Seasonal and Spatial Dynamics of Freshwater Snails and Schistosomiasis in Mizan Aman, Southwest Ethiopia
Abstract
:1. Introduction
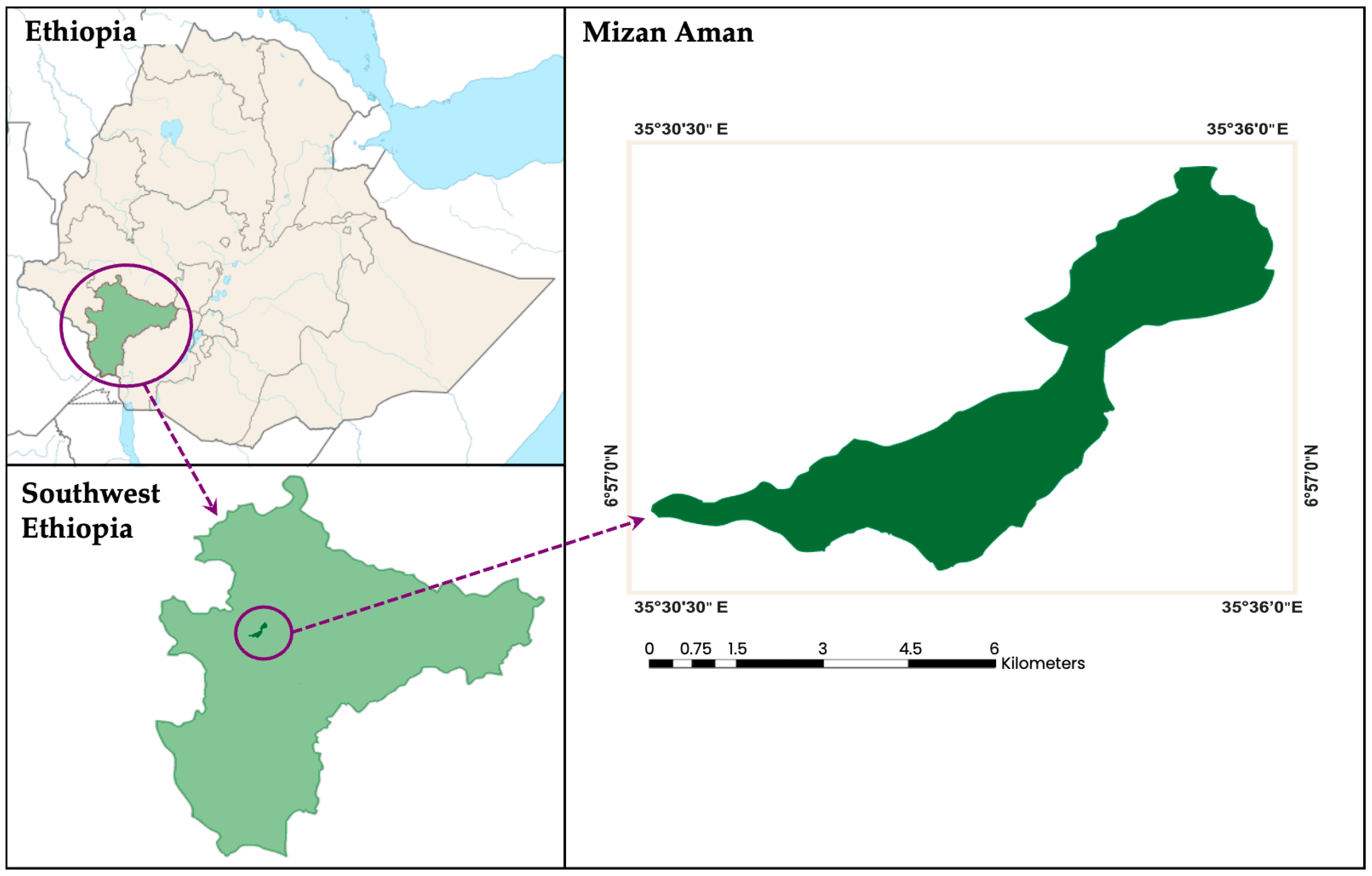
2. Materials and Methods
Sampling and Data Collection
3. Results
3.1. Spatial Variation in Snail Abundance and Species Composition
3.2. Snail Composition Shows Mild Shifts Between Seasons
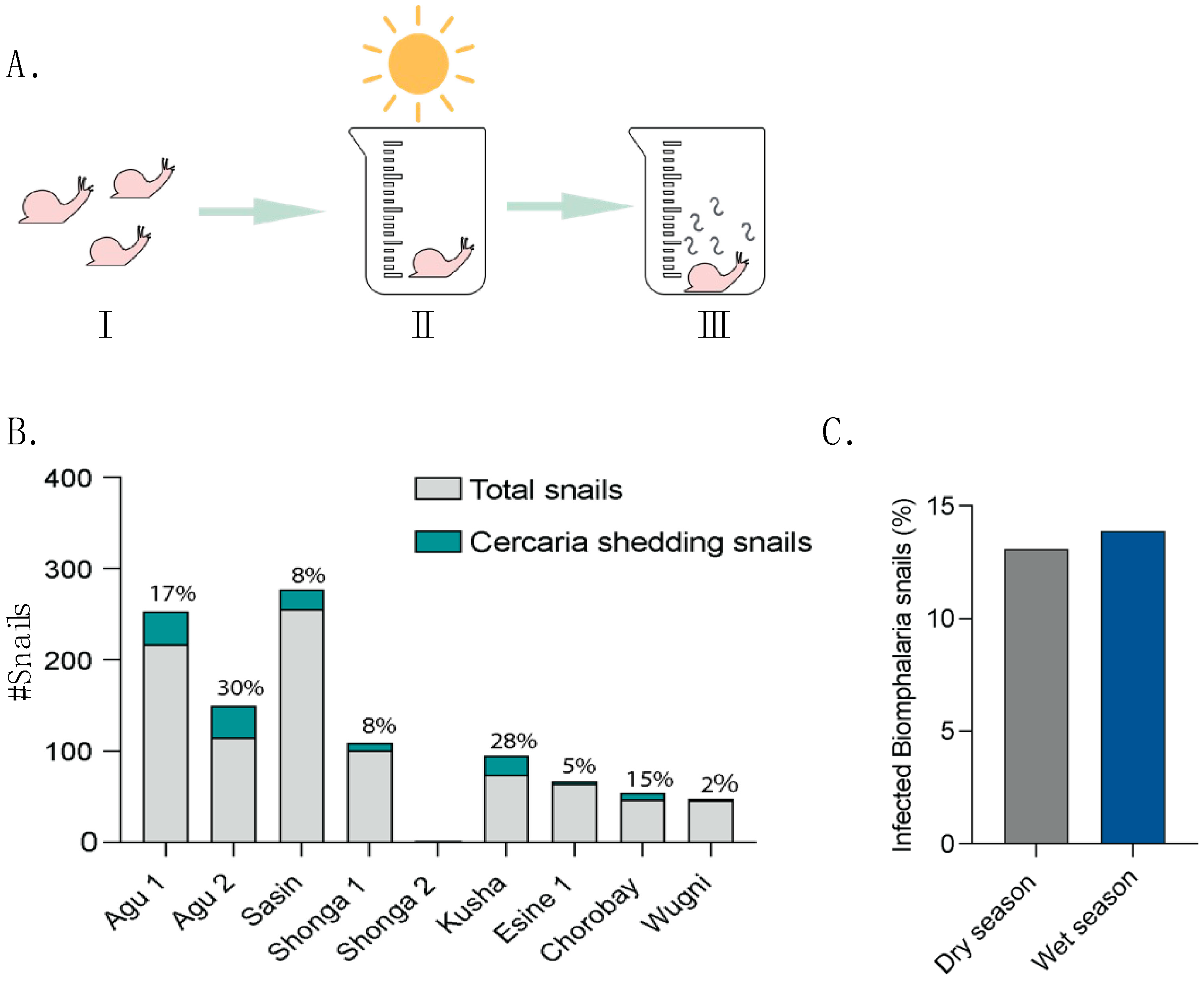
3.3. Spatial Variation in Biomphalaria Snail Infection Rates Across Sites
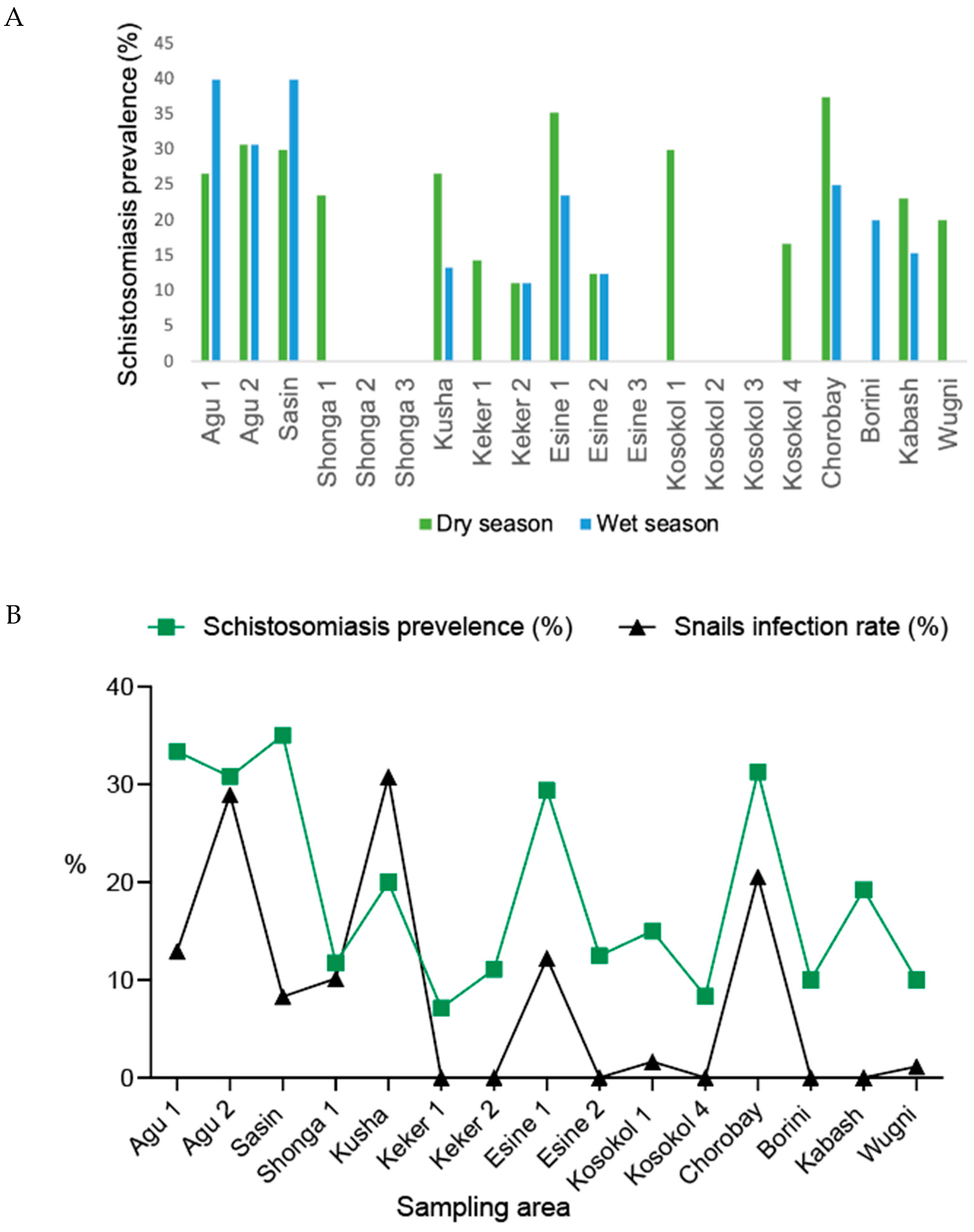
3.4. Seasonal and Spatial Distribution of Schistosomiasis Infection
4. Discussion
4.1. Snail Abundance and Species Composition
4.2. Biomphalaria Snail Infection Rates and Schistosomiasis Cases
4.3. Correlation Between Infected Snails and Schistosomiasis Cases
4.4. Environmental Factors and Snail Distribution
4.5. Community-Led Vegetation Clearance
4.6. Conclusion and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaye, P.M.; Doucouré, S.; Sow, D.; Sokhna, C.; Ranque, S. Freshwater snail-borne parasitic diseases in Africa. Trop. Med. Health 2024, 52, 61. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.H.; Condemine, C.; Christiansen, R.; LaCourse, E.J.; Makaula, P.; Stanton, M.C.; Juziwelo, L.; Kayuni, S.; Stothard, J.R. Biomphalaria pfeifferi Snails and Intestinal Schistosomiasis, Lake Malawi, Africa, 2017–2018. Emerg. Infect. Dis. 2019, 25, 613–615. [Google Scholar] [CrossRef]
- Hailegebriel, T.; Nibret, E.; Munshea, A. Distribution and seasonal abundance of Biomphalaria snails and their infection status with Schistosoma mansoni in and around Lake Tana, northwest Ethiopia. Sci. Rep. 2022, 12, 17055. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.E.; Croll, D.; E Harrison, W.