Development of a Simple Cell Harvesting Method to Maximise DNA Recovery from Historic Microscope Slides for Sexual Assault Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microscope Slide Samples
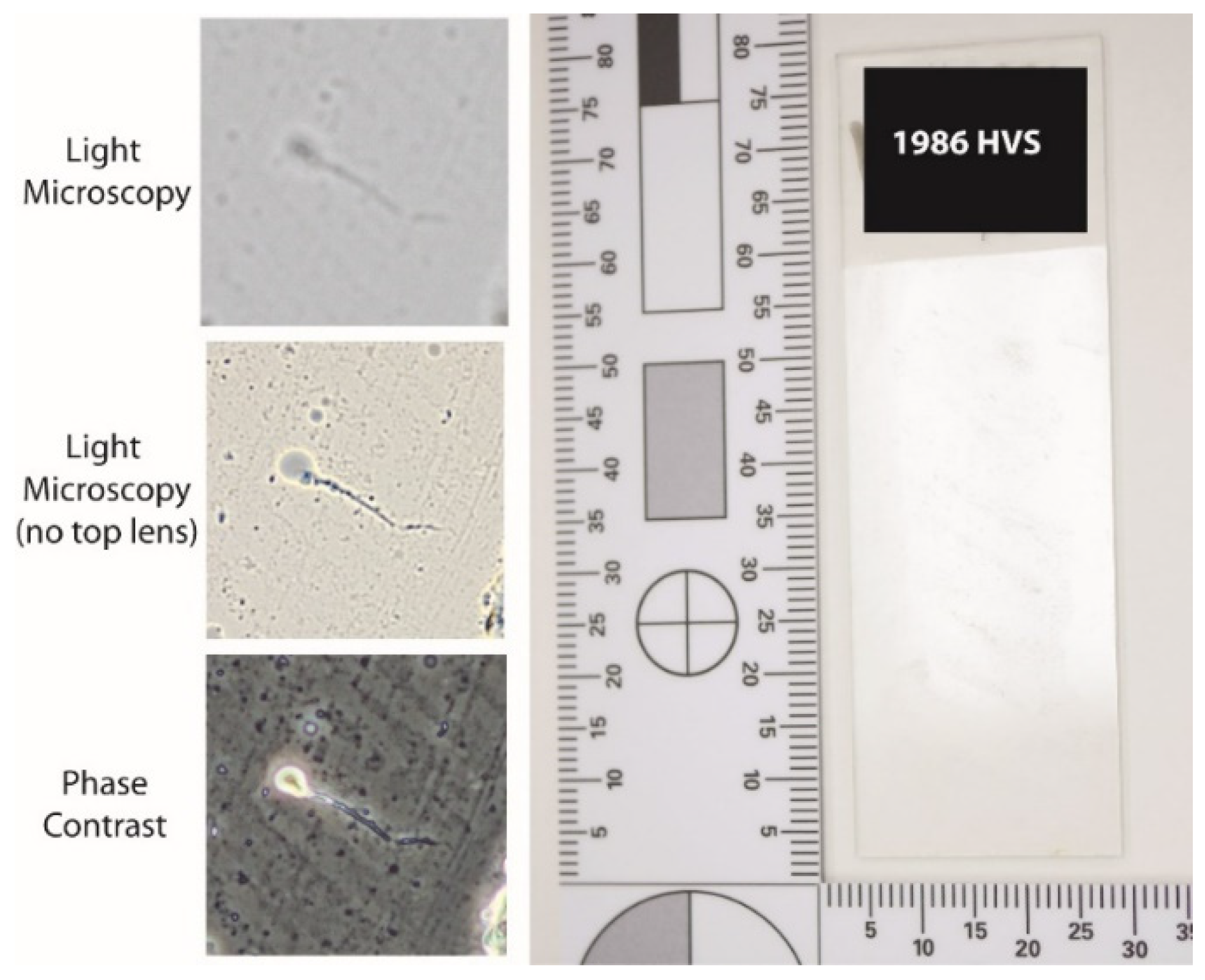
2.2. Light Microscopy
2.3. Microscope Slide Preparation
2.4. Microscope Slide Cell Harvest
2.5. i-sep® Differential Separation (DS)
2.6. Direct Extraction Method
2.7. DNA Extraction
2.8. DNA Quantification and STR Analysis
3. Results
3.1. Phase 1—Evaluation of DNA Recovery and DNA Quality of Scraped Cellular Material from Cover Slipped Crystal Violet and Christmas Tree Stained Mock Sexual Assault Microscope Slide Smears of Decreasing Sperm Scores Prepared in 2010
3.2. Phase 2—Evaluation of DNA Recovery and DNA Quality of Scraped Cellular Material from Historic Casework Sexual Assault Microscope Slide Smears from the 1990s
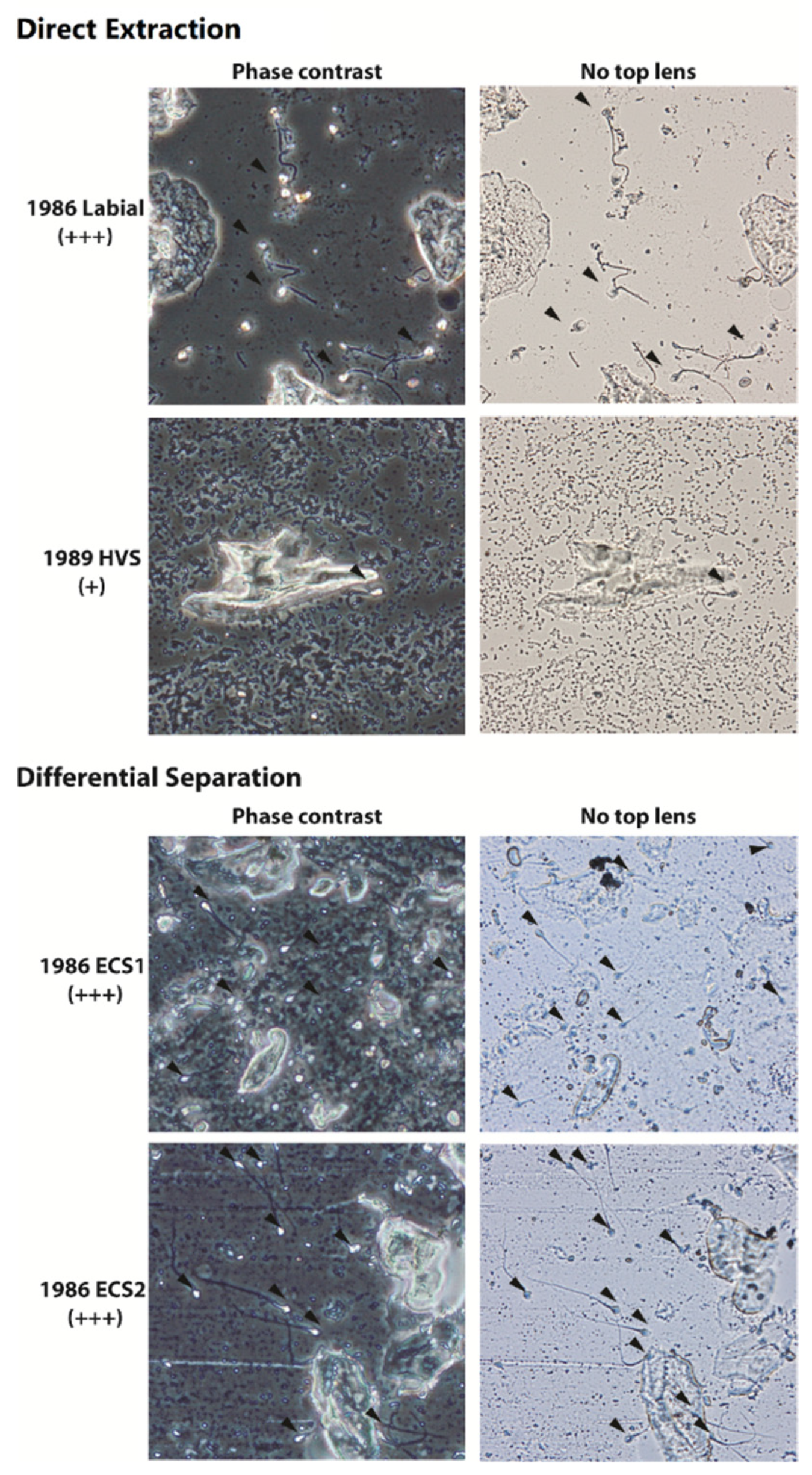
3.3. Phase 3—Evaluation of DNA Recovery and DNA Quality of Scraped Cellular Material from Historic Casework Sexual Assault Microscope Slide Smears from the 1980s
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos, P.; Handt, O.; Taylor, D. Investigating the position and level of DNA transfer to undergarments during digital sexual assault. Forensic Sci. Int. Genet. 2020, 47, 102316. [Google Scholar] [CrossRef] [PubMed]
