Abstract
Toxoplasmosis and chlamydiosis remain among the primary causes of abortion and fetal loss in small ruminants. Consequently, they are a source of considerable economic losses for farmers. The objectives of this study were to determine the seroprevalence and highlight the risk factors associated with Toxoplasma gondii and Chlamydophila abortus infection in small ruminants in Cameroon. A cross-sectional study was conducted in 200 small ruminant farms during a period from April to October 2021. A total of 1061 small ruminants were sampled, and the sera obtained were analyzed using the indirect ruminant multi-species ELISA test for specific IgG antibody detection for T. gondii and C. abortus at the National Veterinary Laboratory. A questionnaire was constructed to collect information about flock management and risk factors possibly associated with T. gondii and C. abortus infection in goats and sheep. Overall, 329 small ruminants tested positive for T. gondii-specific IgG antibodies and 45 for C. abortus-specific IgG antibodies with a seroprevalence of 31.1% (95% CI: 28.2–33.8) for T. gondii and 4.2% (95% CI: 3.0–5.5) for C. abortus, respectively. However, a significant co-infection of 1.8% (95% CI: 0.37–3.3) was obtained between T. gondii and C. abortus (p = 0.02). Linear regression model analysis revealed that, the seroprevalence of T. gondii at the flock level was significantly correlated with the region (0.22 CI: 0.17; 0.26) the breeding objectives (0.36 CI: 0.17; 0.56) the level of hygiene (0.11 CI: 0.01; 0.21) and presence abortions (0.55 CI: 0.46; 0.64) on the farms and that of C. abortus was significantly correlated with the regions (0.01 CI: 0.00–0.02) and degree of abortion on the farms (−0.26 CI: −039; −0.14). At the individual level, a significant correlation was observed between the seroprevalence of T. gondii and region (−0.09 CI: −0.14; −0.04), species (0.07 CI: 0.01–0.14), sex (−0.11 CI: −0.18; −0.03), age (0.08 CI: 0.04–0.12) and physiological status (0.21 CI: 0.18; 0.24). For C. abortus seroprevalence, a significant correlation was observed with the regions (0.02 CI: 0.00; 0.03) and breed (0.01 CI: 0.01; 0.02) of small ruminants reared in the northern regions of Cameroon. These results could be used to implement efficient management measures to prevent and control T. gondii and C. abortus infection in goats and sheep in Cameroon.
1. Introduction
Small ruminant production plays an important role in Cameroon’s pastoral economy, since it accounts for 20% of the protein needs of Cameroonians [1]. Despite numerous government efforts, this production remains insufficient to meet the demand of the national population [2,3] because it faces many constraints such as socio-cultural constraints, dietary factors, poorly performing genetic material and the pathological factor [4,5,6,7]. The latter is responsible for 30% of livestock losses in Cameroon [5]. Among these multiple pathologies, abortive pathologies are a source of significant numerical and economic losses on farms [8]. Among the infectious causes of abortion in small ruminants, toxoplasmosis and chlamydiosis appear to be the most important causes of abortion [9,10,11].
Several studies have investigated the seroprevalence of T. gondii and C. abortus in sheep and goats [12,13,14,15,16,17,18,19,20,21,22]. These studies point to the multiplicity and complexity of abortifacient infections, either at the individual or herd level. The clinical manifestations and epidemiological characteristics of abortions are not specific. At best, these elements can point to a suspicion, hence the need for laboratory analysis. Analytical diagnosis uses direct methods with a search for the causal agent or indirect methods (search for antibodies) [15,23].
In Cameroon, seroprevalence surveys of T. gondii infection have only been conducted among pregnant women [24,25]. A seroprevalence study of C. abortus infection was carried out in cattle in 1985 [26]. However, to date, no study has reported the seroprevalence of these two infections in small ruminants in Cameroon. Determining the seroprevalence of T. gondii and C. abortus and the risk factors associated with infection could contribute to the control of these diseases in animals and indirectly in humans in Cameroon.
2. Results
2.1. Seroprevalence of T. gondii and C. abortus at Flock and Individual (Animal) Level
A total of 200 small ruminant flocks in the northern regions of Cameroon were included in the study. Overall, 74 flocks tested positive for T. gondii and 6 flocks tested positive for C. abortus, with a flock-level prevalence of 37% (95% CI: 30.3–43.7) and 3% (95% CI: 0.64–5.4), respectively (Table 1). At the individual level, 1061 small ruminants over one month of age were analyzed. In total, 329 animals tested positive for T. gondii and 45 for C. abortus, with an individual prevalence of 31.01% (95% CI: 28.2–33.8) for T. gondii and 4.24% (95% CI: 3.0–5.5) for C. abortus, respectively. However, a co-infection of 1.82% (95% CI: 0.37–3.3) was obtained between T. gondii and C. abortus, with a significant difference (p = 0.02) in the univariate analysis (Table 2).

