Discovering Halite Traces on a Victim’s Clothing through a Forensic Geoscience Analytical Approach: A Suspicious Case in Italy
Abstract
:1. Introduction
A Brief Overview of the Case
2. Materials and Methods
2.1. Optical Stereomicroscope
2.2. SEM-EDS
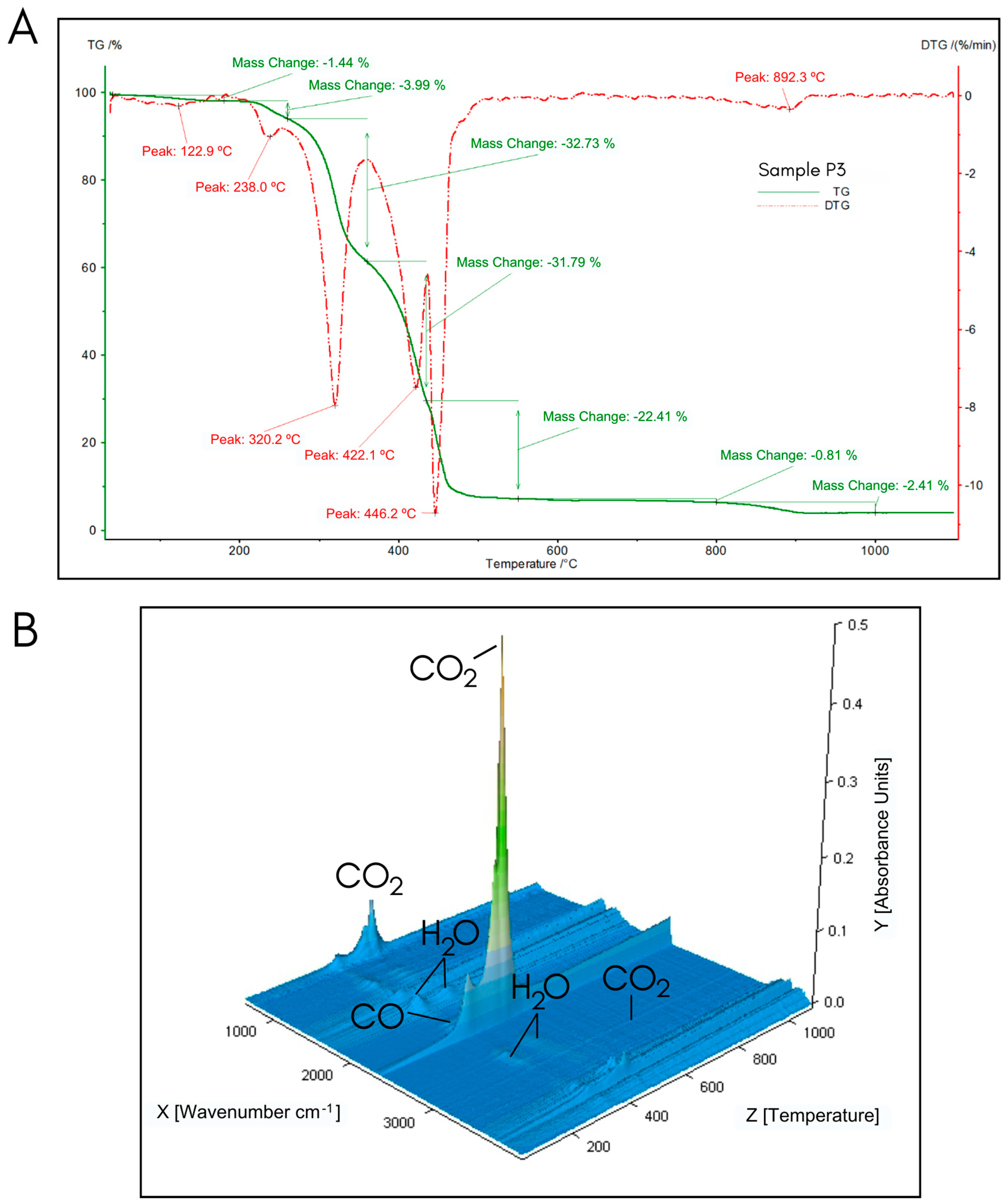
2.3. Simultaneous Thermal Analyses (STA)
3. Results and Discussion
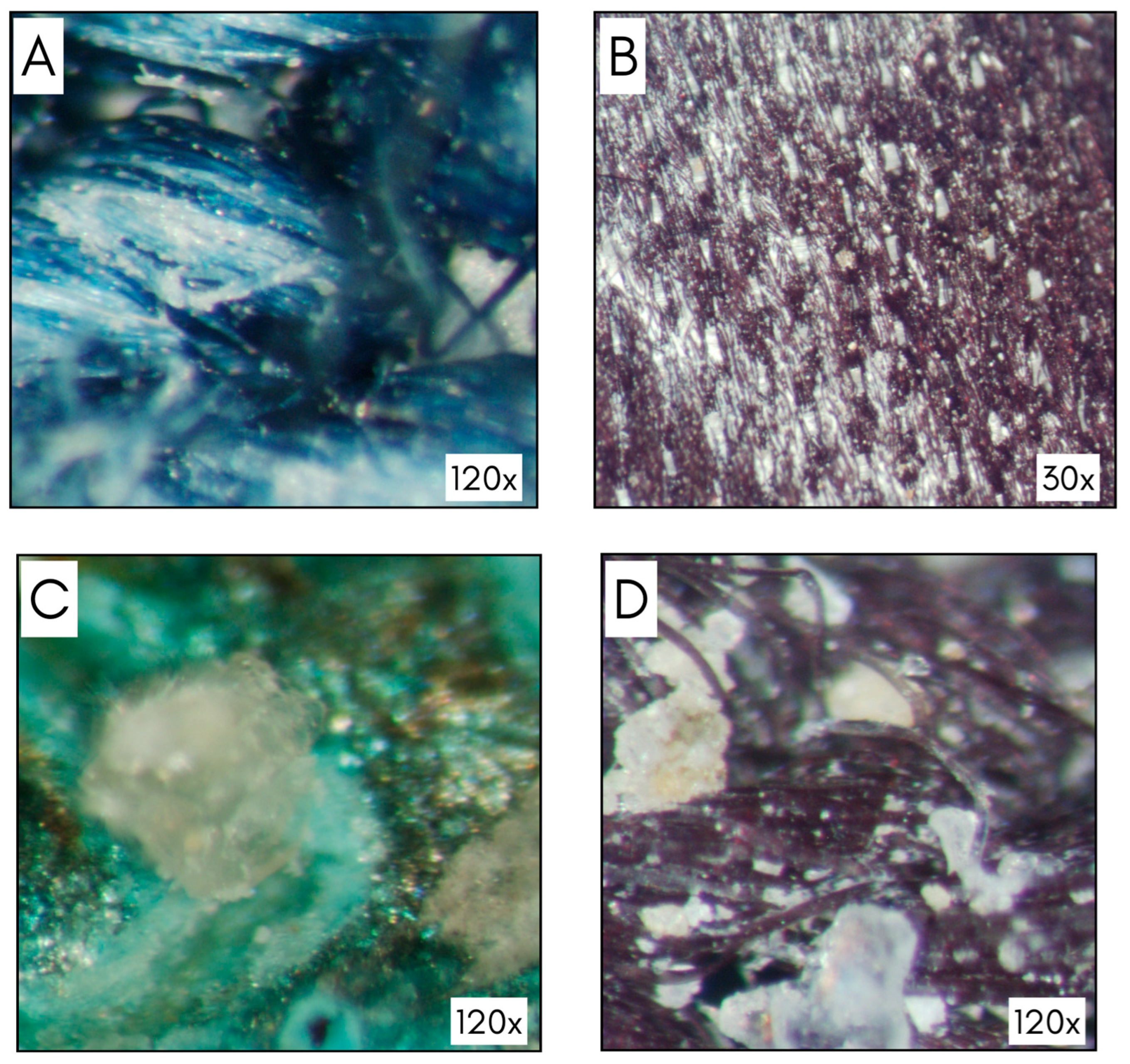
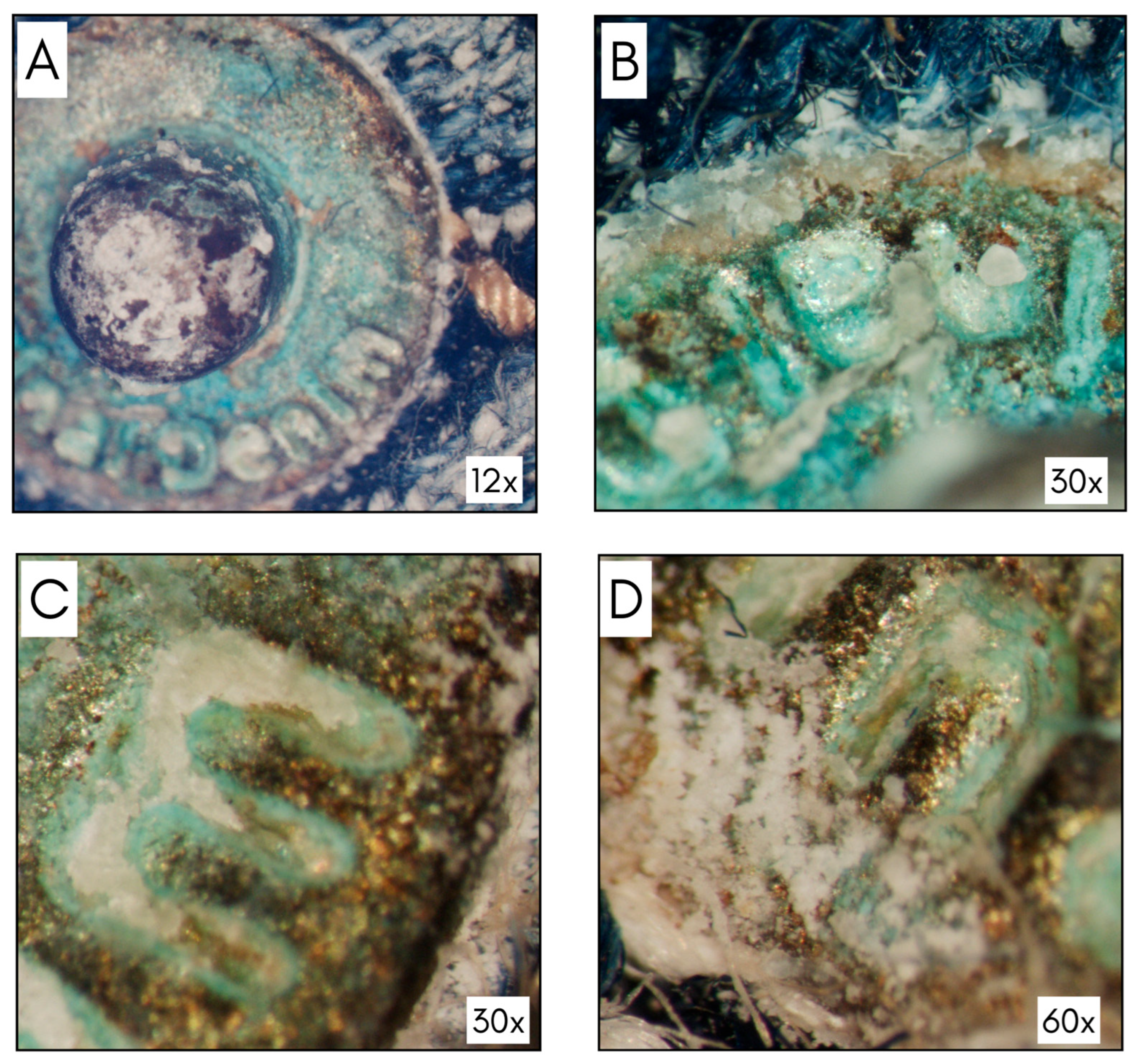
3.1. Optical Stereomicroscope
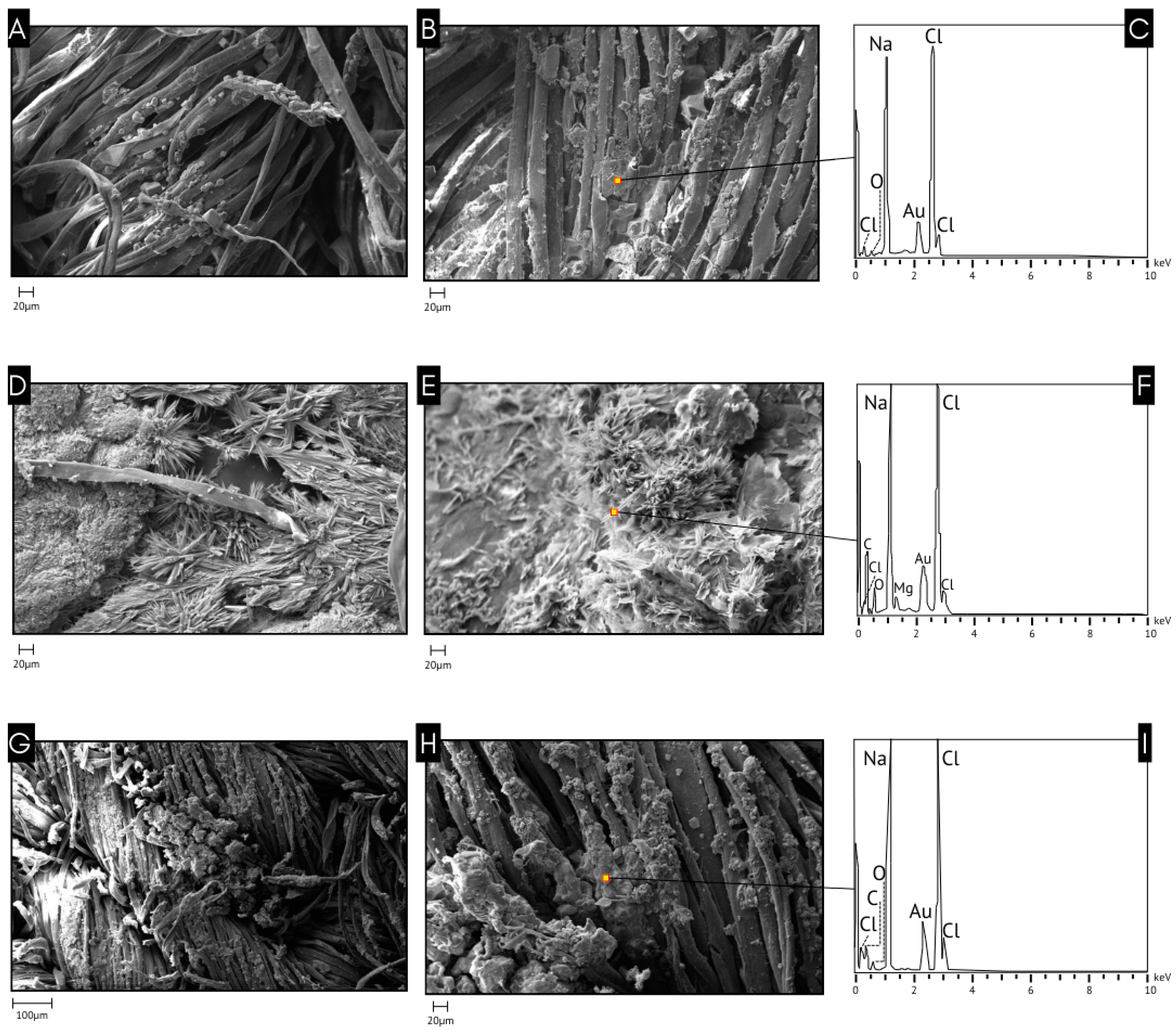
3.2. SEM-EDS
3.3. STA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobus, H.; Robertson, J. The importance of forensic soil science and geology being connected to mainstream forensic science. Geol. Soc. Lond. Spec. Publ. 2021, 492, 33–38. [Google Scholar] [CrossRef]
- Vanderkolk, J.R. Forensic Comparative Science: Qualitative Quantitative Source Determination of Unique Impressions, Images, and Objects; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Donnelly, L.J. Introduction. In A Guide to Forensic Geology; Donnelly, L.J., Pirrie, D., Harrison, M., Ruffell, A., Dawson, L., Eds.; Geological Society: London, UK, 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Fitzpatrick, R.W.; Donnelly, L.J. An introduction to forensic soil science and forensic geology: A synthesis. Geol. Soc. Lond. Spec. Publ. 2021, 492, 1–32. [Google Scholar] [CrossRef]
- Ruffell, A.; McKinley, J. Background to the Work, Organization of the Text and History of Research. In Geoforensics; Ruffell, A., McKinley, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Lombardi, G. The contribution of forensic geology and other trace evidence analysis to the investigation of the killing of Italian Prime Minister Aldo Moro. J. Forensic Sci. 1999, 44, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Povilauskas, L.K.; Tranchida, M.C. Palynology and mycology as biological evidence in a homicide case. J. Forensic Sci. 2023, 68, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.A.S.; Nogueira e Silva, M.P.; Mascarenhas, R.O.; Rumbelsperger, A.M.B. The application of forensic geology to investigate the substitution of zinc ingots between China and Brazil. Geol. Soc. Lond. Spec. Publ. 2021, 492, 147–154. [Google Scholar] [CrossRef]