- Connell, M. Expert testimony in sexual assault cases: Alcohol intoxication and memory. Int. J. Law Psychiatry 2015, 42–43, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, S.R.; Garrett, B.L.; Fenn, K.M.; Loftus, E. Convicting with confidence? Why we should not over-rely on eyewitness confidence. Memory 2022, 30, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.C.; Wells, W. DNA testing in sexual assault cases: When do the benefits outweigh the costs? Forensic Sci. Int. 2019, 299, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sekiguchi, K.; Mizuno, N.; Kasai, K.; Sakai, I.; Sato, H.; Seta, S. The modified method of two-step differential extraction of sperm and vaginal epithelial cell DNA from vaginal fluid mixed with semen. Forensic Sci. Int. 1995, 72, 25–33. [Google Scholar] [CrossRef]
- Lounsbury, J.A.; Nambiar, S.M.; Karlsson, A.; Cunniffe, H.; Norris, J.V.; Ferrance, J.P.; Landers, J.P. Enhanced recovery of spermatozoa and comprehensive lysis of epithelial cells from sexual assault samples having a low cell counts or aged up to one year. Forensic Sci. Int. Genet. 2014, 8, 84–89. [Google Scholar] [CrossRef]
- Timken, M.D.; Klein, S.B.; Kubala, S.; Scharnhorst, G.; Buoncristiani, M.R.; Miller, K.W.P. Automation of the standard DNA differential extraction on the Hamilton AutoLys STAR system: A proof-of-concept study. Forensic Sci. Int. Genet. 2019, 40, 96–104. [Google Scholar] [CrossRef]
- Sinha, S.K.; Brown, H.; Holt, H.; Khan, M.R.; Brown, R.; Sgueglia, J.B.; Loftus, A.; Murphy, G.; Montgomery, A. Development and validation of a novel method “SpermX” for high throughput differential extraction processing of sexual assault kits (SAKs) for DNA analysis. Forensic Sci. Int. Genet. 2022, 59, 102690. [Google Scholar] [CrossRef]