Table 1.
Seroprevalence of T. gondii and C. abortus infections on small ruminant farms in the northern regions of Cameroon. (Adamawa, North and Far North).

Table 2.
Univariate analysis of co-infection between T. gondii and C. abortus in small ruminants in the northern regions of Cameroon (Adamawa, North and Far North).
2.2. Risk Factor Analysis
The flock and individual (animal) risk factors associated with seropositivity in sheep and goats in a univariate analysis at p < 0.05 are presented in Tables S1 and S2. The seroprevalence of T. gondii at the flock level was significantly correlated with the region, (p = 0.03), the breeding objectives (p = 0.0001) and the level of hygiene (p = 0.04) on the farms, and that of C. abortus was significantly correlated with the regions (p = 0.0001), the breeding objectives (p = 0.00) and the degree of abortion on the farms (p = 0.0003). A significant correlation was observed between the seroprevalence of T. gondii at the individual level and region (p = 0.0001), species (p = 0.0001), sex (p = 0.0002), age (p = 0.0002), physiological status (p = 0.04) and breed (p = 0.01). For C. abortus seroprevalence, a significant correlation was observed with the regions (p = 0.04) and breed (p = 0.0001) of small ruminants reared in the northern regions of Cameroon.
Table 3 showed flock and animal level risk factors associated with T. gondii and C. abortus seropositivity in a Linear regression model analysis at p < 0.05 and p < 0.01. At the flock level, the T. gondii and C. abortus seropositivity was significantly correlated with the region (0.22 CI: 0.17; 0.26, p = 0.00), the breeding objectives (0.36 CI: 0.17; 0.56, p = 0.00), the level of hygiene (0.11 CI: 0.01; 0.21, p = 0.03) and the presence of abortions (0.55 CI: 0.46; 0.64, p = 0.00) on the farms, and that of C. abortus was significantly correlated with the regions (0.01 CI: 0.00–0.02, p = 0.02) and the presence of abortions on the farms (−0.26 CI:,−039; −0.14, p = 0.0001). At the individual level, a significant correlation was observed between the seroprevalence of T. gondii and region (−0.09 CI: −0.14; −0.04, p = 0.00), species (0.07 CI: 0.01–0.14, p = 0.03), sex (−0.11 CI: −0.18; −0.03, p = 0.005), age (0.08 CI: 0.04–0.12, p = 0.0001) and physiological status (0.21 CI: 0.18; 0.24, p = 0.00). For C. abortus seroprevalence, a significant correlation was observed with the regions (0.02 CI: 0.00; 0.03, p = 0.04) and breed (0.01 CI: 0.01; 0.02, p = 0.001) of small ruminants reared in the northern regions of Cameroon.

Table 3.
Model analysis of flock and animal risk factors association with C. abortus and T. gondii seropositivity among sheep and goats sampled from flocks in the northern regions of Cameroon (Adamawa, North and Far North).
3. Discussion
Small ruminant breeding appears to be an opportunity for income generation in the context of poverty alleviation in some African countries [27,28,29,30,31]. This is the case in Cameroon, where small ruminants (sheep and goats) play a very important role in the pastoral economy, accounting for 20% of the protein needs of Cameroonians [1]. This production is mainly concentrated in the northern regions of Cameroon [32]. In this study, T. gondii has a wide distribution in these areas, unlike C. abortus, and is very harmful to both human and animal health. This study provides substantial epidemiological data on the seroprevalence of T. gondii and C. abortus in small ruminant livestock in Cameroon.
A co-infection of 1.82% (95% CI: 0.37–3.27) was obtained between T. gondii and C. abortus in the present study. The presence of C. abortus in small ruminant farms would significantly (p = 0.02) increase the risk of T. gondii infestation. This co-infection was reported in the 2013 study by Romano and Coppens, where it was shown that Toxoplasma and Chlamydiophila tend to conform to their respective intracellular developmental programs, regardless of the presence of the other organism in the cell. The normal growth of each pathogen is highly dependent on the ability of the pathogen to maintain a threshold level of interaction between its vacuole and host cell organelles [33]. However, an infection with C. abortus could create a depressed state in the animals, thus allowing a T. gondii infestation.
