Manufacturing Functional Polymer Surfaces by Direct Laser Interference Patterning (DLIP): A Polymer Science View
Abstract
:1. Introduction
1.1. Theory of Formation of Light Patterns by Interference
1.2. Interaction of Light with Polymer Materials
2. Direct Laser Interference Patterning (DLIP) of Polymer Materials
2.1. Homopolymers
2.1.1. Poly(ethyleneterephthalate) (PET) and Related Polyesters
2.1.2. Polyimide (PI)
2.1.3. Polystyrene (PS)
2.1.4. Polycarbonate (PC)
2.1.5. Polymethylmethacrylate (PMMA) and Related Acrylates
2.1.6. Poly(etheretherketone) (PEEK)
2.1.7. Triazene Polymer
2.2. Copolymers
2.2.1. Polyurethane
 ) [119]. Changing the groups linked to the urethane (R1, R2), it is possible to produce materials with a variety of properties, from flexible foams, to tough engineering thermoplastics [120]. The structuring of the surface is relevant for the extensive application of polyurethanes in biomedical devices [121].
) [119]. Changing the groups linked to the urethane (R1, R2), it is possible to produce materials with a variety of properties, from flexible foams, to tough engineering thermoplastics [120]. The structuring of the surface is relevant for the extensive application of polyurethanes in biomedical devices [121].2.2.2. Poly(styrene-co-acrylonitrile) (SAN)
2.2.3. Poly(methylmethacrylate-co-styrene) (P(MMA-co-S)
2.2.4. Poly(styrene-co-glycidylmethacrylate) P(S-co-GMA)
2.3. Hydrogels
2.3.1. PNIPAM
2.3.2. Poly(hydroxyethylmethacrylate), PHEMA
2.3.3. Safrofilcon A
2.4. Conducting Polymers
2.4.1. Polyaniline (PANI)
2.4.2. Polypyrrole (PPy)
2.4.3. Poly(3,4-ethylenedioxythiophene) Poly(styrenesulfonate) (PEDOT:PSS)
3. Conclusions
4. Future Endeavors
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | poly(acrylonitrile-butadiene-styrene) |
| CE | cellulose |
| CHI | chitosan |
| CMC | carboxymethylcellulose |
| CP | conductive polymer |
| c-PAA | cross-linked poly(acrylic acid) |
| c-PAMPS | poly(2-acrylamido-2-methylpropanesulfonic acid) |
| c-PDAMAC | cross-linked polydiallyldimethylammonium chloride |
| DOE | diffractive optical element |
| EDS | energy dispersive X-ray spectroscopy |
| GC | glassy carbon |
| GMA | glycidylmethacrylate |
| HPC | hydroxypropylcellulose |
| ITO | indium tin oxide |
| LIFT | laser-induced forward transfer |
| LIPSS | laser-induced periodic surface structures |
| MMA | methylmethacrylate |
| MMI | modified Michelson interferometer |
| NCel | nitrocellulose |
| NIR | near-infrared range (800–2400 nm) |
| NMP | N-methylpyrrolidone |
| P(α-MeS) | poly(α-methylstyrene) |
| PA | poly(amide) (nylon 66) |
| PAN | polyacrylonitrile |
| PBT | polybutylene terephthalate |
| PC (BPA) | polycarbonate (with bisphenol A as diol) |
| PC | polycarbonate |
| PCBz | Polycarbazole |
| PDA | poly(dopamine) |
| PDMS | poly(dimethylsiloxane) |
| PE | polyethylene |
| PEDOT | poly(ethylenedioxythiophene) |
| PEEK | poly(ether ether ketone) |
| PEG | poly(ethyleneglycol) |
| PEN | poly(ethylene 2,6-naphthalate) |
| PEO | poly(ethyleneoxide) |
| PET | poly(ethyleneterephthalate) |
| PHEMA | poly(hydroxyethylmethacrylate) |
| PI | polyimide |
| PLA | polylactic acid |
| PMAA | poly(methacrylic acid) |
| PMMA | poly(methylmethacrylate) |
| PNIPAM | poly(N-isopropylacrylamide) |
| POAP | poly(o-aminophenol) |
| POM | polyoxymethylene |
| PP | polypropylene |
| PPD | poly(phenylenediamine) |
| PPV | poly(phenylenevinylene) |
| PPy | polypyrrole |
| PS | polystyrene |
| PSS | poly(styrene sulfonate) |
| PTh | polythiophene |
| PTPA | poly(triphenylamine) |
| PTT | poly(trimethyleneterephthalate) |
| PVA | poly(vinyl alcohol) |
| PVAc | poly(vinylacetate) |
| SAN | poly(styrene-co-acrylonitrile) |
| UV | ultraviolet range (200–380 nm) |
| Vis | visible range (380–800 nm) |
| α | coefficient of light intensity attenuation (cm−1) |
| ε | molar extinction coefficients (M−1 cm−1) |
References
- Voisiat, B.; Wang, W.; Holzhey, M.; Lasagni, A.F. Improving the homogeneity of diffraction based colours by fabricating periodic patterns with gradient spatial period using Direct Laser Interference Patterning. Sci. Rep. 2019, 9, 7801. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, C.W.J.; Škereň, M.; Najdek, D.; Černý, F. Superhydrophobic surface structures in thermoplastic polymers by interference lithography and thermal imprinting. Appl. Surf. Sci. 2009, 255, 9305–9310. [Google Scholar] [CrossRef]
- Samal, S.; Tyc, O.; Heller, L.; Šittner, P.; Malik, M.; Poddar, P.; Catauro, M.; Blanco, I. Study of interfacial adhesion between nickel-titanium shape memory alloy and a polymer matrix by laser surface pattern. Appl. Sci. 2020, 10, 2172. [Google Scholar] [CrossRef] [Green Version]
- Lomba, M.; Oriol, L.; Sánchez-Somolinos, C.; Grazú, V.; Moros, M.; Serrano, J.L.; De La Fuente, J.M. Cell adhesion on surface patterns generated by the photocrosslinking of hyperbranched polyesters with a trisdiazonium salt. React. Funct. Polym. 2013, 73, 499–507. [Google Scholar] [CrossRef]
- Alikhani, A.; Fathollahzadeh, M.; Hajihosseini, H.; Fathipour, M. An interesting route using electron-beam lithography and photolithography to pattern submicron interdigitated electrodes array for sensing applications. J. Iran. Chem. Soc. 2020, 17, 187–194. [Google Scholar] [CrossRef]
- Deku, F.; Cohen, Y.; Joshi-Imre, A.; Kanneganti, A.; Gardner, T.J.; Cogan, S.F. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J. Neural Eng. 2018, 15, 016007. [Google Scholar] [CrossRef]
- Proust, V.; Kirscher, Q.; Nguyen, T.K.N.; Obringer, L.; Ishii, K.; Rault, L.; Demange, V.; Berthebaud, D.; Ohashi, N.; Uchikoshi, T.; et al. Hafnium Oxide Nanostructured Thin Films: Electrophoretic Deposition Process and DUV Photolithography Patterning. Nanomaterials 2022, 12, 2334. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, E.A.; Han, J.; Choi, Y.-H.; Hahm, D.; Kang, C.J.; Bae, W.K.; Lim, J.; Cho, S.-Y. Nondestructive Direct Photolithography for Patterning Quantum Dot Films by Atomic Layer Deposition of ZnO. Adv. Mater. Interfaces 2022, 9, 2200835. [Google Scholar] [CrossRef]
