The Influence of Alkanethiols on the Production of Hydrophobic Gold Nanoparticles via Pulsed Laser Ablation in Liquids
Abstract
:1. Introduction
2. Materials and Methods
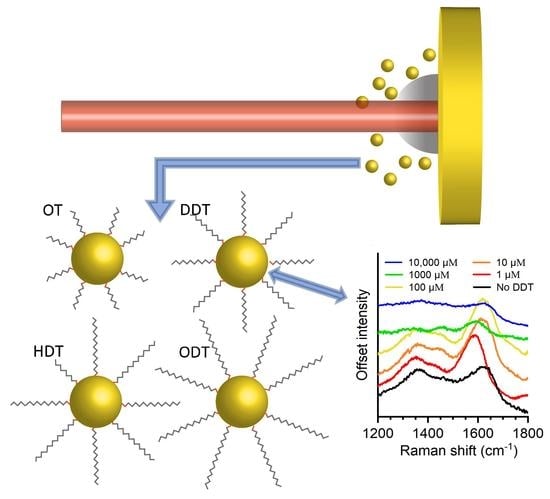
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.-H.; Cho, H.-Y.; Choi, H.K.; Lee, J.-Y.; Choi, J.-W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull 2010, 43, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Jeong, E.H.; Jung, G.; Hong, C.A.; Lee, H. Gold nanoparticle (AuNP)-Based drug delivery and molecular imaging for biomedical applications. Arch. Pharm. Res. 2014, 37, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Tian, H.; Deng, M.; Chen, X. Gold Nanoparticles for Cancer Theranostics. Chin. J. Chem. 2015, 33, 1001–1010. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Kister, T.; Monego, D.; Mulvaney, P.; Widmer-Cooper, A.; Kraus, T. Colloidal Stability of Apolar Nanoparticles: The Role of Particle Size and Ligand Shell Structure. ACS Nano 2018, 12, 5969–5977. [Google Scholar] [CrossRef] [Green Version]
- Monego, D.; Kister, T.; Kirkwood, N.; Mulvaney, P.; Widmer-Cooper, A.; Kraus, T. Colloidal Stability of Apolar Nanoparticles: Role of Ligand Length. Langmuir 2018, 34, 12982–12989. [Google Scholar] [CrossRef] [Green Version]
- Raveendran, A.; DeWolf, C.E.; Bu, W.; McWhirter, S.; Meron, M.; Lin, B.; Meli, M.-V. Langmuir Films of n-Alkanethiol-Capped Gold Nanoparticles and n-Alkanes: Interfacial Mixing Scenarios Assessed by X-ray Reflectivity and Grazing Incidence Diffraction. J. Phys. Chem. C 2018, 122, 2975–2982. [Google Scholar] [CrossRef]
- Martin, J.E.; Wilcoxon, J.P.; Odinek, A.J.; Provencio, P. Control of the Interparticle Spacing in Gold Nanoparticle Superlattices. J. Phys. Chem. B 2000, 104, 9475–9486. [Google Scholar] [CrossRef] [Green Version]
- Kanehara, M.; Oumi, Y.; Sano, T.; Teranishi, T. Formation of Low-Symmetric 2D Superlattices of Gold Nanoparticles through Surface Modification by Acid—Base Interaction. J. Am. Chem. Soc. 2003, 125, 8708–8709. [Google Scholar] [CrossRef]
- Chen, C.-F.; Tzeng, S.-D.; Chen, H.-Y.; Lin, K.-J.; Gwo, S. Tunable Plasmonic Response from Alkanethiolate-Stabilized Gold Nanoparticle Superlattices: Evidence of Near-Field Coupling. J. Am. Chem. Soc. 2008, 130, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Fisher, E.A.; Meli, M.-V.; Trudel, S. Tuning the magnetism of gold nanoparticles by changing the thiol coating. Nanoscale 2020, 12, 19797–19803. [Google Scholar] [CrossRef]
- Mathiyazhakan, M.; Wiraja, C.; Xu, C. A Concise Review of Gold Nanoparticles-Based Photo-Responsive Liposomes for Controlled Drug Delivery. Nano-Micro Lett. 2018, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- DiSalvo, G.M.; Robinson, A.R.; Aly, M.S.; Hoglund, E.R.; O’Malley, S.M.; Griepenburg, J.C. Polymersome Poration and Rupture Mediated by Plasmonic Nanoparticles in Response to Single-Pulse Irradiation. Polymers 2020, 12, 2381. [Google Scholar] [CrossRef] [PubMed]