A logistic regression model analysis of this study showed that, at the flock level, the T. gondii and C. abortus seropositivity was significantly correlated with the region (p = 0.00), the breeding objectives (p = 0.00), the level of hygiene (p = 0.03) and the presence of abortions (p = 0.00) on the farms, and that of C. abortus was significantly correlated with the regions (p = 0.02) and presence of abortions on the farms (p = 0.0001). At the individual level, a significant correlation was observed between the seroprevalence of T. gondii and region (p = 0.00), species (p = 0.03), sex (p = 0.005), age (p = 0.0001) and physiological status (p = 0.00). For C. abortus seroprevalence, a significant correlation was observed with the regions (p = 0.04) and breed (p = 0.001) of small ruminants reared in the northern regions of Cameroon. The Adamawa region (46.04% (95% CI: 39.2–52.9) had significantly (p = 0.0001) high T. gondii seroprevalence. This region has a savannah climate with a dry winter according to the Köppen–Geiger classification, unlike the other regions in the study (North and Far North). Rainfall in this region is much higher in summer than in winter. Over the year, the average temperature in Adamawa is 21.8 °C and the average rainfall is 951.9 mm [34]. This type of climate favors the development and expansion of this pest [12,21]. The sheep species was significantly more infected (35.93% (95% CI: 31.9–40, p = 0.0001)) with T. gondii than the goats in these areas. This result was similar to those obtained in Nigeria [35], in Southern Spain [36] and in Tunisia [14]. Sheep are generally associated with large cattle for grazing, unlike goats, which favor greater contact with this infectious agent [37]. Tests revealed that females (33.94% (95% CI: 30.6–37.3)) were significantly more infested with T. gondii than males, as observed in the Lahmar study in Tunisia [14]; this is probably due to greater sensitivity to protozoan pathogen in females than in males [38].
Small ruminants aged 3-5 years were significantly more infested with T. gondii than young animals (41.90% (95% CI: 36.3–47.6)). This observation was also highlighted by Qin et al. (2015) and was associated with continuous and increasing exposure to infectious oocytes in the environment [12]. The Oudah breed in the sheep species was significantly more infected with T. gondii (38.38%) than the other breeds, and the Sahel goat in the goat species was more infected with C. abortus (33.33%). These breeds were used in sedentary, transhumant or nomadic farming