- Guo, H.; Yao, Y.; Li, Y.; Wang, P.; Hu, C.; Yuan, M.; Mei, H.; Zhu, J. A case study in forensic soil comparison. J. Forensic Sci. 2022, 67, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, R.W.; Raven, M.D. The forensic comparison of trace amounts of soil on a pyjama top with hypersulphidic subaqueous soil from a river as evidence in a homicide cold case. In Forensic Soil Science and Geology; Fitzpatrick, R.W., Donnelly, L.J., Eds.; Geological Society of London: London, UK, 2021; Special Publications; Volume 492, pp. 285–302. [Google Scholar] [CrossRef]
- Fitzpatrick, R.W.; Raven, M.D.; Forrester, S.T. A Systematic Approach to Soil Forensics: Criminal Case Studies Involving Transference from Crime Scene to Forensic Evidence. In Criminal and Environmental Soil Forensics; Ritz, K., Dawson, L., Miller, D., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Dawson, L.A.; Mayes, R.W. Criminal and Environmental Soil Forensics: Soil as Physical Evidence in Forensic Investigations. In Introduction to Environmental Forensics, 3rd ed.; Murphy, B.L., Morrison, R.D., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 457–486. [Google Scholar] [CrossRef]
- Chauhan, R.; Singh, A.; Sharma, A.; Kumar, V.; Singh, J. Soil forensics: A spectroscopic examination of trace evidence. Microchem. J. 2018, 139, 74–84. [Google Scholar] [CrossRef]
- Corrêa, R.S.; Melo, V.F.; Abreu, G.G.F.; Sousa, M.H.; Chaker, J.A.; Gomes, J.A. Soil forensics: How far can soil clay analysis distinguish between soil vestiges? Sci. Justice 2018, 58, 138–144. [Google Scholar] [CrossRef]
- Ogilvie, R.H.; Lednev, I.K. Soil from footwear is a newly rediscovered type of forensic evidence due to the application of modern analytical techniques: A review. Trends Anal. Chem. 2023, 163, 117081. [Google Scholar] [CrossRef]