- Gill, P.; Jeffreys, A.J.; Werrett, D.J. Forensic application of DNA ‘fingerprints’. Nature 1985, 318, 577–579. [Google Scholar] [CrossRef]
- Virkler, K.; Lednev, I.K. Analysis of body fluids for forensic purposes: From laboratory testing to non-destructive rapid confirmatory identification at a crime scene. Forensic Sci. Int. 2009, 188, 1–17. [Google Scholar] [CrossRef]
- Golomingi, R.; Haas, C.; Dobay, A.; Kottner, S.; Ebert, L. Sperm hunting on optical microscope slides for forensic analysis with deep convolutional networks—A feasibility study. Forensic Sci. Int. Genet. 2022, 56, 102602. [Google Scholar] [CrossRef] [PubMed]
- Westring, C.G.; Wiuf, M.; Nielsen, S.J.; Fogleman, J.C.; Old, J.B.; Lenz, C.; Reich, K.A.; Morling, N. SPERM HY-LITER for the identification of spermatozoa from sexual assault evidence. Forensic Sci. Int. Genet. 2014, 12, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.L. Analysis of Body Fluids in Sexual Assault Cases; Forensic Science Handbook; CRC Press: Boca Raton, FL, USA, 2020; pp. 613–706. [Google Scholar]
- White, T.J.; Rye, M.S.; Tay, J.W. Developmental validation of an efficient differential separation method incorporating the i-sep(®) DL spin column with high sperm DNA recovery for the processing of sexual assault samples. J. Forensic Sci. 2022, 67, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Correia-de-Sa, P.; Porto, M.J.; Caine, L. The Use of