Precursor-Based Syntheses of Mo(C,N,O)x, Molybdenum Carbide, Nitride, and Oxide Applying a Microjet Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
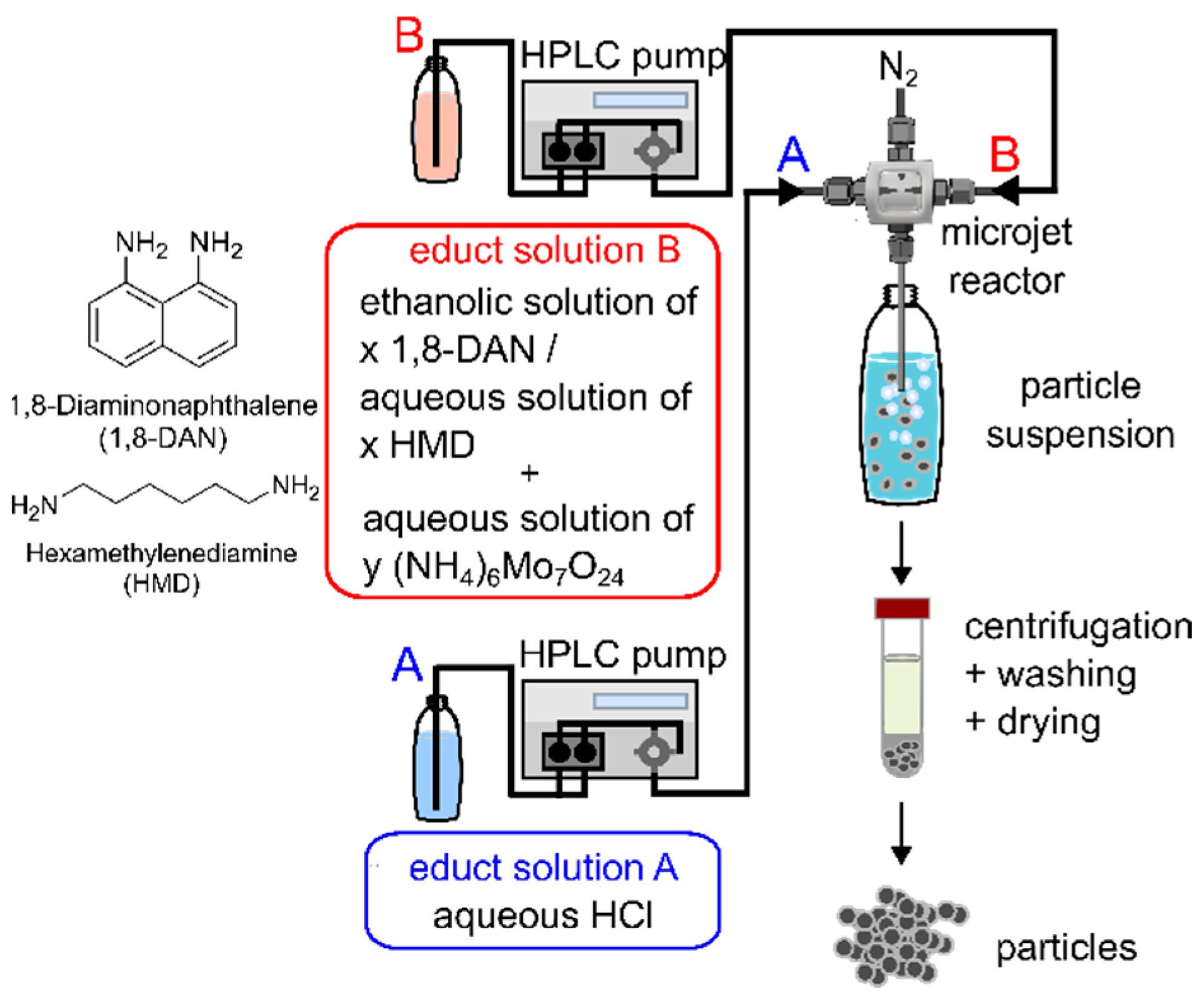
2.2. Syntheses—Microjet Synthesis of Precipitates
2.3. Characterization
3. Results and Discussion
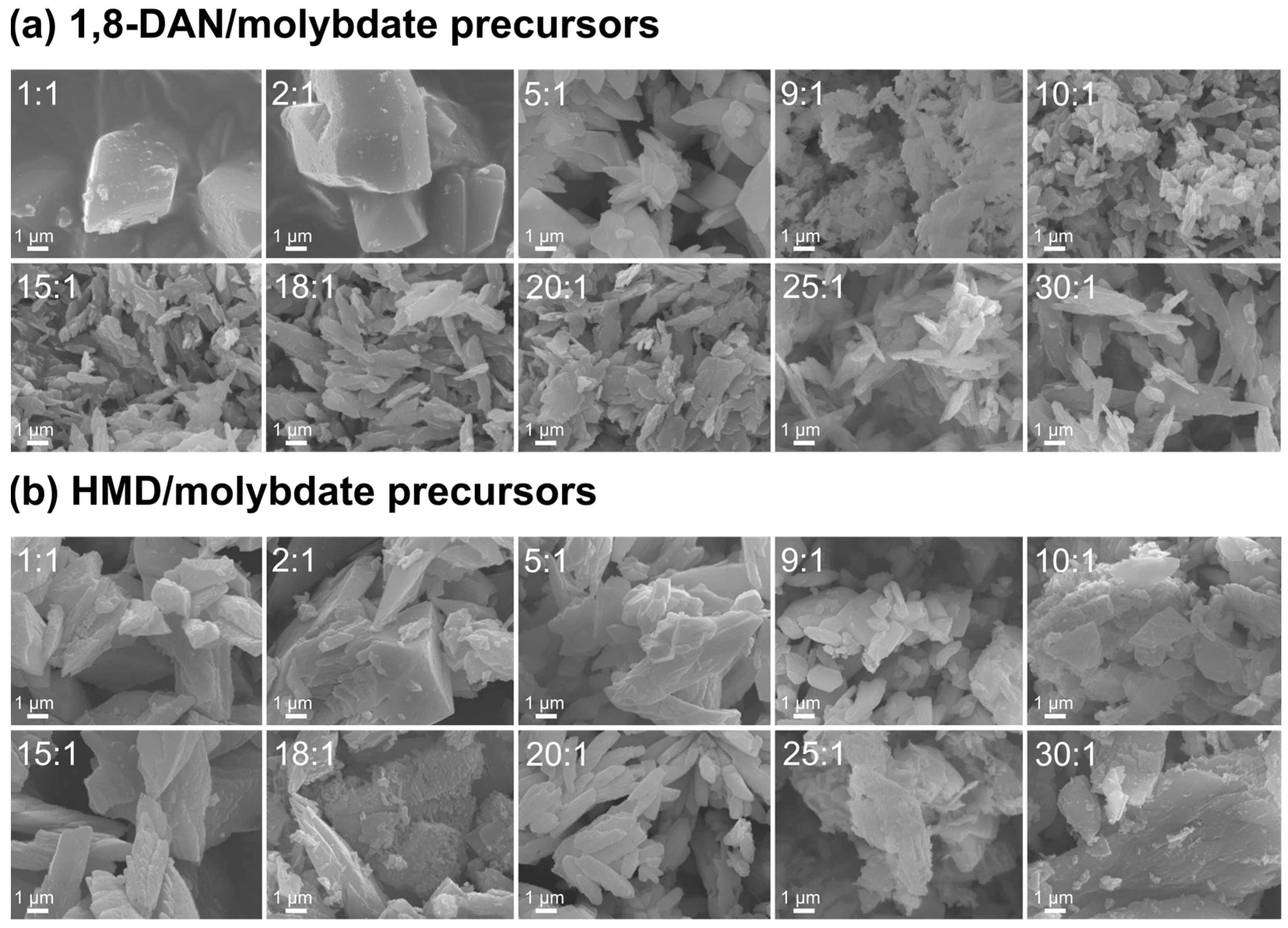
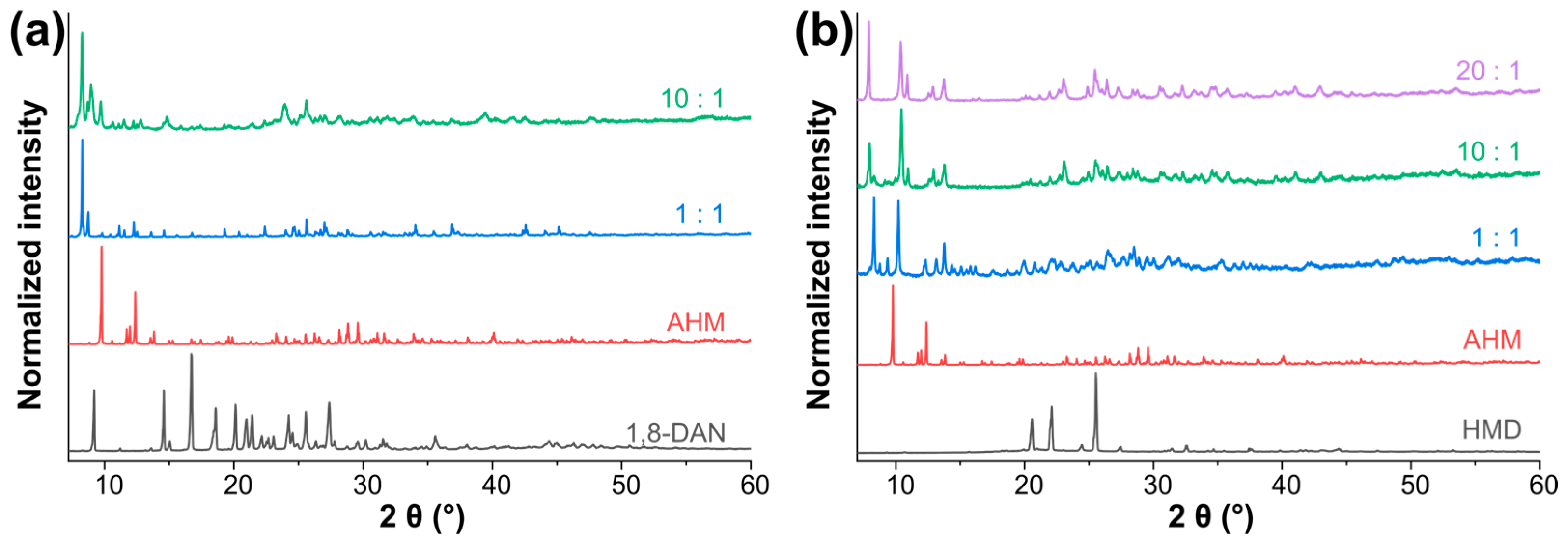
3.1. Synthesis/Precipitation Reaction

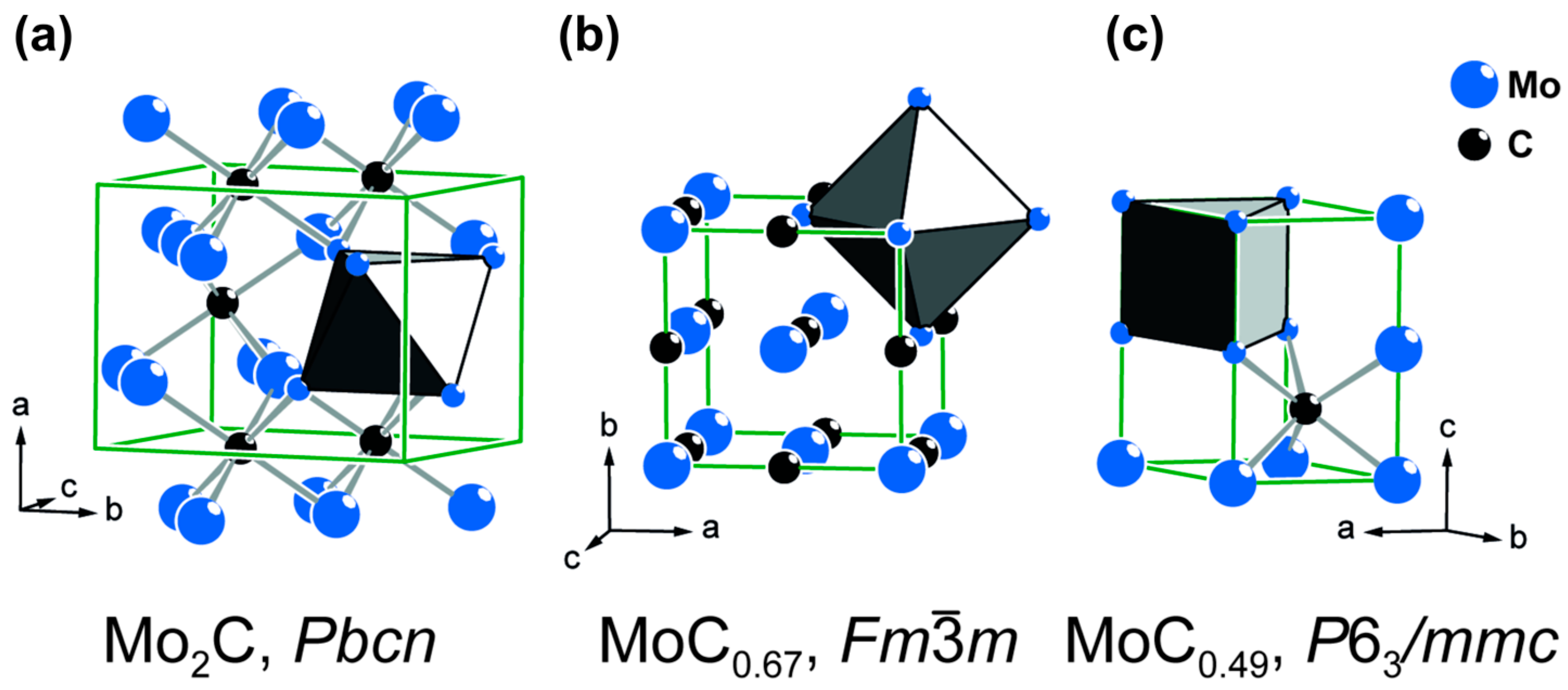
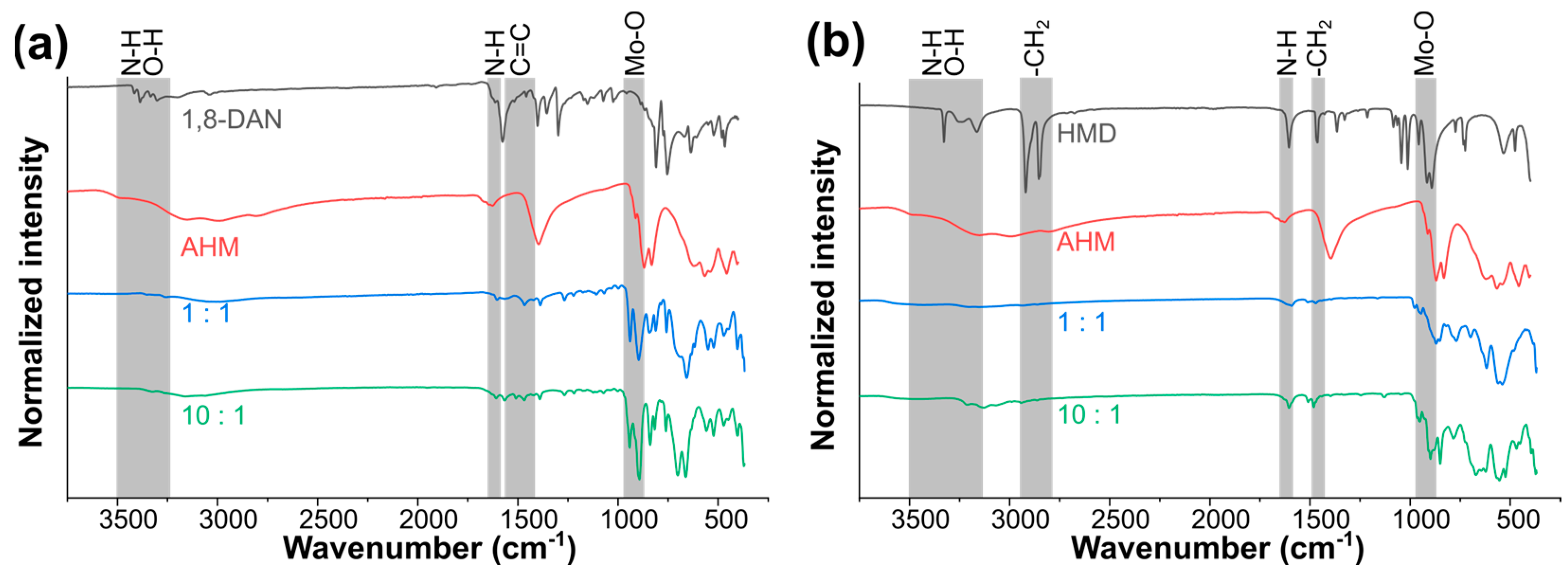
3.2. Thermal Decomposition Behavior of the Precursors
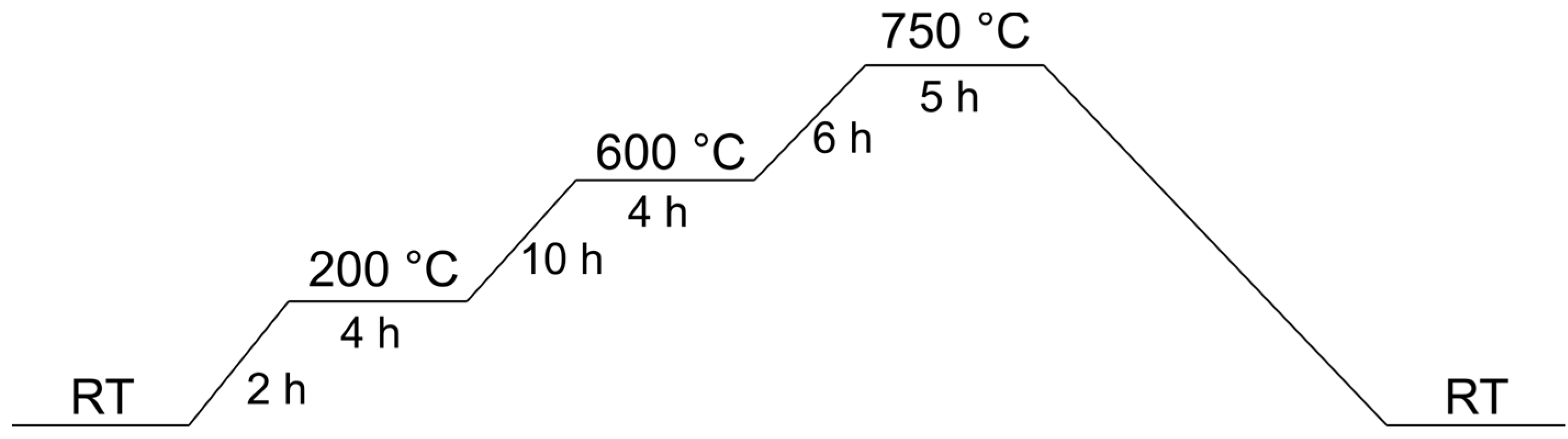
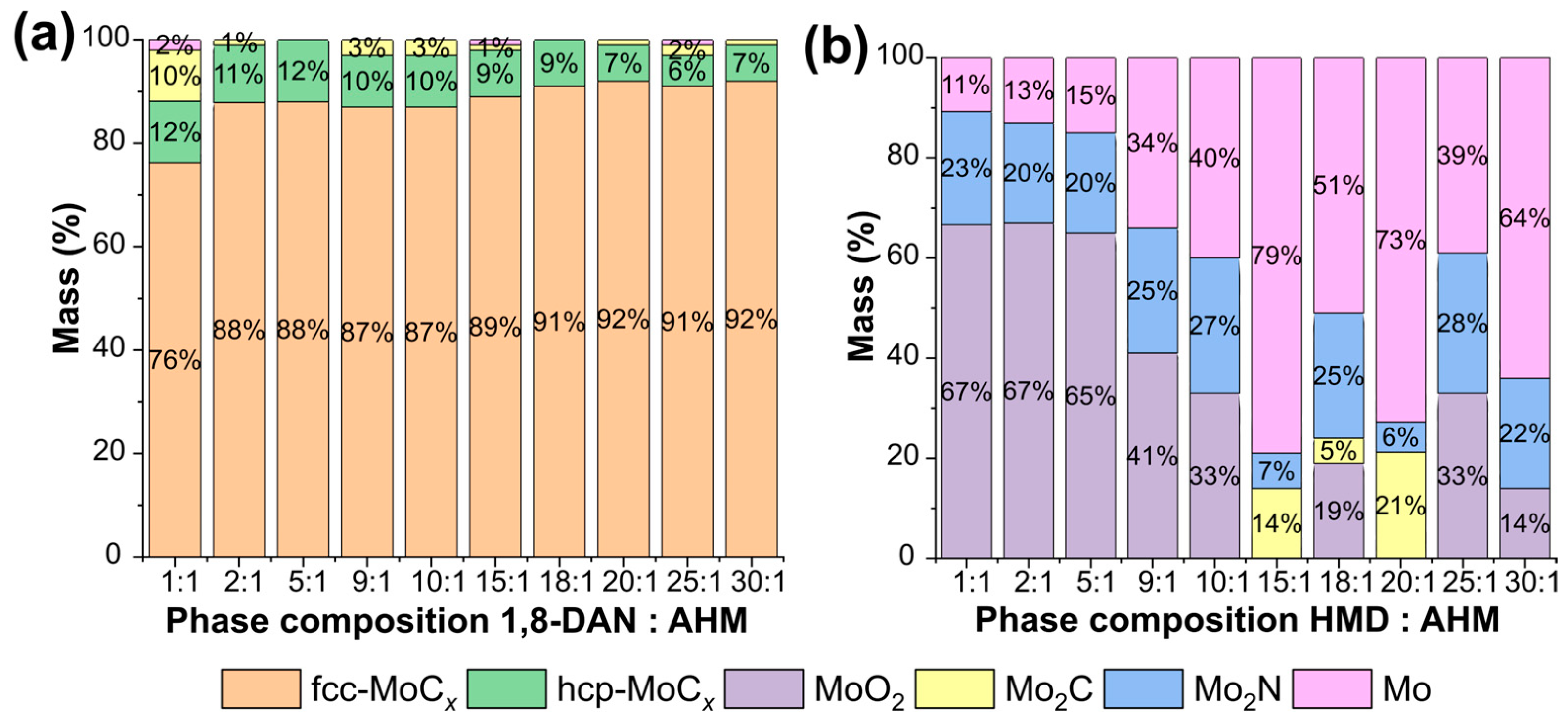
3.3. Pyrolysis of the Precipitation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiu, Z.; Kim, D.; Alfaruqi, M.H.; Song, J.; Kim, S.; Duong, P.T.; Mathew, V.; Baboo, J.P.; Kim, J. Ultrafine molybdenum oxycarbide nanoparticles embedded in N-doped carbon as a superior anode material for lithium-ion batteries. J. Alloys Compd. 2017, 696, 143–149. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Preiss, H.; Meyer, B.; Olschewski, C. Preparation of molybdenum and tungsten carbides from solution derived precursors. J. Mater. Sci. 1998, 33, 713–722. [Google Scholar] [CrossRef]