- Bull, P.A.; Morgan, R.M. Sediment fingerprints: A forensic technique using quartz sand grains. Sci. Justice 2006, 46, 107–124. [Google Scholar] [CrossRef]
- Morgan, R.M.; Bull, P.A. The use of grain size distribution analysis of sediments and soils in forensic enquiry. Sci. Justice 2007, 47, 125–135. [Google Scholar] [CrossRef]
- Essefi, E. Advances in Forensic Sedimentology. In Technologies to Advance Automation in Forensic Science and Criminal Investigation; IGI Global: Hershey, PA, USA, 2022; pp. 37–47. [Google Scholar] [CrossRef]
- Pirrie, D.; Pidduck, A.J.; Crean, D.E.; Nicholls, T.M.; Awbery, R.P. Identification and analysis of man-made geological product particles to aid forensic investigation of provenance in the built environment. Forensic Sci. Int. 2019, 305, 109974. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.F.P.D.; Guimarães-Cestaro, L.; Serrão, J.E.; Message, D.; Martins, M.F.; Alves, M.L.T.M.F.; Teixeira, É.W. Using palynological evidence from royal jelly to mediate the spread of Paenibacillus larvae in Brazil. Hoehnea 2018, 45, 512–539. [Google Scholar] [CrossRef]
- Laurence, A.R.; Bryant, V.M. Forensic palynology and the search for geolocation: Factors for analysis and the Baby Doe case. Forensic Sci. Int. 2019, 302, 109903. [Google Scholar] [CrossRef] [PubMed]
- Ezegbogu, M.O. Identifying the scene of a crime through pollen analysis. Sci. Justice 2021, 61, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Isphording, W.C. Forensic use of heavy minerals in civil and criminal investigations. Dev. Sedimentol. 2007, 58, 963–982. [Google Scholar] [CrossRef]
- Pitts, K.M.; Clarke, R.M. The forensic discrimination of quartz sands from the Swan Coastal Plain, Western Australia. Forensic Sci. Int. Rep. 2020, 2, 100130. [Google Scholar] [CrossRef]
- Getenet, M.; García-Ruiz, J.M.; Otálora, F.; Emmerling, F.; Al-Sabbagh, D.; Verdugo-Escamilla, C. A comprehensive methodology for monitoring evaporitic mineral precipitation and hydrochemical evolution of saline lakes: The case of Lake Magadi soda brine (East African Rift Valley, Kenya). Cryst. Growth Des. 2022, 22, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.K. Basic Mineralogy. In Introduction to Mineralogy and Petrology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 109–143. [Google Scholar] [CrossRef]
