Heartworm (Dirofilaria immitis) Prevalence in Dogs Determined by In-House ELISA Based on Filaria-Specific Antibodies in Tropical and Temperate Regions of Mexico
Abstract
1. Introduction
2. Results
2.1. Determination of Positive and Negative Samples via Direct ELISA
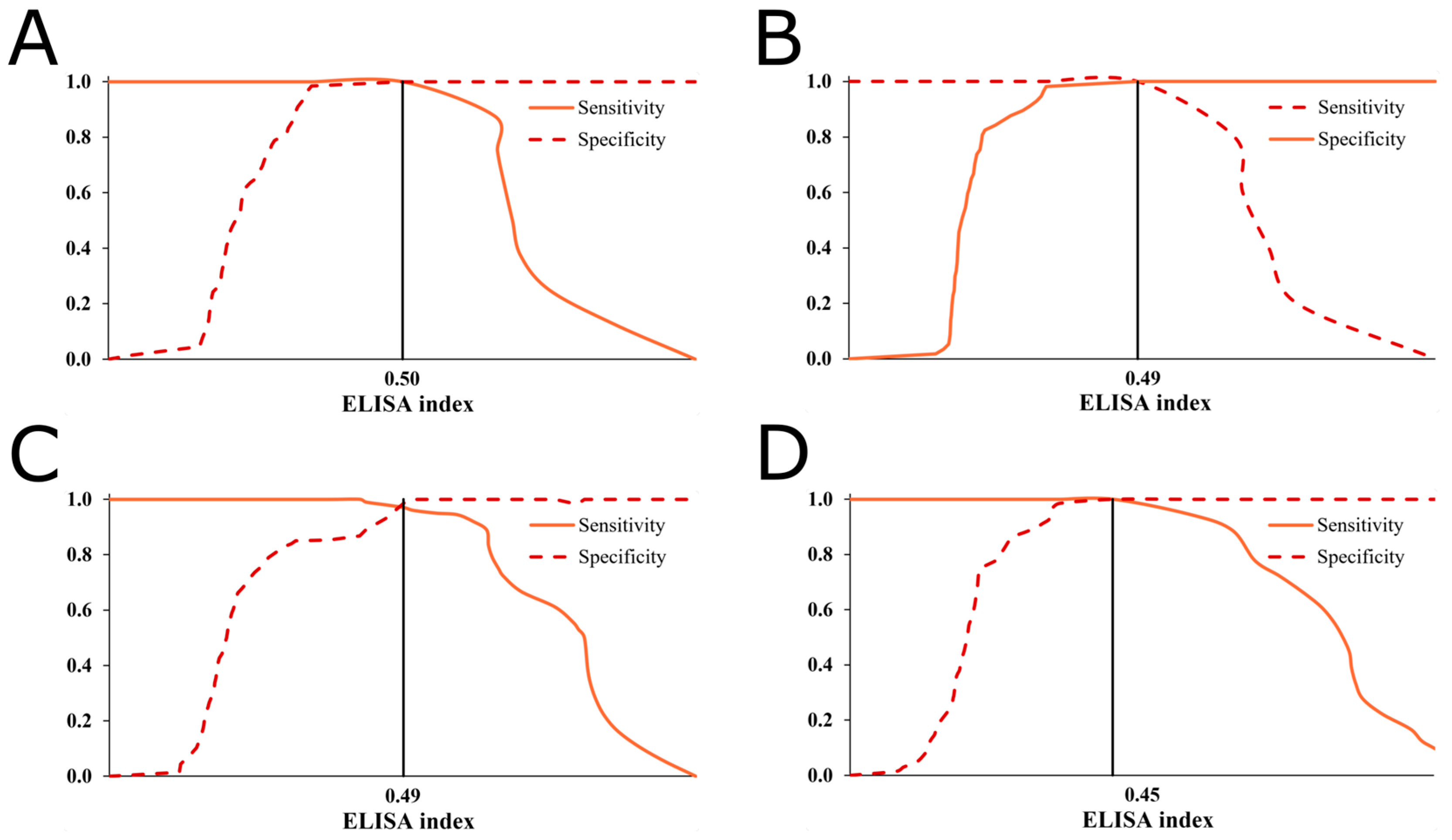
2.2. Prevalence of D. immitis and the ROC Analysis
3. Discussion
4. Materials and Methods
4.1. Collection of Positive and Negative Samples of D. immitis
4.2. Modified Knott’s Test
4.3. Study Area and Collection of Samples for Analysis
4.4. Optimisation of Test Serum Concentration and Secondary Antibodies by Direct ELISA
4.5. Direct ELISA with Test Sera and Positive and Negative Controls
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef]
- Atkinson, P.J.; O’Handley, R.; Nielsen, T.; Caraguel, C.G. Relative diagnostic accuracy of point-of-care tests to rule-in Dirofilaria immitis infection in clinically suspect dogs: A systematic review and meta-analysis. Prev. Vet. Med. 2023, 217, 105970. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Kramer, L.H. The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef]
- Thilakarathne, S.S.; Yuen, N.K.Y.; Hassan, M.M.; Yahathugoda, T.C.; Abdullah, S. Animal and Human Dirofilariasis in India and Sri Lanka: A Systematic Review and Meta-Analysis. Animals 2023, 13, 1551. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Dirofilariosis in the Americas: A more virulent Dirofilaria immitis? Parasites Vectors 2013, 6, 288. [Google Scholar] [CrossRef]
- Panarese, R.; Iatta, R.; Mendoza-Roldan, J.A.; Szlosek, D.; Braff, J.; Liu, J.; Beugnet, F.; Dantas-Torres, F.; Beall, M.J.; Otranto, D. Comparison of Diagnostic Tools for the Detection of Dirofilaria immitis Infection in Dogs. Pathogens 2020, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Sonnberger, K.; Fuehrer, H.P.; Sonnberger, B.W.; Leschnik, M. The Incidence of Dirofilaria immitis in Shelter Dogs and Mosquitoes in Austria. Pathogens 2021, 10, 550. [Google Scholar] [CrossRef]
- Noack, S.; Harrington, J.; Carithers, D.S.; Kaminsky, R.; Selzer, P.M. Heartworm disease—Overview, intervention, and industry perspective. Int. J. Parasitol. Drugs Drug Resist. 2021, 16, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Mendoza, M.V.; Arcila-Quiceno, V.; Albarracin-Navas, J.; Hernandez, I.; Flechas-Alarcon, M.C.; Morchon, R. Current Situation of the Presence of Dirofilaria immitis in Dogs and Humans in Bucaramanga, Colombia. Front. Vet. Sci. 2020, 7, 488. [Google Scholar] [CrossRef] [PubMed]