Laser Microdissection in Forensic Sexual Assault Casework: Pros and Cons Compared to Standard Methods. J. Forensic Sci. 2017, 62, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Dimo-Simonin, N.; Grange, F.; Brandt-Casadevall, C. PCR-based forensic testing of DNA from stained cytological smears. J. Forensic Sci. 1997, 42, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhuri, S.; Chow, C.W.; Kane, M.K.; Yao, H.; Wistuba, I.I.; Krishnamurthy, S.; Stewart, J.; Staerkel, G. Optimizing the DNA yield for molecular analysis from cytologic preparations. Cancer Cytopathol. 2016, 124, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Dejmek, A.; Zendehrokh, N.; Tomaszewska, M.; Edsjö, A. Preparation of DNA from cytological material: Effects of fixation, staining, and mounting medium on DNA yield and quality. Cancer Cytopathol. 2013, 121, 344–353. [Google Scholar] [CrossRef]
- Clark, M.; Gill, J.; Sasinouski, K.; McGuire, A. Cold Case Homicides: DNA Testing of Retained Autopsy Sexual Assault Smears. J. Forensic Sci. 2019, 64, 1100–1104. [Google Scholar] [CrossRef]
- Oppitz, E. Eine neue Färbemethode zum Nachweis der Spermien bei Sittlichkeitsdelikten. J. Arch. Kriminol. 1969, 144, 145–148. [Google Scholar]
- Fischer, R. The Gram-staining behavior of spermatozoa. Experientia 1953, 9, 335–336. [Google Scholar] [CrossRef]
- Applied Biosystems Quantifiler™ HP and Trio DNA Quantification Kits Uer Guide: Publication Number 4485354 Revision G; Thermofisher Scientific: Waltham, MA, USA, 2017.
