Life-History Traits and Genetic Characterization of Polystoma borellii (Monogenea, Polystomatidae), a Parasite of Pleurodema borellii (Anura, Leptodactylidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Treatment of Hosts and Parasites
2.2. Optical Microscopy
2.3. Scanning Electron Microscopy (SEM)
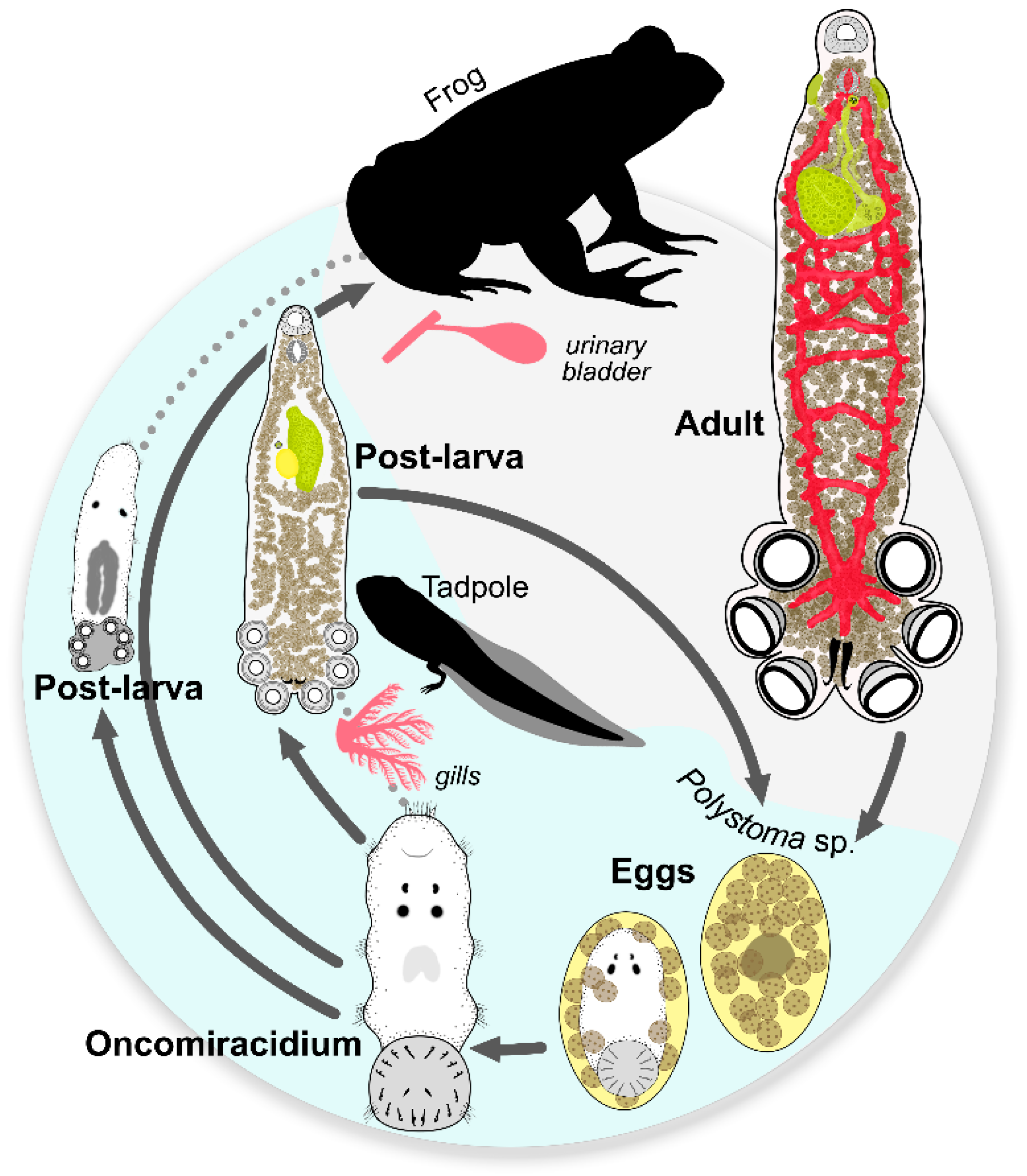
2.4. Life Cycle
2.5. Molecular, Phylogenetic, and Distance Analyses
3. Results
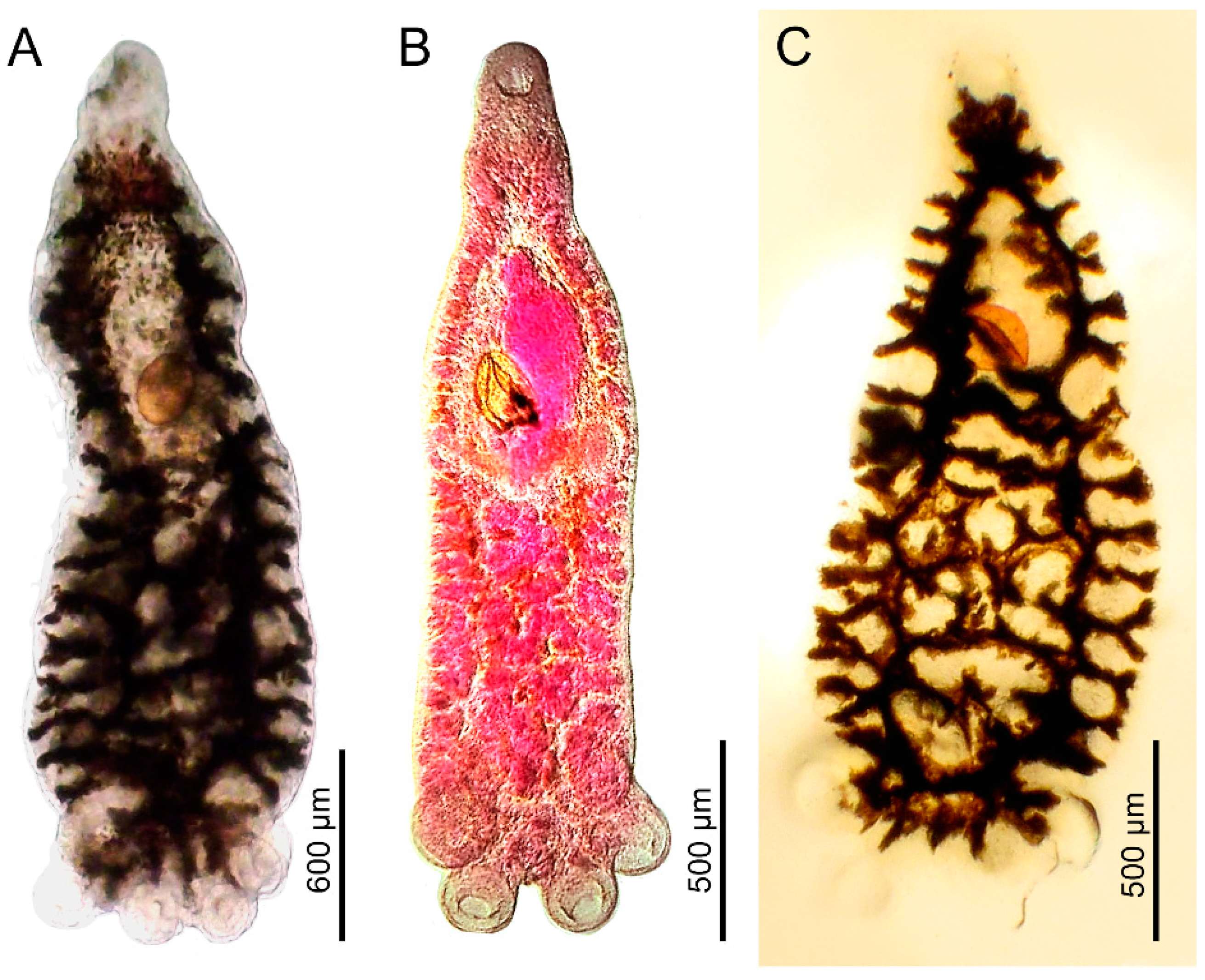
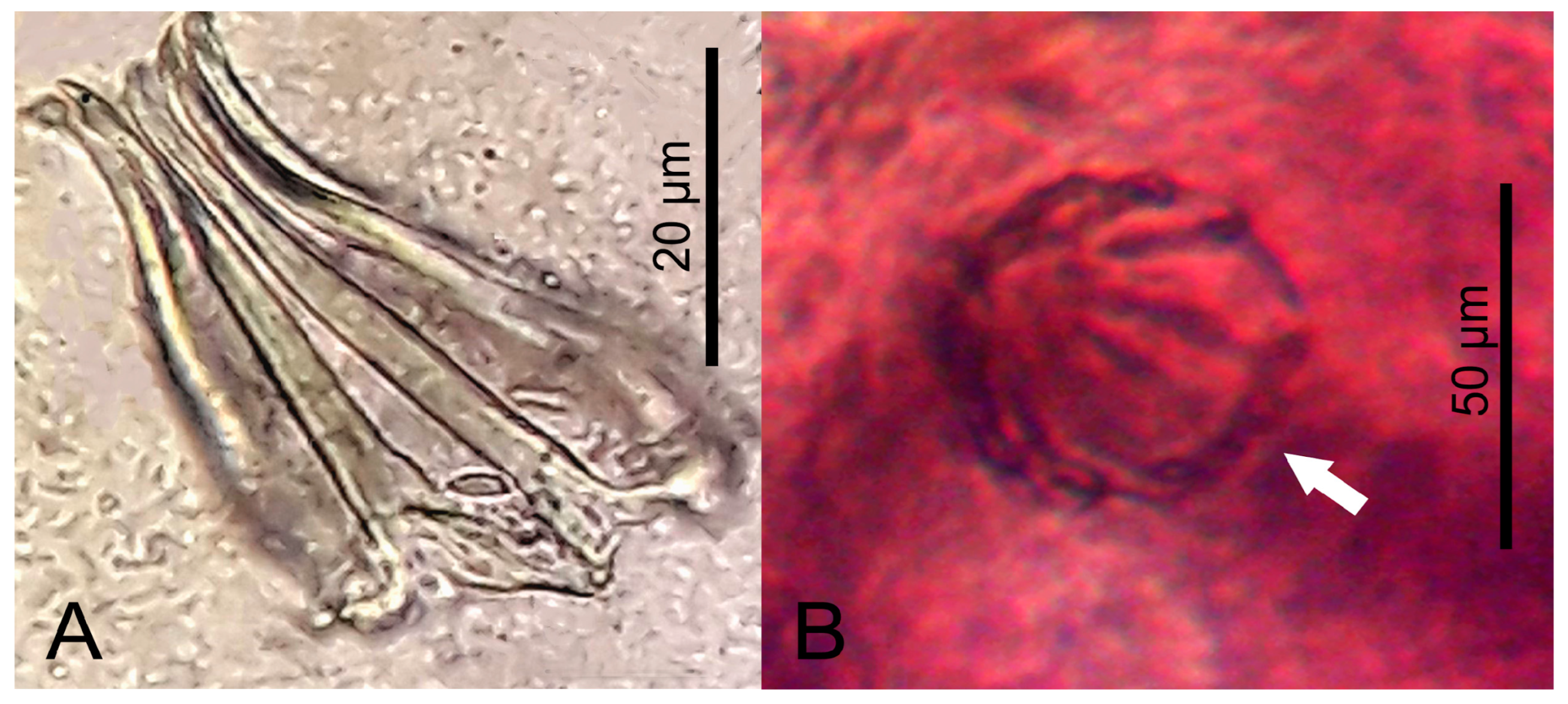
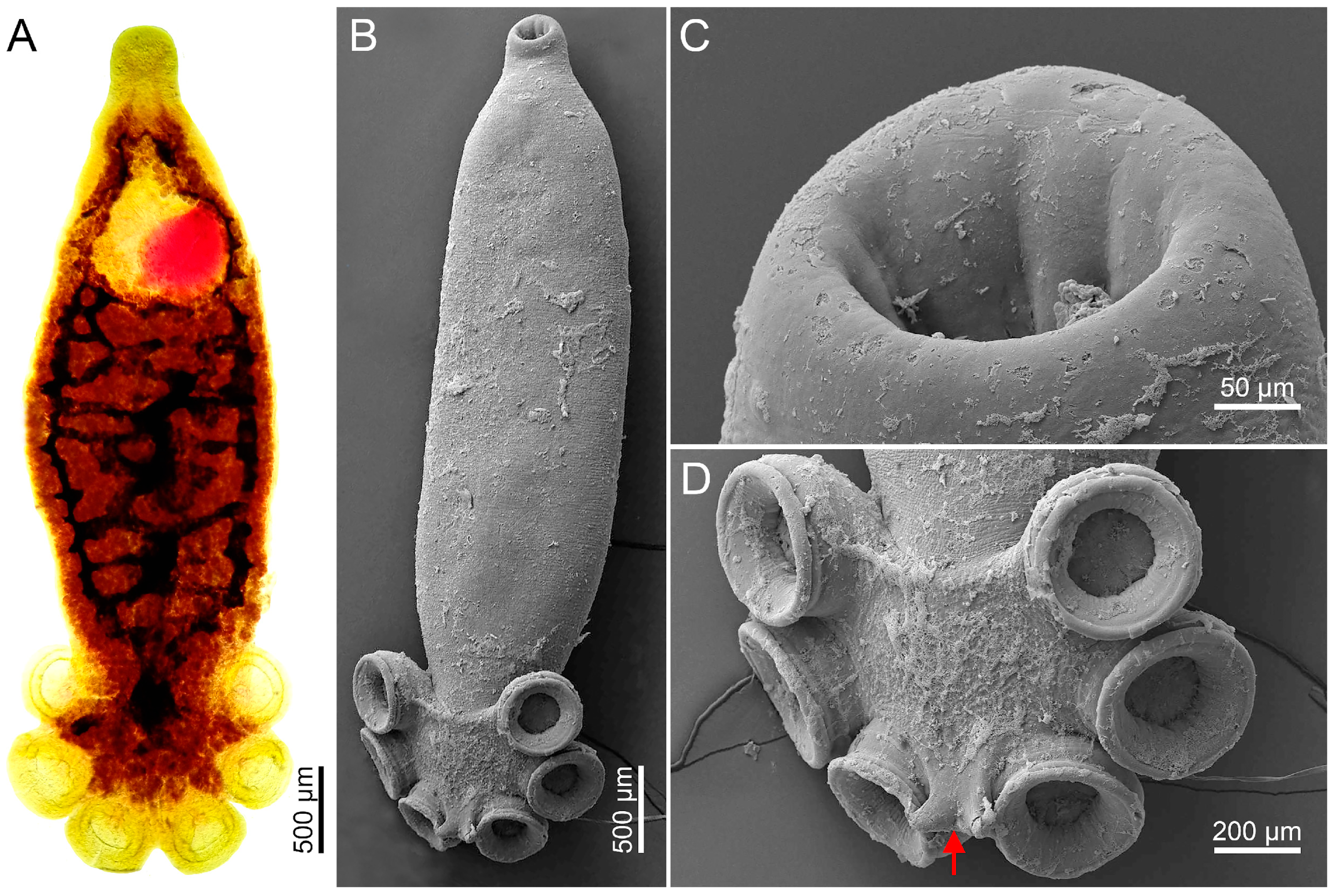
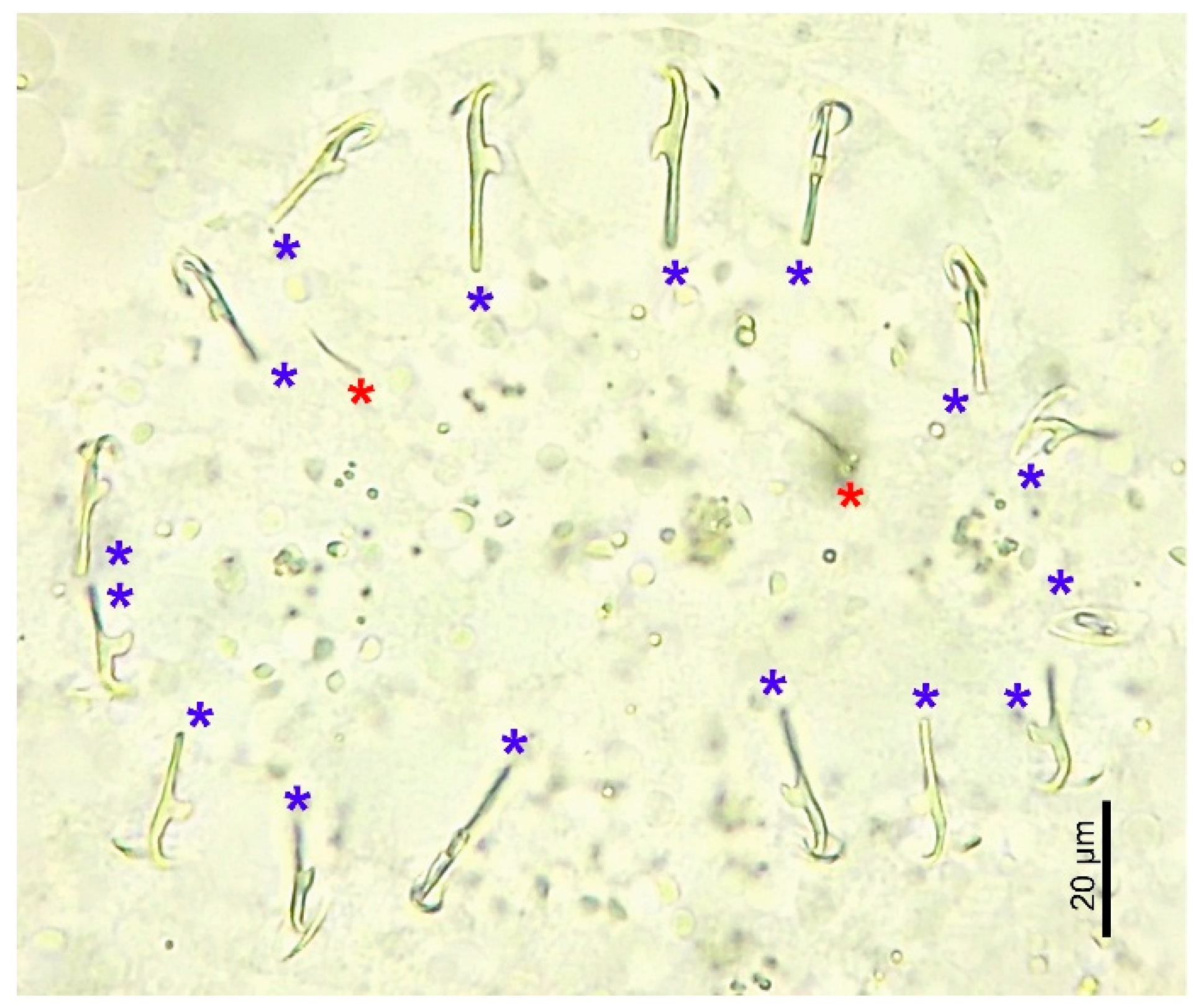
3.1. Morphological Analysis by Optical Microscopy and SEM
3.2. Life Cycle
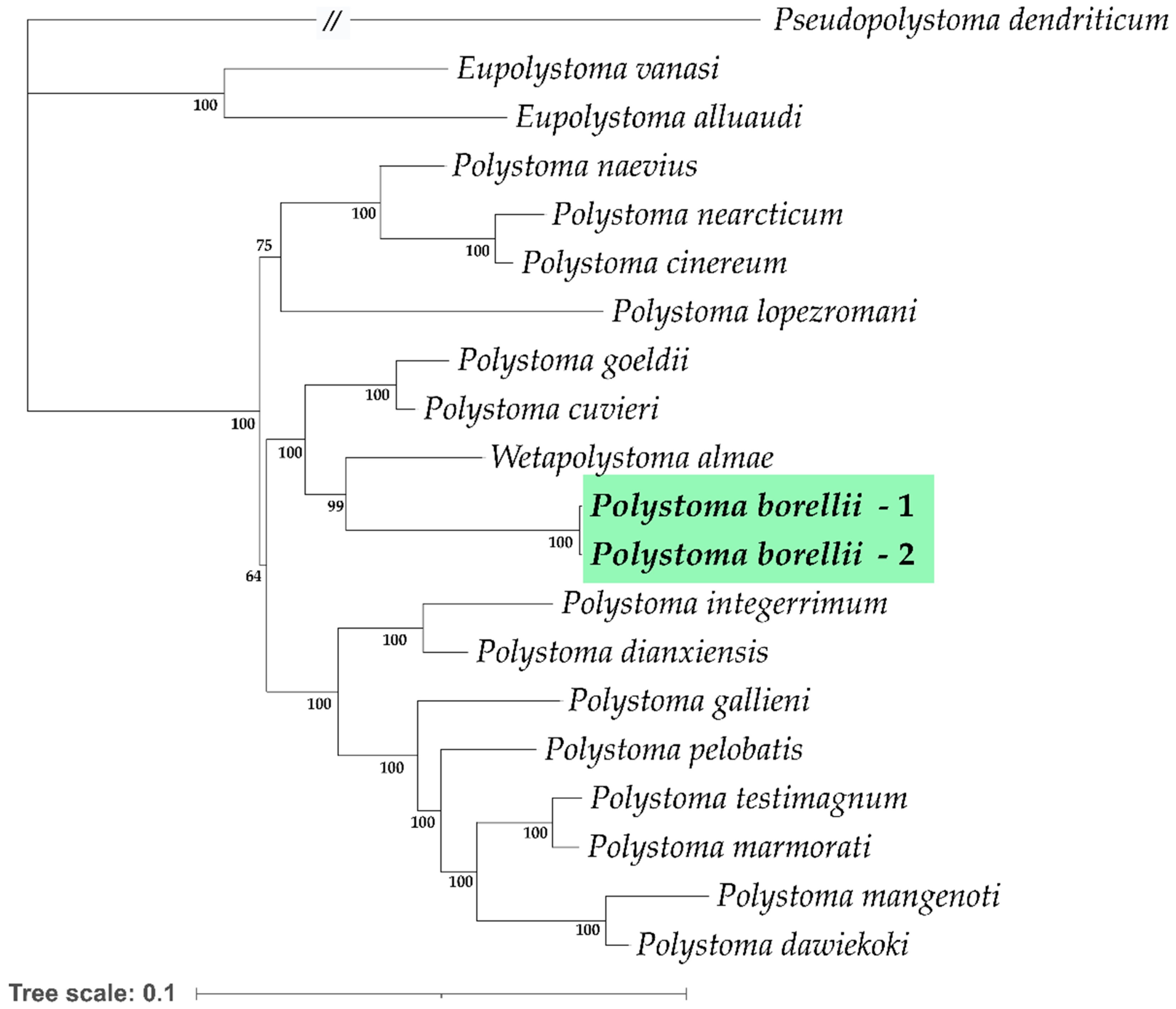
3.3. Molecular, Phylogenetic, and Distance Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BI | Bayesian Inference |
| COI | Cytochrome Oxidase I |
| ITS1 | Internal Transcribed Spacer 1 |
References
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef]
- Mozer, A.; Prost, S. An introduction to illegal wildlife trade and its effects on biodiversity and society. Forensic Sci. Int. Anim. Environ. 2023, 3, 100064. [Google Scholar] [CrossRef]
- Schoeman, A.L.; Du Preez, L.H.; Kmentová, N.; Vanhove, M.P.M. A monogenean parasite reveals the widespread translocation of the African clawed frog in its native range. J. Appl. Ecol. 2022, 59, 2670–2687. [Google Scholar] [CrossRef]
- Verneau, O.; Bentz, S.; Sinnappah, N.D.; Du Preez, L.; Whittington, I.; Combes, C. A view of early vertebrate evolution inferred from the phylogeny of polystome parasites (Monogenea: Polystomatidae). Proc. Biol. Sci. Lond. Ser. B 2002, 269, 535–543. [Google Scholar] [CrossRef]