- Younes, L.; Barre-Cardi, H.; Bedjaoui, S.; Ayhan, N.; Varloud, M.; Mediannikov, O.; Otranto, D.; Davoust, B. Dirofilaria immitis and Dirofilaria repens in mosquitoes from Corsica Island, France. Parasites Vectors 2021, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Bolio-Gonzalez, M.E.; Rodriguez-Vivas, R.I.; Sauri-Arceo, C.H.; Gutierrez-Blanco, E.; Ortega-Pacheco, A.; Colin-Flores, R.F. Prevalence of the Dirofilaria immitis infection in dogs from Merida, Yucatan, Mexico. Vet. Parasitol. 2007, 148, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Caro-Gonzalez, J.A.; Bolio-Gonzalez, M.E.; Escobedo-Ortegon, F.J.; Manrique-Saide, P.; Rodriguez-Vivas, R.I.; Rodriguez-Buenfil, J.C.; Sauri-Arceo, C.H. Prevalence of Dirofilaria immitis infection in dogs from Celestun, Mexico, using polymerase chain reaction test. Vector Borne Zoonotic Dis. 2011, 11, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Movilla, R.; García, C.; Siebert, S.; Roura, X. Countrywide serological evaluation of canine prevalence for Anaplasma spp., Borrelia burgdorferi (sensu lato), Dirofilaria immitis and Ehrlichia canis in Mexico. Parasites Vectors 2016, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- González-Morteo, C.; De la Cruz-Moreno, O.; Alvarez-Guerrero, C.; Peña-Parra, B.; Carrillo-Díaz, F.; Borrayo-González, J. Dirofilaria immitis Prevalence in eleven municipalities of Nayarit. Abanico Vet. 2016, 5, 42–48. [Google Scholar]
- Rodríguez, P.R.; García, E.; Santos, M.C.; Burgos, B.P.; Valladolid, G.O.; Ruiz, P.H.; Ponce-Covarrubias, J. Prevalencia de Dirofilaria immitis en caninos domésticos de dos municipios del trópico de Guerrero, México. Abanico Vet. 2019, 9, 1–11. [Google Scholar]
- Anvari, D.; Narouei, E.; Daryani, A.; Sarvi, S.; Moosazadeh, M.; Ziaei Hezarjaribi, H.; Narouei, M.R.; Gholami, S. The global status of Dirofilaria immitis in dogs: A systematic review and meta-analysis based on published articles. Res. Vet. Sci. 2020, 131, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Trancoso, T.A.L.; Lima, N.d.C.; Barbosa, A.S.; Leles, D.; Fonseca, A.B.M.; Labarthe, N.V.; Bastos, O.M.P.; Uchôa, C.M.A. Detection of Dirofilaria immitis using microscopic, serological and molecular techniques among dogs in Cabo Frio, RJ, Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e017219. [Google Scholar] [CrossRef]
- Constantinoiu, C.; Croton, C.; Paterson, M.B.; Knott, L.; Henning, J.; Mallyon, J.; Coleman, G.T. Prevalence of canine heartworm infection in Queensland, Australia: Comparison of diagnostic methods and investigation of factors associated with reduction in antigen detection. Parasites Vectors 2023, 16, 63. [Google Scholar] [CrossRef]