systems, which favor their permanent contact with these pathogens [39]. The occurrence of abortions on small ruminant farms in our study was strongly associated with C. abortus infection, as mentioned in the report of the observatory and monitoring of the causes of abortions in ruminants report, 2019 [40]. This could be justified by the fact that C. abortus is the leading cause of abortion in small ruminants (the second being Campylobacter), although its seroprevalence was low in this study [41].
4. Materials and Methods
4.1. Study Area
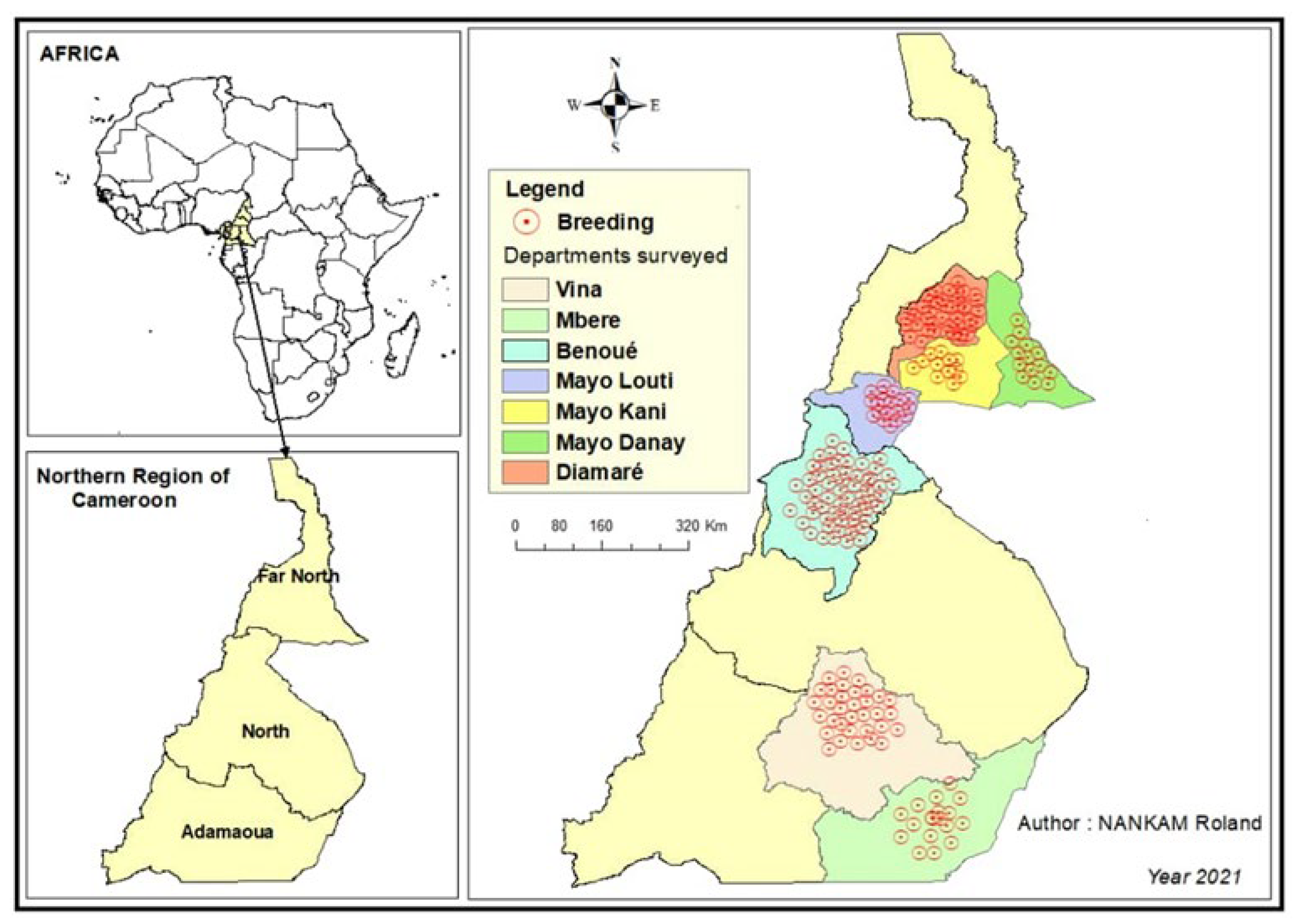
The study was carried out in the northern regions of Cameroon, namely the Adamawa, North and Far North regions (Figure 1). These regions are well known for their involvement in ruminant production in Cameroon, as more than 75% of the national small ruminant population is located in these regions. The production system of small ruminants in these areas was mainly extensive, and they were mainly raised for meat production. This part of Cameroon represents a prime area for improving small ruminant production in Cameroon. The Adamawa region has an area of 64,000 km2, which makes it the 3rd largest region in Cameroon. The land is poor and sparsely populated. The main economic activity is cattle breeding. It has 5 departments (Djérem, Faro and Déo, Mayo Banyo, Mbéré, Vina) and stretches from 6°30′0″ Latitude North to 13°30′0″ Longitude East. The high altitude of the region results in a relatively cool climate of between 22 and 25 degrees. In the southern part of the region, the climate is equatorial and Guinean, with four seasons. The North Cameroon region is made up of four departments: Benue, Mayo-Louti, Faro and Mayo-Rey. It extends between 8° and 10° North latitude and 12° and 16° East longitude, with an area of 6,557,600 ha and a population density of 26 inhabitants/km². The climate is hot and semi-arid, with a rainy season from mid-May to September, while the rest of the year is characterized by heat and drought. The Far North region includes 6 departments (Diamaré, Logone and Chari, Mayo Danay, Mayo Kani, Mayo Sava and Mayo Tsanaga). It covers an area of 34,263 km² and borders Chad and Nigeria. Its relief is dominated by steppe and grassy savannah, interspersed with massifs with strange and tormented shapes. Far North has a desert climate according to the classification of Köppen–Geiger. Over the year, the average temperature in Far North is 28.7 °C and the average rainfall is 726.2 mm.
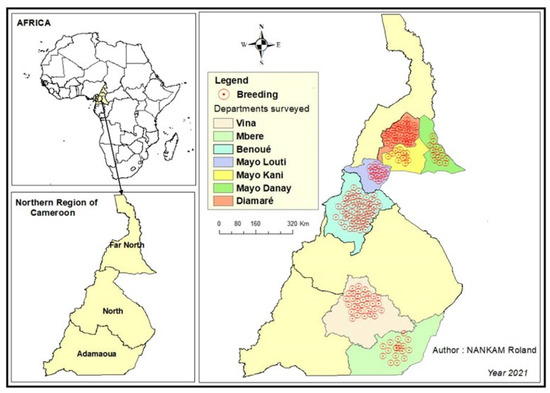
Figure 1.