- Du Preez, L.H.; Landman, W.J.; Verneau, O. Polystomatid Flatworms: State of Knowledge and Future Trends, 1st ed.; Feldhaar, H., Schmidt-Rhaesa, A., Eds.; Springer: Cham, Switzerland, 2023; pp. 29–59, 209–369. [Google Scholar] [CrossRef]
- Combes, C. Biologie, écologie des cycles et biogéographie de Digènes et Monogènes d’Amphibiens dans l’est des Pyrénées. Mém. Mus. Natl. Hist. Nat. Sér. A Zool. 1968, 51, 1–195. [Google Scholar]
- Badets, M.; Verneau, O. Origin and evolution of alternative developmental strategies in amphibious sarcopterygian parasites (Platyhelminthes, Monogenea, Polystomatidae). Org. Divers. Evol. 2009, 9, 155–164. [Google Scholar] [CrossRef]
- Badets, M.; Du Preez, L.H.; Verneau, O. Alternative development in Polystoma gallieni (Platyhelminthes, Monogenea) and life cycle evolution. Exp. Parasitol. 2013, 135, 283–286. [Google Scholar] [CrossRef]
- Du Preez, L.H.; Dominguez, M.V. Polystoma knoffi n. sp. and Polystoma travassosi n. sp. (Monogenea: Polystomatidae): Naming museum-archived specimens from Brazil. Syst. Parasitol. 2019, 96, 755–765. [Google Scholar] [CrossRef]
- Sales, A.N.; Du Preez, L.; Verneau, O.; Domingues, M.V. Morphology and molecular characterization of Polystoma goeldii n. sp. (Monogenea, Polystomatidae) parasite from the urinary bladder of Physalaemus ephippifer (Steindachner) (Anura, Leptodactylidae). Parasitol. Int. 2023, 92, 102685. [Google Scholar] [CrossRef]
- Combes, C.; Laurent, R.F. Polystoma borellii n. sp. (Monogenea, Polystomatidae) parasite de Pleurodema borellii Peracca (Anura, Leptodactylidae) en Republique Argentine. Acta Zool. Lilloana 1974, 31, 57–64. [Google Scholar]
