Point-Defect Segregation and Space-Charge Potentials at the Σ5(310)[001] Grain Boundary in Ceria
Abstract
1. Introduction
2. Computational Methods
2.1. Atomistic Simulations
| Pair | Ref. | |||
|---|---|---|---|---|
| [67] | ||||
| [67] | ||||
| [67] | ||||
| – | [68] | |||
| – | [68] | |||
| – | [69] |

2.2. Structural Analysis
2.3. Continuum Modelling of Space-Charge Layers
3. Results
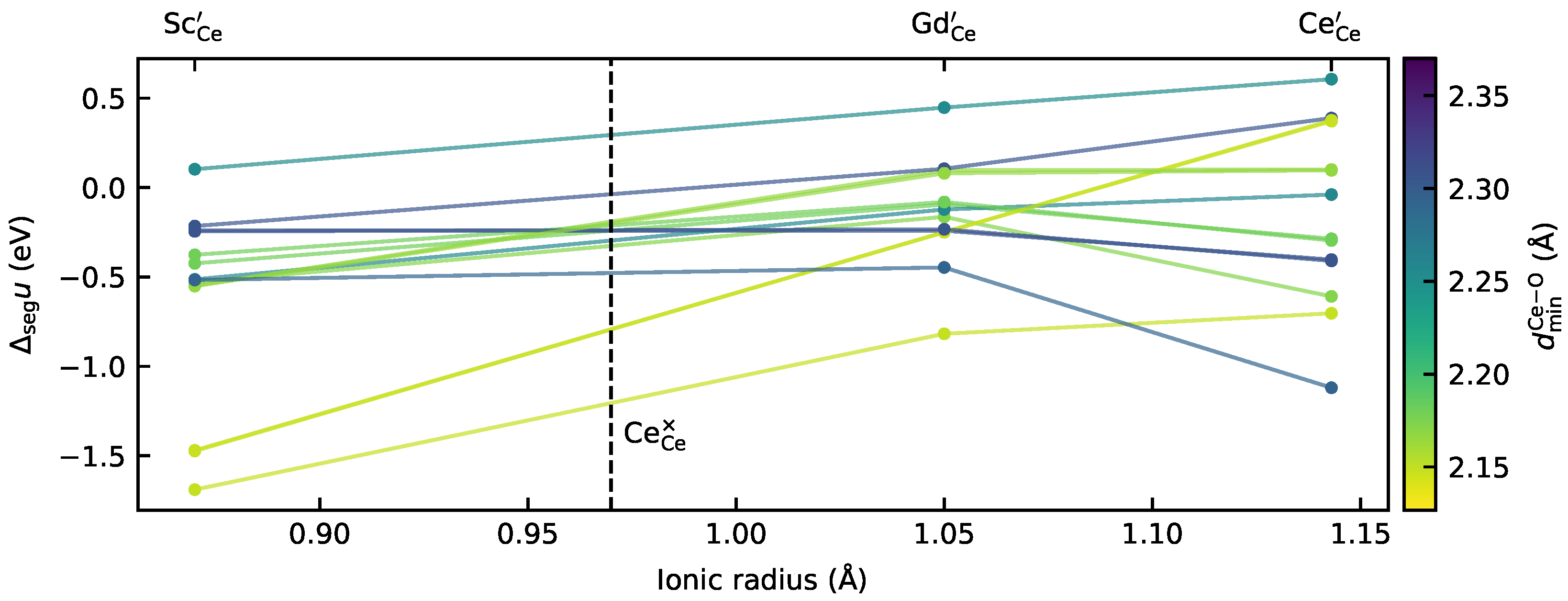
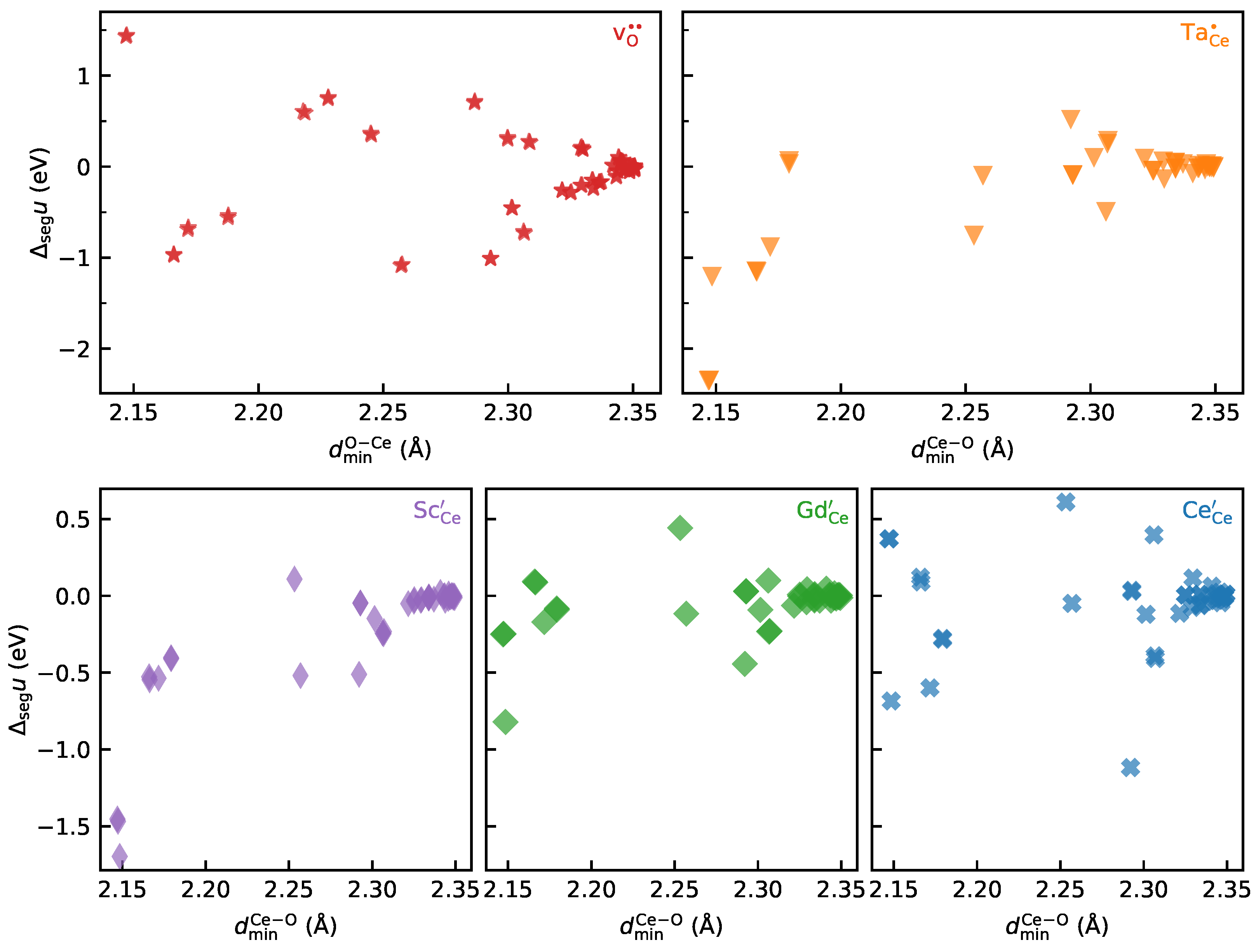
3.1. Analysing the Correlations between Local Structure and Segregation Energies
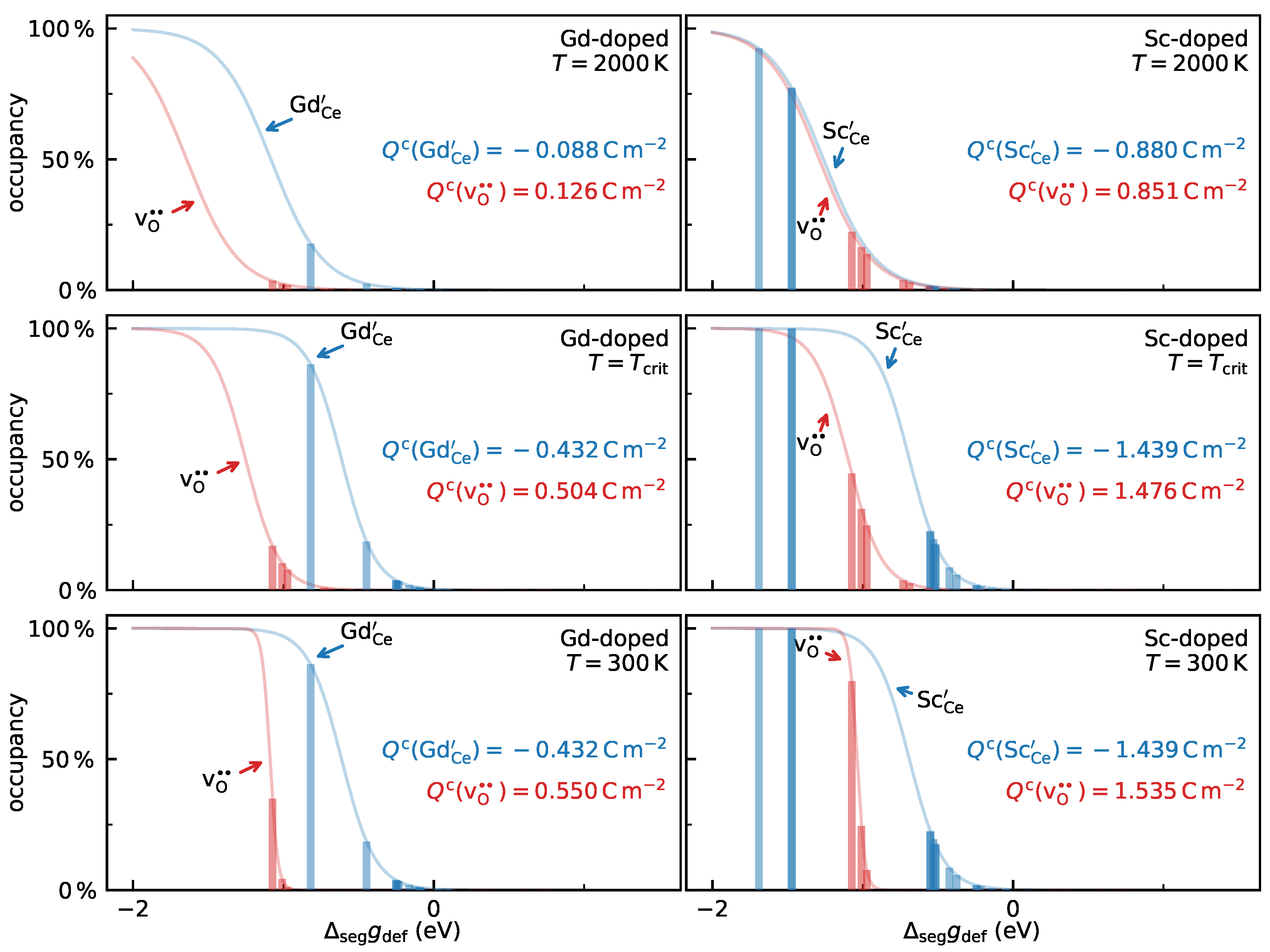
3.2. The Effects of Different Segregation Energies on Space-Charge Behaviour
4. Conclusions
- The segregation energetics of point defects, the interatomic distances, and the coordination numbers all exhibit a similar length scale on which they deviate from bulk values. Although the precise cutoff between bulk and grain-boundary regions is somewhat arbitrary, all three quantities yield for this grain boundary in this system a grain-boundary width of ca. on either side of the interface, i.e., ca. in total.
- Even for a symmetric, tilt grain boundary, there is no clear relationship between simple structural descriptors (such as the ionic size mismatch, nearest-neighbour distances, and effective coordination numbers) and defect energetics.
- In a space-charge model, most segregation energies (especially the positive values) can safely be neglected and the complexity of the model can in this manner be strongly reduced. To avoid oversimplification, however, one must consider that (i) a correct prediction of the behaviour (both quantitatively and qualitatively) can only be achieved if site-exclusion effects are taken into account; (ii) segregation energies for acceptors () must not be disregarded, even though the sign of is in most cases governed by oxygen-vacancy segregation, rather than by acceptor segregation; (iii) when a simple, empirical space-charge model (with ) is fitted to experimental data, the obtained grain-boundary parameters ( and ) are effective values that are not directly comparable to atomistic results since they incorporate the effects of acceptor segregation. Nevertheless, in our model system, we found such a fit to yield good estimates of together with good extrapolating capabilities of the behaviour within the restricted-equilibrium regime.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feighery, A.J.; Irvine, J.T.S. Effect of alumina additions upon electrical properties of 8 mol% yttria-stabilised zirconia. Solid State Ion. 1999, 121, 209–216. [Google Scholar] [CrossRef]