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasites Vectors 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Wepnje, G.B.; Peters, M.K.; Green, A.E.; Nkuizin, T.E.; Kenko, D.B.N.; Dzekashu, F.F.; Kimbi, H.K.; Anchang-Kimbi, J.K. Seasonal and environmental dynamics of intra-urban freshwater habitats and their influence on the abundance of Bulinus snail host of Schistosoma haematobium in the Tiko endemic focus, Mount Cameroon region. PLoS ONE 2023, 18, e0292943. [Google Scholar] [CrossRef]
- Magero, V.; Kisara, S.; A Suleman, M.; Wade, C.M. Distribution of the schistosome intermediate snail host Biomphalaria pfeifferi in East Africa’s river systems and the prevalence of Schistosoma mansoni infection. Trans. R. Soc. Trop. Med. Hyg. 2024, 119, 1–13. [Google Scholar] [CrossRef]
- Kalinda, C.; Chimbari, M.J.; Mukaratirwa, S. Effect of temperature on the Bulinus globosus—Schistosoma haematobium system. Infect. Dis. Poverty 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Odongo-Aginya, E.M.; Kironde, F.; Kabatereine, N.; Kategere, P.; Kazibwe, F. Effect of Seasonal Rainfall and Other Environmental Changes, on Snail Density and Infection Rates with Schistosoma mansoni Fifteen Years After the Last Snails’ Study In Kigungu, Entebbe, Uganda. East. Afr. Med. J. 2008, 85, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, M.A.; Pukuma, M.S.; Adebote, D.; Abubakar, S.; Inuwa, Y.; Umar, S. Distribution, abundance and infection rate of water snails in Hadejia River Valley, Jigawa State, Nigeria. Dutse J. Pure Appl. Sci. 2023, 9, 227–243. [Google Scholar]
- Tekalign, E.; Sebeta, A.; Nureye, D.; Duguma, T.; Tesfaye, T. Intestinal parasitic infections among children aged 7–14 years in Mizan-Aman city, Southwest Ethiopia: A community-based cross-sectional study. Front. Public. Health 2024, 12, 1478293. [Google Scholar] [CrossRef]