- Duay, J.; Goran, J.M.; Stevenson, K.J. Facile fabrication of carbon ultramicro- to nanoelectrode arrays with tunable voltammetric response. Anal. Chem. 2014, 86, 11528–11532. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Kim, D.S. Robust topographical micro-patterning of nanofibrillar collagen gel by in situ photochemical crosslinking-assisted collagen embossing. Nanomaterials 2020, 10, 2574. [Google Scholar] [CrossRef]
- Fu, Y.; Soldera, M.; Wang, W.; Voisiat, B.; Lasagni, A.F. Picosecond laser interference patterning of periodical micro-architectures on metallic molds for hot embossing. Materials 2019, 12, 3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajfutdinow, M.; Uhlig, K.; Prager, A.; Schneider, C.; Abel, B.; Smith, D.M. Nanoscale patterning of self-assembled monolayer (SAM)-functionalised substrates with single molecule contact printing. Nanoscale 2017, 9, 15098–15106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, V.; Roch, T.; Lasagni, A.F. World record in high speed laser surface microstructuring of polymer and steel using direct laser interference patterning. Proc. SPIE Int. Soc. Opt. Eng. 2016, 9736, 97360Z. [Google Scholar] [CrossRef] [Green Version]
- Nebel, C.E. Laser interference structuring of α-Si:H. Mat. Res. Soc. Sym. Proc. 1996, 420, 117–128. [Google Scholar] [CrossRef]
- Müller, D.W.; Fox, T.; Grützmacher, P.G.; Suarez, S.; Mücklich, F. Applying Ultrashort Pulsed Direct Laser Interference Patterning for Functional Surfaces. Sci. Reps. 2020, 10, 3647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabris, D.; Lasagni, A.F.; Fredel, M.C.; Henriques, B. Direct laser interference patterning of bioceramics: A short review. Ceramics 2019, 2, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Ilcisin, K.J.; Fedosejevs, R. Direct production of gratings on plastic substrates using 248-nm krf laser radiation. Appl. Opt. 1987, 26, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Labeyrie, A.; Flamand, J. Spectrographic performance of holographically made diffraction gratings. Opt. Commun. 1969, 1, 5–8. [Google Scholar] [CrossRef]
- Lasagni, A.F.; Yuan, D.; Das, S. Layer-by-layer interference lithography of three-dimensional microstructures in SU. Adv. Eng. Mater. 2009, 11, 408–411. [Google Scholar] [CrossRef]
- Jurkevičiute, A.; Armakavičius, N.; Virganavičius, D.; Šimatonis, L.; Tamulevičius, T.; Tamulevičius, S. Fabrication and characterization of one- and two-dimensional regular patterns produced employing multiple exposure holographic lithography. J. Optoelectron. Adv. M 2017, 19, 119–126. [Google Scholar]
- Rößler, F.; Lang, V.; Günther, D.; Lasagni, A.F. Fabricating Three-Dimensional Periodic Micro Patterns on Photo-Resists Using Laser Interference Lithography. Adv. Eng. Mater. 2017, 19, 1600855. [Google Scholar] [CrossRef]
- Lee, D.; Kim, B.-Y.; Park, C.H.; Jeong, G.; Park, S.-D.; Yoo, M.J.; Yang, H.; Lee, W.S. Photocurable Three-Dimensional Printing Resin to Enable Laser-Assisted Selective Electroless Metallization for Customized Electronics. ACS Appl. Polym. Mater. 2021, 3, 4735–4745. [Google Scholar] [CrossRef]
- Mulko, L.; Soldera, M.; Lasagni, A.F. Structuring and functionalization of non-metallic materials using direct laser interference patterning: A review. Nanophotonics 2022, 11, 203–240. [Google Scholar] [CrossRef]
- Rebollar, E.; Castillejo, M.; Ezquerra, T.A. Laser induced periodic surface structures on polymer films: From fundamentals to applications. Eur. Polym. J. 2015, 73, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, A.; Rebollar, E.; Soccio, M.; Ezquerra, T.A.; Rueda, D.R.; Garcia-Ramos, J.V.; Castillejo, M.; Garcia-Gutierrez, M.-C. Laser-Induced Periodic Surface Structures on Conjugated Polymers: Poly(3-hexylthiophene). Macromolecules 2015, 48, 4024–4031. [Google Scholar] [CrossRef] [Green Version]
- Mezera, M.; Alamri, S.; Hendriks, W.A.P.M.; Hertwig, A.; Elert, A.M.; Bonse, J.; Kunze, T.; Lasagni, A.F.; Römer, G.-W.R.B.E. Hierarchical micro-/nano-structures on polycarbonate via uv pulsed laser processing. Nanomaterials 2020, 10, 1184. [Google Scholar] [CrossRef]
- Philipp, H.R.; Cole, H.S.; Liu, Y.S.; Sitnik, T.A. Optical absorption of some polymers in the region 240–170 nm. Appl. Phys. Lett. 1986, 48, 192–194. [Google Scholar] [CrossRef] [Green Version]
- Chuang, T.J.; Hiraoka, H.; Mödl, A. Laser-photoetching characteristics of polymers with dopants. Appl. Phys. A Sol. Surf. 1988, 45, 277–288. [Google Scholar] [CrossRef]
- Aleksandrov, A.P.; Bityurin, N.M.; Genkin, V.N.; Rukhman, I.V. Polymethylmethacrylate photoetching kinetics and modification at 200–300 nm. Sov. Microelect. 1989, 18, 16–19. [Google Scholar]
- Ouchi, I.; Nakai, I.; Kamada, M. Anisotropic absorption spectra of polyester films in the ultraviolet and vacuum ultraviolet regions. Nucl. Instrum. Methods Phys. Res. B 2003, 199, 270–274. [Google Scholar] [CrossRef]
- Costela, A.; Figuera, J.M.; Florido, F.; García-Moreno, I.; Collar, E.P.; Sastre, R. Ablation of poly(methyl methacrylate) and poly(2-hydroxyethyl methacrylate) by 308, 222 and 193 nm excimer-laser radiation. Appl. Phys. A Mater. Sci. Process 1995, 60, 261–270. [Google Scholar] [CrossRef]
- Lippert, T.; Yabe, A.; Wokaun, A. Laser ablation of doped polymer systems. Adv. Mater. 1997, 9, 105–119. [Google Scholar] [CrossRef]
- Kosmidis, C.E.; Skordoulis, C.D. Dopant-enhanced ablation of nitrocellulose by a nitrogen laser. Appl. Phys. A Sol. Surf. 1993, 56, 64–68. [Google Scholar] [CrossRef]