- Combes, C.; Laurent, R.F. Deux nouveaux Polystomatidae (Monogenea) de Republique Argentine. Acta Zool. Lilloana 1978, 33, 85–91. [Google Scholar]
- Vaira, M. Population morphological variation of the monogenean Polystoma andinum, parasitic in Melanophryniscus rubriventris (Anura, Bufonidae). Acta Parasitol. 2004, 49, 281–291. [Google Scholar]
- Ferraro, D.; Casagranda, M. Geographic distribution of the genus Pleurodema in Argentina (Anura: Leiuperidae). Zootaxa 2009, 2024, 33–55. [Google Scholar] [CrossRef]
- Vaira, M.; Akmentins, M.S.; Attademo, M.; Baldo, D.; Barrasso, D.; Barrionuevo, S.; Basso, N.; Blotto, B.; Cairo, S.; Cajade, R.; et al. Categorización del estado de conservación de los anfibios de la República Argentina. Cuad. Herpetol. 2012, 26, 131–159. [Google Scholar]
- Du Preez, L.H.; Maritz, M.F. Demonstrating morphometric protocols using polystome marginal hooklet measurements. Syst. Parasitol. 2006, 63, 1–15. [Google Scholar] [CrossRef]
- Du Preez, L.H.; Raharivololoniaina, L.; Verneau, O.; Vences, M. A new genus of polystomatid parasitic flatworm (Monogenea: Polystomatidae) without free-swimming life stage from the Malagasy poison frogs. Zootaxa 2010, 2722, 54–68. [Google Scholar] [CrossRef]
- Bentz, S.; Leroy, S.; Du Preez, L.; Mariaux, J.; Vaucher, C.; Verneau, O. Origin and evolution of African Polystoma (Monogenea: Polystomatidae) assessed by molecular methods. Int. J. Parasitol. 2001, 31, 697–705. [Google Scholar] [CrossRef]
- Littlewood, D.T.J.; Rohde, K.; Clough, K.A. Parasite speciation within or between host species? Phylogenetic evidence from site-specific polystome monogeneans. Int. J. Parasitol. 1997, 27, 1289–1297. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martínez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Moritz, C.; Schneider, C.J.; Wake, D.B. Evolutionary relationships within the Ensatina eschscholtzii complex confirm the ring species interpretation. Syst. Biol. 1992, 41, 273–291. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position–specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rubtsova, N.Y.; Heckmann, R.A. Morphological and structural differences of normal adult and sub-adult bladder forms of Polystoma integerrimum (Fröhlich, 1798) (Monogenea: Polystomatidae) from the common frog. Rana temporaria. Sci. Parasitol. 2017, 18, 38–53. [Google Scholar]
- MacDonald, S.; Combes, C. The hatching rhythm of Polystoma integerrimum, a monogenean from the frog Rana temporaria. Chronobiol. 1978, 5, 277–285. [Google Scholar]
- Kok, D.J. Polystoma umthakathi (Monogenea): Establishment, mortality and reproduction of neotenic parasites in experimentally infected Natalobatrachus bonebergi tadpoles. S. Afr. J. Zool. 1990, 25, 26–30. [Google Scholar] [CrossRef]
- Héritier, L.; Badets, M.; Du Preez, L.H.; Aisien, M.S.; Lixian, F.; Combes, C.; Verneau, O. Evolutionary processes involved in the diversification of chelonian and mammal polystomatid parasites (Platyhelminthes, Monogenea, Polystomatidae) revealed by palaeoecology of their hosts. Mol. Phylogenet. Evol. 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Bentz, S.; Sinnappah-Kang, N.D.; Lim, L.H.S.; Lebedev, B.; Combes, C.; Verneau, O. Historical biogeography of amphibian parasites, genus Polystoma (Monogenea: Polystomatidae). J. Biogeog. 2006, 33, 742–749. [Google Scholar] [CrossRef]