- Meleko, A.; Li, S.; Turgeman, D.B.; Bruck, M.; Kesete, N.Z.; Zaadnoordijk, W.; Rollinson, D.; Sabar, G.; Bentwich, Z.; Golan, R. Schistosomiasis Control in Ethiopia: The Role of Snail Mapping in Endemic Communities. Trop. Med. Infect. Dis. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Mandahl-Barth, G. Key to the identification of east and central African freshwater snails of medical and veterinary importance. Bull. World Health Organ. 1962, 27, 135–150. [Google Scholar]
- Min, F.; Wang, J.; Liu, X.; Yuan, Y.; Guo, Y.; Zhu, K.; Chai, Z.; Zhang, Y.; Li, S. Environmental Factors Affecting Freshwater Snail Intermediate Hosts in Shenzhen and Adjacent Region, South China. Trop. Med. Infect. Dis. 2022, 7, 426. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.R.; Lv, S.; Rollinson, D.; Zhou, X.-N. Invasion and Dispersal of Biomphalaria Species: Increased Vigilance Needed to Prevent the Introduction and Spread of Schistosomiasis. Front. Med. 2021, 8, 614797. [Google Scholar] [CrossRef]
- Mutuku, M.W.; Dweni, C.K.; Mwangi, M.; Kinuthia, J.M.; Mwangi, I.N.; Maina, G.M.; E Agola, L.; Zhang, S.-M.; Maranga, R.; Loker, E.S.; et al. Field-derived Schistosoma mansoni and Biomphalaria pfeifferi in Kenya: A compatible association characterized by lack of strong local adaptation, and presence of some snails able to persistently produce cercariae for over a year. Parasites Vectors 2014, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Lu, L.; Laidemitt, M.R.; Zhang, S.-M.; Mutuku, M.; Mkoji, G.; Steinauer, M.; Loker, E.S. A genome sequence for Biomphalaria pfeifferi, the major vector snail for the human-infecting parasite Schistosoma mansoni. PLoS Negl. Trop. Dis. 2023, 17, e0011208. [Google Scholar] [CrossRef]
