Optimizing In Vitro Metacyclogenesis: Strain-Specific Variability in Trypanosoma cruzi Responses to Nutritional and pH Stress
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
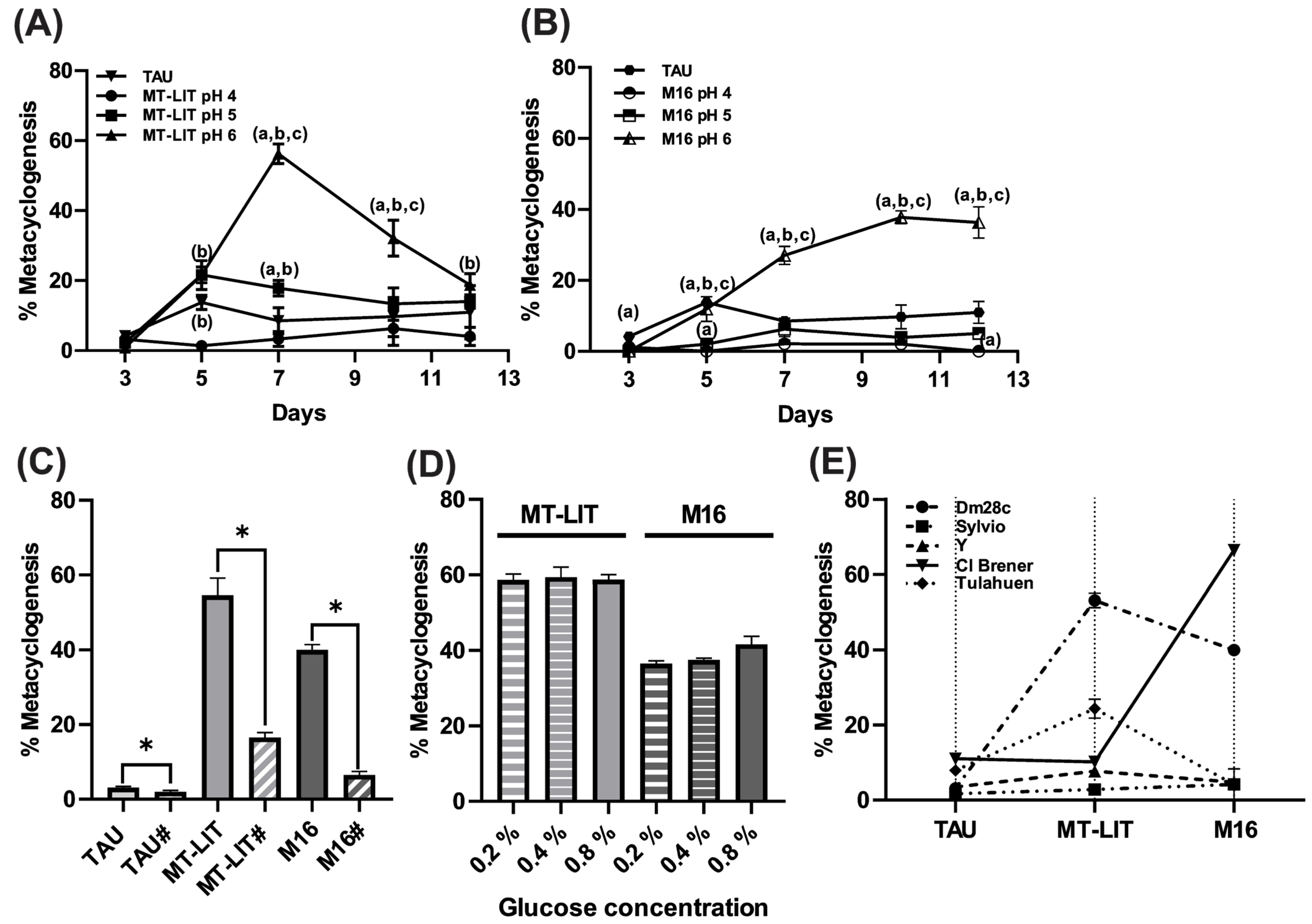
3.1. Trypanosoma cruzi In Vitro Metacyclogenesis Is Modulated by pH, Glucose, and Agitation
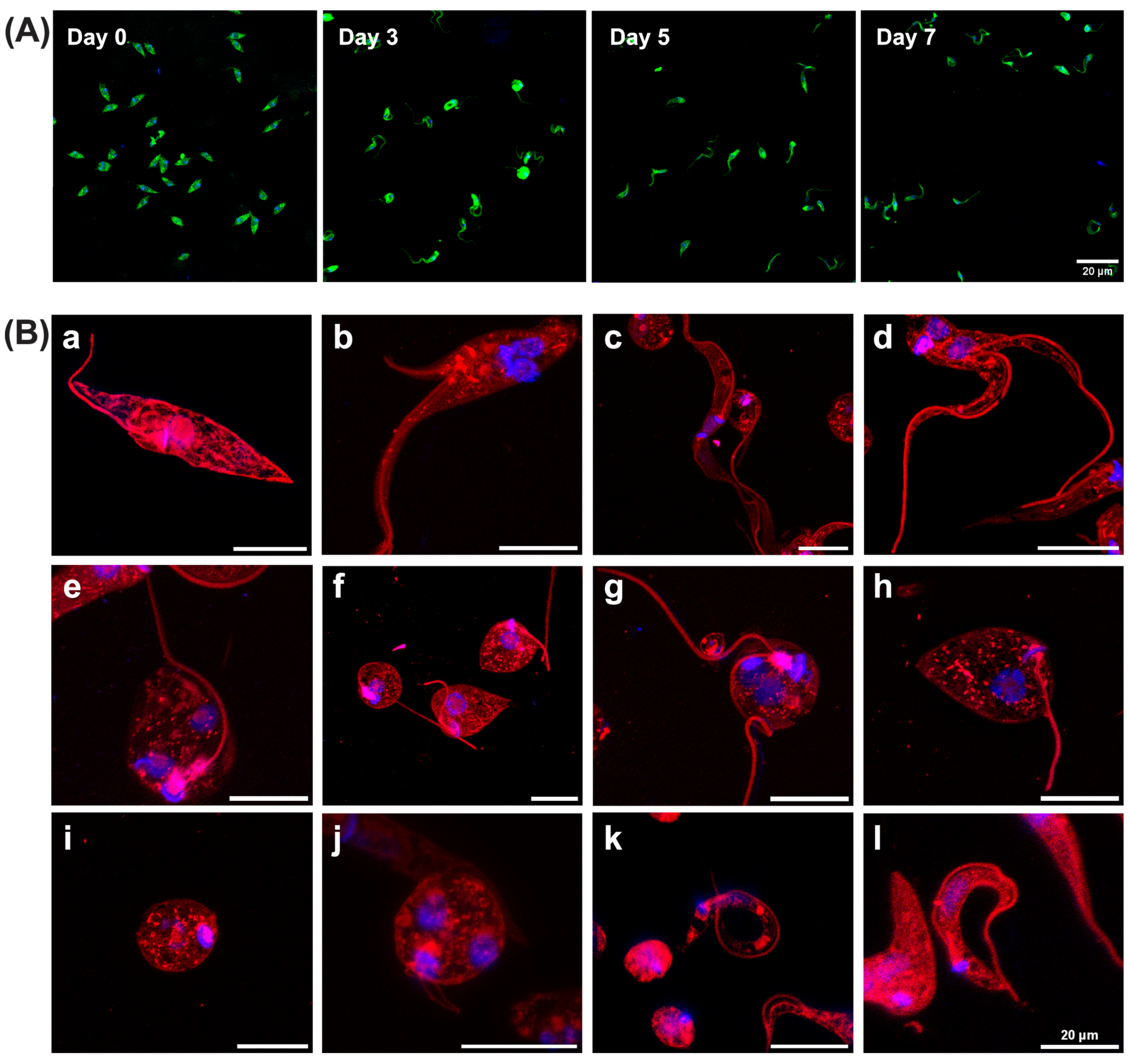
3.2. In Vitro Metacyclogenesis of Trypanosoma cruzi in MT-LIT or M16 Medium Generates Intermediate Morphological Forms Bearing Resemblance to Those Reported in the Digestive Tract of Triatomine Vectors
3.3. MT Obtained Through In Vitro Metacyclogenesis in MT-LIT or M16 Media Exhibit High Infectivity Rates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Chagas disease |
| DTU | Discrete typing units |
| LIT | Liver infusion tryptose medium |
| MT | Metacyclic trypomastigotes |
| MT-LIT | Metacyclic trypomastigotes—liver infusion tryptose medium |
| RT | Room temperature |
| TAU | Triatomine artificial urine |
| T. cruzi | Trypanosoma cruzi |
References
- Arsuaga, M.; De Miguel Buckley, R.; De La Calle-Prieto, F.; Diaz-Menendez, M. Imported infectious diseases in migrants from Latin America: A retrospective study from a referral centre for tropical diseases in Spain, 2017–2022. Travel. Med. Infect. Dis. 2024, 59, 102708. [Google Scholar] [CrossRef]
- Giancola, M.L.; Angheben, A.; Scorzolini, L.; Carrara, S.; Petrone, A.; Vulcano, A.; Lionetti, R.; Corpolongo, A.; Marrone, R.; Faraglia, F.; et al. Chagas Disease in the Non-Endemic Area of Rome, Italy: Ten Years of Experience and a Brief Overview. Infect. Dis. Rep. 2024, 16, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, N.S.; Montgomery, S.P. Chagas Disease. Ann. Intern. Med. 2023, 176, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Y.; Pinto, A.; Pett, S. Chagas disease in Australia and New Zealand: Risks and needs for public health interventions. Trop. Med. Int. Health 2014, 19, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.; Agudelo Higuita, N.I.; Hamer, S.A.; Ibarra-Cerdena, C.N.; Valdez-Tah, A.; Stigler Granados, P.; Hamer, G.L.; Vingiello, M.; Beatty, N.L. Climate change and Trypanosoma cruzi transmission in North and central America. Lancet Microbe 2024, 5, 100946. [Google Scholar] [CrossRef]