- Acevedo, D.F.; Miras, M.C.; Barbero, C.A. Combinatorial synthesis and screening of photochromic dyes and modified conducting polymers. In Combinatorial and High-Throughput Discovery and Optimization of Catalysts and Materials; Potyrailo, R.A., Maier, W.F., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 239–256. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Saleh, M.A. Thermal degradation of azobenzene dyes. Results Chem. 2020, 2, 100085. [Google Scholar] [CrossRef]
- Fujiwara, H.; Fukumura, H.; Masuhara, H. Laser ablation of a pyrene-doped poly(methyl methacrylate) film: Dynamics of pyrene transient species by spectroscopic measurements. J. Phys. Chem. 1995, 99, 11844–11853. [Google Scholar] [CrossRef]
- Stoll, L.; Rech, R.; Flôres, S.H.; Nachtigall, S.M.B.; de Oliveira Rios, A. Carotenoids extracts as natural colorants in poly(lactic acid) films. J. Appl. Polym. Sci. 2018, 135, 46585. [Google Scholar] [CrossRef]
- Smith, C.P., Jr. Review of methods for coloring nonwovens. Text. Chem. Color. 1990, 22, 25–29. [Google Scholar]
- Xu, J.; Zhang, G.; Wu, C.; Liu, W.; Zhang, T.; Huang, Y.; Rong, Y. Organic solvent assisted laser processing of transparent polymer films based on the swelling and penetration behavior. Opt. Laser Technol. 2022, 150, 107937. [Google Scholar] [CrossRef]
- D’Couto, G.C.; Babu, S.V.; Egitto, F.D.; Davis, C.R. Excimer laser a.blation of polyimide-doped poly(tetrafluoroethylene) at 248 and 308 nm. J. Appl. Phys. 1993, 74, 5972–5980. [Google Scholar] [CrossRef]
- Jiang, L.J.; Maruo, S.; Osellame, R.; Xiong, W.; Campbell, J.H.; Lu, Y.F. Femtosecond laser direct writing in transparent materials based on nonlinear absorption. MRS Bull. 2016, 41, 975–983. [Google Scholar] [CrossRef]
- Raudino, A.; Fragalà, M.E.; Compagnini, G.; Puglisi, O. Modeling of low-temperature depolymerization of poly (methyl methacrylate) promoted by ion beam. J. Chem. Phys. 1999, 111, 1721–1731. [Google Scholar] [CrossRef]
- Stump, B.L.; Snyder, W.J. Thermal Degradation of Polyimides by Thermogravimetric Analysis. High Perform. Polym. 1989, 1, 247–262. [Google Scholar] [CrossRef]
- Lasagni, A.; Cornejo, M.; Lasagni, F.; Muecklich, F. Laser ablation modeling of periodic pattern formation on polymer substrates. Adv. Eng. Mater. 2008, 10, 488–493. [Google Scholar] [CrossRef]
- Baumann, R.; Alamri, S.; Aguilar-Morales, A.I.; Lasagni, A.F.; Kunze, T. Advanced remote laser cutting of battery foils using an interference approach. Mater. Lett. X 2022, 14, 100138. [Google Scholar] [CrossRef]
- Lippert, T.H.; Wokaun, A.; Stebani, J.; Nuyken, O.; Ihlemann, J. Triazene polymers designed for excimer laser ablation. Angew. Makromolek. Chem. 1993, 206, 97–110. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Wang, Z. Improvement of the frequency-doubling efficiency of high-average-power lasers using multicrystal scheme with opposite thermal properties. Results Phys. 2017, 7, 3530–3536. Available online: https://velocimetry.net/laser%20catalogues/quantel/Brilliant%20B%20Qswitched%20NdYAG%20oscillator.pdf (accessed on 13 October 2022). [CrossRef]
- Kuhnke, M.; Cramer, L.; Dyer, P.E.; Dickinson, J.T.; Lippert, T.; Niino, H.; Pervolaraki, M.; Walton, C.D.; Wokaun, A. F2 excimer laser (157 nm) ablation of polymers: Relation of neutral and ionic fragment detection and absorption. J. Phys. Conf. Ser. 2007, 59, 625–631. [Google Scholar] [CrossRef]
- Ishi-Hayase, J.; Ishihara, T. Fundamental optical properties of photonic crystal slabs in the strong coupling regime. Semicon. Sci. Technol. 2003, 18, S411–S418. [Google Scholar] [CrossRef]
- Burnett, J.H.; Gupta, R.; Griesmann, U. Absolute refractive indices and thermal coefficients of CaF2, SrF2, BaF2, and LiF near 157 nm. Appl. Opt. 2002, 41, 2508–2513. [Google Scholar] [CrossRef]
- Phillips, H.M.; Callahan, D.L.; Sauerbrey, R.; Szabó, G.; Bor, Z. Sub-100 nm lines produced by direct laser ablation in polyimide. Appl. Phys. Lett. 1991, 58, 2761–2763. [Google Scholar] [CrossRef]
- Phillips, H.M.; Callahan, D.L.; Sauerbrey, R.; Szabo, G.; Bor, Z. Direct laser ablation of sub-100 nm line structures into polyimide. Appl. Phys. A. Sol. Surf. 1992, 54, 158–165. [Google Scholar] [CrossRef]
- Dyer, P.E.; Farley, R.J.; Giedl, R.; Karnakis, D.M. Excimer laser ablation of polymers and glasses for grating fabrication. Appl. Surf. Sci. 1996, 96–98, 537–549. [Google Scholar] [CrossRef]
- Lippert, T.; Gerber, T.; Wokaun, A.; Funk, D.J.; Fukumura, H.; Goto, M. Single pulse nm-size grating formation in polymers using laser ablation with an irradiation wavelength of 355 nm. Appl. Phys. Lett. 1999, 75, 1018–1020. [Google Scholar] [CrossRef]
- Klein-Wiele, J.-H.; Simon, P. Fabrication of periodic nanostructures by phase-controlled multiple-beam interference. Appl. Phys. Lett. 2003, 83, 4707–4709. [Google Scholar] [CrossRef]
- Li, P.; Bakowsky, U.; Yu, F.; Loehbach, C.; Muecklich, F.; Lehr, C.-M. Laser ablation patterning by interference induces directional cell growth. IEEE Trans. Nanobio. 2003, 2, 138–145. [Google Scholar] [CrossRef]
- Yu, F.; Li, P.; Shen, H.; Mathur, S.; Lehr, C.-M.; Bakowsky, U.; Mücklich, F. Laser interference lithography as a new and efficient technique for micropatterning of biopolymer surface. Biomaterials 2005, 26, 2307–2312. [Google Scholar] [CrossRef]
- Müller-Meskamp, L.; Kim, Y.H.; Roch, T.; Hofmann, S.; Scholz, R.; Eckardt, S.; Leo, K.; Lasagni, A.F. Efficiency enhancement of organic solar cells by fabricating periodic surface textures using direct laser interference patterning. Adv. Mater. 2012, 24, 906–910. [Google Scholar] [CrossRef]
- Perez-Hernandez, H.; Lasagni, A.F. Fast and efficient manufacturing method of one- and two-dimensional polyethylene terephthalate transmission diffraction gratings by direct laser interference patterning. Polym. Eng. Sci. 2012, 52, 1903–1908. [Google Scholar] [CrossRef]
