Pyrethroid-Resistant and Susceptible Triatoma infestans (Klug, 1834) (Hemiptera, Triatominae): Analysis of Their Vectorial Characteristics by Metacyclogenesis, Feeding/Defecation Patterns, and Parasite Load
Abstract
:1. Introduction
2. Results
2.1. Analysis of Feeding and Defecation Behavior
2.2. Metacyclogenesis
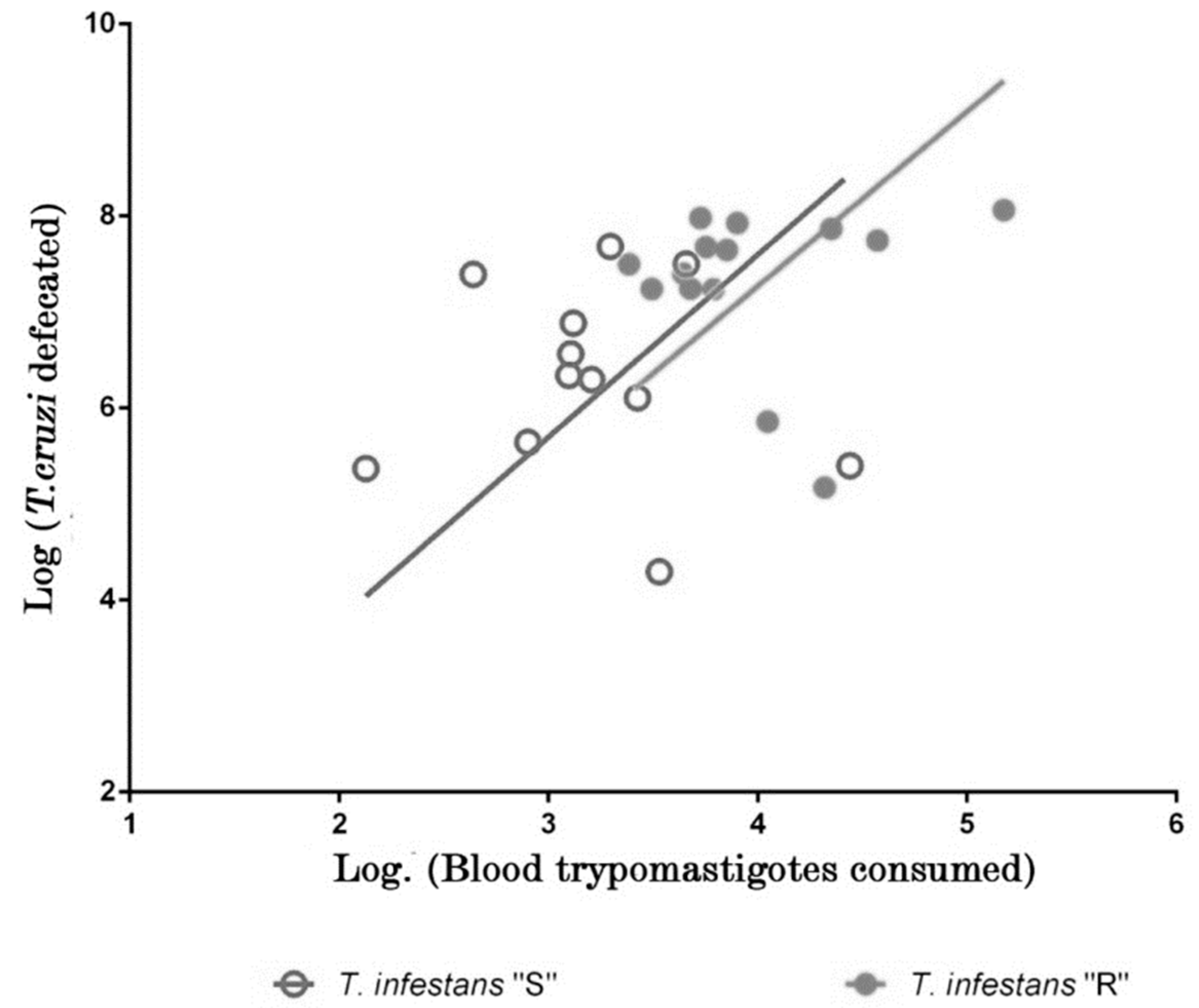
2.3. Molecular Analysis
3. Discussion
4. Materials and Methods
4.1. Triatomine Nymphs
4.2. Parasites
4.3. Mice
4.4. Study Design
4.5. Feeding and Defecation Patterns
4.6. Metacyclogenesis and Parasite Load in Feces of Nymphs
4.7. Quantification of Parasite Load by PCR
4.8. Calibration Curves
4.9. qPCR
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherlock, I. Vetores. In Trypanosoma cruzi e Doença de Chagas; Brener, Z., Andrade, Z., Barral Neto, M., Eds.; Guanabara Kooga: Rio de Janeiro, Brazil, 2000; Volume 21. [Google Scholar]
- Garcia, E.S.; Azambuja, P. Development and interactions of Trypanosoma cruzi within the insect vector. Parasitol. Today 1991, 7, 240–244. [Google Scholar] [CrossRef]
- Garcia, E.S.; Genta, F.A.; de Azambuja, P.; Schaub, G.A. Interactions between intestinal compounds of triatomines and Trypanosoma cruzi. Trends Parasitol. 2010, 26, 499–505. [Google Scholar] [CrossRef]
- Kollien, A.H.; Schaub, G.A. The development of Trypanosoma cruzi in triatominae. Parasitol. Today 2000, 16, 381–387. [Google Scholar] [CrossRef]
- Garcia, E.S.; Ratcliffe, N.A.; Whitten, M.M.; Gonzalez, M.S.; Azambuja, P. Exploring the role of insect host factors in the dynamics of Trypanosoma cruzi–Rhodnius prolixus interactions. J. Insect Physiol. 2007, 53, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; RatcliffeII, N.A.; Garcia, E.S. Towards an understanding of the interactions of Trypanosoma cruzi and Trypanosoma rangeli with in the reduviid insect host Rhodnius prolixus. An. Acad. Bras. Cienc. 