- Cho, Y.H.; Cho, P.S.; Auchterlonie, G.; Kim, D.K.; Lee, J.H.; Kim, D.Y.; Park, H.M.; Drennan, J. Enhancement of grain-boundary conduction in gadolinia-doped ceria by the scavenging of highly resistive siliceous phase. Acta Mater. 2007, 55, 4807–4815. [Google Scholar] [CrossRef]
- Yan, M.; Luo, S.D.; Schaffer, G.B.; Qian, M. Impurity (Fe, Cl, and P)-Induced Grain Boundary and Secondary Phases in Commercially Pure Titanium (CP-Ti). Metall. Mater. Trans. A 2013, 44, 3961–3969. [Google Scholar] [CrossRef]
- Tschoepe, A.; Birringer, R. Grain size dependence of electrical conductivity in polycrystalline cerium oxide. J. Electroceram. 2001, 7, 169–177. [Google Scholar] [CrossRef]
- Guo, X.; Maier, J. Grain Boundary Blocking Effect in Zirconia: A Schottky Barrier Analysis. J. Electrochem. Soc. 2001, 148, E121. [Google Scholar] [CrossRef]
- De Souza, R.A. The formation of equilibrium space-charge zones at grain boundaries in the perovskite oxide SrTiO3. Phys. Chem. Chem. Phys. 2009, 11, 9939–9969. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Waser, R. Space charge concept for acceptor-doped zirconia and ceria and experimental evidences. Solid State Ion. 2004, 173, 63–67. [Google Scholar] [CrossRef]
- Tong, X.; Mebane, D.S.; De Souza, R.A. Analyzing the grain-boundary resistance of oxide-ion conducting electrolytes: Poisson-Cahn vs. Poisson-Boltzmann theories. J. Am. Ceram. Soc. 2019, 103, 5–22. [Google Scholar] [CrossRef]
- Helgee, E.E.; Lindman, A.; Wahnstrom, G. Origin of Space Charge in Grain Boundaries of Proton-Conducting BaZrO3. Fuel Cells 2013, 13, 19–28. [Google Scholar] [CrossRef]
- Shirpour, M.; Merkle, R.; Maier, J. Space charge depletion in grain boundaries of BaZrO3 proton conductors. Solid State Ion. 2012, 225, 304–307. [Google Scholar] [CrossRef]
- Gellert, M.; Gries, K.I.; Yada, C.; Rosciano, F.; Volz, K.; Roling, B. Grain Boundaries in a Lithium Aluminum Titanium Phosphate-Type Fast Lithium Ion Conducting Glass Ceramic: Microstructure and Nonlinear Ion Transport Properties. J. Phys. Chem. C 2012, 116, 22675–22678. [Google Scholar] [CrossRef]
- Genreith-Schriever, A.R.; Parras, J.P.; Heelweg, H.J.; De Souza, R.A. The Intrinsic Structural Resistance of a Grain Boundary to Transverse Ionic Conduction. ChemElectroChem 2020, 7, 4718–4723. [Google Scholar] [CrossRef]