- WHO. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 6 January 2025).
- Melo, R.F.P.; Guarneri, A.A.; Silber, A.M. The Influence of Environmental Cues on the Development of Trypanosoma cruzi in Triatominae Vector. Front. Cell Infect. Microbiol. 2020, 10, 27. [Google Scholar] [CrossRef]
- Loshouarn, H.; Guarneri, A.A. The interplay between temperature, Trypanosoma cruzi parasite load, and nutrition: Their effects on the development and life-cycle of the Chagas disease vector Rhodnius prolixus. PLoS Negl. Trop. Dis. 2024, 18, e0011937. [Google Scholar] [CrossRef]
- Pedra-Rezende, Y.; Fernandes, M.C.; Mesquita-Rodrigues, C.; Stiebler, R.; Bombaca, A.C.S.; Pinho, N.; Cuervo, P.; De Castro, S.L.; Menna-Barreto, R.F.S. Starvation and pH stress conditions induced mitochondrial dysfunction, ROS production and autophagy in Trypanosoma cruzi epimastigotes. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166028. [Google Scholar] [CrossRef]
- Garcia, E.S.; Gonzalez, M.S.; de Azambuja, P.; Baralle, F.E.; Fraidenraich, D.; Torres, H.N.; Flawia, M.M. Induction of Trypanosoma cruzi metacyclogenesis in the gut of the hematophagous insect vector, Rhodnius prolixus, by hemoglobin and peptides carrying alpha D-globin sequences. Exp. Parasitol. 1995, 81, 255–261. [Google Scholar] [CrossRef]
- De Lima, A.R.; Navarro, M.C.; Arteaga, R.Y.; Contreras, V.T. Cultivation of Trypanosoma cruzi epimastigotes in low glucose axenic media shifts its competence to differentiate at metacyclic trypomastigotes. Exp. Parasitol. 2008, 119, 336–342. [Google Scholar] [CrossRef]