- Vizcayno, C.; Garcia-Gonzalez, M.T.; Gutierrez, M.; Rodriguez, R. Mineralogical, chemical and morphological features of salt accumulations in the Flumen-Monegros district, NE Spain. Geoderma 1995, 68, 193–210. [Google Scholar] [CrossRef]
- Aquilano, D.; Otálora, F.; Pastero, L.; García-Ruiz, J.M. Three study cases of growth morphology in minerals: Halite, calcite and gypsum. Prog. Cryst. Growth Charact. Mater. 2016, 62, 227–251. [Google Scholar] [CrossRef]
- Sirota, I.; Enzel, Y.; Mor, Z.; Ben Moshe, L.; Eyal, H.; Lowenstein, T.K.; Lensky, N.G. Sedimentology and stratigraphy of a modern halite sequence formed under Dead Sea level fall. Sedimentology 2021, 68, 1069–1090. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Otálora, F. Crystal growth in geology: Patterns on the rocks. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–43. [Google Scholar] [CrossRef]
- Zago, G.P.; Penha, F.M.; Seckler, M.M. Product characteristics in simultaneous crystallization of NaCl and CaSO4 from aqueous solution under different evaporation rates. Desalination 2019, 457, 85–95. [Google Scholar] [CrossRef]
- Xia, Z.; Lin, Y.; Wei, H.; Hu, Z.; Liu, C.; Li, W. Reconstruct hydrological history of terrestrial saline lakes using Mg isotopes in halite: A case study of the Quaternary Dalangtan playa in Qaidam Basin, NW China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 587, 110804. [Google Scholar] [CrossRef]
- Galamay, A.R.; Bukowski, K.; Zinchuk, I.M.; Meng, F. The Temperature of Halite Crystallization in the Badenian Saline Basins, in the Context of Paleoclimate Reconstruction of the Carpathian Area. Minerals 2021, 11, 831. [Google Scholar] [CrossRef]
- Licsandru, G.; Noiriel, C.; Duru, P.; Geoffroy, S.; Abou-Chakra, A.; Prat, M. Evaporative destabilization of a salt crust with branched pattern formation. Sci. Rep. 2023, 13, 5132. [Google Scholar] [CrossRef] [PubMed]