- Tuller, H.L.; Nowick, A.S. Small Polaron Electron-Transport in Reduced CeO2 Single-Crystals. J. Phys. Chem. Solids 1977, 38, 859–867. [Google Scholar] [CrossRef]
- Steele, B. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ion. 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Fuentes, R.O.; Baker, R.T. Synthesis and properties of Gadolinium-doped ceria solid solutions for IT-SOFC electrolytes. Int. J. Hydrog. Energy 2008, 33, 3480–3484. [Google Scholar] [CrossRef]
- Shajahan, I.; Ahn, J.; Nair, P.; Medisetti, S.; Patil, S.; Niveditha, V.; Uday Bhaskar Babu, G.; Dasari, H.P.; Lee, J.H. Praseodymium doped ceria as electrolyte material for IT-SOFC applications. Mater. Chem. Phys. 2018, 216, 136–142. [Google Scholar] [CrossRef]
- Wang, D.Y.; Nowick, A.S. The Grain-Boundary Effect in Doped Ceria Solid Electrolytes. J. Solid State Chem. 1980, 35, 325–333. [Google Scholar] [CrossRef]
- Gerhardt, R.; Nowick, A.S. Grain-boundary effect in ceria doped with trivalent cations: I, electrical measurements. J. Am. Ceram. Soc. 1986, 69, 641–646. [Google Scholar] [CrossRef]
- Gerhardt, R.; Nowick, A.S.; Mochel, M.E.; Dumler, I. Grain-boundary effect in ceria doped with trivalent cations: II, Microstructure and microanalysis. J. Am. Ceram. Soc. 1986, 69, 647–651. [Google Scholar] [CrossRef]
- Tanaka, J.; Baumard, J.F.; Abelard, P. Nonlinear electrical properties of grain boundaries in an oxygen-ion conductor (CeO2 · Y2O3). J. Am. Ceram. Soc. 1987, 70, 637–643. [Google Scholar] [CrossRef]
- Christie, G.M.; Van Berkel, F.P.F. Microstructure–ionic conductivity relationships in ceria-gadolinia electrolytes. Solid State Ion. 1996, 83, 17–27. [Google Scholar] [CrossRef]
- Aoki, M.; Chiang, Y.M.; Kosacki, I.; Lee, L.J.R.; Tuller, H.; Liu, Y. Solute Segregation and Grain-Boundary Impedance in High-Purity Stabilized Zirconia. J. Am. Ceram. Soc. 1996, 79, 1169–1180. [Google Scholar] [CrossRef]
- Guo, X.; Sigle, W.; Maier, J. Blocking grain boundaries in yttria-doped and undoped ceria ceramics of high purity. J. Am. Ceram. Soc. 2003, 86, 77–87. [Google Scholar] [CrossRef]
- Tschoepe, A. Grain size-dependent electrical conductivity of polycrystalline cerium oxide II: Space charge model. Solid State Ion. 2001, 139, 267–280. [Google Scholar] [CrossRef]
- Tschoepe, A.; Kilassonia, S.; Birringer, R. The grain boundary effect in heavily doped cerium oxide. Solid State Ion. 2004, 173, 57–61. [Google Scholar] [CrossRef]
