Metazoan Marine Parasites of Costa Rica: A Review
Abstract
:1. Introduction
2. Results
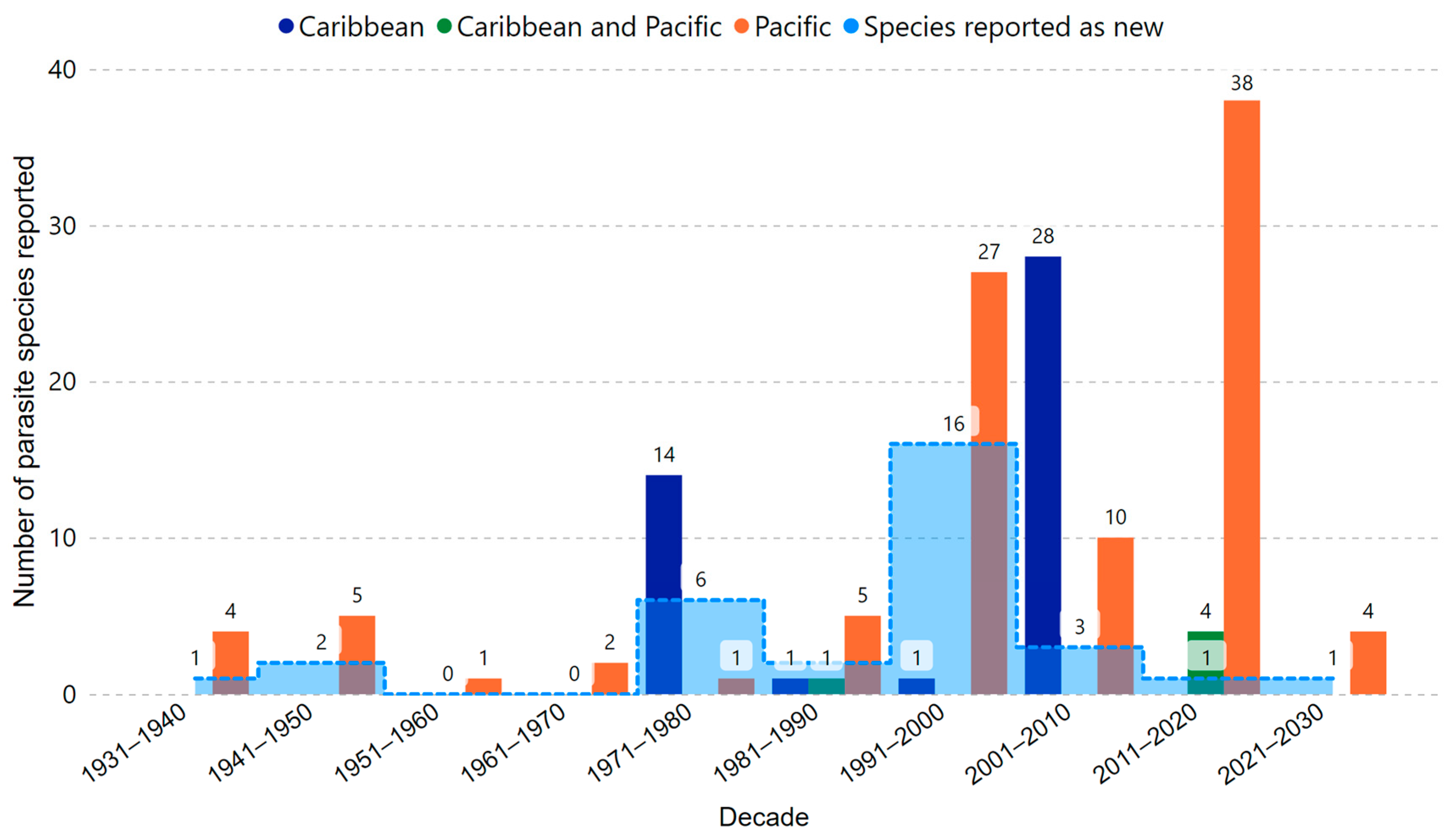
2.1. Brief History of Marine Parasites Research in Costa Rica
2.2. Costa Rican Marine Parasite Diversity
2.3. Platyhelminthes
2.3.1. Trematodes
2.3.2. Trematdoes from Sea Turtles
2.3.3. Monogeneans
2.3.4. Cestodes
2.4. Nematodes
2.5. Acanthocephalans
2.6. Hirudinean Annelids
2.7. Parasitic Crustaceans
2.7.1. Parasitic Malacostracan Crustaceans
2.7.2. Copepodes
2.7.3. Cirripedians
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parasite Species | Class/Order | Host Species | Reporting Reference | Location |
|---|---|---|---|---|
| Acanthobothrium nicoyaensea | Cestoda: Tetraphyllidea | Aetobatus narinari | [22] | Punta Morales, Pacific Coast |
| Acanthobothrium costarricensea | Cestoda: Tetraphyllidea | Dasyatis longus | [23] | Punta Morales, Pacific Coast |
| Acanthobothrium cimari a | Cestoda: Tetraphyllidea | Dasyatis longus | [23] | Punta Morales, Pacific Coast |
| Acanthobothrium puntarenasense a | Cestoda: Tetraphyllidea | Dasyatis longus | [23] | Punta Morales, Pacific Coast |
| Acanthobothrium vargasi a | Cestoda: Tetraphyllidea | Dasyatis longus | [23] | Punta Morales, Pacific Coast |
| Acanthobothrium campbelli a | Cestoda: Tetraphyllidea | Urotrygon chilensis | [23] | Punta Morales, Pacific Coast |
| Acanthobothrium franus a | Cestoda: Tetraphyllidea | Narcine entemedor | [25] | Cuajiniquil, Gulf of Santa Elena, Guanacaste |
| Acanthobothrium inbiorium a | Cestoda: Tetraphyllidea | Narcine entemedor | [25] | Cuajiniquil, Gulf of Santa Elena, Guanacaste |
| Acanthobothroides pacificus a | Cestoda: Tetraphyllidea | Himantura pacifica | [24] | Playa Panamá, Guanacaste |
| Scalithrium geminum a | Cestoda: Tetraphyllidea | Himantura pacifica | [24] | Playa Panamá, Guanacaste |
| Cylindrophorus hypoprioni | Cestoda: Tetraphyllidea | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Triloculatum triloculatum | Cestoda: Tetraphyllidea | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Escherbothrium molinae a,b | Cestoda: Tetraphyllidea | Urotrygon chilensis | [57] | Punta Morales, Puntarenas |
| Anthobothrium pristis | Cestoda: Tetraphyllidea | Pristis perotteti | [19] | Barra del Colorado, Caribbean Coast |
| Anthobothrium laciniatum | Cestoda: Tetraphyllidea | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Pterobothrioides carvajali a,b | Cestoda: Trypanorhyncha | Dasyatis longus | [26] | Cuajiniquil, Gulf of Santa Elena, Guanacaste |
| Dasyrhynchus varioucinnatus | Cestoda: Trypanorhyncha | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Callitetrarhynchus gracilis | Cestoda: Trypanorhyncha | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Heteronybelinia estigmena | Cestoda: Trypanorhyncha | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Otobothrium penetrans | Cestoda: Trypanorhyncha | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Cathetocephalus thatcheri | Cestoda: Cathetocephalidea | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Tetrabothrius forsteri | Cestoda: Tetrabothriidea | Stenella coeruleoalba Stenella attenuata | [49] | Playa hermosa, Guanacaste |
| Strobilocephalus triangularis | Cestoda: Tetrabothriidea | Stenella coeruleoalba | [49] | undefined location |
| Trigonocotyle sp. | Cestoda: Tetrabothriidea | Stenella longirostris | [49] | Tamarindo, Guanacaste |
| Phyllobothrium delphini | Cestoda: Phyllobothridea | Stenella coeruleoalba | [49] | undefined location |
| Phyllobothrium pristis a | Cestoda: Phyllobothridea | Pristis perotteti | [19] | Barra del Colorado, Caribbean Coast |
| Paraorygmatobothrium nicaraguensis a | Cestoda: Phyllobothridea | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Monorygma grimaldii | Cestoda: Phyllobothridea | Stenella coeruleoalba Stenella attenuata Tursiops truncatus | [49] | undefined location |
| Scyphophyllidium leuci a | Cestoda: Phyllobothridea | Charcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Hargicola oligoplites | Monogenea: Mazocraeidea | Oligoplites altus | [50] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Mazocraes sp. | Monogenea: Mazocraeidea | Anchovia macrolepidota | [50] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Neohexostoma euthynni | Monogenea: Mazocraeidea | Euthynnus lineatus | [50] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Ahpua piscicola | Monogenea: Mazocraeidea | Caranx Caballus, Oligoplites altus | [32] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Pseudomazocraes monsivaisae | Monogenea: Mazocraeidea | Selene peruviana | [32] | Puntarenas |
| Cemocotylella elongata a | Monogenea: Mazocraeidea | Caranx caballus | [12] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Hargicotyle louisianensis | Monogenea: Mazocraeidea | Menticirrhus sp. | [32] | Puntarenas |
| Heterobothrium ecuadori | Monogenea: Mazocraeidea | Sphoeroides sp. | [18] | Puntarenas |