- Çelik, M.Y.; Aygün, A. The effect of salt crystallization on degradation of volcanic building stones by sodium sulfates and sodium chlorides. Bull. Eng. Geol. Environ. 2019, 78, 3509–3529. [Google Scholar] [CrossRef]
- Granneman, S.J.; Lubelli, B.; van Hees, R.P. Mitigating salt damage in building materials by the use of crystallization modifiers—A review and outlook. J. Cult. Herit. 2019, 40, 183–194. [Google Scholar] [CrossRef]
- Balksten, K.; Strandberg-de Bruijn, P. Understanding deterioration due to salt and ice crystallization in scandinavian massive brick masonry. Heritage 2021, 4, 349–370. [Google Scholar] [CrossRef]
- Bai, Y.; Thompson, G.E.; Martinez-Ramirez, S.; Brüeggerhoff, S. Mineralogical study of salt crusts formed on historic building stones. Sci. Total Environ. 2023, 302, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Siedel, H. Salt efflorescence as indicator for sources of damaging salts on historic buildings and monuments: A statistical approach. Environ. Earth Sci. 2018, 77, 572. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, G.; Liu, L.; Shi, P.; Zhang, H.; Han, X.; Xue, K.; Hu, X.; Zhang, J.; Xiang, M.; et al. Effects of efflorescence and subflorescence by different salts on soil physical properties and aeolian erosion. Catena 2022, 215, 106323. [Google Scholar] [CrossRef]
- Gibson, M.E.; Benison, K.C. It’s a trap!: Modern and ancient halite as Lagerstätten. J. Sediment. Res. 2023, 93, 642–655. [Google Scholar] [CrossRef]
- Murray, R.C. Evidence from the Earth: Forensic Geology and Criminal Investigation; Mountain Press Publishing: Missoula, MT, USA, 2004; ISBN 978-0-87842-577-8. [Google Scholar]
- Murray, R.C. Forensic examination of soils. In Forensic Chemistry Handbook; Kobilinsky, L., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 109–130. [Google Scholar] [CrossRef]