Map showing the study regions (Adamawa, North, Far North) in Cameroon.
4.2. Study Design, Sample and Data Collection
A cross-sectional study was conducted in 200 small ruminant farms during a period from April to October 2021. A total of 1061 small ruminants were obtained according to the formula proposed by Musallam et al. (2015) [27], with 50% expected seroprevalence at the flock level and at the individual level for C. abortus and T. gondii. An absolute precision of 5% was applied with a 95% confidence interval (CI). The sheep and goat flocks were not selected strictly at random, but a certain representativeness of the national herd was sought by diversifying the types of flock (sedentary or transhumant) and the geographical areas. On this occasion, a questionnaire was filled in with the aim of bringing out information on the herd (the number of adult females, the recent introduction of animals (including partial returns from transhumance), the number of abortions and stillbirths during the year and the pathology of the young from birth to one month). This questionnaire also allowed us to trace the reproductive career of the female in chronology, with emphasis on any abortive episodes and their clinical consequences. During the survey, the investigator(s), through technical observations of the farms, characterized the hygiene level of the farms studied on a scale of 1 to 4 (4—Very clean, 3—Clean, 2—Dirty, 1—Very dirty) [42]. Observations were based on the average internal score (measures to reduce the within-flock spread of pathogens such as housing cleaning and disinfection, removal of manure and abortion animals). For each feature rated, a score (1–4) was attributed to farmers based on the level of measures applied with respect to the sub-categories. The overall score was the average of the hygiene level scores. A blood sample was then taken during data collection. The blood collected in dry tubes was left to rest for 30 min. The fresh serum obtained was collected with a micropipette and divided into aliquots in identified cryotubes, which were then transported to the laboratory in a cooler containing carbohydrate ice and stored in a freezer at −20 °C pending analysis. The analysis was performed one (1) week after the end of the collection.
4.3. Serological Examination
Serum samples were assayed with the indirect multi-species ELISA for T. gondii (ID.vet, Grabels, France), which detects IgG antibodies to T. gondii and C. abortus (Indirect multi-species ELISA kit for the detection of anti-MOMP antibodies). Briefly, serum samples and controls were diluted 1:10 and tested. The microplate ELISA reader measured the optical densities (OD) at 450 nm. Interpretation of OD values was based on the ID.vet kit instructions. The ODs obtained were used to calculate the Substrate/Product percentage (S/P%) via the following equations: S/P (%) = (OD sample/DOpc) × 100 and S/P (%) = (OD sample − DONC/DOPC − DONC) × 100, respectively, for C. abortus and T. gondii. For C. abortus, any sample with an S/P% > 60% was considered seropositive; if the S/P% was between ≥50 and ≤60, the result was considered doubtful, while any sample with a S/P% < 50% was classified as seronegative. Furthermore, for the interpretation of the ODs obtained for T. gondii, samples with a S/P% less than or equal to 40% were considered negative. If the S/P% was between 40% and 50%, the result was considered doubtful and considered positive if the S/P% was greater than or equal to 50%.
4.4. Statistical Analysis
The data were analyzed using the R Foundation for Statistical Computing package: R i386 4.1.2 software (Copyright 2021). The prevalence and 95% confidence intervals per pathogen species (T. gondii and C. abortus) were calculated using this software. The chi-squared test and logistic regression analysis were used to compare the proportions of detected sample positivity indifferent regions and among different animals to identify risk factors for T. gondii and C. abortus. The differences were considered to be statistically significant when the resulting p-values were lower than 0.05 and 0.01.
5. Conclusions
This study reports on the seroprevalence of T. gondii and C. abortus on small ruminant farms in Cameroon. These two zoonotic pathogens are not well known to the populations of these regions, and therefore represent a potential danger to farmers, their families, health professionals and consumers of animal protein in these regions. Although some farmers are aware of the risks associated with these diseases, there is a need for targeted community health education programs to minimize the transmission of zoonotic pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/parasitologia2030017/s1, Table S1: Univariate analyses of the association between risk factors and flock-level seropositivity of T. gondii and C. abortus infections on small ruminant farms in the northern regions of Cameroon (Adamawa, North and Far North), Table S2: Univariate analysis of the association between risk factors and individual seropositivity to T. gondii and C. abortus infections on small ruminant farms in the northern regions of Cameroon (Adamawa, North and Far North).