2005, 77, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, C.S.; Ávila, A.R.; de Souza, W.; Motta, M.C.M.; Cavalcanti, D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasites Vectors 2018, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Schuster, J.P.; Schaub, G.A. Trypanosoma cruzi: Skin penetration kinetics of vector-derived metacyclic trypomastigotes. Int. J. Parasitol. 2000, 30, 1475–1479. [Google Scholar] [CrossRef]
- Dujardin, J.P.; Schofield, C.J.; Panzera, F. Los Vectores de la Enfermedad de Chagas; Academie Royale des Sciences d’Outre-Mer: Brussels, Belgium, 2002; Volume 25, pp. 17–23. [Google Scholar]
- Zeledón, R.; Alvarado, R.; Jiron, L.F. Observations on the feeding and defecation patterns of three triatomin especies (Hemiptera: Reduviidae). Actatropica 1977, 34, 65–77. [Google Scholar]
- Higgs, S.; Beaty, B.J. Natural cycles of vector-borne pathogens. In Biology of Disease Vectors, 2nd ed.; Marquardt, W.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 167–185. [Google Scholar]
- Vieira, C.B.; Praça, Y.R.; Bentes, K.; Santiago, P.B.; Silva, S.; Silva, G.; Motta, F.N.; Bastos, I.; de Santana, J.M.; de Araújo, C.N. Triatominos: Tripanosomátidos, bacterias y vectores potenciales de virus? Front. Microbiol. Cel. Infecc. 2018, 8, 405. [Google Scholar] [CrossRef]
- Quintero Gil, D.C.; Osorio Benitez, J.E.; Martinez Gutierrez, M. Competencia vectorial: Consideraciones entomológicas y su influencia sobre la epidemiología del dengue. Iatreia 2010, 23, 137–145. [Google Scholar]
- Zerba, E.N. Past and Present of Chagas Vector Control and Future Needs. 1999. Available online: https://www.who.int/publications/i/item/who-cds-whopes-cgdpp-99.1 (accessed on 12 August 2022).