- Mebane, D.S.; De Souza, R.A. A generalised space-charge theory for extended defects in oxygen-ion conducting electrolytes: From dilute to concentrated solid solutions. Energy Environ. Sci. 2015, 8, 2935–2940. [Google Scholar] [CrossRef]
- Parras, J.P.; Cao, C.; Ma, Z.; Mücke, R.; Jin, L.; Dunin-Borkowski, R.; Guillon, O.; De Souza, R.A. The grain-boundary resistance of CeO2 ceramics: A combined microscopy–spectroscopy–simulation study of a dilute solution. J. Am. Ceram. Soc. 2020, 103, 1755–1764. [Google Scholar] [CrossRef]
- Lei, Y.; Ito, Y.; Browning, N.D.; Mazanec, T.J. Segregation Effects at Grain Boundaries in Fluorite-Structured Ceramics. J. Am. Ceram. Soc. 2002, 85, 2359–2363. [Google Scholar] [CrossRef]
- Hojo, H.; Mizoguchi, T.; Ohta, H.; Findlay, S.D.; Shibata, N.; Yamamoto, T.; Ikuhara, Y. Atomic Structure of a CeO2 Grain Boundary: The Role of Oxygen Vacancies. Nano Lett. 2010, 10, 4668–4672. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Jung, H.J.; Lee, M.H.; Kim, Y.B.; Park, J.S.; Sinclair, R.; Prinz, F.B. Oxygen surface exchange at grain boundaries of oxide ion conductors. Adv. Funct. Mater. 2012, 22, 965–971. [Google Scholar] [CrossRef]
- Lee, H.B.; Prinz, F.B.; Cai, W. Atomistic simulations of grain boundary segregation in nanocrystalline yttria-stabilized zirconia and gadolinia-doped ceria solid oxide electrolytes. Acta Mater. 2013, 61, 3872–3887. [Google Scholar] [CrossRef]
- Parras, J.P.; De Souza, R.A. Grain-boundary diffusion of cations in fluorite-type oxides is faster but not always easier. Acta Mater. 2020, 195, 383–391. [Google Scholar] [CrossRef]
- Beschnitt, S.; De Souza, R.A. Impurity diffusion of Hf and Zr in Gd-doped CeO2. Solid State Ion. 2017, 305, 23–29. [Google Scholar] [CrossRef]
- Aidhy, D.S.; Zhang, Y.W.; Weber, W.J. Impact of segregation energetics on oxygen conductivity at ionic grain boundaries. J. Mater. Chem. A 2014, 2, 1704–1709. [Google Scholar] [CrossRef]
- Arora, G.; Aidhy, D.S. Segregation and binding energetics at grain boundaries in fluorite oxides. J. Mater. Chem. A 2017, 5, 4026–4035. [Google Scholar] [CrossRef]
- Wu, L.; Aguiar, J.A.; Dholabhai, P.P.; Holesinger, T.; Aoki, T.; Uberuaga, B.P.; Castro, R.H. Interface energies of nanocrystalline doped ceria: Effects of manganese segregation. J. Phys. Chem. C 2015, 119, 27855–27864. [Google Scholar] [CrossRef]
- Yuan, F.; Liu, B.; Zhang, Y.; Weber, W.J. Segregation and Migration of the Oxygen Vacancies in the Σ3 (111) Tilt Grain Boundaries of Ceria. J. Phys. Chem. C 2016, 120, 6625–6632. [Google Scholar] [CrossRef]