Author Contributions
J.K. and F.N. designed, structured and coordinated the study. R.C.N. coordinated the fieldwork but also collected data with G.J.T.T., and S.V.G., R.C.N. and M.F.D. extracted and analyzed the data. R.C.N. and A.P.K.K. prepared the first draft of the manuscript. J.K., F.N. and R.N.G.N. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Universite des Montagnes (N° UdM-BUR-CPR-2021/006) for studies involving animals. The regional delegations in charge of animal health allowed the survey in the three northern regions of Cameroon Adamawa (N° 0020/21/RA/DREPIA/SRSV), North (N° 0025/21/RN/DREPIA/SRSV) and Far North (N° 0017/21/MINEPIA/SG/DREN). Small ruminant farmers were informed of the purpose of the study and the approximate duration of the interview, and their informed consent was sought prior to their participation in the survey.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the agronomic and veterinary department of the Université des Montagnes, Mohamed Moctar Mouiche Mouliom and Félicité F. Djuikwo Teukeng for their technical and logistical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Njoya, A. Strengthening the Resilience and Adaptive Capacity of Livestock Farmers to Climate Variability and Extreme Events in the Sahel and Savannah Regions of West and Central Africa. 2010, p. 96. Available online: https://www.apess.org/wp-content/uploads/2019/05/Rapport-final-1.pdf (accessed on 17 April 2022).
- MINEPIA. Support for the Improvement of the Control of Transboundary Diseases of Livestock in Trade. 2011, pp. 1–32. Available online: https://www.standardsfacility.org/sites/default/files/STDF_PG_336_Application_May-12.pdf (accessed on 17 April 2022).
- MINEPIA. National Plan for the Control and Eradication of Peste des Petits Ruminants. 2018, p. 57. Available online: https://www.prodel.cm/wp-content/uploads/2019/04/Plan-national-de-contrôle-et-déradication-de-la-PPR-au-Cameroun-Phase-2018-2023.pdf (accessed on 17 April 2022).
- FAO. Support for the Improvement of Transboundary Livestock Disease Control in Cameroon. 2018, pp. 1–46. Available online: http://www.standardsfacility.org/sites/default/files/STDF_PG_336_Evaluation_Report_Final_FR.pdf (accessed on 17 April 2022).
- MINEPIA. Environmental and Social Management Framework (CGES) of the Livestock Development Project (PRODEL) in the Working Group for the Preparation of the Livestock Development Project (PRODEL). 2018, p. 124. Available online: https://documents1.worldbank.org/curated/en/291221472106313475/pdf/SFG2401-EA-FRENCH-P154908-Box396304B–PUBLIC-Disclosed-8-24-2016.pdf (accessed on 17 April 2022).
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 564. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis of Animals and Humans, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 336. [Google Scholar] [CrossRef]
- Menzies, P. Control of Important Causes of Infectious Abortion in Sheep and Goats. Vet. Clin. Food Anim. Pract. 2011, 27, 81–93. [Google Scholar] [CrossRef]
- Cremoux, R.; Pouget, C.; Lacz, C. Differential diagnosis of abortions in small ruminants in Midi-Pyrénées. Bull. GTV 2017, 1, 73–82. [Google Scholar]
- Elandalousi, R.B.; Ghram, A.; Maaroufi, A.; Mnif, W. Seroprevalence of zoonotic abortifacient diseases in ruminants in northern Tunisia. Research 2015, 2, 1419. [Google Scholar] [CrossRef]
- Khammassi-Khabou, M.; Hammami, S.; Cherif, A.; Majok, A. Seroprevalence of Major Infectious Diseases Causing Abortion in Small Ruminants. 2009, pp. 5–29. Available online: https://www.researchgate.net/publication/279978031_Seroprevalence_des_majeures_maladies_infectieuses_causant_l’avortement_chez_les_petits_ruminants (accessed on 31 July 2020).