- Gray, M.E. Wetapolystoma almae n. gen., n. sp. (Monogenea: Polystomatidae) Parasite of Bufo typhonius (Linnaeus, 1758) (Amphibia: Bufonidae) from Tropical Peru. Trans. Kans. Acad. Sci. 1993, 96, 181–185. [Google Scholar] [CrossRef]
- Pereyra, M.O.; Blotto, B.L.; Baldo, D.; Chaparro, J.C.; Ron, S.R.; Elias-Costa, A.J.; Iglesias, P.P.; Venegas, P.J.; Thomé, M.T.C.; Ospina-Sarria, J.J.; et al. Evolution in the genus Rhinella: A total evidence phylogenetic analysis of Neotropical True Toads (Anura: Bufonidae). Bull. Am. Mus. Nat. Hist. 2021, 447, 1–156. [Google Scholar] [CrossRef]
- Blasco-Costa, I.; Cutmore, S.C.; Miller, T.L.; Nolan, M.J. Molecular approaches to trematode systematics: ‘best practice’ and implications for future study. Syst. Parasitol. 2016, 93, 295–306. [Google Scholar] [CrossRef]
- Du Preez, L.H.; Verneau, O.; Gross, T.S. Polystoma floridana n. sp. (Monogenea: Polystomatidae) a parasite in the green tree frog, Hyla cinerea (Schneider), of North America. Zootaxa 2007, 1663, 33–45. [Google Scholar] [CrossRef]


| Taxon | Host Species | Geographic Origin | GenBank Accession Number | |||
|---|---|---|---|---|---|---|
| COI | ITS1 | 28S | 18S | |||
| Pseudopolystoma dendriticum | Onychodactylus japonicus | Japan | KR856180 | --- | FM992707 | FM992700 |
| Eupolystoma alluaudi | Sclerophrys regularis | Kenya | FR667558 | AJ301695 | AM157199 | AM051066 |
| Eupolystoma vanasi | Schismaderma carens | South Africa | FR667559 | --- | AM157200 | AM157185 |
| Metapolystoma cachani | Ptychadena longirostris | Ivory Coast | KR856163 | --- | FM897262 | FM897280 |
| Polystoma borellii 1 | Pleurodema borellii | Argentina | OK489454 | OK491390 | --- | --- |
| Polystoma borellii 2 | Pleurodema borellii | Argentina | OK489455 | OK491391 | --- | --- |