- Lardeux, F.; Depickère, S.; Duchon, S.; Chavez, T. Insecticide resistance of Triatoma infestans (Hemiptera, Reduviidae) vector of Chagas disease in Bolivia. Tropical medicine & international health: TM IH 2010, 15, 1037–1048. [Google Scholar]
- Picollo, M.I.; Vassena, C.; Santo Orihuela, P.; Barrios, S.; Zaidemberg, M.; Zerba, E. High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera: Reduviidae) from Northern Argentina. J. Med. Entomol. 2005, 42, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Depickere, S.; Buitrago, R.; Sinani, E.; Baune, M.; Monje, M.; Lopez, R.; Waleckx, E.; Chavez, T.; Breniere, S.F. Susceptibility and resistance to deltamethrin of wild and domestic populations of Triatoma infestans (Reduviidae: Triatominae) in Bolivia: New discoveries. Mem. Inst. Oswaldo Cruz 2012, 107, 1042–1047. [Google Scholar] [CrossRef]
- Casimiro, G. Efecto de la Resistencia a Insecticidas Piretroides sobre Algunos Parámetros del Desarrollo de Triatoma infestans; Facultad de Ciencias Naturales, Universidad Nacional de Salta: Salta, Argentina, 2012. [Google Scholar]
- Panzera, F.; Ferreiro, M.J.; Pita, S.; Calleros, L.; Perez, R.; Basmadjian, Y.; Guevara, Y.; Breniere, S.F.; Panzera, Y. Evolutionary and dispersal history of Triatoma infestans, main vector of Chagas disease, by chromosomal markers. Infect. Genet. Evol. 2014, 27, 105–113. [Google Scholar] [CrossRef]
- Pedrini, N.; Mijailovsky, S.J.; Girotti, J.R.; Stariolo, R.; Cardozo, R.M.; Gentile, A.; Juarez, M.P. Control of pyrethroid-resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl. Trop. Dis. 2009, 3, e434. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.E.; Gürtler, R.E. Modeling household transmission of American trypanosomiasis. Science 2001, 293, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.C.; Silveira, A.C.; Schofield, C.J. The impact of Chagas disease control in Latin America: A review. Mem. Inst. Oswaldo Cruz 2002, 97, 603–612. [Google Scholar] [CrossRef] [Green Version]
- Lardeux, F.; Aliaga, C.; Depickère, S. Bias due to methods of parasite detection when estimating prevalence of infection of Triatoma infestans by Trypanosoma cruzi. J. Vector Ecol. 2016, 41, 285–291. [Google Scholar] [CrossRef]
- Moser, D.R.; Kirchhoff, L.V.; Donelson, J.E. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J. Clin. Microbiol. 1989, 27, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, V.L.; da Silva, S.C.; Sartori, A.M.; Bezerra, R.C.; Westphalen, E.V.; Molina, T.D.; Teixeira, A.R.; Ibrahim, K.Y.; Shikanai-Yasuda, M.A. Real-time PCR in HIV/Trypanosoma cruzi coinfection with and without Chagas disease reactivation: Association with HIV viral load and CD4 level. PLoS Negl. Trop. Dis. 2011, 5, e1277. [Google Scholar] [CrossRef] [Green Version]