- Symington, A.R.; Molinari, M.; Statham, J.; Wu, J.; Parker, S.C. The role of dopant segregation on the oxygen vacancy distribution and oxygen diffusion in CeO2 grain boundaries. JPhys Energy 2019, 1, 042005. [Google Scholar] [CrossRef]
- Fisher, C.A.J.; Matsubara, H. Molecular dynamics investigations of grain boundary phenomena in cubic zirconia. Comput. Mater. Sci. 1999, 14, 177–184. [Google Scholar] [CrossRef]
- Mao, Z.G.; Sinnott, S.B.; Dickey, E.C. Ab initio calculations of pristine and doped zirconia Σ5(310)/[001] tilt grain boundaries. J. Am. Ceram. Soc. 2002, 85, 1594–1600. [Google Scholar] [CrossRef]
- Oyama, T.; Yoshiya, M.; Matsubara, H.; Matsunaga, K. Numerical analysis of solute segregation at Σ 5(310)/[001] symmetric tilt grain boundaries in Y2O3-doped ZrO2. Phys. Rev. B 2005, 71, 224105. [Google Scholar] [CrossRef]
- Nerikar, P.V.; Rudman, K.; Desai, T.G.; Byler, D.; Unal, C.; McClellan, K.J.; Phillpot, S.R.; Sinnott, S.B.; Peralta, P.; Uberuaga, B.P.; et al. Grain Boundaries in Uranium Dioxide: Scanning Electron Microscopy Experiments and Atomistic Simulations. J. Am. Ceram. Soc. 2011, 94, 1893–1900. [Google Scholar] [CrossRef]
- Yoshiya, M.; Oyama, T. Impurity and vacancy segregation at symmetric tilt grain boundaries in Y2O3-doped ZrO2. J. Mater. Sci. 2011, 46, 4176–4190. [Google Scholar] [CrossRef]
- Hong, M.K.; Uberuaga, B.P.; Phillpot, S.R.; Andersson, D.A.; Stanek, C.R.; Sinnott, S.B. The role of charge and ionic radius on fission product segregation to a model UO2 grain boundary. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Williams, N.R.; Molinari, M.; Parker, S.C.; Storr, M.T. Atomistic investigation of the structure and transport properties of tilt grain boundaries of UO2. J. Nucl. Mater. 2015, 458, 45–55. [Google Scholar] [CrossRef]
- Dholabhai, P.P.; Aguiar, J.A.; Wu, L.J.; Holesinger, T.G.; Aoki, T.; Castro, R.H.R.; Uberuaga, B.P. Structure and segregation of dopant-defect complexes at grain boundaries in nanocrystalline doped ceria. Phys. Chem. Chem. Phys. 2015, 17, 15375–15385. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Sugiyama, I.; Hojo, H.; Ohta, H.; Shibata, N.; Ikuhara, Y. Atomic structures and oxygen dynamics of CeO2 grain boundaries. Sci. Rep. 2016, 6, 20288. [Google Scholar] [CrossRef] [PubMed]
- Usler, A.L.; Ketter, F.; De Souza, R.A. How space-charge behaviour at grain boundaries in electroceramic oxides is modified by two restricted equilibria. Phys. Chem. Chem. Phys. 2024, 26, 8287–8298. [Google Scholar] [CrossRef] [PubMed]