- Alaeddini, R.; Walsh, S.J.; Abbas, A. Forensic implications of genetic analyses from degraded DNA—A review. Forensic Sci. Int. Genet. 2010, 4, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.G.; Domijan, K.; MacNeill, S.; Rizet, D.; O’Connell, D.; Ryan, J. The Persistence of Sperm and the Development of Time Since Intercourse (TSI) Guidelines in Sexual Assault Cases at Forensic Science Ireland, Dublin, Ireland. J. Forensic Sci. 2017, 62, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, G.M.G.; Gibb, A.; Lin Paul, K.T.; Rolf, B.; Forat, S.; Jäger, R. DNA profiling of single sperm cells after whole genome amplification. Forensic Sci. Int. Rep. 2021, 4, 100240. [Google Scholar] [CrossRef]
- Elliott, K.; Hill, D.S.; Lambert, C.; Burroughes, T.R.; Gill, P. Use of laser microdissection greatly improves the recovery of DNA from sperm on microscope slides. Forensic Sci. Int. 2003, 137, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.; Sanchez, N.; Ballantyne, J.; Peterson, D. Laser Microdissection Separation of Pure Spermatozoa from Epithelial Cells for Short Tandem Repeat Analysis*. J. Forensic Sci. 2006, 51, 748–757. [Google Scholar] [CrossRef]
- Meredith, M.; Bright, J.A.; Cockerton, S.; Vintiner, S. Development of a one-tube extraction and amplification method for DNA analysis of sperm and epithelial cells recovered from forensic samples by laser microdissection. Forensic Sci. Int. Genet. 2012, 6, 91–96. [Google Scholar] [CrossRef]
- Clark, C.; Turiello, R.; Cotton, R.; Landers, J.P. Analytical approaches to differential extraction for sexual assault evidence. Anal. Chim. Acta. 2021, 1141, 230–245. [Google Scholar] [CrossRef]
- Zhao, X.C.; Wang, L.; Sun, J.; Jiang, B.W.; Zhang, E.L.; Ye, J. Isolating Sperm from Cell Mixtures Using Magnetic Beads Coupled with an Anti-PH-20 Antibody for Forensic DNA Analysis. PLoS ONE 2016, 11, e0159401. [Google Scholar] [CrossRef]

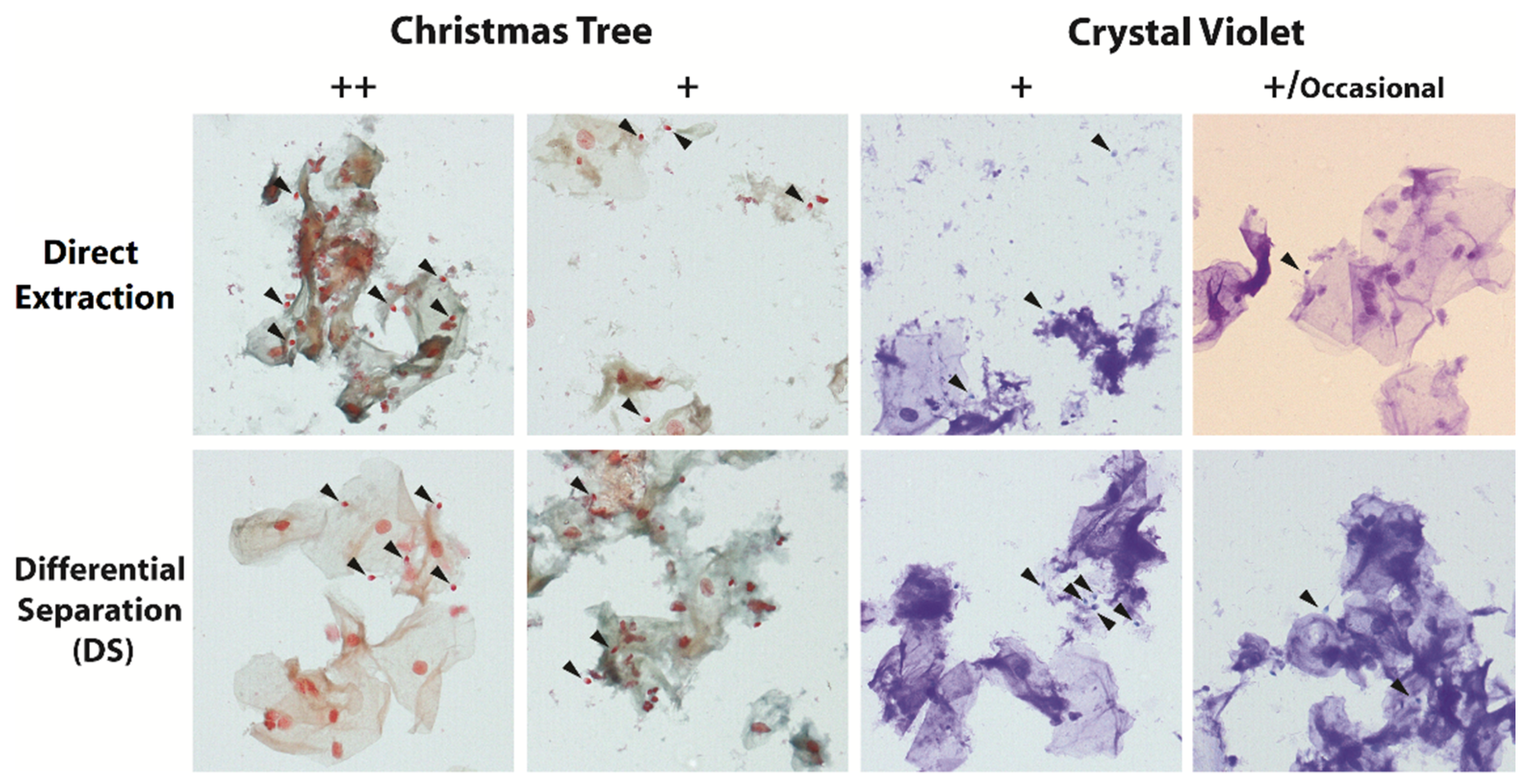

| Sperm Observed at 40× Field for Christmas Tree Staining | Sperm Observed at 100× Field for Crystal Violet Staining | Recorded Result |
|---|---|---|
| >60 per 40× field | 13 or more per 100× field | Many |
| 31–60 per 40× field | 6–12 per 100× field | +++ |