- Negron-Perez, V.M.; Fausnacht, D.W.; Rhoads, M.L. Invited review: Management strategies capable of improving the reproductive performance of heat-stressed dairy cattle. J. Dairy. Sci. 2019, 102, 10695–10710. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Cold-stress response of engorged females of Rhipicephalus sanguineus. Exp. Appl. Acarol. 2011, 54, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Mendes-de-Almeida, F.; Alves, L.C.; do Amaral Fernandes, P.; de Menezes Leivas, R.; Labarthe, N. Infection with Dirofilaria immitis and Other Infections in Cats and Dogs from Rio de Janeiro, Brazil: The Need for Prophylactic Enforcement. Acta Parasitol. 2021, 66, 962–968. [Google Scholar] [CrossRef]
- Soares, H.S.; Camargo, L.M.A.; Gennari, S.M.; Labruna, M.B. Survey of canine tick-borne diseases in Lábrea, Brazilian Amazon: ‘accidental’ findings of Dirofilaria immitis infection. Rev. Bras. Parasitol. Vet. 2014, 23, 473–480. [Google Scholar] [CrossRef]
- Labarthe, N.V.; Pereira Paiva, J.; Reifur, L.; Mendes-de-Almeida, F.; Merlo, A.; Carvalho Pinto, C.J.; Juliani, P.S.; Ornelas de Almeida, M.A.; Câmara Alves, L. Updated canine infection rates for Dirofilaria immitis in areas of Brazil previously identified as having a high incidence of heartworm-infected dogs. Parasites Vectors 2014, 7, 493. [Google Scholar] [CrossRef] [PubMed]
- Zumaquero, L.; Simón, F.; Carretón, E.; Hernández, I.; Sandoval, C.; Morchón, R. Prevalence of canine and human dirofilariosis in Puebla, Mexico. Vet. Parasitol. 2020, 282, 109098. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Bowman, D.; Drake, J. Canine heartworm disease (Dirofilaria immitis) in Western Europe: Survey of veterinary awareness and perceptions. Parasites Vectors 2014, 7, 206. [Google Scholar] [CrossRef]
- Genchi, M.; Rinaldi, L.; Venco, L.; Cringoli, G.; Vismarra, A.; Kramer, L. Dirofilaria immitis and D. repens in dog and cat: A questionnaire study in Italy. Vet. Parasitol. 2019, 267, 26–31. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Juste, M.; Mellado, I.; Morchón, R.; Simón, F. Epidemiological survey of canine heartworm disease on the island of Gran Canaria (Canary Islands–Spain) between 2000 and 2008. Vet. Parasitol. 