| Polystoma cinereum | Dryophytes cinereus | USA | AM913869 | --- | AM157211 | AM157188 |
| Polystoma cuvieri | Physalaemus cuvieri | Paraguay | AM913862 | AJ301691 | AM157203 | AM051068 |
| Polystoma dawiekoki | Ptychadena anchietae | South Africa | AM913856 | AJ310405 | AM157204 | AM051069 |
| Polystoma gallieni | Hyla meridionalis | France | JF699305 | AJ301687 | AM157205 | AM051070 |
| Polystoma goeldii | Physalaemus ephippifer | Brazil | OP537251 | --- | --- | --- |
| Polystoma mangenoti | Ptychadena superciliaris | Ivory Coast | --- | AJ310408 | --- | --- |
| Polystoma dianxiensis | Rana chaochiaoensis | China | KR856167 | --- | KR856143 | KR856125 |
| Polystoma integerrimum | Rana temporaria | Europe | JF699306 | AJ301688 | AM157206 | AM051071 |
| Polystoma lopezromani | Trachycephalus typhonius | Paraguay | AM913863 | AJ301690 | AM157207 | AM051072 |
| Polystoma marmorati | Hyperolius marmoratus | South Africa | AM913858 | AJ310396 | AM157208 | AM051073 |
| Polystoma naevius | Smilisca baudinii | Costa Rica | AM913864 | --- | AM157209 | AM157187 |
| Polystoma nearcticum | Dryophytes versicolor | USA | AM913865 | AJ301692 | AM157210 | AM051074 |
| Polystoma pelobatis | Pelobates cultripes | France | KR856168 | FR821519 | KR856144 | AM051076 |
| Polystoma testimagnum | Strongylopus fasciatus | South Africa | AM913860 | AJ310397 | AM157217 | AM157194 |
| Wetapolystoma almae | Rhinella margaritifera | French Guiana | AM913866 | AJ301693 | AM157220 | AM051081 |
| Adults | Post-Larvae | Oncomiracidia | ||
|---|---|---|---|---|
| Character | This study a | Combes and Laurent, 1974 [11] | This study | This study |
| Body length (BL) | 5040 | 5100 (4200–5600) | 1962 ± 414 (1440–2650) | 259 ± 5.67 (255–265) |