- Hagege, S.; Carter, C.B.; Cosandey, F.; Sass, S.L. The variation of grain boundary structural width with misorientation angle and boundary plane. Phil. Mag. A 1982, 45, 723–740. [Google Scholar] [CrossRef]
- Wynblatt, P.; Rohrer, G.S.; Papillon, F. Grain boundary segregation in oxide ceramics. J. Eur. Ceram. Soc. 2003, 23, 2841–2848. [Google Scholar] [CrossRef]
- Kingery, W.D. Plausible Concepts Necessary and Sufficient for Interpretation of Ceramic Grain-Boundary Phenomena-II, Solute Segregation, Grain-Boundary Diffusion, and General Discussion. J. Am. Ceram. Soc. 1974, 57, 74–83. [Google Scholar] [CrossRef]
- Kingery, W.D. Segregation phenomena at surfaces and at grain boundaries in oxides and carbides. Solid State Ion. 1984, 12, 299–307. [Google Scholar] [CrossRef]
- Avila-Paredes, H.J.; Choi, K.; Chen, C.T.; Kim, S. Dopant-concentration dependence of grain-boundary conductivity in ceria: A space-charge analysis. J. Mater. Chem. 2009, 19, 4837–4842. [Google Scholar] [CrossRef]
- Vollman, M.; Waser, R. Grain Boundary Defect Chemistry of Acceptor-Doped Titanates: Space Charge Layer Width. J. Am. Ceram. Soc. 1994, 77, 235–243. [Google Scholar] [CrossRef]
- Peterson, N.L. Grain-boundary diffusion in metals. Int. Met. Rev. 1983, 28, 65–91. [Google Scholar] [CrossRef]
- Mistler, R.E.; Coble, R.L. Grain-boundary diffusion and boundary widths in metals and ceramics. J. Appl. Phys. 1974, 45, 1507–1509. [Google Scholar] [CrossRef]
- Zacherle, T.; Schriever, A.; De Souza, R.A.; Martin, M. Ab initio analysis of the defect structure of ceria. Phys. Rev. B 2013, 87, 134104-1–134104-11. [Google Scholar] [CrossRef]
- Tuller, H.L.; Nowick, A.S. Doped ceria as a solid oxide electrolyte. J. Electrochem. Soc. 1975, 122, 255. [Google Scholar] [CrossRef]
- Tuller, H.L.; Nowick, A.S. Defect Structure and Electrical-Properties of Nonstoichiometric CeO2 Single-Crystals. J. Electrochem. Soc. 1979, 126, 209–217. [Google Scholar] [CrossRef]
- Stratton, T.G.; Tuller, H.L. Thermodynamic and transport studies of mixed oxides. The CeO2–UO2 system. J. Chem. Soc. Faraday Trans. 2 1987, 83, 1143–1156. [Google Scholar] [CrossRef]
- Göbel, M.C.; Gregori, G.; Maier, J. Electronically blocking grain boundaries in donor doped cerium dioxide. Solid State Ion. 2012, 215, 45–51. [Google Scholar] [CrossRef]
- Waldow, S.P.; Wardenga, H.; Beschnitt, S.; Klein, A.; De Souza, R.A. Concentration and Diffusivity of Oxygen Interstitials in Niobia-Doped Ceria. J. Phys. Chem. C 2019, 123, 6340–6350. [Google Scholar] [CrossRef]