| Polymicrocotyle manteri | Monogenea: Mazocraeidea | Lutjanus colorado | [32] | Puntarenas |
| Jaliscia caballeroi | Monogenea: Mazocraeidea | Lepidochelys olivacea | [46,47] | undefined location |
| Dermophthirius maccallumi a | Monogenea: Mazocraeidea | Carcharhinus leucas | [19] | Barra del Colorado, Caribbean |
| Neomicrocotyle pacifica | Monogenea: Mazocraeidea | Caranx sexfasciatus | [14] | Bahía Culebra, Guanacaste |
| Heteronchocotyle leucas a | Monogenea: Diclybothriidea | Carcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Erpocotyle carcharhini a | Monogenea: Diclybothriidea | Carcharhinus leucas | [19] | Barra del Colorado, Caribbean Coast |
| Alloinfundiburictus costaricae a | Trematoda: Plagiorchiida | Haemulopsis axillaris | [13] | Bahía Culebra, Guanacaste |
| Bucephalus gorgon a | Trematoda: Plagiorchiida | Caranx hippos and Seriola | [13] | Bahía Culebra, Guanacaste |
| Prosorhynchoides sp. | Trematoda: Plagiorchiida | Caranx caballus | [46,47] | undefined location |
| Manteria brachyderus | Trematoda: Plagiorchiida | Caranx caballus/Oligoplites altus | [21] | undefined location |
| Brachycladium palliatum | Trematoda: Plagiorchiida | Stenella coeruleoalba | [49] | undefined location |
| Brachycladium pacificum | Trematoda: Plagiorchiida | Stenella longirostris | [49] | undefined location |
| Nasitrema globicephalae | Trematoda: Plagiorchiida | Stenella attenuata | [49] | undefined location |
| Oschmarinella albamarina | Trematoda: Plagiorchiida | Ziphius cavirostris | [49] | undefined location |
| Homalometron lesliorum a | Trematoda: Plagiorchiida | Eucinostomus currani | [28] | Cuajiniquil, Gulf of Santa Elena, Guanacaste |
| Theletrum lamothei a | Trematoda: Plagiorchiida | Echidna nocturna | [27] | Cuajiniquil, Gulf of Santa Elena, Guanacaste |
| Ectenurus virgula | Trematoda: Plagiorchiida | Fistularia commersonii | [27] | Ocotal, Guanacaste |
| Lecithochirium microstomum | Trematoda: Plagiorchiida | Fistularia commersonii | [27] | Ocotal, Guanacaste |
| Lecithochirium monticelli | Trematoda: Plagiorchiida | Syhodus sp. | [18] | Ocotal, Guanacaste |
| Mecoderus oligoplitis | Trematoda: Plagiorchiida | Oligoplites altus, Oligoplites refulgens, Oligoplites sp. | [21] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Ekuarhuni papillatus a,b | Trematoda: Plagiorchiida | Mugil curema | [52] | Punta Dominical, Puntarenas |
| Forticulcita isabelae a | Trematoda: Plagiorchiida | Mugil curema | [52] | Punta Dominical, Puntarenas |
| Trifoliovarium sp. | Trematoda: Plagiorchiida | Echidna nocturna | [27] | Cuajiniquil, Gulf of Santa Elena, Guanacaste |
| Bianium plicitum | Trematoda: Plagiorchiida | Arothron hispidus, Sphoeroides sp. | [16] | Bahía prieta, Guanacaste and Mata de Limón, Puntarenas |
| Hypocreadium myohelicatum | Trematoda: Plagiorchiida | Epinephelus itajara | [27] | Cuajiniquil, Gulf of Santa Elena, Guanacaste |
| Stephanostomum casum | Trematoda: Plagiorchiida | Caranx caballus | [27] | Playa Ocotal, Guanacaste, Pacific Coast |
| Tergestia laticollis | Trematoda: Plagiorchiida | Caranx caballus | [27] | Playa Ocotal, Guanacaste, Pacific Coast |
| Plesiochorus cymbiformis | Trematoda: Plagiorchiida | Lepidochelys olivacea | [38] | Ostional National Wildlife Refuge, Nicoya Peninsula, North Pacific Coast |
| Enodiotrema megachondrus | Trematoda: Plagiorchiida | Lepidochelys olivacea | [38] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Enodiotrema reductum | Trematoda: Plagiorchiida | Eretmochelys imbricata | [41] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Pachypsolus irroratus | Trematoda: Plagiorchiida | Lepidochelys olivacea | [38] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Microscaphidium reticulare | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Microscaphidium warui | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Deuterobaris intestinalis | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Polyangium linguatula | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Octangium hyphalum | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Schizamphistomum scleroporum | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Schizamphistomum erratum | Trematoda: Plagiorchiida | Chelonya mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Helicometra fasciata | Trematoda: Plagiorchiida | Muraena sp. | [13] | Bahía Culebra, Guanacaste |
| Cricocephalus resectus | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Cricocephalus megastomus | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Cricocephalus albus | Trematoda: Plagiorchiida | Chelonia mydas, Eretmochelys imbricata | [33,41] | Tortuguero National Park, North Caribbean Coast, Gulf of Nicoya |
| Desmogonius desmogonius | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Pronocephalus obliquus | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Charaxicephaloides polyorchis | Trematoda: Plagiorchiida | Chelonia mydas | [36] | Tortuguero National Park, North Caribbean Coast |