- Soldera, M.; Alamri, S.; Storm, S.; Kunze, T.; Lasagni, A.F. Maximizing the efficiency of laser-fabricated diffraction gratings on PET using direct laser interference patterning. Proc. SPIE 2020, 11268, 112680Z. [Google Scholar] [CrossRef]
- Guenther, D.; Valle, J.; Burgui, S.; Gil, C.; Solano, C.; Toledo-Arana, A.; Helbig, R.; Werner, C.; Lasa, I.; Lasagni, A.F. Direct laser interference patterning for decreased bacterial attachment. Proc. SPIE 2016, 9736, 973611. [Google Scholar] [CrossRef]
- Alamri, S.; Fraggelakis, F.; Kunze, T.; Krupop, B.; Mincuzzi, G.; Kling, R.; Lasagni, A.F. On the interplay of DLIP and LIPSS upon ultra-short laser pulse irradiation. Materials 2019, 12, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuello, E.A.; Mulko, L.E.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Development of micropatterning polyimide films for enhanced antifouling and antibacterial properties . Colloids Surf. B 2020, 188, 110801. [Google Scholar] [CrossRef]
- Acevedo, D.F.; Martínez, G.; Arana, J.T.; Yslas, E.I.; Mücklich, F.; Barbero, C.; Salavagione, H.J. Easy way to fabricate nanostructures on a reactive polymer surface. J. Phys. Chem. B 2009, 113, 14661–14666. [Google Scholar] [CrossRef]
- Valle, J.; Burgui, S.; Langheinrich, D.; Gil, C.; Solano, C.; Toledo-Arana, A.; Helbig, R.; Lasagni, A.; Lasa, I. Evaluation of Surface Microtopography Engineered by Direct Laser Interference for Bacterial Anti-Biofouling. Macromol. Biosci. 2015, 15, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ränke, F.; Baumann, R.; Voisiat, B.; Fabián Lasagni, A. High throughput laser surface micro-structuring of polystyrene by combining direct laser interference patterning with polygon scanner technology. Mater. Lett. X 2022, 14, 100144. [Google Scholar] [CrossRef]
- Martín-Fabiani, I.; Riedel, S.; Rueda, D.R.; Siegel, J.; Boneberg, J.; Ezquerra, T.A.; Nogales, A. Micro- and submicrostructuring thin polymer films with two and three-beam single pulse laser interference lithography. Langmuir 2014, 30, 8973–8979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, D.; Salavagione, H.; Lasagni, A.; Morallõn, E.; Mücklich, F.; Barbero, C. SERS active surface in two steps, patterning and metallization. Adv. Eng. Mater. 2013, 15, 325–329. [Google Scholar] [CrossRef]
- Alamri, S.; Lasagni, A.F. Direct laser interference patterning of transparent and colored polymer substrates: Ablation, swelling, and the development of a simulation model. Proc. SPIE 2017, 10092, 1009219. [Google Scholar] [CrossRef]
- Alamri, S.; Lasagni, A.F. Development of a general model for direct laser interference patterning of polymers. Opt. Express 2017, 25, 9603–9616. [Google Scholar] [CrossRef] [PubMed]
- Günther, K.; Sonntag, F.; Moritzer, E.; Hirsch, A.; Klotzbach, U.; Lasagni, A.F. Universal micromachining platform and basic technologies for the manufacture and marking of microphysiological systems. Micromachines 2017, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, D.; Lasagni, A.; Barbero, C.; Muecklich, F. Micro/nano fabrication of surface architectures on polymers and copolymers using direct laser interference patterning. Mater. Res. Symp. Proc. 2008, 1054, 7–12. [Google Scholar] [CrossRef]
- Broglia, M.F.; Suarez, S.; Soldera, F.; Mücklich, F.; Barbero, C.A.; Bellingeri, R.; Alustiza, F.; Acevedo, D. Direct laser interference patterning of polystyrene films doped with azo dyes, using 355 nm laser light. Appl. Surf. Sci. 2014, 300, 86–90. [Google Scholar] [CrossRef]
- Bremus-Koebberling, E.A.; Beckemper, S.; Koch, B.; Gillner, A. Nano structures via laser interference patterning for guided cell growth of neuronal cells. J. Laser App. 2012, 24, 042013. [Google Scholar] [CrossRef] [Green Version]
- Mulko, L.E.; Rossa, M.; Aranguren-Abrate, J.P.; Pino, G.A. Micropatterning of fluorescent silver nanoclusters in polymer films by Laser Interference. Appl. Surf. Sci. 2019, 485, 141–146. [Google Scholar] [CrossRef]
- Hauschwitz, P.; Jagdheesh, R.; Alamri, S.; Rostohar, D.; Kunze, T.; Brajer, J.; Kopeček, J.; Mocek, T. Fabrication of functional superhydrophobic surfaces on carbon fibre reinforced plastics by IR and UV direct laser interference patterning. Appl. Surf. Sci. 2020, 508, 144817. [Google Scholar] [CrossRef]
- Nuyken, O.; Stebani, J.; Lippert, T.; Wokaun, A.; Stasko, A. Photolysis, thermolysis, and protolytic decomposition of a triazene polymer in solution. Macromol. Chem. Phys. 1995, 196, 751–761. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, S.; Li, Y. High ultraviolet sensitivity of phthalic acid esters with environmental friendliness after modification through pharmacophore modeling associated with the solvation effect. Pol. J. Environ. Stud. 2020, 29, 2303–2316. [Google Scholar] [CrossRef]
- Dziȩcioł, M.; Trzeszczyński, J. Volatile products of poly(ethylene terephthalate) thermal degradation in nitrogen atmosphere. J. Appl. Polym. Sci. 2000, 77, 1894–1901. [Google Scholar] [CrossRef]
- Novis, Y.; Pireaux, J.J.; Brezini, A.; Petit, E.; Caudano, R.; Lutgen, P.; Feyder, G.; Lazare, S. Structural origin of surface morphological modifications developed on poly(ethylene terephthalate) by excimer laser photoablation. J. Appl. Phys. 1988, 64, 365–370. [Google Scholar] [CrossRef]
- Sangkhawasi, M.; Remsungnen, T.; Vangnai, A.S.; Maitarad, P.; Rungrotmongkol, T. Prediction of the Glass Transition Temperature in Polyethylene Terephthalate/Polyethylene Vanillate (PET/PEV) Blends: A Molecular Dynamics Study. Polymers 2022, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- Morales-Huerta, J.C.; De Ilarduya, A.M.; Muñoz-Guerra, S. Poly(alkylene 2,5-furandicarboxylate)s (PEF and PBF) by ring opening polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Ishida, H.; Wellinghoff, S.T.; Baer, E.; Koenig, J.L. Spectroscopic Studies of Poly[N,N'-bis(phenoxyphenyl)pyromellitimide]. Structures of the Polyimide and Three Model Compounds. Macromolecules 1980, 13, 826–834. [Google Scholar] [CrossRef]