| 11–30 per 40× field | 3–6 per 100× field | ++ |
| 1–10 per 40× field | 1–2 per 100× field | + |
| 1–10 per 3–6 40× fields | 1–2 per 3–6 100× fields | Occasional |
| 1–10 per 6–12 40× fields | 1–2 per 6–12 100× fields | Very Occasional |
| No sperm seen | No sperm seen | Negative |
| Extraction Method | Stain | Original Sperm Scoring | Fraction | SA (ng/μL) | Y (ng/μL) | DI | Passing Loci | Mixture |
|---|---|---|---|---|---|---|---|---|
| Direct extraction | Christmas Tree | ++ | - | 1.83 | 0.182 | 8.29 | 13/21 | Yes |
| Christmas Tree | + | - | 0.264 | 0.0976 | 3.98 | 14/21 | Yes | |
| Crystal Violet | + | - | 0.103 | 0.0256 | 19.42 | 8/21 | Yes | |
| Crystal Violet | +/Occasional | - | 0.155 | 0.0227 | 17.75 | 9/21 | Yes | |
| i-sep® differential separation | Christmas Tree | ++ | Non-sperm | 1.131 | 0.007 | 3.325 | 15/21 | No |
| Sperm | 0.054 | 0.054 | 3.283 | 16/21 | No | |||
| Christmas Tree | + | Non-sperm | 0.697 | 0.025 | 3.554 | 14/21 | Yes | |
| Sperm | 0.072 | 0.092 | 2.260 | 19/21 | No | |||
| Crystal Violet | + | Non-sperm | 0.415 | 0.011 | 4.201 | 11/21 | Yes | |
| Sperm | 0.061 | 0.058 | 5.917 | 10/21 | No | |||
| Crystal Violet | +/Occasional | Non-sperm | 0.100 | 0.007 | 5.178 | 13/21 | Yes | |
| Sperm | 0.045 | 0.040 | 8.403 | 12/21 | Yes |
| Extraction Type | Year of Preparation | Type of Smear | Original Microscopy Score | Fraction | SA (ng/μL) | Y (ng/μL) | DI | Passing Loci | Mixture |
|---|---|---|---|---|---|---|---|---|---|
| Direct extraction | 1995 | Endocervical | ++ | - | 0.031 | 0.022 | 34.01 | 8/21 | Yes |
| 1995 | High Vaginal | ++ | - | 0.068 | 0.027 | 7.130 | 10/21 | Yes | |
| 1993 | Low Vaginal | + | - | 0.0006 | 0.0002 | 10.74 | 0/21 | - | |
| 1993 | Vulva | Occ | - | N/D | N/D | N/A | 0/21 | - | |
| i-sep® differential separation | 1995 | Endocervical | ++ | non-sperm | 0.0074 | 0.003 | 6.648 | 8/21 | Yes |
| sperm | 0.183 | 0.179 | 3.266 | 16/21 | No | ||||
| 1995 | High Vaginal | ++ | non-sperm | 0.346 | 0.0067 | 16.41 | 11/21 | Yes | |
| sperm | 0.161 | 0.206 | 5.972 | 13/21 | No | ||||
| 1993 | Labial | Occ | non-sperm | 0.0002 | N/D | - | 0/21 | - | |
| sperm | N/D | N/D | - | 0/21 | - | ||||