- Saadat, S.; Pandey, G.; Tharmavaram, M. Microscopy for forensic investigations. In Technology in Forensic Science: Sampling, Analysis, Data and Regulations; Rawtani, D., Tharmavaram, C.M., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 101–127. [Google Scholar] [CrossRef]
- Di Maggio, R.M.; Salvador, F.A.S. Optical Microscopy Applied to Forensics. In Mineralogical Analysis Applied to Forensics. Soil Forensics; Mercurio, M., Langella, A., Di Maggio, R.M., Cappelletti, P., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Cengiz, S.; Karaca, A.C.; Çakır, İ.; Üner, H.B.; Sevindik, A. SEM–EDS analysis and discrimination of forensic soil. Forensic Sci. Int. 2004, 141, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Pirrie, D.; Power, M.R.; Rollinson, G.K.; Wiltshire, P.E.; Newberry, J.; Campbell, H.E. Automated SEM-EDS (QEMSCAN®) mineral analysis in forensic soil investigations: Testing instrumental reproducibility. In Criminal and Environmental Soil Forensics; Ritz, K., Dawson, L., Miller, D., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 411–430. [Google Scholar] [CrossRef]
- Pirrie, D.; Butcher, A.R.; Power, M.R.; Gottlieb, P.; Miller, G.L. Rapid quantitative mineral and phase analysis using automated scanning electron microscopy (QemSCAN); potential applications in forensic geoscience. Geol. Soc. Lond. Spec. Publ. 2004, 232, 123–136. [Google Scholar] [CrossRef]
- Di Maggio, R.M. Preventing art and antiquities crimes using forensic geology. In Multidisciplinary Approaches to Forensic Archaeology: Topics Discussed during the European Meetings on Forensic Archaeology (EMFA); Barone, P.M., Groen, W.J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 239–249. [Google Scholar] [CrossRef]
- Pirrie, D.; Crean, D.E.; Pidduck, A.J.; Nicholls, T.M.; Awbery, R.P.; Shail, R.K. Automated mineralogical profiling of soils as an indicator of local bedrock lithology: A tool for predictive forensic geolocation. In Forensic Soil Science and Geology; Fitzpatrick, R.W., Donnelly, L.J., Eds.; Geological Society of London: London, UK, 2021; Special Publications. [Google Scholar] [CrossRef]