- Wainszelbaum, M.J.; Belaunzaran, M.L.; Lammel, E.M.; Florin-Christensen, M.; Florin-Christensen, J.; Isola, E.L. Free fatty acids induce cell differentiation to infective forms in Trypanosoma cruzi. Biochem. J. 2003, 375 Pt 3, 705–712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamedi, A.; Botelho, L.; Britto, C.; Fragoso, S.P.; Umaki, A.C.; Goldenberg, S.; Bottu, G.; Salmon, D. In vitro metacyclogenesis of Trypanosoma cruzi induced by starvation correlates with a transient adenylyl cyclase stimulation as well as with a constitutive upregulation of adenylyl cyclase expression. Mol. Biochem. Parasitol. 2015, 200, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.C.B.Q.; Rosa, D.S.; Soares, M.J. Differentiation of Trypanosoma cruzi epimastigotes: Metacyclogenesis and Adhesion to Substrate Are Triggered by Nutritional Stress. J. Parasitol. 2000, 86, 1213–1218. [Google Scholar] [CrossRef]
- Garcia-Huertas, P.; Cuesta-Astroz, Y.; Araque-Ruiz, V.; Cardona-Castro, N. Transcriptional changes during metacyclogenesis of a Colombian Trypanosoma cruzi strain. Parasitol. Res. 2023, 122, 625–634. [Google Scholar] [CrossRef]
- Losinno, A.D.; Martinez, S.J.; Labriola, C.A.; Carrillo, C.; Romano, P.S. Induction of autophagy increases the proteolytic activity of reservosomes during Trypanosoma cruzi metacyclogenesis. Autophagy 2021, 17, 439–456. [Google Scholar] [CrossRef]
- Rodriguez Duran, J.; Munoz-Calderon, A.; Gomez, K.A.; Potenza, M. In vitro differentiation of Trypanosoma cruzi epimastigotes into metacyclic trypomastigotes using a biphasic medium. STAR Protoc. 2021, 2, 100703. [Google Scholar] [CrossRef]
- Contreras, V.T.; Salles, J.M.; Thomas, N.; Morel, C.M.; Goldenberg, S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem. Parasitol. 1985, 16, 315–327. [Google Scholar] [CrossRef]
- Goncalves, C.S.; Avila, A.R.; de Souza, W.; Motta, M.C.M.; Cavalcanti, D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasit. Vectors 2018, 11, 83. [Google Scholar] [CrossRef]
- Alarcon, M.; Colasante, C.; Araujo, S.; Gutierrez-Marin, R.; Cazorla-Perfetti, D.; Sandoval-Ramirez, C.M. Metacyclogenesis of Trypanosoma cruzi in B. ferroae (Reduviidae: Triatominae) and feces infectivity under laboratory conditions. Biomedica 2021, 41, 179–186. [Google Scholar] [CrossRef]