- Akinyi, C.; Longun, J.; Chen, S.; Iroh, J.O. Decomposition and flammability of polyimide graphene composites. Minerals 2021, 11, 168. [Google Scholar] [CrossRef]
- Ortelli, E.E.; Geiger, F.; Lippert, T.; Wei, J.; Wokaun, A. UV-laser-induced decomposition of Kapton studied by infrared spectroscopy. Macromolecules 2000, 33, 5090–5097. [Google Scholar] [CrossRef]
- Braun, R.; Nowak, R.; Hess, P.; Oetzmann, H.; Schmidt, C. Photoablation of polyimide with IR and UV laser radiation. Appl. Surf. Sci. 1989, 43, 352–357. [Google Scholar] [CrossRef]
- Workman, J.J., Jr.; Weye, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC: Boca Raton, FL, USA, 2008; p. 218. [Google Scholar]
- Johnson, S.L.; Schriver, K.E.; Haglund, R.F.; Bubb, D.M. Effects of the absorption coefficient on resonant infrared laser ablation of poly(ethylene glycol). J. Appl. Phys. 2009, 105, 024901. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Li, T.; Zhou, C.; Jiang, M. UV absorption spectra of polystyrene. Polym. Bull. 1991, 25, 211–216. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Vijayakumar, R.P. Studies on biological degradation of polystyrene by pure fungal cultures. Environ. Dev. Sustain. 2020, 22, 4495–4508. [Google Scholar] [CrossRef]
- Lemoine, P.; Blau, W.; Drury, A.; Keely, C. Molecular weight effects on the 248-nm photoablation of polystyrene spun films. Polymer 1993, 34, 5020–5028. [Google Scholar] [CrossRef]
- Su, Z.; Bedolla-Valdez, Z.I.; Wang, L.; Rho, Y.; Chen, S.; Gonel, G.; Taurone, E.N.; Moulé, A.J.; Grigoropoulos, C.P. High-Speed Photothermal Patterning of Doped Polymer Films. ACS Appl. Mater. Interfaces 2019, 11, 41717–41725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bityurin, N. Studies on laser ablation of polymers. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2005, 101, 216–247. [Google Scholar] [CrossRef]
- Beinhorn, F.; Ihlemann, J.; Luther, K.; Troe, J. Micro-lens arrays generated by UV laser irradiation of doped PMMA. Appl. Phys. A Mater. Sci. Process 1999, 68, 709–713. [Google Scholar] [CrossRef]
- Mullen, P.A.; Searle, N.Z. The ultraviolet activation spectrum of polycarbonate. J. Appl. Polym. Sci. 1970, 14, 765–776. [Google Scholar] [CrossRef]
- Artham, T.; Doble, M. Biodegradation of aliphatic and aromatic polycarbonates. Macromol. Biosci. 2008, 8, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.N.; Wilkie, C.A.A. TGA/FTIR and mass spectral study on the thermal degradation of bisphenol A polycarbonate. Polym. Degrad. Stab. 2004, 86, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Kunz, T.H.; Stebani, J.; Ihlemann, J.; Wokaun, A. Photoablation and microstructuring of polyestercarbonates and their blends with a XeCl excimer laser. Appl. Phys. A Mater. Sci. Proces. 1998, 67, 347–352. [Google Scholar] [CrossRef]
- Cardona, M.; Güntherodt, G. Light Scattering in Solids IV: Electronics Scattering, Spin Effects, SERS, and Morphic Effects. In Topics in Applied Physics; Springer: Berlin, Germany, 1984. [Google Scholar]
- Madzharova, F.; Heiner, Z.; Kneipp, J. Surface-Enhanced Hyper Raman Spectra of Aromatic Thiols on Gold and Silver Nanoparticles. J. Phys. Chem. C 2020, 124, 6233. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Xu, J.; Feng, E.; Song, J. Renaissance of aliphatic polycarbonates: New techniques and biomedical applications. J. Appl. Polym. Sci. 2014, 131, 39822. [Google Scholar] [CrossRef] [Green Version]
- El-Newehy, M.H.; Kim, H.Y.; Khattab, T.A.; El-Naggar, M.E. Production of photoluminescent transparent poly(methyl methacrylate) for smart windows. Luminescence 2022, 37, 97–107. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lim, H.S.; Lee, N.E.; Cho, S.O. Biocompatible UV-absorbing polymer nanoparticles prepared by electron irradiation for application in sunscreen. RSC Adv. 2019, 10, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-S.; Kim, S.G.; Kim, J.-S. Thermal degradation of poly(methyl methacrylate) polymers: Kinetics and recovery of monomers using a fluidized bed reactor. J. Anal. Appl. Pyrol. 2008, 81, 7–13. [Google Scholar] [CrossRef]
- Srinivasan, R.; Braren, B.; Dreyfus, R.W.; Hadel, L.; Seeger, D.E. Mechanism of the ultraviolet laser ablation of polymethyl methacrylate at 193 and 248 nm: Laser-induced fluorescence analysis, chemical analysis, and doping studies. J.Opt. Soc. B 1986, 3, 785–791. [Google Scholar] [CrossRef]
- Lippert, T. Interaction of photons with polymers: From surface modification to ablation. Plasma Process. Polym. 2005, 2, 525–546. [Google Scholar] [CrossRef]
- Monerris, M.; Broglia, M.F.; Yslas, E.I.; Barbero, C.A.; Rivarola, C.R. Highly effective antimicrobial nanocomposites based on hydrogel matrix and silver nanoparticles: Long-lasting bactericidal and bacteriostatic effects. Soft Matter 2019, 15, 8059–8066. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Fouracre, R.A.; Given, M.J.; Banford, H.M.; Wysocki, S.; Karolczak, S. The effects on polyetheretherketone and polyethersulfone of electron and γ irradiation. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 295–303. [Google Scholar] [CrossRef]
- Patel, P.; Hull, T.R.; McCabe, R.W.; Flath, D.; Grasmeder, J.; Percy, M. Mechanism of thermal decomposition of poly(ether ether ketone) (PEEK) from a review of decomposition studies. Polym. Degrad. Stab. 2010, 95, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Babu, S.V.; D’Couto, G.C.; Egitto, F.D. Excimer laser induced ablation of polyetheretherketone, polyimide, and polytetrafluoroethylene. J. Appl. Phys. 1992, 72, 692–698. [Google Scholar] [CrossRef]