- Pirrie, D.; Dawson, L.; Graham, G. Predictive geolocation: Forensic soil analysis for provenance determination. Epis. J. Int. Geosci. 2017, 40, 141–147. [Google Scholar] [CrossRef]
- Petrosino, P.; Pirrie, D.; Santoro, L.; de Gennaro, R. Scanning Electron Microscopy (SEM) in Forensic Geoscience. In Mineralogical Analysis Applied to Forensics. Soil Forensics; Mercurio, M., Langella, A., Di Maggio, R.M., Cappelletti, P., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Ozawa, T. Thermal analysis—Review and prospect. Thermochim. Acta 2000, 355, 35–42. [Google Scholar] [CrossRef]
- Mercurio, M.; Izzo, F.; Langella, A.; Sarkar, B. Simultaneous Thermal Analysis (STA): A Powerful Tool for Forensic Investigation of Geomaterials. In Mineralogical Analysis Applied to Forensics. Soil Forensics; Mercurio, M., Langella, A., Di Maggio, R.M., Cappelletti, P., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, R.; Diwan, P.K.; Sharma, V. Thermogravimetric analysis and chemometric based methods for soil examination: Application to soil forensics. Forensic Chem. 2020, 17, 100191. [Google Scholar] [CrossRef]
- Germinario, C.; Cultrone, G.; Cavassa, L.; de Bonis, A.; Izzo, F.; Langella, A.; Mercurio, M.; Morra, V.; Munzi, P.; Grifa, C. Local production and imitations of late Roman pottery from a well in the Roman necropolis of Cuma in Naples, Italy. Geoarchaeology 2019, 34, 62–79. [Google Scholar] [CrossRef]
- Karavoltsos, S.; Sakellari, A.; Bakeas, E.; Bekiaris, G.; Plavšić, M.; Proestos, C.; Zinelis, S.; Koukoulakis, K.; Diakos, I.; Dassenakis, M.; et al. Trace elements, polycyclic aromatic hydrocarbons, mineral composition, and FT-IR characterization of unrefined sea and rock salts: Environmental interactions. Environ. Sci. Pollut. Res. 2020, 27, 10857–10868. [Google Scholar] [CrossRef]
- Picco, P. Climatological Atlas of the Western Mediterranean; Santa Teresa Centre for Energy and Environmental Research: La Spezia, Italy, 1990. [Google Scholar]
- Pilson, M.E. An Introduction to the Chemistry of the Sea, 1st ed.; Prentice Hall: Hoboken, NJ, USA, 1998. [Google Scholar]