- Trout, C.J.; Clapp, J.A.; Griepenburg, J.C. Plasmonic carriers responsive to pulsed laser irradiation: A review of mechanisms, design, and applications. New J. Chem. 2021. [Google Scholar] [CrossRef]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Schulz, F.; Homolka, T.; Bastús, N.G.; Puntes, V.; Weller, H.; Vossmeyer, T. Little Adjustments Significantly Improve the Turkevich Synthesis of Gold Nanoparticles. Langmuir 2014, 30, 10779–10784. [Google Scholar] [CrossRef]
- Perala, S.R.K.; Kumar, S. On the Mechanism of Metal Nanoparticle Synthesis in the Brust–Schiffrin Method. Langmuir 2013, 29, 9863–9873. [Google Scholar] [CrossRef]
- Tomko, J.; O’Malley, S.M.; Trout, C.; Naddeo, J.; Jimenez, R.; Griepenburg, J.C.; Soliman, W.; Bubb, D.M. Cavitation bubble dynamics and nanoparticle size distributions in laser ablation in liquids. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 368–372. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805–3821. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Meneghetti, M. What controls the composition and the structure of nanomaterials generated by laser ablation in liquid solution? Phys. Chem. Chem. Phys. 2013, 15, 3027–3046. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Jakobi, J.; Hörtinger, A.; Barcikowski, S. In situ conjugation—Tailored nanoparticle-conjugates by laser ablation in liquids. J. Laser Micro Nanoeng 2009, 4, 71–74. [Google Scholar] [CrossRef]
- Walter, J.G.; Petersen, S.; Stahl, F.; Scheper, T.; Barcikowski, S. Laser ablation-based one-step generation and bio-functionalization of gold nanoparticles conjugated with aptamers. J. Nanobiotechnol. 2010, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcikowski, S.; Jakobi, J.; Petersen, S.; Hahn, A.; Bärsch, N.; Chichkov, B. Adding functionality to metal nanoparticles during femtosecond laser ablation in liquids. ICALEO 2007, 404. [Google Scholar] [CrossRef]
- Compagnini, G.; Scalisi, A.A.; Puglisi, O.; Spinella, C. Synthesis of gold colloids by laser ablation in thiol-alkane solutions. J. Mater. Res. 2004, 19, 2795–2798. [Google Scholar] [CrossRef]
- Letzel, A.; Reich, S.; Rolo, T.D.S.; Kanitz, A.; Hoppius, J.; Rack, A.; Olbinado, M.P.; Ostendorf, A.; Gokce, B.; Plech, A.; et al. Time and Mechanism of Nanoparticle Functionalization by Macromolecular Ligands during Pulsed Laser Ablation in Liquids. Langmuir 2019, 35, 3038–3047. [Google Scholar] [CrossRef]
- Kurita, H.; Takami, A.; Koda, S. Size reduction of gold particles in aqueous solution by pulsed laser irradiation. Appl. Phys. Lett. 1998, 72, 789–791. [Google Scholar] [CrossRef]
- González-Rubio, G.; Guerrero-Martínez, A.; Liz-Marzán, L.M. Reshaping, Fragmentation, and Assembly of Gold Nanoparticles Assisted by Pulse Lasers. Acc. Chem. Res. 2016, 49, 678–686. [Google Scholar] [CrossRef] [Green Version]
- Fales, A.M.; Vogt, W.C.; Pfefer, T.J.; Ilev, I.K. Quantitative Evaluation of Nanosecond Pulsed Laser-Induced Photomodification of Plasmonic Gold Nanoparticles. Sci. Rep. 1570. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Polizzi, S.; Meneghetti, M.; Amendola, V. Laser ablation synthesis of gold nanoparticles in organic solvents. J. Phys. Chem. B 2006, 110, 7232–7237. [Google Scholar] [CrossRef]