- Lippert, T.H.; Wokaun, A.; Stebani, J.; Nuyken, O.; Ihlemann, J. Dopant-induced laser ablation of PMMA at 308 nm: Influence of the molecular weight of PMMA and of the photochemical activity of added chromophores. Angew. Makromol. Chem. 1993, 213, 127–155. [Google Scholar] [CrossRef]
- Bhatnagar, M.S. A Textbook of Polymer Chemistry; Chand Publishing: New Dehli, India, 2004. [Google Scholar]
- Da Silva, A.C.; Semeano, A.T.S.; Dourado, A.H.B.; Ulrich, H.; De Torresi, S.I.C. Novel Conducting and Biodegradable Copolymers with Noncytotoxic Properties toward Embryonic Stem Cells. ACS Omega 2018, 3, 5593–5604. [Google Scholar] [CrossRef]
- Estevam-Alves, R.; Günther, D.; Dani, S.; Eckhardt, S.; Roch, T.; Mendonca, C.R.; Cestari, I.N.; Lasagni, A.F. UV Direct Laser Interference Patterning of polyurethane substrates as tool for tuning its surface wettability. Appl. Surf. Sci. 2016, 374, 222–228. [Google Scholar] [CrossRef]
- Broglia, M.F.; Acevedo, D.F.; Langheinrich, D.; Perez-Hernandez, H.R.; Barbero, C.A.; Lasagni, A.F. Rapid fabrication of periodic patterns on poly(styrene-co-acrylonitrile) surfaces using direct laser interference patterning. Int. J. Polym. Sci. 2015, 2015, 721035. [Google Scholar] [CrossRef] [Green Version]
- Ates, M.; Karadag, S.; Eker, A.A.; Eker, B. Polyurethane foam materials and their industrial applications. Polym. Int. 2022, 71, 1157–1163. [Google Scholar] [CrossRef]
- Somarathna, H.M.C.C.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K.H. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Sikdar, P.; Dip, T.M.; Dhar, A.K.; Bhattacharjee, M.; Hoque, M.S.; Ali, S.B. Polyurethane (PU) based multifunctional materials: Emerging paradigm for functional textiles, smart, and biomedical applications. J. Appl. Polym. Sci. 2022, 139, e52832. [Google Scholar] [CrossRef]
- Senevirathna, S.R.; Amarasinghe, D.A.S.; Karunaratne, V.; Koneswaran, M.; Karunanayake, L. Effect of microstructural arrangement of MDI-based polyurethanes on their photoproperties. J. Appl. Polym. Sci. 2019, 136, 47431. [Google Scholar] [CrossRef]
- Allan, D.; Daly, J.; Liggat, J.J. Thermal volatilisation analysis of TDI-based flexible polyurethane foam. Polym. Degrad. Stab. 2013, 98, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Zafiropulos, V.; Petrakis, J.; Fotakis, C. Photoablation of polyurethane films using UV laser pulses. Opt. Quantum Electron. 1995, 27, 1359–1376. [Google Scholar] [CrossRef]
- Cassales, A.; Ramos, L.A.; Frollini, E. Synthesis of bio-based polyurethanes from Kraft lignin and castor oil with simultaneous film formation. Int. J. Biol. Macromol. 2020, 145, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Gürses, C.; Karaaslan-Tunç, M.G.; Keleştemur, Ü.; Balcıoğlu, S.; Gülgen, S.; Köytepe, S.; Ateş, B. Aliphatic Polyurethane Films Based on Hexamethylene Diisocyanate and Saccharides for Biocompatible Transparent Coating on Optic Medical Devices. Starch/Staerke 2022, 74, 2100214. [Google Scholar] [CrossRef]
- Priddy, D.B. Thermal discoloration chemistry of styrene-co-acrylonitrile. Adv. Polym. Sci. 1995, 121, 122–154. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Khan, S.; F Didar, T. Bio-functionalization of microfluidic platforms made of thermoplastic materials: A review. Anal. Chim. Acta 2022, 1209, 339283. [Google Scholar] [CrossRef]
- Friedlander, H.N.; Peebles, L.H., Jr.; Brandrup, J.; Kirby, J.R. On the chromophore of polyacrylonitrile. VI. Mechanism of color formation in polyacrylonitrile. Macromolecules 1968, 1, 79–86. [Google Scholar] [CrossRef]
- Xue, T.J.; McKinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Nishio, S.; Chiba, T.; Matsuzaki, A.; Sato, H. Preparation of polymer films by laser ablation of polyacrylonitrile: Significant dependence on ablation wavelengths. Appl. Surf. Sci. 1996, 106, 132–136. [Google Scholar] [CrossRef]
- Krajnovich, D.J. Incubation and photoablation of poly(methyl methacrylate) at 248 nm. New insight into the reaction mechanism using photofragment translational spectroscopy. J. Phys. Chem. A 1997, 101, 2033–2039. [Google Scholar] [CrossRef]
- Rogers, M.T. The Electric Moments and Ultraviolet Absorption Spectra of Some Derivatives of Cyclopropane and of Ethylene Oxide. J. Amer. Chem. Soc. 1947, 69, 2544–2548. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Broglia, M.F.; Acevedo, D.F.; Barbero, C.A. Smart surfaces: Reversible switching of a polymeric hydrogel topography. Soft Matter 2012, 8, 307–310. [Google Scholar] [CrossRef]
- Sola, D.; Milles, S.; Lasagni, A.F. Direct laser interference patterning of diffraction gratings in safrofilcon-a hydrogel: Fabrication and hydration assessment. Polymers 2021, 3, 679. [Google Scholar] [CrossRef]
- Mulko, L.E.; Cuello, E.A.; Barbero, C.A.; Pino, G.A.; Molina, M.; Rossa, M. Remote radiofrequency triggering of topography changes in a surface micropatterned PANI@PNIPAM nanocomposite. Appl. Surf. Sci. 2020, 509, 145370. [Google Scholar] [CrossRef]
- Sola, D.; Alamri, S.; Lasagni, A.F. UV Direct Laser Interference Patterning of Diffraction Gratings in Poly-Hydroxyethyl-Methacrylate Ophthalmic Polymers. J. Laser Micro Nanoeng. 2020, 15, 186–190. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Al Momani, F.A.; Örmeci, B. Measurement of polyacrylamide polymers in water and wastewater using an in-line UV-vis spectrophotometer. J. Environ. Chem. Eng. 2014, 2, 765–772. [Google Scholar] [CrossRef]
- Rivarola, C.R.; Biasutti, M.A.; Barbero, C.A. A visible light photoinitiator system to produce acrylamide based smart hydrogels: Ru(bpy)+2 as photopolymerization initiator and molecular probe of hydrogel microenvironments. Polymer 2009, 50, 3145–3152. [Google Scholar] [CrossRef]
- Molina, M.A.; Rivarola, C.R.; Barbero, C.A. Evidence of hydrophobic interactions controlling mobile ions release from smart hydrogels. Mol. Cryst. Liq. Cryst. 2010, 521, 265–271. [Google Scholar] [CrossRef]