| Body at maximum width | 1325 | 2500 (2000–3200) | 506 ± 82 (412–638) | 96 ± 9 (86–103) |
| Haptor length (HL) | 1584 | 1500 (1300–1700) | 425 ± 111 (265–658) | 73 ± 6 (69–81) |
| Haptor width | 1642 | 2600 (2300–3200) | 568 ± 88 (432–697) | 83 ± 4 (81–88) |
| HL/BL ratio | 0.31 | 0.29 b | 0.16–0.28 | 0.28 |
| Haptoral suckers | 6 | 6 | 6 | --- |
| Haptoral suckers’ average length | 476 | 533 c | 164 ± 26 (118–209) | --- |
| Haptoral suckers’ average width | 457 | --- | 164 ± 26 (118–209) | --- |
| Hamulus length | 412 | 430 (350–530) | 96 ± 32 (51–137) | --- |
| Internal root length | --- | --- | 91 ± 29 (51–127) | --- |
| External root length | --- | --- | 96 ± 32 (51–137) | --- |
| Hamulus tip length | 39 | --- | 46 ± 8 (37–61) | --- |
| Hooklet length | --- | --- | 24 ± 2 (20–27) | --- |
| Sickle length of hooklets | --- | --- | 11 ± 1 (10–12) | --- |
| Oral sucker length | 285 | ---- | 138 ± 29 (98–184) | --- |
| Oral sucker width | 334 | --- | 139 ± 30 (98–189) | --- |
| Pharynx length | 236 | 250 (229–274) | 114 ± 20 (81–145) | --- |
| Pharynx width | 177 | 240 (200–285) | 106 ± 17 (73–122) | --- |
| Intestinal anastomoses | 6 | Reticulated | 4–6 | --- |
| Ovary length (OL) | 638 | --- | 367 ± 62 (265–461) | --- |
| Ovary width (OW) | 511 | --- | 133 ± 47 (42–184) | --- |
| OL/BL ratio | 0.126 | --- | 0.174–0.184 | --- |
| Vagina | 2 | 2 | 0 | --- |
| No. of genital spines | 10 | 8 | 10 | --- |
| Genital spine length | --- | 54 | 35 (24–49) | --- |
| Egg length carmine-stained | --- | 230 | 164 ± 14 (147–184) | --- |
| Egg width carmine-stained d | --- | 120 | 120 ± 11 (103–135) | --- |