- Kröger, F.; Vink, H. Relations between the concentrations of imperfections in crystalline solids. Solid State Phys. 1956, 3, 307–435. [Google Scholar] [CrossRef]
- Norby, T. A Kröger–Vink compatible notation for defects in inherently defective sublattices. J. Korean Ceram. Soc. 2010, 47, 19–25. [Google Scholar] [CrossRef]
- De Souza, R.; Harrington, G. Revisiting point defects in ionic solids and semiconductors. Nat. Mater. 2023, 22, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Born, M.; Mayer, J.E. Zur Gittertheorie der Ionenkristalle. Z. Phys. 1932, 75, 1–18. [Google Scholar] [CrossRef]
- Balducci, G.; Kaspar, J.; Fornasiero, P.; Graziani, M.; Islam, M.S.; Gale, J.D. Computer simulation studies of bulk reduction and oxygen migration in CeO2-ZrO2 solid solutions. J. Phys. Chem. B 1997, 101, 1750–1753. [Google Scholar] [CrossRef]
- Lewis, G.V.; Catlow, C.R.A. Potential models for ionic oxides. J. Phys. C Solid State Phys. 1985, 18, 1149. [Google Scholar] [CrossRef]
- Exner, M.; Donnerberg, H.; Catlow, C.R.A.; Schirmer, O.F. Computer simulation of defects in KTaO3. Phys. Rev. B 1995, 52, 3930. [Google Scholar] [CrossRef] [PubMed]
- Sayle, T.X.T.; Parker, S.C.; Sayle, D.C. Oxidising CO to CO2 using ceria nanoparticles. Phys. Chem. Chem. Phys. 2005, 7, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Sayle, T.X.T.; Parker, S.C.; Sayle, D.C. Ionic conductivity in nano-scale CeO2/YSZ heterolayers. J. Mater. Chem. 2006, 16, 1067–1081. [Google Scholar] [CrossRef]
- Sayle, T.X.T.; Parker, S.C.; Sayle, D.C. Oxygen transport in unreduced, reduced and Rh(III)-doped CeO2 nanocrystals. Faraday Disc. 2007, 134, 377–397. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.A.; Metlenko, V.; Park, D.; Weirich, T.E. Behavior of oxygen vacancies in single-crystal SrTiO3: Equilibrium distribution and diffusion kinetics. Phys. Rev. B 2012, 85, 174109. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular-Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Wojdyr, M.; Khalil, S.; Liu, Y.; Szlufarska, I. Energetics and structure of (001) tilt grain boundaries in SiC. Model. Simul. Mater. Sci. Eng. 2010, 18, 075009. [Google Scholar] [CrossRef]
- Yang, L.; Wirth, B.D. Tilt grain boundary stability in uranium dioxide and effect on xenon segregation. J. Nucl. Mater. 2023, 577, 154302. [Google Scholar] [CrossRef]
- Heinz, M.V.; Usler, A.L.; Bonkowski, A.; Feldmann, G. Molara. 2024. Available online: https://zenodo.org/records/11120926 (accessed on 7 May 2024).