- Rivero, R.; Alustiza, F.; Capella, V.; Liaudat, C.; Rodriguez, N.; Bosch, P.; Barbero, C.; Rivarola, C. Physicochemical properties of ionic and non-ionic biocompatible hydrogels in water and cell culture conditions: Relation with type of morphologies of bovine fetal fibroblasts in contact with the surfaces. Colloids Surf. B 2017, 158, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. PHEMA: An overview for biomedical applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Monge, N.E.; Miras, M.C.; Barbero, C.A. High-throughput screening method to detect amphiphilic counterions able to solubilize conducting polymers. J. Comb. Chem. 2010, 12, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Planes, G.A.; Rodríguez, J.L.; Miras, M.C.; García, G.; Pastor, E.; Barbero, C.A. Spectroscopic evidence for intermediate species formed during aniline polymerization and polyaniline degradation. PCCP 2010, 12, 10584–10593. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, D.A.; Lasagni, A.F.; Barbero, C.A.; Mücklich, F. Simple fabrication method of conductive polymeric arrays by using direct laser interference micro-/nanopatterning. Adv. Mater. 2007, 19, 1272–1275. [Google Scholar] [CrossRef]
- Lasagni, A.; Acevedo, D.; Barbero, C.; Muecklich, F. Fabrication of conductive polymeric arrays using direct laser interference micro/nano patterning. Mater. Res. Symp. Proc. 2008, 1030, 61–66. [Google Scholar] [CrossRef]
- Lasagni, A.F.; Hendricks, J.L.; Shaw, C.M.; Yuan, D.; Martin, D.C.; Das, S. Direct laser interference patterning of poly(3,4-ethylene dioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS) thin films. Appl. Surf. Sci. 2009, 255, 9186–9192. [Google Scholar] [CrossRef]
- Acevedo, D.F.; Frontera, E.; Broglia, M.F.; Mücklich, F.; Miras, M.C.; Barbero, C.A. One step lithography of polypyrrole. Adv. Eng. Mater. 2011, 13, 405–410. [Google Scholar] [CrossRef]
- Abel, S.B.; Gallarato, L.A.; Dardanelli, M.S.; Barbero, C.A.; Rivarola, C.R.; Yslas, E.I. Photothermal lysis of Pseudomonas aeruginosa by polyaniline nanoparticles under near infrared irradiation. Biomed. Phys. Eng. Express 2018, 4, 045037. [Google Scholar] [CrossRef] [Green Version]
- Barbero, C.; Tucceri, R.I.; Posadas, D.; Silber, J.J.; Sereno, L. Impedance characteristics of poly-o-aminophenol electrodes. Electrochim. Acta 1995, 40, 1037–1040. [Google Scholar] [CrossRef]
- Tucceri, R.I.; Barbero, C.; Silber, J.J.; Sereno, L.; Posadas, D. Spectroelectrochemical study of poly-o-aminophenol. Electrochim. Acta 1997, 42, 919–927. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Rashid, S.A.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Singla, E.; Agnihotri, P.K. Modulation of optical properties of electrochromic device. J. Mater. Sci. Mater. Electron. 2022, 33, 21935–21954. [Google Scholar] [CrossRef]
- Barbero, C.; Kötz, R. Nanoscale Dimensional Changes and Optical Properties of Polyaniline Measured by in Situ Spectroscopic Ellipsometry. J. Electrochem. Soc. 1994, 141, 859–865. [Google Scholar] [CrossRef]
- Kulkarni, V.G.; Campbell, L.D.; Mathew, W.R. Thermal stability of polyaniline. Synth. Met. 1989, 30, 321–325. [Google Scholar] [CrossRef]
- Lippert, T.; Raimondi, F.; Wambach, J.; Wei, J.; Wokaun, A. Surface modification and structuring of electrical conducting and isolating polyaniline films. Appl. Phys. A Mater. Sci. Process 1999, 69, S291–S293. [Google Scholar] [CrossRef]
- Cavallo, P.; Coneo Rodriguez, R.; Broglia, M.; Acevedo, D.F.; Barbero, C.A. Simple fabrication of active electrodes using direct laser transference. Electrochim. Acta 2014, 116, 194–202. [Google Scholar] [CrossRef]
- Nogueira Pedroza Dias Mello, H.J.; Mulato, M. Impedimetric and Capacitive Transducer Platform for Chemical Sensors Based on Electrodeposited Polyaniline Thin Films. J. Phys. Chem. C 2022, 126, 12222–12229. [Google Scholar] [CrossRef]
- Huang, W.S.; MacDiarmid, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Cavallo, P.; Acevedo, D.F.; Fuertes, M.C.; Soler-Illia, G.J.A.A.; Barbero, C.A. Understanding the sensing mechanism of polyaniline resistive sensors. Effect of humidity on sensing of organic volatiles. Sens. Actuators B Chem. 2015, 210, 574–580. [Google Scholar] [CrossRef]
- Lasagni, A.F.; Acevedo, D.F.; Barbero, C.A.; Mücklich, F. Advanced design of conductive polymeric arrays with controlled electrical resistance using direct laser interference patterning. Appl. Phys. A 2008, 91, 369–373. [Google Scholar] [CrossRef]
- Joshi Anil, G.; Sarma, G.H. SEM studies on laser trimmed resistors. IETE J. Res. 1982, 28, 494–497. [Google Scholar] [CrossRef]
- Yslas, E.I.; Cavallo, P.; Acevedo, D.F.; Barbero, C.A.; Rivarola, V.A. Cysteine modified polyaniline films improve biocompatibility for two cell lines. Mater. Sci. Eng. C 2015, 51, 51–56. [Google Scholar] [CrossRef]
- Gallarato, L.A.; Mulko, L.E.; Dardanelli, M.S.; Barbero, C.A.; Acevedo, D.F.; Yslas, E.I. Synergistic effect of polyaniline coverage and surface microstructure on the inhibition of Pseudomonas aeruginosa biofilm formation. Colloids Surf. B 2017, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel approaches to combat medical device-associated biofilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Barbero, C.; Zerbino, J.; Sereno, L.; Posadas, D. Optical properties of electropolymerized orthoaminophenol. Electrochim. Acta 1987, 32, 693–697. [Google Scholar] [CrossRef]
- AlQattan, B.; Butt, H.; Sabouri, A.; Yetisen, A.K.; Ahmed, R.; Mahmoodi, N. Holographic direct pulsed laser writing of two-dimensional nanostructures. RSC Adv. 2016, 6, 111269–111275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, D.F.; Salavagione, H.J.; Lasagni, A.F.; Morallón, E.; Mücklich, F.; Barbero, C. Fabrication of highly ordered arrays of platinum nanoparticles using direct laser interference patterning. ACS Appl. Mater. Interfaces 2009, 1, 549–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planes, G.A.; Rodríguez, J.L.; Pastor, E.; Barbero, C. Evidence of a free Pt surface under electrodeposited polyaniline (PANI) films: CO adsorption and methanol oxidation at PANI/Pt without metal particles. Langmuir 2003, 19, 8137–8140. [Google Scholar] [CrossRef]