- Qin, S.Y.; Huang, S.Y.; Yin, M.Y.; Tan, Q.D.; Liu, G.X.; Zhou, D.H.; Zhu, X.Q.; Zhou, J.Z.; Qian, A.D. Seroprevalence and risk factors of Chlamydophila abortus infection in free-ranging white yaks in China. BMC Vet. Res. 2015, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, H.L.; Zahida, T. Seroprevalence of Toxoplasmosis in Sheep in Southern Punjab, Pakistan. Pak. Vet. J. 2010, 30, 91–94. [Google Scholar]
- Lahmar, I.; Lachkhem, A.; Slama, D.; Sakly, W.; Haouas, N.; Gorcii, M.; Pfaff, A.W.; Candolfi, E.; Babba, H. Prevalence of Toxoplasmosis in Sheep, Goats and Cattle in Southern Tunisia. J. Bacteriol. Parasitol. 2015, 6, 10–14. [Google Scholar] [CrossRef]
- Galván-Ramírez, M.D.L.L.; Charles-Niño, C.; Pedroza-Roldán, C.; Salazar-Reveles, C.; Ocampo-Figueroa, K.L.; Rodríguez-Pérez, L.R.; Paez-Magallán, V.M. Prevalence of Toxoplasma gondii Measured by Western Blot, ELISA and DNA Analysis, by PCR, in Cats of Western Mexico. Pathogens 2022, 11, 109. [Google Scholar] [CrossRef]
- Li, G.; Zheng, W.; Yang, J.; Qi, T.; He, Y.; Chen, W.; Ma, H.; Sun, Y.; Li, Y.; Kang, M.; et al. Seroprevalence and epidemiology of toxoplasma gondii in animals in the qinghai-tibetan plateau area, china. Pathogens 2021, 10, 432. [Google Scholar] [CrossRef]
- Sidibe, S.; Coulibaly, K.; Sery, A.; Fofana, M.; Sidibe, S.; Kanoute, M. Prevalence of brucellosis, chlamydia and toxoplasmosis in small ruminants in Mali: Results of a sero-epidemiological survey. Rev. Mali. D’infect. Microbiol. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Malal, M.E.; Karagül, M.S.; Akar, K. Serological investigation of ovine chlamydiosis in small ruminants in Western Turkey. Acta Vet. Brno 2020, 89, 255–261. [Google Scholar] [CrossRef]
- Al-Qudah, K.M.; Sharif, L.A.; Raouf, R.Y.; Hailat, N.Q.; Al-Domy, F.M. Seroprevalence of antibodies to Chlamydophila abortus shown in Awassi sheep and local goats in Jordan. Vet. Med. 2004, 49, 460–466. [Google Scholar] [CrossRef]
- Bamba, S.; Faye, B.; Tarnagda, Z.; Boly, N.; Guiguemdé, T.; Villena, I. Séroprévalence de la toxoplasmose chez les ovins à Bobo-Dioulasso, Burkina Faso. Rev. D’élevage Méd. Vét. Pays Trop. 2012, 65, 63. [Google Scholar] [CrossRef][Green Version]
- Fayez, M.; Elmoslemany, A.; Alorabi, M.; Alkafafy, M.; Qasim, I.; Al-Marri, T.; Elsohaby, I. Seroprevalence and risk factors associated with Chlamydophila abortus infection in sheep and goats in eastern Saudi Arabia. Pathogens 2021, 10, 489. [Google Scholar] [CrossRef]
- Li, M.-H.; Yang, B.-T.; Yin, Z.-W.; Wang, W.; Zhao, Q.; Jiang, J. A Seroepidemiological Survey of Toxoplasma gondii and Chlamydia Infection in Chickens, Ducks, and Geese in Jilin Province, Northeastern China. Vector-Borne Zoonotic Dis. 2020, 20, 825–830. [Google Scholar] [CrossRef]
- Sachse, K.; Hotzel, H.; Slickers, P.; Ellinger, T.; Ehricht, R. DNA microarray-based detection and identification of Chlamydia and Chlamydophila spp. Mol. Cell. Probes 2005, 19, 41–50. [Google Scholar] [CrossRef]
- Todjom, F.G.; Tsapi, E.M.; Gamago, G.; Vignoles, P.; Pone, J.W.; Teukeng, F.D. Seroprevalence of Toxoplasmosis and associated risk factors in pregnant women at the Protestant Hospital, Mbouo-Bandjoun, Cameroon. Afr. J. Clin. Exp. Microbiol. 2019, 20, 221. [Google Scholar] [CrossRef]
- Njunda, A.L.; Assob, J.C.N.; Nsagha, D.S.; Kamga, H.L.; Nde, P.F.; Yugah, V.C. Seroprevalence of toxoplasma gondii infection among pregnant women in Cameroon. J. Public Health Afr. 2011, 2, 98–101. [Google Scholar] [CrossRef]
