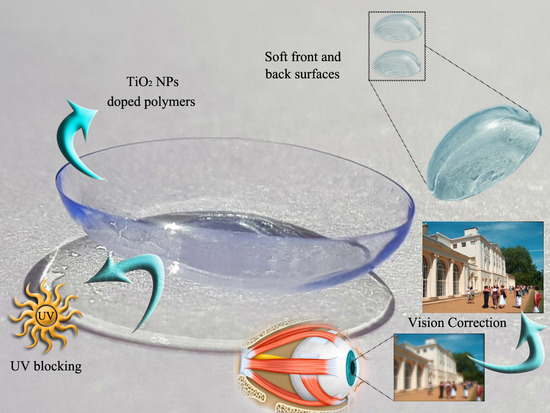
Nano-Titanium Oxide in Polymeric Contact Lenses: Short Communication
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, P.B.; Efron, N.; Woods, C.A.; Santodomingo-Rubido, J. International survey of orthokeratology contact lens fitting. Contact Lens Anterior Eye 2018, 42, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Alipour, F.; Khaheshi, S.; Soleimanzadeh, M.; Heidarzadeh, S.; Heydarzadeh, S. Contact Lens-related Complications: A Review. J. Ophthalmic Vis. Res. 2017, 12, 193–204. [Google Scholar] [CrossRef]
- Ohta, K.; Shimamura, I.; Shiraishi, A.; Ohashi, Y. Confocal Microscopic Observations of Stromal Keratocytes in Soft and Rigid Contact Lens Wearers. Cornea 2012, 31, 66–73. [Google Scholar] [CrossRef]
- Liesegang, T.J. Physiologic changes of the cornea with contact lens wear. CLAO J. 2002, 28, 12–27. [Google Scholar]
- Bennett, E.S.; Weissman, B.A. Clinical Contact Lens Practice; Lippincott Williams & Wilkins: Philadeplhia, PA, USA, 2005; pp. 475–477. [Google Scholar]
- Bennett, E.S.; Vinita, A.H. Clinical Manual of Contact Lenses, 4th ed.; Lippincott Williams & Wilkins: Philadeplhia, PA, USA, 2015. [Google Scholar]
- Brennan, N.A.; Efron, N.; Bruce, A.S.; Duldig, D.I.; Russo, N.J. Dehydration of hydrogel lenses: Environmental influences during normal wear. Am. J. Optom. Physiol. Opt. 1988, 65, 277–281. [Google Scholar] [CrossRef]
- Turhan, S.; Toker, E. Optical coherence tomography to evaluate the interaction of different edge designs of four different silicone hydrogel lenses with the ocular surface. Clin. Ophthalmol. 2015, 9, 935–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richdale, K.; Sinnott, L.T.; Skadahl, E.; Nichols, J.J. Frequency of and Factors Associated with Contact Lens Dissatisfaction and Discontinuation. Cornea 2007, 26, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaker, L.M.; Al-Amiery, A.A.; Kadhum, A.A.H.; Takriff, M.S. Manufacture of Contact Lens of Nanoparticle-Doped Polymer Complemented with ZEMAX. Nanomaterials 2020, 10, 2028. [Google Scholar] [CrossRef]
- Demir, M.M.; Koynov, K.; Akbey, Ü.; Bubeck, C.; Park, I.; Lieberwirth, A.I.; Wegner, G. Optical Properties of Composites of PMMA and Surface-Modified Zincite Nanoparticles. Macromolecules 2007, 40, 1089–1100. [Google Scholar] [CrossRef] [Green Version]
- Al-Obaidi, A.A.; Salman, A.J.; Yousif, A.R.; Al-Mamoori, D.H.; Mussa, M.H.; Gaaz, T.S.; Kadhum, A.A.H.; Takriff, M.S.; Al-Amiery, A.A. Characterization the effects of nanofluids and heating on flow in a baffled vertical channel. Int. J. Mech. Mater. Eng. 2019, 14, 11. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Zhu, W.; Cui, Y. Synthesis of Polymeric Nanocomposite Hydrogels Containing the Pendant ZnS Nanoparticles: Approach to Higher Refractive Index Optical Polymeric Nanocomposites. Macromolecules 2018, 51, 2672–2681. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, H.; Wan, L.; Zhao, Q.; Jiang, T.; Wang, B.; Wang, S. Potential application of functional porous TiO2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater. 2015, 13, 354–363. [Google Scholar] [CrossRef]
- Carlander, U.; Li, D.; Jolliet, O.; Emond, C.; Johanson, G. Toward a general physiologically-based pharmacokinetic model for intravenously injected nanoparticles. Int. J. Nanomed. 2016, 11, 625–640. [Google Scholar] [CrossRef] [Green Version]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Hautala, J.; Kääriäinen, T.; Hoppu, P.; Kemell, M.; Heinämäki, J.; Cameron, D.; George, S.; Juppo, A.M. Atomic layer deposition—A novel method for the ultrathin coating of minitablets. Int. J. Pharm. 2017, 531, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Razavi, H.; Janfaza, S. Medical nanobiosensors: A tutorial review. Nanomed. J. 2015, 2, 74–87. Available online: http://nmj.mums.ac.ir (accessed on 24 June 2022).