| Charaxicephalus robustus | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Pleurogonius longiusculus | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Pleurogonius sindhii | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Pleurogonius sp. | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Pleurogonius tortugueroi a | Trematoda: Plagiorchiida | Chelonia mydas | [34] | Tortuguero National Park, North Caribbean Coast |
| Pleurogonius linearis c | Trematoda: Plagiorchiida | Chelonia mydas, Eretmochelys imbricata | [33] | Tortuguero National Park, North Caribbean Coast, Gulf of Nicoya |
| Pleurogonius solidus | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Pleurogonius lobatus | Trematoda: Plagiorchiida | Chelonia mydas, Eretmochelys imbricata | [33,41] | Tortuguero National Park, North Caribbean Coast, Gulf of Nicoya |
| Pleurogonius trigonocephalus c | Trematoda: Plagiorchiida | Eretmochelys imbricata | [41] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Rameshwarotrema uterocrescens | Trematoda: Plagiorchiida | Chelonia mydas | [35] | Tortuguero National Park, North Caribbean Coast |
| Adenogaster serialis c | Trematoda: Plagiorchiida | Eretmochelys imbricata | [41] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Pyelosomum crassum c | Trematoda: Plagiorchiida | Eretmochelys imbricata | [41] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Pyelosomum cochlear | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Pyelosomum posterorchis c | Trematoda: Plagiorchiida | Eretmochelys imbricata | [41] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Rhytidodoides similis | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Rhytidodoides intestinalis | Trematoda: Plagiorchiida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Clinostomum complanatum | Trematoda: Diplostomida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Learedius learedi | Trematoda: Diplostomida | Chelonia mydas | [33,37] | Tortuguero National Park, North Caribbean Coast |
| Hapalotrema postorchis | Trematoda: Diplostomida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Monticellius indicus | Trematoda: Diplostomida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Amphiorchis solus | Trematoda: Diplostomida | Chelonia mydas | [33] | Tortuguero National Park, North Caribbean Coast |
| Carettacola stunkardi c | Trematoda: Diplostomida | Eretmochelys imbricata | [41] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Neospirorchis sp. | Trematoda: Diplostomida | Chelonia mydas | [37] | Tortuguero National Park, North Caribbean Coast |
| Halocercus lagenorhynchi | Nematoda: Strongylida | Stenella coeruleoalba | [49] | Playa Panamá, Guanacaste |
| Halocercus sp. | Nematoda: Strongylida | Stenella attenuata Stenella longirostris | [49] | undefined location |
| Echinocephalus janzeni a | Nematoda: Rabditida | Himantura pacifica | [59] | undefined location |
| Crassicauda anthonyi | Nematoda: Rhabditida | Ziphius cavirostris | [49] | Matapalo, Puntarenas, Pacific Coast |
| Anisakis spp. | Nematoda: Rhabditida | Stenella coeruleoalba Stenella attenuata Stenella longirostris Ziphius cavirostris | [49] | undefined location |
| Koronacantha pectinaria | Palaeoacanthocephala: Echinorhynchida | Microlepidotus brevipinnis | [60] | Bahía Culebra, Guanacaste |
| Floridosentis pacifica | Eoacanthocephala: Neoechinorhynchida | Mugil curema | [51] | Punta Dominical, Puntarenas and Gulf of Nicoya |
| Neoechinorhynchus mamesi | Eoacanthocephala: Neoechinorhynchida | Dormitator maculatus, Dormitator latifrons | [62] | Playa Grande, Guanacaste |
| Ozobranchus branchiatus | Hirudinea: Rhynchobdellida | Chelonia mydas, Lepidochelys olivacea | [37,39,40] | Tortuguero National Park, North Caribbean Coast, Ostional wildlife refuge, Nicoya penninsula, north Pacific coast |
| Ozobranchus margoi | Hirudinea: Rhynchobdellida | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Cymothoa exigua | Crustacea: Isopoda | Lutjanus colorado, L. jordani and L. peru | [44] | undefined location |
| Probopyrus pandalicola | Crustacea: Isopoda | Palaemon hiltoni | [29] | Gulf of Nicoya, Puntarenas, North Pacific Coast |
| Aporobopyrus bourdonis a | Crustacea: Isopoda | Petrolisthes edwardsii | [45] | Junquillal, Area of conservation, Guanacaste |
| Aporobopyrus aduliticus | Crustacea: Isopoda | Petrolisthes sp. | [31] | Punta Dominical, Puntarenas and Gulf of Nicoya |
| Aporobopyrus trilobatus | Crustacea: Isopoda | Petrolisthes ortmanni | [45] | Punta Dominical, Puntarenas and Gulf of Nicoya |
| Podocerus chelonophilus | Crustacea: Amphipoda | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Planes sp. | Crustacea: Decapoda | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Anelasma squalicola c | Crustacea-Cirripedia: Pollicipedomorpha | Etmopterus benchleyi | [53] | Off Pacific Coast |
| Paralepas quadrata | Crustacea-Cirripedia: Scalpellomorpha | Panulirus penicillatus | [30] | Chatam Bay, Isla del Coco |