- Chen, W.-F.; Wang, C.-H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Liao, L.; Wang, S.; Xiao, J.; Bian, X.; Zhang, Y.; Scanlon, M.D.; Hu, X.; Tang, Y.; Liu, B.; Girault, H.H. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 387–392. [Google Scholar] [CrossRef]
- Ge, C.; Jiang, P.; Cui, W.; Pu, Z.; Xing, Z.; Asiri, A.M.; Obaid, A.Y.; Sun, X.; Tian, J. Shape-controllable synthesis of Mo2C nanostructures as hydrogen evolution reaction electrocatalysts with high activity. Electrochim. Acta 2014, 134, 182–186. [Google Scholar] [CrossRef]
- Pang, M.; Wang, X.; Xia, W.; Muhler, M.; Liang, C. Mo(VI)-Melamine Hybrid As Single-Source Precursor to Pure-Phase β-Mo2C for the Selective Hydrogenation of Naphthalene to Tetralin. Ind. Eng. Chem. Res. 2013, 52, 4564–4571. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Li, G.-D.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with Nitrogen-Rich Nanocarbon Leads to Efficient Hydrogen-Evolution Electrocatalytic Sites. Angew. Chem. 2015, 127, 10902–10907. [Google Scholar] [CrossRef]
- Giordano, C.; Erpen, C.; Yao, W.; Antonietti, M. Synthesis of Mo and W Carbide and Nitride Nanoparticles via a Simple “Urea Glass” Route. Nano Lett. 2008, 8, 4659–4663. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C Nanoparticles Dispersed on Hierarchical Carbon Microflowers for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, C.; Sagaltchik, A.; Pachfule, P.; Zhao, C.; Thomas, A. Metal-Organic Precursor–Derived Mesoporous Carbon Spheres with Homogeneously Distributed Molybdenum Carbide/Nitride Nanoparticles for Efficient Hydrogen Evolution in Alkaline Media. Adv. Funct. Mater. 2019, 29, 1807419. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, X.-H.; Zhang, M.-H.; Du, X.-Y.; Li, W.; Tao, K.-Y. Synthesis of Bulk and Supported Molybdenum Carbide by a Single-Step Thermal Carburization Method. Chem. Mater. 2007, 19, 1801–1807. [Google Scholar] [CrossRef]