- Stensgaard, A.S.; Utzinger, J.; Vounatsou, P.; Hürlimann, E.; Schur, N.; Saarnak, C.F.; Simoonga, C.; Mubita, P.; Kabatereine, N.B.; Tchuenté, L.A.T.; et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Trop. 2013, 128, 378–390. [Google Scholar] [CrossRef]
- Ponpetch, K.; Erko, B.; Bekana, T.; Richards, L.; Liang, S. Biogeographical characteristics of Schistosoma mansoni endemic areas in Ethiopia: A systematic review and meta analysis. Infect. Dis. Poverty 2021, 10, 83. [Google Scholar] [CrossRef]
- Li, E.Y.; Gurarie, D.; Lo, N.C.; Zhu, X.; King, C.H. Improving public health control of schistosomiasis with a modified WHO strategy: A model-based comparison study. Lancet Glob. Health 2019, 7, e1414–e1422. [Google Scholar] [CrossRef]
- Aula, O.P.; McManus, D.P.; Jones, M.K.; Gordon, C.A. Schistosomiasis with a Focus on Africa. Trop. Med. Infect. Dis. 2021, 6, 109. [Google Scholar] [CrossRef]
- Standley, C.J.; Mugisha, L.; Adriko, M.; Arinaitwe, M.; Rukundo, J.; Ajarova, L.; Mopya, S.; Betson, M.; Kabatereine, N.B.; Stothard, J.R. Intestinal schistosomiasis in chimpanzees on Ngamba Island, Uganda: Observations on liver fibrosis, schistosome genetic diversity and praziquantel treatment. Parasitology 2013, 140, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Ekpo, U.F.; Hürlimann, E.; Schur, N.; Oluwole, A.S.; Abe, E.M.; Mafe, M.A.; Nebe, O.J.; Isiyaku, S.; Olamiju, F.; Kadiri, M.; et al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat. Health 2013, 7, 355–366. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization(WHO). Study Group on the Ecology of Intermediate Snail Hosts of Bilharziasis. Paris, 1957. Available online: https://iris.who.int/handle/10665/40374 (accessed on 1 February 2025).