- Amendola, V.; Rizzi, G.A.; Polizzi, S.; Meneghetti, M. Synthesis of Gold Nanoparticles by Laser Ablation in Toluene: Quenching and Recovery of the Surface Plasmon Absorption. J. Phys. Chem. B 2005, 109, 23125–23128. [Google Scholar] [CrossRef] [PubMed]
- Compagnini, G.; Scalisi, A.A.; Puglisi, O. Production of gold nanoparticles by laser ablation in liquid alkanes. J. Appl. Phys. 2003, 94, 7874–7877. [Google Scholar] [CrossRef]
- Giorgetti, E.; Muniz-Miranda, M.; Marsili, P.; Scarpellini, D.; Giammanco, F. Stable gold nanoparticles obtained in pure acetone by laser ablation with different wavelengths. J. Nanopart. Res. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Hendel, T.; Wuithschick, M.; Kettemann, F.; Birnbaum, A.; Rademann, K.; Polte, J. In Situ Determination of Colloidal Gold Concentrations with UV–Vis Spectroscopy: Limitations and Perspectives. Anal. Chem. 2014, 86, 11115–11124. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, C.G.; Buhrman, R.A. Ultrafine metal particles. J. Appl. Phys. 1976, 47, 2200–2219. [Google Scholar] [CrossRef]
- He, Y.Q.; Liu, S.P.; Kong, L.; Liu, Z.F. A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2861–2866. [Google Scholar] [CrossRef]
- Ansar, S.M.; Gadogbe, M.; Siriwardana, K.; Howe, J.Y.; Dogel, S.; Hosseinkhannazer, H.; Collier, W.E.; Rodriguez, J.M.D.; Zou, S.; Zhang, D. Dispersion Stability, Ligand Structure and Conformation, and SERS Activities of 1-Alkanethiol Functionalized Gold and Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 24925–24934. [Google Scholar] [CrossRef]
- Wang, G.; Sun, W. Optical Limiting of Gold Nanoparticle Aggregates Induced by Electrolytes. J. Phys. Chem. B 2006, 110, 20901–20905. [Google Scholar] [CrossRef]
- Takami, A.; Kurita, H.; Koda, S. Laser-Induced Size Reduction of Noble Metal Particles. J. Phys. Chem. B 1999, 103, 1226–1232. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free Silver Nanoparticles Synthesized by Laser Ablation in Organic Solvents and Their Easy Functionalization. Langmuir 2007, 23, 6766–6770. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Terakawa, S.; Hayashi, S.; Asaka, T.; Itoigawa, F.; Ono, S.; Takayanagi, J. Carbonization of Silicon Nanoparticles via Ablation Induced by Femtosecond Laser Pulses in Hexane. Arab. J. Sci. Eng. 2017, 42, 4221–4226. [Google Scholar] [CrossRef]
- Matsue, T.; Yamada, Y.; Kobayashi, Y. Iron carbide nanoparticles produced by laser ablation in organic solvent. Hyperfine Interact. 2012, 205, 31–35. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.P.; Srikanth, A.; Vishwanathan, V.; Chary, K.V.R. Vapor Phase Oxidation of Benzyl Alcohol over Nano Au/SBA-15 Catalysts: Effect of Preparation Methods. Catal. Lett. 2015, 146, 35–46. [Google Scholar] [CrossRef]
- Conte, M.; Miyamura, H.; Kobayashi, S.; Chechik, V. Spin Trapping of Au−H Intermediate in the Alcohol Oxidation by Supported and Unsupported Gold Catalysts. J. Am. Chem. Soc. 2009, 131, 7189–7196. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Efficient Aerobic Oxidation of Alcohols using a Hydrotalcite-Supported Gold Nanoparticle Catalyst. Adv. Synth. Catal. 2009, 351, 1890–1896. [Google Scholar] [CrossRef]