2010, 173, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Oi, M.; Yoshikawa, S.; Ichikawa, Y.; Nakagaki, K.; Matsumoto, J.; Nogami, S. Prevalence of Dirofilaria immitis among shelter dogs in Tokyo, Japan, after a decade: Comparison of 1999–2001 and 2009–2011. Parasite 2014, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Vrhovec, M.G.; Pantchev, N.; Failing, K.; Bauer, C.; Travers-Martin, N.; Zahner, H. Retrospective analysis of canine vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of leishmaniosis. Parasitol. Res. 2017, 116, 131–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diakou, A.; Soubasis, N.; Chochlios, T.; Oikonomidis, I.L.; Tselekis, D.; Koutinas, C.; Karaiosif, R.; Psaralexi, E.; Tsouloufi, T.K.; Brellou, G. Canine and feline dirofilariosis in a highly enzootic area: First report of feline dirofilariosis in Greece. Parasitol. Res. 2019, 118, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.O.; Akande, F.A.; Adenubi, O.T. Canine Dirofilariasis: A Case Report and Review of the Literature. Folia Vet. 2020, 64, 75–81. [Google Scholar] [CrossRef]
- Capelli, G.; Genchi, C.; Baneth, G.; Bourdeau, P.; Brianti, E.; Cardoso, L.; Danesi, P.; Fuehrer, H.-P.; Giannelli, A.; Ionică, A.M. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasites Vectors 2018, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.A.; Matias, I.C.; Silva, S.S.; Ramos, M.E.O.; Silva, A.P.; Barretto, M.L.; Brasil, A.W.; Silva, M.L.C.; Galiza, G.J.; Maia, L.A. Parasitological, serological and molecular diagnosis of Dirofilaria immitis in dogs in Northeastern Brazil. Exp. Parasitol. 2022, 236, 108233. [Google Scholar] [CrossRef]
- Girdan, G.; Anghel, R.G.; Ioniță, M.; Mitrea, I.L. Data on canine heartworm Dirofilaria immitis infection and other vector-borne pathogens in dogs in Bucharest area, Romania. Sci. Work Ser. C Vet. Med. 2015, 61, 146–151. [Google Scholar]
- Simón, F.; González-Miguel, J.; Diosdado, A.; Gómez, P.J.; Morchón, R.; Kartashev, V. The complexity of zoonotic filariasis episystem and its consequences: A multidisciplinary view. BioMed Res. Int. 2017, 2017, 6436130. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Otranto, D. Overview on Dirofilaria immitis in the Americas, with notes on other filarial worms infecting dogs. Vet. Parasitol. 2020, 282, 109113. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Venco, L.; Genchi, M. Guideline for the Laboratory Diagnosis of Canine and Feline Dirofilaria Infections. 2007, 8, 137–144. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20083097549 (accessed on 10 August 2024).
- INEGI. Instituto Nacional de Estadística y Geografía. In Anuario Estadístico y Geográfico de los Estados Unidos Mexicanos; Aguascalientes, México, 2017; Available online: https://www.inegi.org.mx/contenidos/productos/prod_serv/contenidos/espanol/bvinegi/productos/nueva_estruc/AEGEUM_2017/702825097912.pdf (accessed on 10 August 2024).