- Hoppe, R.; Mehlhorn, B. Die Kristallstruktur von K2ZrF6. Z. Anorg. Allg. Chem. 1976, 425, 200–208. [Google Scholar] [CrossRef]
- Hoppe, R. Effective coordination numbers (ECoN) and mean fictive ionic radii (MEFIR). Z. Für Krist.-Cryst. Mater. 1979, 150, 23–52. [Google Scholar] [CrossRef]
- Bentley, J.L. Multidimensional binary search trees used for associative searching. Commun. ACM 1975, 18, 509–517. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ganose, A.M.; Horton, M.; Aykol, M.; Persson, K.A.; Zimmermann, N.E.R.; Jain, A. Benchmarking coordination number prediction algorithms on inorganic crystal structures. Inorg. Chem. 2021, 60, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Brauer, G.; Gingerich, K.; Holtschmidt, U. Über die Oxyde des Cers-IV: Die Sauerstoffzersetzungdrücke im System der Ceroxyde. J. Inorg. Nucl. Chem. 1960, 16, 77–86. [Google Scholar] [CrossRef]
- Shirpour, M.; Gregori, G.; Merkle, R.; Maier, J. On the proton conductivity in pure and gadolinium doped nanocrystalline cerium oxide. Phys. Chem. Chem. Phys. 2011, 13, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Nerikar, P.V.; Parfitt, D.C.; Trujillo, L.A.C.; Andersson, D.A.; Unal, C.; Sinnott, S.B.; Grimes, R.W.; Uberuaga, B.P.; Stanek, C.R. Segregation of xenon to dislocations and grain boundaries in uranium dioxide. Phys. Rev. B 2011, 84. [Google Scholar] [CrossRef]
- Tao, C.; Mutter, D.; Urban, D.F.; Elsässer, C. Atomistic calculations of charged point defects at grain boundaries in SrTiO3. Phys. Rev. B 2021, 104, 054114. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic-Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tschoepe, A. Interface defect chemistry and effective conductivity in polycrystalline cerium oxide. J. Electroceram. 2005, 14, 5–23. [Google Scholar] [CrossRef]
- Guo, X.; Waser, R. Electrical properties of the grain boundaries of oxygen ion conductors: Acceptor-doped zirconia and ceria. Prog. Mater. Sci. 2006, 51, 151–210. [Google Scholar] [CrossRef]
- McCune, R.C.; Wynblatt, P. Calcium segregation to a magnesium oxide (100) surface. J. Am. Ceram. Soc. 1983, 66, 111–117. [Google Scholar] [CrossRef]
- Gouy, M. Sur la constitution de la charge électrique à la surface d’un électrolyte. J. Phys. Theor. Appl. 1910, 9, 457–468. [Google Scholar] [CrossRef]
- Chapman, D.L. A contribution to the theory of electrocapillarity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 25, 475–481. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, D.Y. Space-charge concepts on grain boundary impedance of a high-purity yttria-stabilized tetragonal zirconia polycrystal. J. Mater. Res. 2001, 16, 2739–2751. [Google Scholar] [CrossRef]
- Lindman, A.; Helgee, E.E.; Nyman, B.J.; Wahnström, G. Oxygen vacancy segregation in grain boundaries of BaZrO3 using interatomic potentials. Solid State Ion. 2013, 230, 27–31. [Google Scholar] [CrossRef][Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usler, A.L.; Heelweg, H.J.; De Souza, R.A.; Genreith-Schriever, A.R. Point-Defect Segregation and Space-Charge Potentials at the Σ5(310)[001] Grain Boundary in Ceria. Solids 2024, 5, 404-421. https://doi.org/10.3390/solids5030027
Usler AL, Heelweg HJ, De Souza RA, Genreith-Schriever AR. Point-Defect Segregation and Space-Charge Potentials at the Σ5(310)[001] Grain Boundary in Ceria. Solids. 2024; 5(3):404-421. https://doi.org/10.3390/solids5030027
Chicago/Turabian StyleUsler, Adrian L., Henrik J. Heelweg, Roger A. De Souza, and Annalena R. Genreith-Schriever. 2024. "Point-Defect Segregation and Space-Charge Potentials at the Σ5(310)[001] Grain Boundary in Ceria" Solids 5, no. 3: 404-421. https://doi.org/10.3390/solids5030027
APA StyleUsler, A. L., Heelweg, H. J., De Souza, R. A., & Genreith-Schriever, A. R. (2024). Point-Defect Segregation and Space-Charge Potentials at the Σ5(310)[001] Grain Boundary in Ceria. Solids, 5(3), 404-421. https://doi.org/10.3390/solids5030027