| Egg length in vivo | 237 ± 9 (226–246) | --- | 193 ± 17 (157–226) | --- |
| Egg width in vivo | 164 ± 13 (147–189) | --- | 162 ± 10 (147–179) | --- |
| p-distances COI/ITS | Polystoma borellii | Polystoma cuvieri | Polystoma goeldii |
|---|---|---|---|
| Polystoma cuvieri | 14.2/14.7 | ||
| Polystoma goeldii | 15.3/-- | 2.6/-- | |
| Wetapolystoma almae | 16.4/13.2 | 13.3/13.4 | 12.6/-- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, C.; Lauthier, J.J.; Renfijes, M.M.; Cruz, I.G.; Davies, D. Life-History Traits and Genetic Characterization of Polystoma borellii (Monogenea, Polystomatidae), a Parasite of Pleurodema borellii (Anura, Leptodactylidae). Parasitologia 2025, 5, 17. https://doi.org/10.3390/parasitologia5020017
Davies C, Lauthier JJ, Renfijes MM, Cruz IG, Davies D. Life-History Traits and Genetic Characterization of Polystoma borellii (Monogenea, Polystomatidae), a Parasite of Pleurodema borellii (Anura, Leptodactylidae). Parasitologia. 2025; 5(2):17. https://doi.org/10.3390/parasitologia5020017
Chicago/Turabian StyleDavies, Carolina, Juan José Lauthier, Matías Martín Renfijes, Ivanna Gabriela Cruz, and Dora Davies. 2025. "Life-History Traits and Genetic Characterization of Polystoma borellii (Monogenea, Polystomatidae), a Parasite of Pleurodema borellii (Anura, Leptodactylidae)" Parasitologia 5, no. 2: 17. https://doi.org/10.3390/parasitologia5020017
APA StyleDavies, C., Lauthier, J. J., Renfijes, M. M., Cruz, I. G., & Davies, D. (2025). Life-History Traits and Genetic Characterization of Polystoma borellii (Monogenea, Polystomatidae), a Parasite of Pleurodema borellii (Anura, Leptodactylidae). Parasitologia, 5(2), 17. https://doi.org/10.3390/parasitologia5020017






