08 | 0.76 | −0.04 | 0.56 | 0.74 | 0.97 | 0.86 | −0.68 | 0.39 | 0.74 | 1 | ||||
| Ti | 0.98 | 0.83 | 0.66 | −0.16 | 0.96 | 0.92 | 0.61 | 0.39 | −0.84 | 0.99 | 0.91 | 0.47 | 1 | |||
| Zn | 0.95 | 0.56 | 0.93 | −0.20 | 0.95 | 0.92 | 0.75 | 0.75 | −0.85 | 0.86 | 0.99 | 0.70 | 0.88 | 1 | ||
| EC | 0.89 | 0.51 | 0.66 | 0.12 | 0.88 | 0.91 | 0.80 | 0.53 | −0.75 | 0.85 | 0.85 | 0.71 | 0.88 | 0.80 | 1 | |
| pH | −0.67 | −0.68 | −0.66 | 0.15 | −0.73 | −0.49 | −0.10 | −0.38 | −0.42 | −0.96 | −0.64 | −0.07 | −0.65 | 0.73 | −0.33 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsoud, A.; Mbonimpa, M.; Benzaazoua, M.; Turcotte, S. Evaluation of the Performances of a Monolayer Cover with an Elevated Water Table Used for the Reclamation of the Abandoned Aldermac Mine Site (Québec, Canada). Mining 2022, 2, 65-85. https://doi.org/10.3390/mining2010005
Maqsoud A, Mbonimpa M, Benzaazoua M, Turcotte S. Evaluation of the Performances of a Monolayer Cover with an Elevated Water Table Used for the Reclamation of the Abandoned Aldermac Mine Site (Québec, Canada). Mining. 2022; 2(1):65-85. https://doi.org/10.3390/mining2010005
Chicago/Turabian StyleMaqsoud, Abdelkabir, Mamert Mbonimpa, Mostafa Benzaazoua, and Sophie Turcotte. 2022. "Evaluation of the Performances of a Monolayer Cover with an Elevated Water Table Used for the Reclamation of the Abandoned Aldermac Mine Site (Québec, Canada)" Mining 2, no. 1: 65-85. https://doi.org/10.3390/mining2010005