| Conchoderma auritum | Crustacea: Cirripedia:Scalpellomorpha | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Conchoderma virgatum | Crustacea: Cirripedia:Scalpellomorpha | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Lepas hilli | Crustacea: Cirripedia:Scalpellomorpha | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Ptychascus barnwelli a | Crustacea: Cirripedia:Kentrogonidae | Minuca burgersi, Minuca mordax & Minuca vocator | [67] | Limón, Moin. |
| Stephanolepas muricata | Crustacea: Cirripedia:Balanomorpha | Eretmochelys imbricata, Chelonya mydas | [63] | Golfo Dulce, Pacific Coast and Gulf of Nicoya |
| Chelonibia testudinaria | Crustacea: Cirripedia:Balanomorpha | Lepidochelys olivacea | [39,40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Platylepas decorata | Crustacea: Cirripedia:Balanomorpha | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Platylepas hexastylos | Crustacea: Cirripedia:Balanomorpha | Lepidochelys olivacea | [40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Stomatolepas elegans | Crustacea: Cirripedia:Balanomorpha | Lepidochelys olivacea | [39,40] | Ostional National Wildlife Refuge, Nicoya Peninsula |
| Xenobalanus globicipitis | Crustacea: Cirripedia:Balanomorpha | Stenella attenuata | [49] | Bajamar Puntarenas |
| Pandarus satyrus | Crustacea: Copepda: Siphonostomatoida | Prionace glauca | [43] | undefined location |
| Pennella instructa | Crustacea: Copepda: Siphonostomatoida | Conchoderma virgatum | [15] | Chatam Bay, Isla del Coco |
| Gloiopotes ornatus | Crustacea: Copepda: Siphonostomatoida | sailfish (SNI) | [15] | Chatam Bay, Isla del Coco |
| Caligus mutabilis | Crustacea: Copepda: Siphonostomatoida | Scomberomorus brasiliensis | [20] | undefined location |
| Caligus omissus a | Crustacea: Copepda: Siphonostomatoida | Scomberomorus sierra | [20] | undefined location |
| Caligus chorinemi | Crustacea: Copepda: Siphonostomatoida | Seriola lalandi | [20] | Bahía Culebra, Guanacaste |
| Lepeophtheirus alvaroi a | Crustacea: Copepda: Siphonostomatoida | Unknown | [65] | Bahía Wafer, Isla del Coco |
| Unicolax collateralis a | Crustacea: Copepda: Siphonostomatoida | Euthynnus lineatus | [20] | undefined location |
| Pseudocycnus appendiculatus | Crustacea: Copepda: Siphonostomatoida | Thunnus albacares | [20] | undefined location |
| Cybicola buccatus | Crustacea: Copepda: Siphonostomatoida | Scomberomorus sierra, Scomberomorus brasiliensis | [20] | undefined location |
| Lernanthropus micropterygis | Crustacea: Copepda: Siphonostomatoida | Seriola sp. | [11] | Bahía Culebra, Guanacaste |
| Balaenophilus manatorum | Crustacea: Copepoda:Harpacticoida | Lepidochelys olivacea | [39] | Ostional National Wildlife Refuge, Nicoya Peninsula |
References
- Rohde, K. Ecology of marine parasites. Helgol. Meeresunters 1984, 37, 5–33. [Google Scholar] [CrossRef]
- Carlson, T.A.; Dallas, T.A.; Alexander, L.W.; Phelan, A.L.; Phillips, A.J. What would it take to describe the global diversity of parasites? Proc. R. Soc. B 2020, 287, 20201841. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.; Lafferty, K.D.; Kuris, A.M.; Hechinger, R.F.; Jetz, W. Homage to Linnaeus: How many parasites? How many hosts? Proc. Natl. Acad. Sci. USA 2008, 105, 11482–11489. [Google Scholar] [CrossRef]
- Poulin, R. Parasite biodiversity revisited: Frontiers and constraints. Int. J. Parasitol. 2014, 44, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Morand, S. (macro-) Evolutionary ecology of parasite diversity: From determinants of parasite species richness to host diversification. Int. J. Parasitol. Parasites Wildl. 2015, 4, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Poulin, R. Meta-analysis of seasonal dynamics of parasite infections in aquatic ecosystems. Int. J. Parasitol. 2020, 50, 501–510. [Google Scholar] [CrossRef]
- Santoro, M.; Laccarino, D.; Bellisario, B. Host biological factors and geographic locality influence predictors of parasite communities in sympatric sparid fishes off the southern Italian coast. Sci. Rep. 2020, 10, 13283. [Google Scholar] [CrossRef]
- Hudson, P.J.; Dobson, A.P.; Lafferty, K.D. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 2006, 21, 381–385. [Google Scholar] [CrossRef]
- Cortés, J. Historia de la investigación marino-costera en Bahía Culebra, Pacífico Norte, Guanacaste, Costa Rica. Rev. Biol. Trop. 2012, 60, 19–37. [Google Scholar] [CrossRef]
- Wilson, C.B. Parasitic copepods taken during the Third Hancock Expedition to the Galapagos Islands. Allan Hancock Pac. Exped. 1937, 2, 24–30. [Google Scholar]
- Meserve, F. Some monogenetic trematodes from Galapagos Islands and the neighbouring Pacific. Rep. Hancock Pac. Exped. 1938, 2, 31–89. [Google Scholar]
- Manter, H.W. Digenetic trematodes of fishes from the Galapagos Islands and the neighboring Pacific. Allan Hancock Pac. Exped. 1940, 2, 329–496. [Google Scholar]