- Zoller, M.; Bubnova, R.; Biryukov, Y.; Haussühl, E.; Pöttgen, R.; Janka, O.; Penner, S.; Praty, C.; Fitzek, H.; Winkler, J.; et al. Elucidating the physical properties of the molybdenum oxide Mo4O11 and its tantalum substituted variant Mo2Ta2O11. Z. Krist.-Cryst. Mater. 2020, 235, 143–155. [Google Scholar] [CrossRef]
- Glemser, O.; Lutz, G. Über Molybdänoxyde. Z. Anorg. Allg. Chem. 1950, 263, 2–14. [Google Scholar] [CrossRef]
- Parthé, E.; Sadogopan, V. The Structure of Dimolybdenum Carbide by Neutron Diffraction Technique. Acta Crystallogr. 1963, 16, 202–205. [Google Scholar] [CrossRef]
- Hull, A.W. The positions of atoms in metals. Proc. Am. Inst. Electr. Eng. 1919, 38, 1171–1192. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Yang, H.; Luo, L. Carbothermic Reduction of MoO3 for Direct Alloying Process. J. Iron Steel Res. Int. 2013, 20, 51–56. [Google Scholar] [CrossRef]
- Norlund Christensen, A. A neutron diffraction investigation on a crystal of alpha-Mo2C. Acta Chem. Scand. Ser. A 1977, 31, 509–511. [Google Scholar] [CrossRef]