- Domenech, J.; Trap, D.; Gaumont, R. Cattle in Central Africa: A survey of chlamydia and Q fever. Rev. Elev. Méd. Vét. Pays Trop. 1985, 38, 138–143. [Google Scholar]
- Musallam, I.; Abo-Shehada, M.; Omar, M.; Guitian, J. Cross-sectional study of brucellosis in Jordan: Prevalence, risk factors and spatial distribution in small ruminants and cattle. Prev. Vet. Med. 2015, 118, 387–396. [Google Scholar] [CrossRef]
- Laanen, M.; Beek, J.; Ribbens, S.; Vangroenweghe, F.; Maes, D.; Dewulf, J. Biosecurity on pig herds: Development of an on-line scoring system and the results of the first 99 participating herds. Vlaams Diergeneeskd. Tijdschr. 2010, 79, 302–306. [Google Scholar]
- Duteurtre, G.; Faye, B.; Dutilly-diane, C.; Alary, V. Elevage et dynamique de la pauvreté: L’approche micro-économique. Mémento L’agronome CIRAD GRET Fr Montp. CIRAD 2002, 5, 1–10. [Google Scholar]
- Orskov, E.R. Goat production on a global basis. Small Rumin. Res. 2011, 98, 9–11. [Google Scholar] [CrossRef]
- Iñiguez, L. The challenges of research and development of small ruminant production in dry areas. Small Rumin. Res. 2011, 98, 12–20. [Google Scholar] [CrossRef]
- Dedieu, B.; Aubin, J.; Duteurtre, G.; Alexandre, G.; Vayssieres, J.; Faye, B.; Bommel, P.; Mahieu, A.; Fanchone, A.; Tourrand, J.-F.; et al. Design and Evaluation of Sustainable Livestock Systems in Hot Regions. 2011. Available online: https://agritrop.cirad.fr/560828/1/document_560828.pdf (accessed on 24 March 2020).
- INS. Chapitre 14: Breeding and Fishing. In Annuary Statistics of the Cameroun; National Statistical Institute: Yaounde, Cameroun, 2020; pp. 209–219. Available online: https://ins-cameroun.cm/wp-content/uploads/2021/02/0CHAPITRE-14_PECHE-ET-ELEVAGE.pdf (accessed on 24 February 2022).
- García-Bocanegra, I.; Cabezón, O.; Hernández, E.; Martinez-Cruz, M.S.; Martinez-Moreno, A.; Martinez-Moreno, J. Toxoplasma gondii in ruminant species (cattle, sheep, and goats) from southern Spain. J. Parasitol. 2013, 99, 438–440. [Google Scholar] [CrossRef]
- Kamani, J.; Mani, A.U.; Egwu, G.O. Seroprevalence of Toxoplasma gondii infection in domestic sheep and goats in Borno state, Nigeria. Trop. Anim. Health Prod. 2010, 42, 793–797. [Google Scholar] [CrossRef]
- Sah, R.P.; Talukder, H.; Rahman, A.A.; Alam, M.Z.; Ward, M.P. Seroprevalence of Toxoplasma gondii infection in ruminants in selected districts in Bangladesh. Vet. Parasitol. Reg. Stud. Rep. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Romano, J.D.; Coppens, I. Host Organelle Hijackers: A similar modus operandi for Toxoplasma gondii and Chlamydia trachomatis: Co-infection model as a tool to investigate pathogenesis. Pathog. Dis. 2013, 69, 72–86. [Google Scholar] [CrossRef]
- Données-Mondiale. Climate: Adamawa, Cameroon. World Data. Available online: https://www.worlddata.info/africa/cameroon/index.php (accessed on 24 February 2022).
- Alexander, J.; Stimson, W.H. Sex hormones and the course of parasitic infection. Parasitol. Today 1988, 4, 189–193. [Google Scholar] [CrossRef]
- Meyer, C.; Faye, B.; Karembe, H. Mediterranean and Tropical Sheep Breeding Guide; CIRAD-EMVT: Montpellier, France, 2004; pp. 92–116. [Google Scholar]
- SCAR. Observatory and Monitoring of the Causes of Abortions in Ruminants Report 2019. 2020, p. 80. Available online: https://www.plateforme-esa.fr/sites/default/files/RésultatsOSCAR2019_VF.pdf (accessed on 24 February 2022).
- Guillaume, B.; Maud, G. Form of Resistance in the External Environment. Biología 2017, 11, 3. Available online: https://m.20-bal.com/biolog/16568/index.html (accessed on 24 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).