- Van Cleave, H.J. The Acanthocephala collected by the Allan Hancock Pacific Expedition, 1934. Rep. Collect. Obtained Allan Hancock Pac. Exped. 1932–1948 1940, 2, 501–527. [Google Scholar]
- Schmitt, W.L. Decapod and other Crustacea collected on the presidential cruise of 1938. Smithson. Misc. Collec. 1939, 98, 1–29. [Google Scholar] [CrossRef]
- Caballero, E.; Brenes, R.R.; Jiménez-Quirós, O. Helmintos de la República de Costa Rica IV. Algunos trematodos de animales domésticos y silvestres. Rev. Biol. Trop. 1957, 5, 135–155. [Google Scholar]
- Brenes, R.R. Catálogo de los helmintos parásitos de Costa Rica. Rev. Biol. Trop. 1961, 9, 67–95. [Google Scholar] [CrossRef]
- Bravo-Hollis, M.; Arroyo, G. Helmintos de peces costarricenses. An. Inst. Biol. Univ. Nal. Autón. Méx. Ser. Zool 1962, 33, 79–95. [Google Scholar]
- Watson., D.E.; Thorson, T.B. Helminths from elasmobranchs in Central American fresh waters. In Investigations of the Ichthyofauna of Nicaraguan Lakes; Thorson, T.B., Ed.; University of Nebraska-Lincoln: Lincoln, NE, USA, 1976; pp. 629–640. [Google Scholar]
- Cressey, R.F.; Cressey, H.B. Parasitic copepods of mackerel-and tuna-like fishes (Scombridae) of the world. Smithson. Contrib. Zool. 1980, 311, 1–186. [Google Scholar] [CrossRef]
- Ponciano, R.G. Estudio Taxonómico de Trematodos de Peces Marinos y Dulceacuícolas de México y América Central. Licenciatura; Facultad de Ciencias, Universidad Autónoma de México: México, Mexico, 1986. [Google Scholar]
- Brooks, D.R.; McCorquodale, S. Acanthobothrium nicoyaense n. sp. (Eucestoda: Tetraphyllidea: Onchobothriidae) in Aetobatus narinari (Euphrasen) (Chondrichthyes: Myliobatiformes: Myliobatidae) from the Gulf of Nicoya, Costa Rica. J. Parasitol. 1995, 81, 244–246. [Google Scholar] [CrossRef]
- Marques, F.; Brooks, D.R.; Monks, S. Five new species of Acanthobothrium van Beneden, 1849 (Eucestoda: Tetraphyllidea: Onchobothriidae) in stingrays from the gulf of Nicoya, Costa Rica. J. Parasitol. 1995, 81, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Brooks, D.R.; Ureña, H.M. Two new species of Tetraphyllidean cestodes in Himantura pacifica (Chondrichthyes: Myliobatiformes: Dasyatididae) from the northwest coast of Costa Rica. J. Parasitol. 1996, 82, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Centritto, R.; Stewart, S.A. Two new pecies of Acanthobothrium in Narcine entemedor (Rajiformes: Narcinidae) from the northwest coast of Guanacaste Peninsula, Costa Rica. J. Parasitol. 1997, 83, 927–931. [Google Scholar] [CrossRef]
- Campbell, R.A.; Beveridge, I. Pterobothrioides, a new genus of tapeworms (Cestoda: Trypanorhyncha: Pterobothriidae) from dasyatid stingrays in the Eastern Atlantic and Pacific Oceans. Syst. Parasitol. 1997, 38, 81–91. [Google Scholar] [CrossRef]
- Ponce de León, G.P.P.; León-Règagnon, V.; Scott, M. Theletrum lamothei sp. nov.(Digenea), parasite of Echidna nocturna from cuajiniquil, Guanacaste, and other digenes of marine fishes from Costa Rica. Rev. Biol. Trop. 1998, 46, 345–354. [Google Scholar]
- Parker, J.H.; Curran, S.S.; Overstreet, R.M.; Tkach, V.V. Examination of Homalometron elongatum Manter, 1947 and description of a new congener from Eucinostomus currani Zahuranec, 1980 in the Pacific ocean off Costa Rica. Comp. Parasitol. 2010, 77, 154–163. [Google Scholar] [CrossRef]
- Jiménez, P.; Vargas, M. Probopyrus pandalicola (Isopoda: Bopyridae) infesting Palaemonetes hiltonii (Crustacea: Caridea), along the Pacific coast of Costa Rica. Rev. Biol. Trop. 1990, 38, 457–462. [Google Scholar]
- Zullo, V.A. Zoogeography of the shallow-water cirriped fauna of the Galápagos Islands and adjacent regions in the tropical eastern Pacific. In Galápagos Marine Invertebrates; James, M.J., Ed.; Springer: Boston, MA, USA, 1991; pp. 173–192. [Google Scholar] [CrossRef]
- Markham, J.C. The Isopoda Bopyridae of the eastern Pacific–missing or just hiding. Proc. San Diego Soc. Natl. His. 1992, 17, 1–4. [Google Scholar]
- Brooks, D.R.; Lamothe-Argumedo, R.; Garcia-Prieto, L.; Osorio-Sarabia, D.; de León, G.P.P. Catalogo de la Coleccion Nacional de Helmintos. J. Parasitol. 1998, 84, 907. [Google Scholar] [CrossRef]
- Santoro, M.; Greiner, E.C.; Morales, J.A.; Rodríguez-Ortíz, B. Digenetic trematode community in nesting sea turtles (Chelonia mydas) from Tortuguero National Park, Costa Rica. J. Parasitol. 2006, 92, 1202–1206. [Google Scholar] [CrossRef]
- Santoro, M.; Greiner, E.C.; Morales, J.A.; Rodríguez-Ortíz, B. A new pronocephalid, Pleurogonius tortugueroi n. sp. (Digenea), from the intestine of green sea turtles (Chelonia mydas) in Costa Rica. Parassitologia 2007, 49, 97–100. Available online: https://pubmed.ncbi.nlm.nih.gov/18412051/ (accessed on 13 November 2022). [PubMed]
- Santoro, M.; Morales, J.A.; Stacy, B.; Greiner, E.C. Rameshwarotrema uterocrescens trematode parasitism of the oesophageal glands in green sea turtles (Chelonia mydas). Vet. Rec. 2007, 160, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Brandmayr, P.; Greiner, E.C.; Morales, J.A.; Rodríguez-Ortíz, B. Redescription of Charaxicephaloides polyorchis Groschaft and Tenora 1978 (Digenea: Pronocephalidae) from the green turtle Chelonia mydas in Costa Rica. Helminthologia 2009, 46, 97–99. [Google Scholar] [CrossRef]