- Rudy, E.; Brukl, C.E.; Windisch, S. Constitution of niobium (columbium)-molybdenum-carbon alloys. Trans. Metall. Soc. AIME 1967, 239, 1796–1808. [Google Scholar]
- Kuo, K.; Hägg, G. A New Molybdenum Carbide. Nature 1952, 170, 245–246. [Google Scholar] [CrossRef]
- Odenwald, C.; Kickelbick, G. Additive-free continuous synthesis of silica and ORMOSIL micro- and nanoparticles applying a microjet reactor. J. Sol-Gel Sci. Technol. 2019, 89, 343–353. [Google Scholar] [CrossRef]
- Betke, A.; Kickelbick, G. Bottom-Up, Wet Chemical Technique for the Continuous Synthesis of Inorganic Nanoparticles. Inorganics 2014, 2, 1–15. [Google Scholar] [CrossRef]
- Krüner, B.; Odenwald, C.; Tolosa, A.; Schreiber, A.; Aslan, M.; Kickelbick, G.; Presser, V. Carbide-derived carbon beads with tunable nanopores from continuously produced polysilsesquioxanes for supercapacitor electrodes. Sustain. Energy Fuels 2017, 1, 1588–1600. [Google Scholar] [CrossRef]
- Krüner, B.; Odenwald, C.; Jäckel, N.; Tolosa, A.; Kickelbick, G.; Presser, V. Silicon Oxycarbide Beads from Continuously Produced Polysilsesquioxane as Stable Anode Material for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 2961–2970. [Google Scholar] [CrossRef]
- Krüner, B.; Odenwald, C.; Quade, A.; Kickelbick, G.; Presser, V. Influence of Nitrogen-Doping for Carbide-Derived Carbons on the Supercapacitor Performance in an Organic Electrolyte and an Ionic Liquid. Batter. Supercaps 2018, 1, 135–148. [Google Scholar] [CrossRef]
- Abdirahman Mohamed, M.; Arnold, S.; Janka, O.; Quade, A.; Presser, V.; Kickelbick, G. Self-Activation of Inorganic-Organic Hybrids Derived through Continuous Synthesis of Polyoxomolybdate and para-Phenylenediamine Enables Very High Lithium-Ion Storage Capacity. ChemSusChem 2023, 16, e202202213. [Google Scholar] [CrossRef]
- Abdirahman Mohamed, M.; Arnold, S.; Janka, O.; Quade, A.; Schmauch, J.; Presser, V.; Kickelbick, G. Continuous wet chemical synthesis of Mo(C,N,O)x as anode materials for Li-ion batteries. J. Mater. Chem. A 2023, 11, 19936–19954. [Google Scholar] [CrossRef]
- AXS, Topas 5.1; General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker: Karlsruhe, Germany, 2014.