- Moreira, O.C.; Ramírez, J.D.; Velázquez, E.; Melo, M.F.; Lima-Ferreira, C.; Guhl, F.; Sosa-Estani, S.; Marin-Neto, J.A.; Morillo, C.A.; Britto, C. Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: A substudy from the BENEFIT trial. Acta Trop. 2013, 125, 23–31. [Google Scholar] [CrossRef]
- Crocco, L.B.; Catalá, S.S. Feeding and defaecation patterns in Triatoma sordida. Mem. Inst. Oswaldo Cruz 1996, 91, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Regis, L. The role of the blood meal in egg-laying periodicity and fecundity in Triatoma infestans. Int. J. Invertebr. Reprod. 1979, 1, 187–195. [Google Scholar] [CrossRef]
- Lobbia, P.; Calcagno, J.; Mougabure-Cueto, G. Excretion/defecation patterns in Triatoma infestans populations that are, respectively, susceptible and resistant to deltamethrin. Med. Vet. Entomol. 2018, 32, 311–322. [Google Scholar] [CrossRef]
- Germano, M.D.; Inés Picollo, M. Reproductive and developmental costs of deltamethrin resistance in the Chagas disease vector Triatoma infestans. Journal of vector ecology: J. Soc. Vector Ecol. 2015, 40, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, M.C.; Tublitz, N.J.; O’Donnell, M.J. Anti-diuresis in the blood-feeding insect Rhodnius prolixus Stål: The peptide CAP2b and cyclic GMP inhibit Malpighian tubule fluid secretion. J. Exp. Biol. 1997, 200, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, R.M.; Panzera, F.; Gentile, A.G.; Segura, M.A.; Pérez, R.; Díaz, R.A.; Basombrío, M.A. Inheritance of resistance to pyrethroids in Triatoma infestans, the main Chagas disease vector in South America. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2010, 10, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.C.; Rosa, D.S.; Soares, M.J. Differentiation of Trypanosoma cruzi epimastigotes: Metacyclogenesis and adhesion to substrate are triggered by nutritional stress. J. Parasitol. 2000, 86, 1213–1218. [Google Scholar] [CrossRef]
- Avendaño, A.; Calderón-Arguedas, O.; Valerio, I.; Chinchilla, M. Heterogenity in the in vitro metacyclogenesis of clones derive from a Costa Rica strain of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae). Parasitol. Latinoam. 