- García, A.E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; Instituto de Geografía, Universidad Nacional Autónoma de México (UNAM): Mexico City, Mexico, 2004; pp. 1–92. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]

| State/District/Municipality | Overall | Negative Samples (OD < 0.45) | Positive Samples (OD ≥ 0.45) | ||||
|---|---|---|---|---|---|---|---|
| No. of Dogs | Mean OD ± SD | No. of Samples | Mean OD ± SD | No. of Samples | Mean OD ± SD | Prevalence (%) | |
| Puebla state | |||||||
| Tecamachalco/Quecholac | 62 | 0.24 ± 0.13 | 57 | 0.21 ± 0.05 | 5 | 0.66 ± 0.01 | 8.06 (3.49–17.53) |
| Tecamachalco/Tecamachalco | 74 | 0.29 ± 0.18 | 66 | 0.23 ± 0.06 | 8 | 0.75 ± 0.09 | 10.81 (5.58–19.91 |
| Guerrero state | |||||||
| Las Vigas/Acapulco | 113 | 0.35 ± 0.24 | 85 | 0.23 ± 0.08 | 28 | 0.71 ± 0.15 | 24.78 (17.74–33.48) |
| Chilpancingo/Chilpancingo | 86 | 0.34 ± 0.26 | 68 | 0.21 ± 0.07 | 18 | 0.78 ± 0.12 | 20.93 (13.67–30.68) |
| Climate regions | |||||||
| Temperate (Puebla) | 136 | 0.27 ± 0.16 | 123 | 0.22 ± 0.06 | 13 | 0.72 ± 0.08 | 9.56 a (5.67–15.67) |
| Tropical (Guerrero) | 199 | 0.34 ± 0.24 | 153 | 0.21 ± 0.07 | 46 | 0.74 ± 0.16 | 23.12 a (17.80–29.45) |
| Overall | 335 | 0.31 ± 0.21 | 276 | 0.22 ± 0.06 | 59 | 0.73 ± 0.15 | 17.56 (13.91–28.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Mancera, A.; Castillo-Barojas, M.; Trejo-Campos, A.; Fernández-Meneses, E.; Robles-Robles, M.; Olivares-Pérez, J.; Olmedo-Juárez, A.; Utrera-Quintana, F.; González-Garduño, R.; Pérez-Mendoza, N.; et al. Heartworm (Dirofilaria immitis) Prevalence in Dogs Determined by In-House ELISA Based on Filaria-Specific Antibodies in Tropical and Temperate Regions of Mexico. Parasitologia 2024, 4, 279-287. https://doi.org/10.3390/parasitologia4030024
Villa-Mancera A, Castillo-Barojas M, Trejo-Campos A, Fernández-Meneses E, Robles-Robles M, Olivares-Pérez J, Olmedo-Juárez A, Utrera-Quintana F, González-Garduño R, Pérez-Mendoza N, et al. Heartworm (Dirofilaria immitis) Prevalence in Dogs Determined by In-House ELISA Based on Filaria-Specific Antibodies in Tropical and Temperate Regions of Mexico. Parasitologia. 2024; 4(3):279-287. https://doi.org/10.3390/parasitologia4030024
Chicago/Turabian StyleVilla-Mancera, Abel, Miguel Castillo-Barojas, Alma Trejo-Campos, Erick Fernández-Meneses, Manuel Robles-Robles, Jaime Olivares-Pérez, Agustín Olmedo-Juárez, Fernando Utrera-Quintana, Roberto González-Garduño, Noemi Pérez-Mendoza, and et al. 2024. "Heartworm (Dirofilaria immitis) Prevalence in Dogs Determined by In-House ELISA Based on Filaria-Specific Antibodies in Tropical and Temperate Regions of Mexico" Parasitologia 4, no. 3: 279-287. https://doi.org/10.3390/parasitologia4030024
APA StyleVilla-Mancera, A., Castillo-Barojas, M., Trejo-Campos, A., Fernández-Meneses, E., Robles-Robles, M., Olivares-Pérez, J., Olmedo-Juárez, A., Utrera-Quintana, F., González-Garduño, R., Pérez-Mendoza, N., Campos-García, H., & Ortega-Vargas, S. (2024). Heartworm (Dirofilaria immitis) Prevalence in Dogs Determined by In-House ELISA Based on Filaria-Specific Antibodies in Tropical and Temperate Regions of Mexico. Parasitologia, 4(3), 279-287. https://doi.org/10.3390/parasitologia4030024