- Santoro, M.; Morales, J.A.; Rodríguez-Ortíz, B. Spirorchiidiosis (Digenea: Spirorchiidae) and lesions associated with parasites in Caribbean green turtles (Chelonia mydas). Vet. Rec. 2007, 161, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Morales, J.A. Some digenetic trematodes of the olive ridley sea turtle, Lepidochelys olivacea (Testudines, Cheloniidae) in Costa Rica. Helminthologia 2007, 44, 25–28. [Google Scholar] [CrossRef]
- Robinson, N.J.; Lazo-Wasem, E.M.; Butler, B.O.; Lazo-Wasem, E.A.; Zardus, J.D.; Pinou, T. Spatial distribution of epibionts on olive ridley sea turtles at Playa Ostional, Costa Rica. PLoS ONE 2019, 14, e0218838. [Google Scholar] [CrossRef]
- Majewska, R.; Santoro, M.; Bolaños, F.; Chaves, G.; De Stefano, M. Diatoms and Other Epibionts Associated with Olive Ridley (Lepidochelys olivacea) Sea Turtles from the Pacific Coast of Costa Rica. PLoS ONE 2015, 10, e0130351. [Google Scholar] [CrossRef]
- Santoro, M.; Morales, J.A.; Bolaños, F.; Chaves, G.; De Stefano, M. Helminths of hawksbill turtle (Eretmochelys imbricata) from the Pacific coast of Costa Rica. Helminthologia 2015, 52, 67–70. [Google Scholar] [CrossRef]
- Santoro, M.; Mattiucci, S. Sea Turtle Parasites. In Marine Biodiversity of Costa Rica, Central America; Wehrtmann, I.S., Cortés, J., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2009; Volume 86, pp. 507–519. [Google Scholar] [CrossRef]
- Rojas, J.R.; Rodríguez Solano, O.; Morales-Ramírez, A. Size and distribution of Pandarus satyrus (Copepoda: Pandaridae) on the blue shark Prionace glauca (Carcharhiniformes: Carcharhinidae) in Costa Rica. Rev. Biol. Trop. 2001, 49, 199–201. [Google Scholar]
- Williams, E.H.; Bunkley-Williams, L. New records of fish-parasitic isopods (Cymothoidae) in the eastern Pacific (Galápagos and Costa Rica). Not. Galapagos 2003, 2003, 21–23. [Google Scholar]
- Markham, J.C. New Records of Pseudionine Bopyrid isopods, including two new species and one new genus, infesting porcellanid Crabs (Decapoda: Anomura) on the Pacific coast of North and Central America. Bull. South. Calif. Acad. Sci. 2008, 107, 145–157. [Google Scholar] [CrossRef]
- Rodriguez-Ortíz, B.; García-Prieto, L.; de León, G.P.P. Checklist of the helminth parasites of vertebrates in Costa Rica. Rev. Biol. Trop. 2004, 52, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ortíz, B.; García-Prieto, L.; Herrera-Vázquez, J.; de León, G.P.P. Addendum to the Checklist of the helminth parasites of vertebrates in Costa Rica. Rev. Biol. Trop. 2004, 52, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J. Marine Fish Parasites. In Marine Biodiversity of Costa Rica, Central America; Wehrtmann, I.S., Cortés, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 521–533. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Morales, J.A.; González-Barrientos, R.C.; Hernández-Gamboa, J.; Hernández-Mora, G. Parasites of cetaceans stranded on the Pacific coast of Costa Rica. Vet. Parasitol. 2011, 182, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Aguilar, R.; Marcotegui, P.; Martorelli, S.; García-Prieto, L. New records for three species of monogeneans (Platyhelminthes) of marine fishes in the Gulf of Nicoya, Costa Rica. Bol. Soc. Zool. Urug. 2018, 27, 7–10. [Google Scholar] [CrossRef]
- Rosas-Valdez, R.; Morrone, J.J.; Pinacho-Pinacho, C.D.; Domínguez-Domínguez, O.; García-Varela, M. Genetic diversification of acanthocephalans of the genus Floridosentis Ward 1953 (Acanthocephala: Neoechinorhynchidae), parasites of mullets from the Americas. Infect. Genet. Evol. 2020, 85, 104535. [Google Scholar] [CrossRef]
- Andrade-Gómez, L.; García-Varela, M. Unexpected morphological and molecular diversity of trematode (Haploporidae: Forticulcitinae) parasites of mullets from the ocean Pacific coasts in Middle America. Parasitol. Res. 2021, 120, 55–72. [Google Scholar] [CrossRef]
- Angulo, A.; Sibaja-Cordero, J.A. First record of the parasitic barnacle Anelasma squalicola Darwin, 1852 (Pollicipedomorpha: Pollicipedidae) in the eastern Pacific Ocean and a new host report: Eretmopterus benchleyi Vásquez, Ebert & Long, 2015 (Squaliformes: Etmopteridae). Zootaxa 2021, 5072, 165–172. [Google Scholar] [CrossRef]
- Meira-Filho, M.R.C.; Andrade, M.F.; Domit, C.; Silva-Souza, A.T. A review of helminths of the green turtle (Chelonia mydas) in Brazil. Oecologia Aust. 2017, 21, 17–26. [Google Scholar] [CrossRef]
- Werneck, M.R.; Lima, E.H.S.M.; Pires, T.; Silva, R.J. Helminth parasites of the juvenile Hawksbill turtle Eretmochelys imbricata (Testudines: Cheloniidae) in Brazil. J. Parasitol. 2015, 101, 500–503. [Google Scholar] [CrossRef]