2006, 61, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Vanrell, M.C.; Losinno, A.D.; Cueto, J.A.; Balcazar, D.; Fraccaroli, L.V.; Carrillo, C.; Romano, P.S. The regulation of autophagy differentially affects Trypanosoma cruzi metacyclogenesis. PLoS Negl. Trop. Dis. 2017, 11, e0006049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, F.; Guerra, B.; Vieira, L.R.; Perdomo, H.D.; Gandara, A.C.; Amaral, R.J.; Vollú, R.E.; Gomes, S.A.; Lara, F.A.; Sorgine, M.H.; et al. Monitoring of the Parasite Load in the Digestive Tract of Rhodnius prolixus by Combined qPCR Analysis and Imaging Techniques Provides New Insights into the Trypanosome Life Cycle. PLoS Negl. Trop. Dis. 2015, 9, e0004186. [Google Scholar] [CrossRef] [Green Version]
- Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005, 21, 568–572. [Google Scholar] [CrossRef]
- Zingales, B.; Andrade, S.G.; Briones, M.R.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. Second Satellite Meeting. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef]
- Loza-Murguía, M.; Noireau, F. Vectorial capacity of Triatoma guasayana (Wygodzinsky & Abalos) (Hemiptera: Reduviidae) compared with two other species of epidemic importance. Neotrop. Entomol. 2010, 39, 799–809. [Google Scholar]
- Duffy, T.; Bisio, M.; Altcheh, J.; Burgos, J.M.; Diez, M.; Levin, M.J.; Favaloro, R.R.; Freilij, H.; Schijman, A.G. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in chagas disease patients. PLoS Negl. Trop. Dis. 2009, 3, e419. [Google Scholar] [CrossRef]
- Elias, M.C.; Vargas, N.S.; Zingales, B.; Schenkman, S. Organization of satellite DNA in the genome of Trypanosoma cruzi. Mol. Biochem. Parasitol. 2003, 129, 1–9. [Google Scholar] [CrossRef]
- Davies, C.; Poma, R.H.; Cardozo, R.M.; Mora, M.C.; Ramos, F.; Rajal, V.B.; Basombrío, M.A. Detección de Trypanosoma cruzi en tejido y sangre murina por PCR convencional y en tiempo real. Acta Bioquím. Clín. Latinoam. 2014, 48, 421–428. [Google Scholar]
- Enriquez, G.F.; Bua, J.; Orozco, M.M.; Wirth, S.; Schijman, A.G.; Gürtler, R.E.; Cardinal, M.V. High levels of Trypanosoma cruzi DNA determined by qPCR and infectiousness to Triatoma infestans support dogs and cats are major sources of parasites for domestic transmission. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2014, 25, 36–43. [Google Scholar] [CrossRef]
- Gonzalez, A.; Prediger, E.; Huecas, M.E.; Nogueira, N.; Lizardi, P.M. Minichromosomal repetitive DNA in Trypanosoma cruzi: Its use in a high-sensitivity parasite detection assay. Proc. Natl. Acad. Sci. USA 1984, 81, 3356–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, J.G. Regression through the origin. Teach. Stat. 2003, 25, 76–80. [Google Scholar] [CrossRef]