- Barbosa, F.S.; Barbosa, C.S. The bioecology of snail vectors for schitosomiasis in Brazil. Cad. Saude Publica 1994, 10, 200–209. [Google Scholar] [CrossRef]
- Thiam, F.; Fall, C.B.; Gaye, P.M.; Senghor, B.; Diamanka, A.; Wotodjo, A.N.; Abotsi, K.; Parola, P.; Faye, B.; Sokhna, C.; et al. Study of the behavior of snails intermediate hosts of Schistosoma spp. under different maintenance conditions and their resistance to salinity in an african laboratory environment. Heliyon 2022, 8, e10289. [Google Scholar] [CrossRef]
- Wood, C.L.; Sokolow, S.H.; Jones, I.J.; Chamberlin, A.J.; Lafferty, K.D.; Kuris, A.M.; Jocque, M.; Hopkins, S.; Adams, G.; Buck, J.C.; et al. Precision mapping of snail habitat provides a powerful indicator of human schistosomiasis transmission. Proc. Natl. Acad. Sci. USA 2019, 116, 23182–23191. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Sack, A.; Bakhoum, S.; Barrett, C.B.; Lopez-Carr, D.; Chamberlin, A.J.; Civitello, D.J.; Diatta, C.; Doruska, M.J.; De Leo, G.A.; et al. A planetary health innovation for disease, food and water challenges in Africa. Nature 2023, 619, 782–787. [Google Scholar] [CrossRef]
- Boelee, E.; Laamrani, H. Environmental control of schistosomiasis through community participation in a Moroccan oasis. Trop. Med. Int. Health 2004, 9, 997–1004. [Google Scholar] [CrossRef]


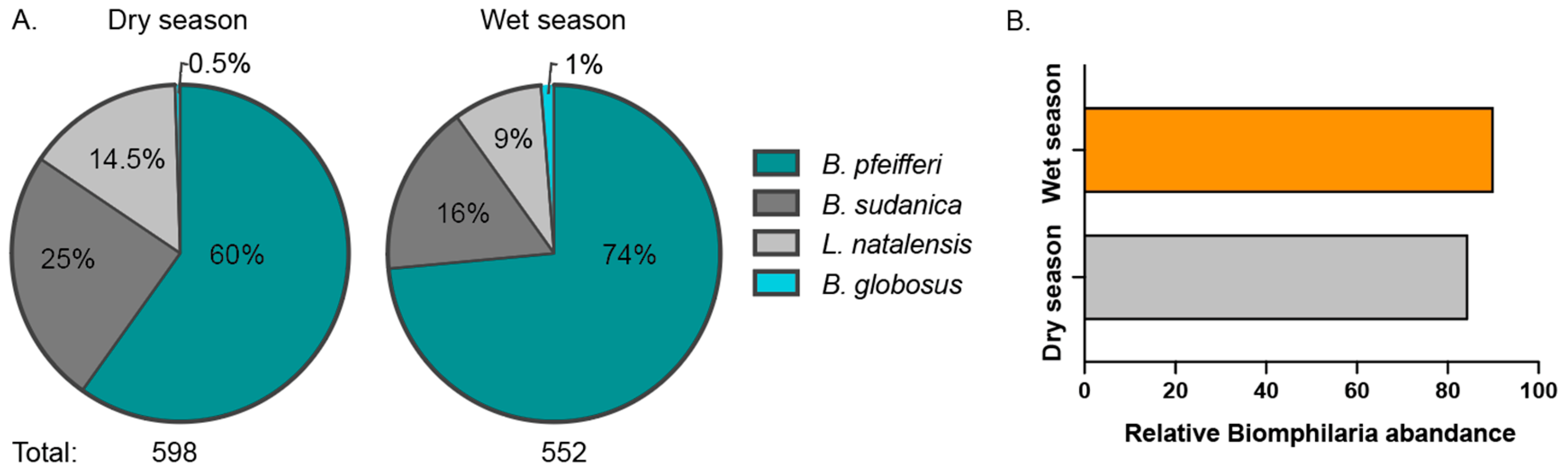
| Water Quality Parameter | Season | Change p-Value | |
|---|---|---|---|
| Dry [11] (December 2021–January 2022) | Wet (June–July 2023) | ||
| Temperature | 24.6 ± 2.7 | 22.1 ± 0.9 | <0.001 * |
| pH | 7.2 ± 0.66 | 6.9 ± 0.24 | 0.010 * |
| Salinity (g/I) | 187.2 ± 182.2 | 156.1 ± 158.8 | 0.160 |