- Werneck, M.R.; Baldassin, P.; D’Azeredo, F.; Trazi, A.; Berger, B. The Hawksbill Sea Turtle Eretmochelys imbricata Linnaeus 1758 (Testudines, Cheloniidae) as new host of Hapalotrema postorchis Rao, 1976 (Digenea: Spirorchiidae). Comp. Parasitol. 2014, 81, 75–78. [Google Scholar] [CrossRef]
- Berman, R.; Brooks, D.R. Escherbothrium molinae n. gen. et n. sp. (Eucestoda: Tetraphyllidea: Triloculariidae) in Urotrygon chilensis (Chondrichthyes: Myliobatiformes: Urolophidae) from the Gulf of Nicoya, Costa Rica. J. Parasitol. 1994, 80, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, T.R.; Caira, J.N.; Cox, A. The cestode order Rhinebothriidea no longer family-less: A molecular phylogenetic investigation with erection of two new families and description of eight new species of Anthocephalum. Zootaxa 2015, 3904, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Hoberg, E.P.; Brooks, D.R.; Molina-Ureña, H.; Erbe, E. Echinocephalus janzeni n. sp. (Nematoda: Gnathostomatidae) in Himantura pacifica (Chondrichthyes: Myliobatiformes) from the Pacific coast of Costa Rica and Mexico, with historical biogeographic analysis of the genus. J. Parasitol. 1998, 84, 571–581. [Google Scholar] [CrossRef]
- Monks, S.; Marques, F.; Leon-Regagnon, V.; de León, G.P.P. Koronacantha pectinaria n. comb. (Acanthocephala: Illiosentidae) from Microlepidotus brevipinnis (Haemulidae) and Redescription of Tegorhynchus brevis. J. Parasitol. 1997, 83, 485–494. [Google Scholar] [CrossRef]
- García-Varela, M.; Nadler, S.A. Phylogenetic relationships among Syndermata inferred from nuclear and mitochondrial gene sequences. Mol. Phylogenet. Evol. 2006, 40, 61–72. [Google Scholar] [CrossRef]
- Pinacho-Pinacho, C.D.; Sereno-Uribe, A.L.; De León, G.P.P.; García-Varela, M. Checklist of the species of Neoechinorhynchus (Acanthocephala: Neoechinorhynchidae) in fishes and turtles in Middle-America, and their delimitation based on sequences of the 28S rDNA. Zootaxa 2015, 3985, 98–116. [Google Scholar] [CrossRef]
- Greenblatt, R.J.; Work, T.M.; Balazs, G.H.; Sutton, C.A.; Casey, R.N.; Casey, J.W. The Ozobranchus leech is a candidate mechanical vector for the fibropapilloma-associated turtle herpesvirus found latently infecting skin tumors on Hawaiian green turtles (Chelonia mydas). Virology 2004, 321, 101–110. [Google Scholar] [CrossRef]
- Rittenburg, L.; Kelley, J.; Mansfield, K.; Savage, A. Marine leech parasitism of sea turtles varies across host species, seasons, and the tumor disease fibropapillomatosis. Dis. Aquat. Organ. 2021, 143, 1–12. [Google Scholar] [CrossRef]
- Suárez-Morales, E.; Gasca, R.A. A new Lepeophtheirus (Copepoda: Siphonostomatoida: Caligidae) from Isla del Coco National Park, Costa Rica, Eastern Tropical Pacific. Rev. Biol. Trop. 2012, 60, 235–242. [Google Scholar] [CrossRef]
- Corrales-Gómez, N.; Herrera-Ulloa, A. first record of the turtle barnacle Stephanolepas muricata from the Pacific coast of Costa Rica. Mar. Turt. Newsl. 2012, 135, 9–10. [Google Scholar]
- Andersen, M.L.; Bohn, M.; Høeg, J.T.; Jensen, P.G. Cyprid ultrastructure and adult morphology in Ptychascus barnwelli, new species, and P. glaber (cirripedia: Rhizocephala), parasites on semiterrestrial crabs. J. Crustac. Biol. 1990, 10, 20–28. [Google Scholar] [CrossRef]
- Holmes, J.C. Helminth communities in marine fishes. In Parasite Communities: Patterns and Processes; Esch, G.W., Bush, A.O., Aho, J.M., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 101–130. [Google Scholar] [CrossRef]
- Kohn, A.; Cohen, S.C.; Salgado-Maldonaldo, G. Checklist of Monogenea Parasites of Freshwater and Marine Fishes, Amphibians and Reptiles from Mexico, Central America and Caribbean; Magnolia Press: Auckland, New Zealand, 2006; pp. 1–114. [Google Scholar]
- Cortés, J. Historia del desarrollo de las ciencias del mar en la Escuela de Biología, Universidad de Costa Rica. Rev. Biol. Trop. 2009, 57, 16–18. [Google Scholar]
- Duarte, R.H. Between the national and the universal: Natural history networks in Latin America in the nineteenth and twentieth centuries. ISIS 2013, 104, 777–787. [Google Scholar] [CrossRef]
- Moravec, F.; Gibbons, L.M. Keys to the nematode parasites of vertebrates: Supplementary volume. Par. Vec. 2010, 3, 9. [Google Scholar] [CrossRef]
- Hernández-Orts, J.S.; Paso-Viola, M.N.; García, N.A.; Crespo, E.A.; González, R.; García-Varela, R.; Kuchta, R. A checklist of the helminth parasites of marine mammals from Argentina. Zootaxa 2015, 3936, 301–334. [Google Scholar] [CrossRef]
- Pereira, F.; González-Solís, D. Review of the parasitic nematodes of marine fishes from off the American continent. Parasitology 2022, 149, 1928–1941. [Google Scholar] [CrossRef]
- Bussing, W.A.; López, M. Marine Fish. In Marine Biodiversity of Costa Rica, Central America; Wehrtmann, I.S., Cortés, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 453–458. [Google Scholar] [CrossRef]
- Rodríguez-Fonseca, J.; Cubero-Pardo, P. Cetacean strandings in Costa Rica (1966–1999). Rev. De Biol. Trop. 2001, 49, 667–672. [Google Scholar]