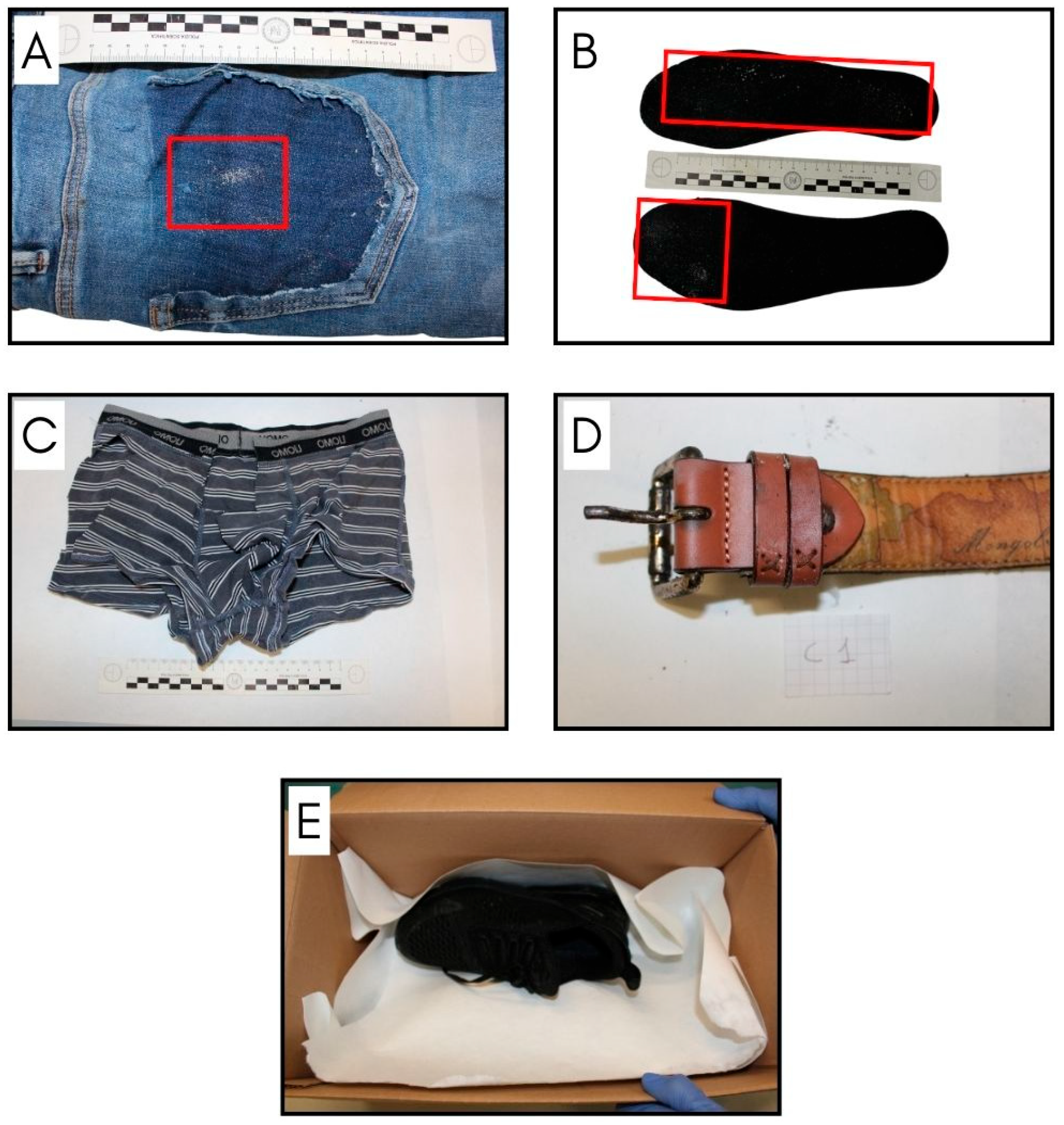
| Sample | Material | Description |
|---|---|---|
| S1 | Briefs | Fabric taken from the left rear area, close to the crotch, where a light-colored trace is visible. |
| S2 | Briefs | Elastic fabric taken from the left rear area. |
| P1 | Trousers | External front side: oxidized metal rivet of the right pocket. |
| P2 | Trousers | External front side: oxidized metal rivet of the left pocket. |
| P3 | Trousers | External front side: fabric around the left pocket. |
| P4 | Trousers | External front side: middle-right leg fabric. |
| P5 | Trousers | External back side: right back pocket fabric. |
| P6 | Trousers | External back side: left back pocket fabric. |
| P7 | Trousers | Internal front side: right leg fabric below the pocket. |
| C1 | Belt | Belt loop close to the buckle. |
| M1 | T-shirt | Fabric from the back of the crew neck. |
| SC1 | Shoe | Inner insole of the right shoe. |
| SC2 | Shoe | Inner insole of the left shoe. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, M.d.S.T.A.; Di Maggio, R.M.; Germinario, C.; Grifa, C.; Izzo, F.; Langella, A.; Mercurio, M. Discovering Halite Traces on a Victim’s Clothing through a Forensic Geoscience Analytical Approach: A Suspicious Case in Italy. Forensic Sci. 2024, 4, 396-408. https://doi.org/10.3390/forensicsci4030024
Lourenço MdSTA, Di Maggio RM, Germinario C, Grifa C, Izzo F, Langella A, Mercurio M. Discovering Halite Traces on a Victim’s Clothing through a Forensic Geoscience Analytical Approach: A Suspicious Case in Italy. Forensic Sciences. 2024; 4(3):396-408. https://doi.org/10.3390/forensicsci4030024
Chicago/Turabian StyleLourenço, Marcelo da Silveira Tortolero Araujo, Rosa Maria Di Maggio, Chiara Germinario, Celestino Grifa, Francesco Izzo, Alessio Langella, and Mariano Mercurio. 2024. "Discovering Halite Traces on a Victim’s Clothing through a Forensic Geoscience Analytical Approach: A Suspicious Case in Italy" Forensic Sciences 4, no. 3: 396-408. https://doi.org/10.3390/forensicsci4030024