| Total dissolved solid (mg/I) | 58.3 ± 54.1 | 44.0 ± 29.9 | 0.213 |
| Conductivity (mS) | 120.2 ± 110.8 | 88.1 ± 59.9 | 0.204 |
| Environmental Parameters | Biomphalaria Snail Abundance | |
|---|---|---|
| Dry [11] | Wet | |
| Temperature | 0.821 | 0.894 |
| pH | 0.873 | 0.819 |
| Salinity (g/I) | 0.013 * | 0.117 |
| Total dissolved solid (mg/I) | 0.979 | 0.071 |
| Conductivity (mS) | 0.21 | 0.071 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meleko, A.; Caplan, N.; Brener Turgeman, D.; Seifu, A.; Bentwich, Z.; Bruck, M.; Kesete, N.Z.; Zaadnoordijk, W.; Dahan, N. Seasonal and Spatial Dynamics of Freshwater Snails and Schistosomiasis in Mizan Aman, Southwest Ethiopia. Parasitologia 2025, 5, 13. https://doi.org/10.3390/parasitologia5020013
Meleko A, Caplan N, Brener Turgeman D, Seifu A, Bentwich Z, Bruck M, Kesete NZ, Zaadnoordijk W, Dahan N. Seasonal and Spatial Dynamics of Freshwater Snails and Schistosomiasis in Mizan Aman, Southwest Ethiopia. Parasitologia. 2025; 5(2):13. https://doi.org/10.3390/parasitologia5020013
Chicago/Turabian StyleMeleko, Asrat, Naomi Caplan, Dorin Brener Turgeman, Azeb Seifu, Zvi Bentwich, Michal Bruck, Nisan Z. Kesete, Willemijn Zaadnoordijk, and Noa Dahan. 2025. "Seasonal and Spatial Dynamics of Freshwater Snails and Schistosomiasis in Mizan Aman, Southwest Ethiopia" Parasitologia 5, no. 2: 13. https://doi.org/10.3390/parasitologia5020013
APA StyleMeleko, A., Caplan, N., Brener Turgeman, D., Seifu, A., Bentwich, Z., Bruck, M., Kesete, N. Z., Zaadnoordijk, W., & Dahan, N. (2025). Seasonal and Spatial Dynamics of Freshwater Snails and Schistosomiasis in Mizan Aman, Southwest Ethiopia. Parasitologia, 5(2), 13. https://doi.org/10.3390/parasitologia5020013