- Zou, X.; Tao, Z.; Asefa, T. Semiconductor and Plasmonic Photocatalysis for Selective Organic Transformations. Curr. Org. Chem. 2013, 17, 1274–1287. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fujita, T.; Takeyasu, N. Plasmon-mediated chemical transformation from alkane to alkene on a silver nanoparticle array under 532 nm excitation. Phys. Chem. Chem. Phys. 2019, 21, 7502–7507. [Google Scholar] [CrossRef]
- Hallett-Tapley, G.L.; Silvero, M.J.; González-Béjar, M.; Grenier, M.; Netto-Ferreira, J.C.; Scaiano, J.C. Plasmon-Mediated Catalytic Oxidation of sec-Phenethyl and Benzyl Alcohols. J. Phys. Chem. C 2011, 115, 10784–10790. [Google Scholar] [CrossRef]
- Battino, R. (Ed.) Oxygen and Ozone, In IUPAC Solubility Data Series; Pergamon Press: Oxford, UK, 1981; Volume 7. [Google Scholar]
- Fogg, P.G.T. (Ed.) Carbon Dioxide in Non-Aqueous Solvents at Pressures Less Than 200 KPA. In UPAC Solubility Data Series; Pergamon Press: Oxford, UK, 1992; Volume 50. [Google Scholar] [CrossRef]
- Matthews, M.J.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Endo, M. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, R6585–R6588. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Jensen, G.C.; Krause, C.E.; Sotzing, G.A.; Rusling, J.F. Inkjet-printed gold nanoparticle electrochemical arrays on plastic. Application to immunodetection of a cancer biomarker protein. Phys. Chem. Chem. Phys. 2011, 13, 4888–4894. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing-Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rizzo, A.; Mazzeo, M.; Carbone, L.; Manna, L.; Cingolani, R.; Gigli, G. White organic light-emitting devices with CdSe/ZnS quantum dots as a red emitter. J. Appl. Phys. Phys. 2005, 97, 113501. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trout, C.J.; Kumpf, P.; Sipps, K.; Griepenburg, J.C.; O’Malley, S.M. The Influence of Alkanethiols on the Production of Hydrophobic Gold Nanoparticles via Pulsed Laser Ablation in Liquids. Nanomanufacturing 2021, 1, 98-108. https://doi.org/10.3390/nanomanufacturing1030009
Trout CJ, Kumpf P, Sipps K, Griepenburg JC, O’Malley SM. The Influence of Alkanethiols on the Production of Hydrophobic Gold Nanoparticles via Pulsed Laser Ablation in Liquids. Nanomanufacturing. 2021; 1(3):98-108. https://doi.org/10.3390/nanomanufacturing1030009
Chicago/Turabian StyleTrout, Cory J., Paul Kumpf, Karli Sipps, Julianne C. Griepenburg, and Sean M. O’Malley. 2021. "The Influence of Alkanethiols on the Production of Hydrophobic Gold Nanoparticles via Pulsed Laser Ablation in Liquids" Nanomanufacturing 1, no. 3: 98-108. https://doi.org/10.3390/nanomanufacturing1030009
APA StyleTrout, C. J., Kumpf, P., Sipps, K., Griepenburg, J. C., & O’Malley, S. M. (2021). The Influence of Alkanethiols on the Production of Hydrophobic Gold Nanoparticles via Pulsed Laser Ablation in Liquids. Nanomanufacturing, 1(3), 98-108. https://doi.org/10.3390/nanomanufacturing1030009