- De Weerdt, J.; Ramos, E.A.; Pouplard, E.; Kochzius, M.; Clapham, P. Cetacean strandings along the Pacific and Caribbean coasts of Nicaragua from 2014 to 2021. Mar. Biodivers. Rec. 2021, 14, 13. [Google Scholar] [CrossRef]
- Blair, D. Keys to the Trematoda; CABI Publishing and The Natural History Museum: London, UK, 2002; Volume 2, p. 521. [Google Scholar]
- Olson, P.D.; Crib, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the digenea (platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- Cribb, T.H.; Cutmore, S.C.; Bray, R.A. The biodiversity of marine trematodes; then, now and in the future. Int. J. Parasitol. 2021, 51, 1085–1097. [Google Scholar] [CrossRef]
- Adnyana, W.; Ladds, P.W.; Blair, D. Prevalence and clinic-pathological studies on trematodiasis in marine turtles in Indonesia. Int. J. Sci. Res. Pub. 2020, 10, 274–295. [Google Scholar] [CrossRef]
- Oliveira, R.E.M.; Rossi, S.; Attademo, F.L.N.; Santoro, T.A.; Revoredo, R.A.; de Farias, D.S.D.; Lima, M.A.; Batista, J.S.; Silva, F.J.L.; Gavilan, S.A.; et al. Colocolic intussusception associated with Octangium sp. (Digenea: Microscaphidiidae) in a green sea turtle Chelonia mydas. J. Aquat. Anim. Health 2021, 33, 17–23. [Google Scholar] [CrossRef]
- Fyler, C.A.; Caira, J.N. Five new species of Acanthobothrium (Tetraphyllidea: Onchobothriidae) from the freshwater stingray Himantura chaophraya (Batoidea: Dasyatidae) in Malaysian Borneo. J. Parasitol. 2006, 92, 105–125. [Google Scholar] [CrossRef]
- Campbell, R.A.; Beveridge, I. The genus Acanthobothrium (Cestoda : Tetraphyllidea : Onchobothriidae) parasitic in Australian elasmobranch fishes. Invertebr. Syst. 2002, 16, 237–344. [Google Scholar] [CrossRef]
- Aznar, F.J.; Agustí, C.; Littlewood, D.T.J.; Raga, J.A.; Olson, P.D. Insight into the role of cetaceans in the life cycle of the tetraphyllideans (Platyhelminthes: Cestoda). Int. J. Parasitol. 2007, 37, 243–255. [Google Scholar] [CrossRef]
- Justine, J.L.; Bonami, J.R. Virus-like particles in a monogenean (platyhelminthes) parasitic in a marine fish. Int. J. Parasitol. 1993, 23, 69–75. [Google Scholar] [CrossRef]
- Kohn, A.; Cohen, S.C. South American Monogenea—List of species, hosts and geographical distribution. Int. J. Parasitol. 1998, 28, 1517–1554. [Google Scholar] [CrossRef]
- Jianying, Z.; Tingbao, Y.; Lin, L.; Xuejuan, D. A list of monogeneans from Chinese marine fishes. Syst. Parasitol. 2003, 54, 111–130. [Google Scholar] [CrossRef]
- Dyer, W.G.; Williams, E.H., Jr.; Williams, L.B. Monogeneans from marine fishes of Okinawa, Japan. Proc. Helminthol. Soc. Wash. 1989, 56, 64–68. [Google Scholar]
- Rohde, K. Diversity gradients of marine Monogenea in the Atlantic and Pacific Oceans. Experientia 1980, 36, 1368–1369. [Google Scholar] [CrossRef]
- Campos, E. Epicarídeos de Baja California : Distribution y notas ecologicas de Probopyrus pandalicola (Packard, 1879) en el Pacifico oriental. Rev. Biol. Trop. 1989, 37, 29–36. [Google Scholar]
- Romero-Rodríguez, J.; Álvarez, F. Bopyrid isopods of the genus Aporobopyrus infesting porcellanid crabs (Decapoda: Anomura) in the Gulf of California, Mexico: New host and parasite records. Proc. Biol. Soc. 2019, 132, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Dojiri, M.; Ho, J.S. Systematics of the Caligidae, Copepods Parasitic on Marine Fishes; Brill: Leiden, The Netherlands; Boston, MA, USA, 2013; p. 460. [Google Scholar]
- Williams, E.H. Conchoderma virgatum (Spengler) (Cirripedia Thoracica) in association with Dinemoura latifolia (Steenstrup & Lutken) (Copepoda, Caligidea), a parasite of the shortfin mako, Isurus oxyrhynchus Rafinesque (Pisces, Chondrichthyes). Crustaceana 1978, 34, 109–110. [Google Scholar]
- Cizauskas, C.A.; Carlson, C.J.; Burgio, K.R.; Clements, C.F.; Dougherty, E.R.; Harris, N.C.; Phillips, A.J. Parasite vulnerability to climate change: An evidence-based functional trait approach. R. Soc. Open Sci. 2017, 4, 160535. [Google Scholar] [CrossRef]
- Rogalski, M.A.; Gowler, C.D.; Shaw, C.L.; Hufbauer, R.A.; Duffy, M.A. Human drivers of ecological and evolutionary dynamics in emerging and disappearing infectious disease systems. Philos. Trans. R. Soc. 2017, 372, 20160043. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano-Barquero, A.; Rojas, A.; Cortés, J. Metazoan Marine Parasites of Costa Rica: A Review. Parasitologia 2023, 3, 116-141. https://doi.org/10.3390/parasitologia3020014
Solano-Barquero A, Rojas A, Cortés J. Metazoan Marine Parasites of Costa Rica: A Review. Parasitologia. 2023; 3(2):116-141. https://doi.org/10.3390/parasitologia3020014
Chicago/Turabian StyleSolano-Barquero, Alberto, Alicia Rojas, and Jorge Cortés. 2023. "Metazoan Marine Parasites of Costa Rica: A Review" Parasitologia 3, no. 2: 116-141. https://doi.org/10.3390/parasitologia3020014
APA StyleSolano-Barquero, A., Rojas, A., & Cortés, J. (2023). Metazoan Marine Parasites of Costa Rica: A Review. Parasitologia, 3(2), 116-141. https://doi.org/10.3390/parasitologia3020014