- Kollien, A.H.; Schaub, G.A. The development of Trypanosoma cruzi in triatominae. Parasitol. Today 2000, 16, 381–387. [Google Scholar] [CrossRef]
- Contreras, V.T.; De Lima, A.R.; Zorrilla, G. Trypanosoma cruzi: Maintenance in culture modify gene and antigenic expression of metacyclic trypomastigotes. Mem. Inst. Oswaldo Cruz 1998, 93, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Rondinelli, E.; Silva, R.; Carvalho, J.F.; de Almeida Soares, C.M.; de Carvalho, E.F.; de Castro, F.T. Trypanosoma cruzi: An in vitro cycle of cell differentiation in axenic culture. Exp. Parasitol. 1988, 66, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Alonso, V.L. Ultrastructure Expansion Microscopy (U-ExM) in Trypanosoma cruzi: Localization of tubulin isoforms and isotypes. Parasitol. Res. 2022, 121, 3019–3024. [Google Scholar] [CrossRef]
- de Hernandez, M.A.; Martinez Peralta, G.; Vena, R.; Alonso, V.L. Ultrastructural Expansion Microscopy in Three In Vitro Life Cycle Stages of Trypanosoma cruzi. J. Vis. Exp. 2023, 12, 195. [Google Scholar] [CrossRef]
- Schaub, G.A. Interaction of Trypanosoma cruzi, Triatomines and the Microbiota of the Vectors-A Review. Microorganisms 2024, 12, 855. [Google Scholar] [CrossRef]
- Perlowagora-Szumlewicz, A.; Moreira, C.J. In vivo differentiation of Trypanosoma cruzi—1. Experimental evidence of the influence of vector species on metacyclogenesis. Mem. Inst. Oswaldo Cruz 1994, 89, 603–618. [Google Scholar] [CrossRef]
- Carvalho-Moreira, C.J.; Spata, M.C.; Coura, J.R.; Garcia, E.S.; Azambuja, P.; Gonzalez, M.S.; Mello, C.B. In vivo and in vitro metacyclogenesis tests of two strains of Trypanosoma cruzi in the triatomine vectors Triatoma pseudomaculata and Rhodnius neglectus: Short/long-term and comparative study. Exp. Parasitol. 2003, 103, 102–111. [Google Scholar] [CrossRef]