- Rabinovich, J.E.; Leal, J.A.; Feliciangeli de Piñero, D. Domiciliary biting frequency and blood ingestion of the Chagas’s disease vector Rhodnius prolixus Ståhl (Hemiptera: Reduviidae), in Venezuela. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 272–283. [Google Scholar] [CrossRef]
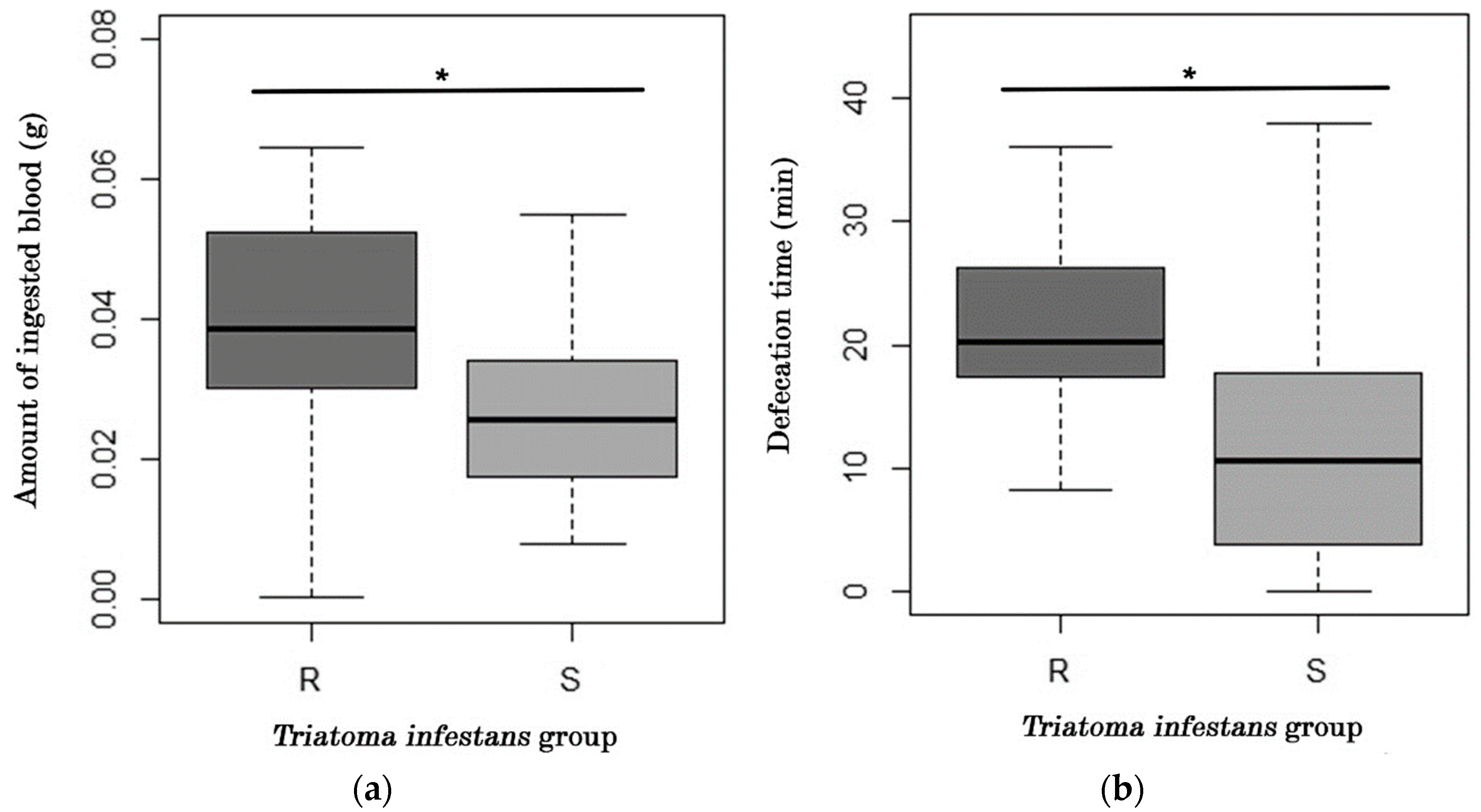
| Parameter | “R” | “S” | p (α = 0.05) |
|---|---|---|---|
| Initial weight (g) | 0.010 ± 0.001 | 0.0084 ± 0.001 | 0.007 |
| Final weight (g) | 0.050 ± 0.004 | 0.031 ± 0.003 | 0.0014 |
| Feeding onset (min) | 3.51 ± 1.02 | 3.24 ± 0.772 | 0.771 |
| Feeding time (min) | 12.6 ± 2.17 | 12.6 ± 0.994 | 0.34 |
| Parameters | “R” | “S” | p (α = 0.05) |
|---|---|---|---|
| % TM | 32.80 ± 5.8 | 72.5 ± 6.77 | 0.001 |
| E (μm) | 31.8 ± 1.40 | 25 ± 2.17 | 0.017 |
| MT (μm) | 27.6 ± 1.17 | 27.1 ± 3.06 | 0.693 |
| “R” | “S” | |
|---|---|---|
| Parasite consumption (parasite-equivalents/mL of blood) | 2.1 × 104 ± 1.0 × 104 | 3.9 × 103 ± 2.2 × 103 |
| Parasite elimination (parasite-equivalents/mL of feces) | 3.8 × 107 ± 9.7 × 106 | 2.1 × 106 ± 4.6 × 106 |
| Slope | 6.6 ± 0.12 | 7.9 ± 0.14 |
| p (α = 0.05) | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guanuco, A.P.; Davies, C.; Poma, H.R.; Gentile, A.G.; Cardozo, R.M. Pyrethroid-Resistant and Susceptible Triatoma infestans (Klug, 1834) (Hemiptera, Triatominae): Analysis of Their Vectorial Characteristics by Metacyclogenesis, Feeding/Defecation Patterns, and Parasite Load. Parasitologia 2022, 2, 255-265. https://doi.org/10.3390/parasitologia2040022
Guanuco AP, Davies C, Poma HR, Gentile AG, Cardozo RM. Pyrethroid-Resistant and Susceptible Triatoma infestans (Klug, 1834) (Hemiptera, Triatominae): Analysis of Their Vectorial Characteristics by Metacyclogenesis, Feeding/Defecation Patterns, and Parasite Load. Parasitologia. 2022; 2(4):255-265. https://doi.org/10.3390/parasitologia2040022
Chicago/Turabian StyleGuanuco, Andrea Paola, Carolina Davies, Hugo Ramiro Poma, Alberto Gerónimo Gentile, and Rubén Marino Cardozo. 2022. "Pyrethroid-Resistant and Susceptible Triatoma infestans (Klug, 1834) (Hemiptera, Triatominae): Analysis of Their Vectorial Characteristics by Metacyclogenesis, Feeding/Defecation Patterns, and Parasite Load" Parasitologia 2, no. 4: 255-265. https://doi.org/10.3390/parasitologia2040022
APA StyleGuanuco, A. P., Davies, C., Poma, H. R., Gentile, A. G., & Cardozo, R. M. (2022). Pyrethroid-Resistant and Susceptible Triatoma infestans (Klug, 1834) (Hemiptera, Triatominae): Analysis of Their Vectorial Characteristics by Metacyclogenesis, Feeding/Defecation Patterns, and Parasite Load. Parasitologia, 2(4), 255-265. https://doi.org/10.3390/parasitologia2040022