| 1993 | High Vaginal | +++ | non-sperm | 0.0228 | 0.0002 | 8.607 | 9/21 | No | |
| sperm | 0.0675 | 0.0167 | 15.50 | 10/21 | Yes |
| Extraction Type | Year of Preparation | Type of Smear | Original Microscopy Score | Fraction | SA (ng/μL) | Y (ng/μL) | DI | |
|---|---|---|---|---|---|---|---|---|
| Single slide | Direct extraction | 1986 | Labial | +++ | - | Undet | Undet | ND |
| 1989 | High Vaginal | + | - | 0.0014 | 0.0005 | ND | ||
| i-sep® | 1986 | Endocervical | +++ | non-sperm | Undet | Undet | ND | |
| sperm | 0.0002 | Undet | ND | |||||
| 1986 | Endocervical | +++ | non-sperm | 0.0012 | Undet | ND | ||
| sperm | 0.013 | Undet | 33.23 | |||||
| Pooled slides | Direct extraction | 1986 | Endocervical /Anal | +++/++ | - | 0.0002 | Undet | ND |
| 1986 | High Vaginal / Low Vaginal | +++/+++ | - | 0.0012 | 0.0002 | ND | ||
| i-sep® | 1988 | Labial / High Vaginal | ++/++ | non-sperm | Undet | Undet | ND | |
| sperm | 0.0006 | Undet | ND | |||||
| 1988 | Endocervical / Low Vaginal | ++/++ | non-sperm | 0.0021 | Undet | ND | ||
| sperm | 0.0014 | Undet | ND | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hymus, C.M.; Egan, S.E.; Tay, J.W. Development of a Simple Cell Harvesting Method to Maximise DNA Recovery from Historic Microscope Slides for Sexual Assault Investigations. Forensic Sci. 2022, 2, 795-807. https://doi.org/10.3390/forensicsci2040057
Hymus CM, Egan SE, Tay JW. Development of a Simple Cell Harvesting Method to Maximise DNA Recovery from Historic Microscope Slides for Sexual Assault Investigations. Forensic Sciences. 2022; 2(4):795-807. https://doi.org/10.3390/forensicsci2040057
Chicago/Turabian StyleHymus, Colby M., Scott E. Egan, and Jasmine W. Tay. 2022. "Development of a Simple Cell Harvesting Method to Maximise DNA Recovery from Historic Microscope Slides for Sexual Assault Investigations" Forensic Sciences 2, no. 4: 795-807. https://doi.org/10.3390/forensicsci2040057
APA StyleHymus, C. M., Egan, S. E., & Tay, J. W. (2022). Development of a Simple Cell Harvesting Method to Maximise DNA Recovery from Historic Microscope Slides for Sexual Assault Investigations. Forensic Sciences, 2(4), 795-807. https://doi.org/10.3390/forensicsci2040057