- Kamil, F.; Hubiter, K.A.; Abed, T.K.; Al-Amiery, A.A. Synthesis of Aluminum and Titanium Oxides Nanoparticles via Sol-Gel Method: Optimization for the Minimum Size. J. Nanosci. Technol. 2016, 2, 37–39. [Google Scholar]
- Schaub, M.; Schwiegerling, J.; Fest, E.C.; Symmons, A.; Shepard, R.H. Molded Optics: Design and Manufacture; Taylor and Francis Group LLC: Oxfordshire, UK, 2011. [Google Scholar]
- Liou, H.-L.; Brennan, N.A. Anatomically accurate, finite model eye for optical modeling. J. Opt. Soc. Am. A 1997, 14, 1684–1695. [Google Scholar] [CrossRef]
- Dua, S.; Acharya, R.; Ng, E.Y.K. Computational Analysis of the Human Eye with Applications; World Scientific: Singapore, 2011. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Jin, T.; Costa, M.; Chen, X. Titanium. In Handbook on the Toxicology of Metals, 5th ed.; Elsevier Inc: Amsterdam, The Netherlands, 2021; pp. 857–868. [Google Scholar]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2014, 45, 289–299. [Google Scholar] [CrossRef]
- Filipecki, J.; Kocela, A.; Korzekwa, P.; Miedzinski, R.; Filipecka-Szymczyk, K.; Golis, E. Structural study of polymer hydrogel contact lenses by means of positron annihilation lifetime spectroscopy and UV–Vis–NIR methods. J. Mater. Sci. Mater. Med. 2013, 24, 1837–1842. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, N.; Hayashi, T. Preparation and characterization of TiO2 and polymer nanocomposite films with high refractive index. J. Appl. Polym. Sci. 2007, 105, 3662–3672. [Google Scholar] [CrossRef]
- Watson, A.B. A formula for the mean human optical modulation transfer function as a function of pupil size Computing the mean radial MTF. J. Vis. 2013, 13, 18. [Google Scholar] [CrossRef] [Green Version]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegel, R.W.; Schadler, L.S. TiO2 nanocomposites with high refractive index and transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Gnanaseelan, M.; Kalita, U.; Janke, A.; Pionteck, J.; Voit, B.; Singha, N.K. All methacrylate block copolymer/TiO2 nanocomposite via ATRP and in-situ sol-gel process. Mater. Today Commun. 2019, 22, 100728. [Google Scholar] [CrossRef]
- Xi, Y.; Qi, Y.; Mao, Z.; Yang, Z.; Zhang, J. Surface hydrophobic modification of TiO2 and its application to preparing PMMA/TiO2 composite cool material with improved hydrophobicity and anti-icing property. Constr. Build. Mater. 2020, 266, 120916. [Google Scholar] [CrossRef]
- Maldonado-Codina, C.; Efron, N. Dynamic wettability of pHEMA-based hydrogel contact lenses. Ophthalmic Physiol. Opt. 2006, 26, 408–418. [Google Scholar] [CrossRef]
- Sugumaran, S.; Bellan, C. Transparent nano composite PVA–TiO2 and PMMA–TiO2 thin films: Optical and dielectric properties. Optik 2014, 125, 5128–5133. [Google Scholar] [CrossRef]
- Tao, P.; Viswanath, A.; Li, Y.; Siegel, R.W.; Benicewicz, B.C.; Schadler, L.S. Bulk transparent epoxy nanocomposites filled with poly(glycidyl methacrylate) brush-grafted TiO2 nanoparticles. Polymer 2013, 54, 1639–1646. [Google Scholar] [CrossRef]
- Opdahl, A.; Kim, S.H.; Koffas, T.S.; Marmo, C.; Somorjai, G.A. Surface mechanical properties of pHEMA contact lenses: Viscoelastic and adhesive property changes on exposure to controlled humidity. J. Biomed. Mater. Res. 2003, 67, 350–356. [Google Scholar] [CrossRef]
- Saptaji, K.; Iza, N.R.; Widianingrum, S.; Mulia, V.K.; Setiawan, I. Poly(2-Hydroxyethyl Methacrylate) Hydrogels for Contact Lens Applications—A Review. Makara J. Sci. 2021, 25, 3. [Google Scholar] [CrossRef]
- Hecht, E. Optics, 5th ed.; Pearson Education Limited: London, UK, 2017. [Google Scholar]
- Castejon-Mochon, J.F.; Lopez-Gil, N.; Benito, A.; Artal, P. Ocular wave-front aberration statistics in a normal young population. Vis. Res. 2002, 42, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- Seinder, L.; Spinelli, H.J.; Ali, M.I.; Weintraub, L. Silicone-Containing Contact Lens Polymers, Oxygen Permeable Contact Lenses and Methods for Making There Lenses and Treating Patients with Visual Impairment. U.S. Patent 5331067, 19 July 1994. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaker, L.M.; Alamiery, A.A.; Takriff, M.; Wan Isahak, W.N.R. Nano-Titanium Oxide in Polymeric Contact Lenses: Short Communication. Nanomanufacturing 2022, 2, 71-81. https://doi.org/10.3390/nanomanufacturing2030006
Shaker LM, Alamiery AA, Takriff M, Wan Isahak WNR. Nano-Titanium Oxide in Polymeric Contact Lenses: Short Communication. Nanomanufacturing. 2022; 2(3):71-81. https://doi.org/10.3390/nanomanufacturing2030006
Chicago/Turabian StyleShaker, Lina Mohammed, Ahmed A. Alamiery, Mohd Takriff, and Wan Nor Roslam Wan Isahak. 2022. "Nano-Titanium Oxide in Polymeric Contact Lenses: Short Communication" Nanomanufacturing 2, no. 3: 71-81. https://doi.org/10.3390/nanomanufacturing2030006
APA StyleShaker, L. M., Alamiery, A. A., Takriff, M., & Wan Isahak, W. N. R. (2022). Nano-Titanium Oxide in Polymeric Contact Lenses: Short Communication. Nanomanufacturing, 2(3), 71-81. https://doi.org/10.3390/nanomanufacturing2030006