- Ettmayer, P. Das System Molybdaen-Stickstoff. Monatshefte Chem. 1970, 101, 127–140. [Google Scholar] [CrossRef]
- Bolzan, A.A.; Kennedy, B.J.; Howard, C.J. Neutron Powder Diffraction Study of Molybdenum and Tungsten Dioxides. Aust. J. Chem. 1995, 48, 1473–1477. [Google Scholar] [CrossRef]
- Häglund, J.; Fernndez Guillermet, A.; Grimvall, G.; Körling, M. Theory of bonding in transition-metal carbides and nitrides. Phys. Rev. B 1993, 48, 11685–11691. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. On the Polymerization of P-Phenylenediamine. Eur. Polym. J. 1996, 32, 43–50. [Google Scholar] [CrossRef]
- Pałys, B.J.; Skompska, M.; Jackowska, K. Sensitivity of poly 1,8-diaminonaphthalene to heavy metal ions-Electrochemical and vibrational spectra studies. J. Electroanal. Chem. 1997, 433, 41–48. [Google Scholar] [CrossRef]
- Ohno, K.; Okimura, M.; Akai, N.; Katsumoto, Y. The effect of cooperative hydrogen bonding on the OH stretching-band shift for water clusters studied by matrix-isolation infrared spectroscopy and density functional theory. Phys. Chem. Chem. Phys. 2005, 7, 3005–3014. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, B.; Mo, Q.; Shao, Z.J.; Nie, K.; Liu, B.; Zhang, H.; Wang, Y.; Zhang, Y.; Gao, Q.; et al. Organic-Inorganic-Hybrid-Derived Molybdenum Carbide Nanoladders: Impacts of Surface Oxidation for Hydrogen Evolution Reaction. ChemNanoMat 2018, 4, 194–202. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, L.; Lu, X.; Mao, J.; Zhang, Y.; Wu, Y.; Tang, Y. Synthesis, characterization and lithium-storage performance of MoO2/carbon hybrid nanowires. J. Mater. Chem. 2010, 20, 2807–2812. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, C.; Xie, S.; Hua, W.; Zhang, Y.; Ren, N.; Xu, H.; Tang, Y. Synthesis of Nanoporous Molybdenum Carbide Nanowires Based on Organic-Inorganic Hybrid Nanocomposites with Sub-Nanometer Periodic Structures. Chem. Mater. 2009, 21, 5560–5562. [Google Scholar] [CrossRef]
- Kasap, E.; Özbay, A.; Özçelik, S. Infrared spectroscopic study of the Hofmann-diam-type clathrates: M(1,6-diaminohexane)Ni(CN)4 C6H6 (M = Ni, Co or Cd). Spectrosc. Lett. 1997, 30, 491–496. [Google Scholar] [CrossRef]
- Wolf, U.; Ernst, F.; Muschik, T.; Finnis, M.W.; Fischmeister, H.F. The influence of grain boundary inclination on the structure and energy of σ = 3 grain boundaries in copper. Philos. Mag. A 1992, 66, 991–1016. [Google Scholar] [CrossRef]
- Hofmann, D.; Ernst, F. Quantitative high-resolution transmission electron microscopy of the incoherent Σ3 (211) boundary in Cu. Ultramicroscopy 1994, 53, 205–221. [Google Scholar] [CrossRef]
- Campbell, G.H.; Chan, D.K.; Medlin, D.L.; Angelo, J.E.; Carter, C.B. Dynamic observation of the FCC to 9R shear transformation in a copper Σ = 3 incoherent twin boundary. Scr. Mater. 1996, 35, 837–842. [Google Scholar] [CrossRef]
- Medlin, D.L.; Campbell, G.H.; Carter, C.B. Stacking defects in the 9R phase at an incoherent twin boundary in copper. Acta Mater. 1998, 46, 5135–5142. [Google Scholar] [CrossRef]