- Silvestrini, M.M.A.; Alessio, G.D.; Frias, B.E.D.; Sales Junior, P.A.; Araujo, M.S.S.; Silvestrini, C.M.A.; Brito Alvim de Melo, G.E.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Martins, H.R. New insights into Trypanosoma cruzi genetic diversity, and its influence on parasite biology and clinical outcomes. Front. Immunol. 2024, 15, 1342431. [Google Scholar] [CrossRef]
- Abegg, C.P.; Abreu, A.P.; Silva, J.L.; Araujo, S.M.; Gomes, M.L.; Ferreira, E.C.; Toledo, M.J. Polymorphisms of blood forms and in vitro metacyclogenesis of Trypanosoma cruzi I, II, and IV. Exp. Parasitol. 2017, 176, 8–15. [Google Scholar] [CrossRef]
- Caceres, T.M.; Cruz-Saavedra, L.; Patino, L.H.; Ramirez, J.D. Comparative analysis of metacyclogenesis and infection curves in different discrete typing units of Trypanosoma cruzi. Parasitol. Res. 2024, 123, 181. [Google Scholar] [CrossRef]
- Urdaneta-Morales, S. Trypanosoma cruzi: Attenuation of virulence by culture in tissues. Ann. Parasitol. Hum. Comp. 1983, 58, 317–324. [Google Scholar] [CrossRef]
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Memórias Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdomo, V.; Boselli, V.; Manarin, R.; Serra, E. Optimizing In Vitro Metacyclogenesis: Strain-Specific Variability in Trypanosoma cruzi Responses to Nutritional and pH Stress. Parasitologia 2025, 5, 20. https://doi.org/10.3390/parasitologia5020020
Perdomo V, Boselli V, Manarin R, Serra E. Optimizing In Vitro Metacyclogenesis: Strain-Specific Variability in Trypanosoma cruzi Responses to Nutritional and pH Stress. Parasitologia. 2025; 5(2):20. https://doi.org/10.3390/parasitologia5020020
Chicago/Turabian StylePerdomo, Virginia, Victoria Boselli, Romina Manarin, and Esteban Serra. 2025. "Optimizing In Vitro Metacyclogenesis: Strain-Specific Variability in Trypanosoma cruzi Responses to Nutritional and pH Stress" Parasitologia 5, no. 2: 20. https://doi.org/10.3390/parasitologia5020020
APA StylePerdomo, V., Boselli, V., Manarin, R., & Serra, E. (2025). Optimizing In Vitro Metacyclogenesis: Strain-Specific Variability in Trypanosoma cruzi Responses to Nutritional and pH Stress. Parasitologia, 5(2), 20. https://doi.org/10.3390/parasitologia5020020