- Wang, R.; Yang, J.; Shi, K.; Wang, B.; Wang, L.; Tian, G.; Bateer, B.; Tian, C.; Shen, P.; Fu, H. Single-step pyrolytic preparation of Mo2C/graphitic carbon nanocomposite as catalyst carrier for the direct liquid-feed fuel cells. RSC Adv. 2013, 3, 4771–4777. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Zhang, Y.; Jiang, W.-J.; Zhang, X.; Dai, Z.; Wan, L.-J.; Hu, J.-S. Pomegranate-like N,P-Doped Mo2C@C Nanospheres as Highly Active Electrocatalysts for Alkaline Hydrogen Evolution. ACS Nano 2016, 10, 8851–8860. [Google Scholar] [CrossRef]
- Varganici, C.D.; Rosu, D.; Barbu-Mic, C.; Rosu, L.; Popovici, D.; Hulubei, C.; Simionescu, B.C. On the thermal stability of some aromatic-aliphatic polyimides. J. Anal. Appl. Pyrolysis 2015, 113, 390–401. [Google Scholar] [CrossRef]
- García, J.M.; Álvarez, J.C.; De La Campa, J.G.; De Abajo, J. Thermal Behavior of Aliphatic-Aromatic Poly(ether-amide)s. J. Appl. Polym. Sci. 1998, 67, 975–981. [Google Scholar] [CrossRef]





| Reaction Number | Ratio (1,8-DAN:AHM) | 1,8-DAN (g) (mmol) | AHM (g) (mmol) | HCl (M) |
|---|---|---|---|---|
| 1 | 1:1 | 0.5 | 3.86 | 0.05 |
| 3.16 | 3.12 | |||
| 2 | 2:1 | 1.0 | 3.86 | 0.05 |
| 6.32 | 3.12 | |||
| 3 | 5:1 | 1.25 | 1.93 | 0.025 |
| 7.90 | 1.56 | |||
| 4 | 9:1 | 2.33 | 1.93 | 0.025 |
| 15 | 1.56 | |||
| 5 | 10:1 | 2.5 | 1.93 | 0.025 |
| 16 | 1.56 | |||
| 6 | 15:1 | 3.75 | 1.93 | 0.05 |
| 24 | 1.56 | |||
| 7 | 18:1 | 4.375 | 1.93 | 0.05 |
| 28 | 1.56 | |||
| 8 | 20:1 | 5.16 | 1.93 | 0.05 |
| 33 | 1.56 | |||
| 9 | 25:1 | 6.09 | 1.93 | 0.075 |
| 38 | 1.56 | |||
| 10 | 30:1 | 7.34 | 1.93 | 0.1 |
| 46 | 1.56 |
| Reaction Number | Ratio (HMD:AHM) | HMD (g) (mmol) | AHM (g) (mmol) | HCl (M) |
|---|---|---|---|---|
| 1 | 1:1 | 0.365 | 3.86 | 0.2 |
| 3.14 | 3.12 | |||
| 2 | 2:1 | 0.725 | 3.86 | 0.2 |
| 6.24 | 3.12 | |||
| 3 | 5:1 | 1.815 | 3.86 | 0.3 |
| 16 | 3.12 | |||
| 4 | 9:1 | 3.265 | 3.86 | 0.3 |
| 28 | 3.12 | |||
| 5 | 10:1 | 3.63 | 3.86 | 0.3 |
| 31 | 3.12 | |||
| 6 | 15:1 | 5.445 | 3.86 | 0.3 |
| 47 | 3.12 | |||
| 7 | 18:1 | 6.535 | 3.86 | 0.4 |
| 56 | 3.12 | |||
| 8 | 20:1 | 7.01 | 3.86 | 0.4 |
| 60 | 3.12 | |||
| 9 | 25:1 | 9.075 | 3.86 | 0.6 |
| 78 | 3.12 | |||
| 10 | 30:1 | 10.89 | 3.86 | 0.6 |
| 97 | 3.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdirahman Mohamed, M.; Janka, O.; Harling, S.; Kickelbick, G. Precursor-Based Syntheses of Mo(C,N,O)x, Molybdenum Carbide, Nitride, and Oxide Applying a Microjet Reactor. Solids 2024, 5, 443-459. https://doi.org/10.3390/solids5030030
Abdirahman Mohamed M, Janka O, Harling S, Kickelbick G. Precursor-Based Syntheses of Mo(C,N,O)x, Molybdenum Carbide, Nitride, and Oxide Applying a Microjet Reactor. Solids. 2024; 5(3):443-459. https://doi.org/10.3390/solids5030030
Chicago/Turabian StyleAbdirahman Mohamed, Mana, Oliver Janka, Susanne Harling, and Guido Kickelbick. 2024. "Precursor-Based Syntheses of Mo(C,N,O)x, Molybdenum Carbide, Nitride, and Oxide Applying a Microjet Reactor" Solids 5, no. 3: 443-459. https://doi.org/10.3390/solids5030030
APA StyleAbdirahman Mohamed, M., Janka, O., Harling, S., & Kickelbick, G. (2024). Precursor-Based Syntheses of Mo(C,N,O)x, Molybdenum Carbide, Nitride, and Oxide Applying a Microjet Reactor. Solids, 5(3), 443-459. https://doi.org/10.3390/solids5030030