- Vernitskaya, T.V. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Liang, Y.; Goh, J.C.-H. Polypyrrole-Incorporated Conducting Constructs for Tissue Engineering Applications: A Review. Bioelectricity 2020, 2, 101–119. [Google Scholar] [CrossRef]
- Yakushi, K.; Lauchlan, L.J.; Clarke, T.C.; Street, G.B. Optical study of polypyrrole perchlorate. J. Chem. Phys. 1983, 79, 4774–4778. [Google Scholar] [CrossRef]
- Omastova, M.; Rychlý, J.; Trchová, M.; Kovářová, J. Properties and thermal decomposition of polypyrrole prepared in the presence of sodium bis(2-ethylhexyl) sulfosuccinate. Des. Monomers Polym. 2004, 7, 633–646. [Google Scholar] [CrossRef]
- Abel, S.B.; Rivarola, C.R.; Barbero, C.A.; Molina, M. Electromagnetic radiation driving of volume changes in nanocomposites made of a thermosensitive hydrogel polymerized around conducting polymer nanoparticles. RSC Adv. 2020, 10, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Harada, H.; Fuchigami, T.; Nonaka, T. Degradation and its prevention, and the deactivation and reactivation of electroactive polythiophene films during oxidation/reduction cycles. J. Electroanal. Chem. 1991, 303, 139–150. [Google Scholar] [CrossRef]
- Yemata, T.A.; Zheng, Y.; Kyaw, A.K.K.; Wang, X.; Song, J.; Chin, W.S.; Xu, J. Improved Thermoelectric Properties and Environmental Stability of Conducting PEDOT:PSS Films Post-treated with Imidazolium Ionic Liquids. Front. Chem. 2020, 7, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepien, L.; Roch, A.; Tkachov, R.; Leupolt, B.; Han, L.; van Ngo, N.; Leyens, C. Thermal operating window for PEDOT:PSS films and its related thermoelectric properties. Synth. Met. 2017, 225, 49–54. [Google Scholar] [CrossRef]
- Karnakis, D.; Kearsley, A.; Knowles, M. Ultrafast laser patterning of OLEDs on flexible substrate for solid-state lighting. J. Laser Micro Nanoeng. 2009, 4, 218–223. [Google Scholar] [CrossRef]
- Gustafsson, J.C.; Liedberg, B.; Inganäs, O. In situ spectroscopic investigations of electrochromism and ion transport in a poly (3,4-ethylenedioxythiophene) electrode in a solid state electrochemical cell. Solid State Ion. 1994, 69, 145–152. [Google Scholar] [CrossRef]
- McDonald, J.P.; Hendricks, J.L.; Mistry, V.R.; Martin, D.C.; Yalisove, S.M. Femtosecond pulsed laser patterning of poly(3,4-ethylene dioxythiophene)-poly(styrenesulfonate) thin films on gold/palladium substrates. J. Appl. Phys. 2007, 102, 013107. [Google Scholar] [CrossRef]
- Hiraoka, H.; Lazare, S.; Chuang, T.J.; Rettner, C.T.; Hunziker, H.E. Laser photoetching of doped poly(tetrafluoroethylene); substituted-PTFE, and polyimide films. Microelectron. Eng. 1991, 13, 429–432. [Google Scholar] [CrossRef]
- Hemmerlin, M.; Mermet, J.M.; Bertucci, M.; Zydowicz, P. Determination of additives in PVC material by UV laser ablation inductively coupled plasma atomic emission spectrometry. Spectrochim. Acta-Part B At. Spectrosc. 1997, 52, 421–430. [Google Scholar] [CrossRef]
- Skordoulis, C.D.; Makropoulou, M.; Serafetinides, A.A. Ablation of nylon-6,6 with UV and IR lasers. Appl. Surf. Sci. 1995, 86, 239–244. [Google Scholar] [CrossRef]
- Burrel, M.C.; Liu, Y.S.; Cole, H.S. An X-ray photoelectron spectroscopy study of poly(methylmethacrylate) and poly(α-methylstyrene) surfaces irradiated by excimer lasers. J. Vac. Sci. Technol. A 1986, 4, 2459–2462. [Google Scholar] [CrossRef]
- See, T.L.; Liu, Z.; Li, L.; Zhong, X.L. A comparison of the characteristics of excimer and femtosecond laser ablation of acrylonitrile butadiene styrene (ABS). Appl. Surf. Sci. 2016, 364, 467–476. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Ho, C.Y.; Chiou, Y.J. Effect analysis of material properties of picosecond laser ablation for ABS/PVC. Proc. SPIE 2017, 10449, 69–74. [Google Scholar] [CrossRef]
- Kim, G.D.; Rundel, J.T.; Paul, B.K. UV laser ablation of polyetherimide embossing tools for the packaging of membranes and microchannels using sealing bosses. Int. J. Precis. Eng. Manuf. 2010, 11, 665–671. [Google Scholar] [CrossRef]
- Belaud, V.; Valette, S.; Stremsdoerfer, G.; Beaugiraud, B.; Audouard, E.; Benayoun, S. Femtosecond laser ablation of polypropylene: A statistical approach of morphological data. Scanning 2014, 36, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lutey, A.H.A.; Moroni, F. Pulsed laser texturing for improved adhesive-bonded polyethylene (PE) joints. Int. J. Adhes. Adhes. 2020, 102, 102676. [Google Scholar] [CrossRef]
- Hu, X.; Wong, T.K.S.; Gao, S.; Liu, H.M.; Lam, Y.L.; Chan, Y.C.; Xu, F.L. Direct patterning of electro-deposited polythiophene thin films by ultraviolet laser ablation. Proc. SPIE 1997, 3183, 57–65. [Google Scholar] [CrossRef]
- Aguilar, C.A.; Lu, Y.; Mao, S.; Chen, S. Direct micro-patterning of biodegradable polymers using ultraviolet and femtosecond lasers. Biomaterials 2005, 26, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Johnson, J.; Fei, Z.; Farson, D.F.; Lannutti, J.J.; Choi, H.W.; Lee, L.J. Micropatterning and characterization of electrospun PCL/gelatin nanofiber tissue scaffolds by femtosecond laser ablation. ICALEO 2009, 2009, 1114–1123. [Google Scholar] [CrossRef]
- Paun, I.A.; Zamfirescu, M.; Mihailescu, M.; Luculescu, C.R.; Mustaciosu, C.C.; Dorobantu, I.; Calenic, B.; Dinescu, M. Laser micro-patterning of biodegradable polymer blends for tissue engineering. J. Mater. Sci. 2014, 50, 923–936. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Wang, C.; Luo, X.; Kim, J.; Wang, Z.; Yamauchi, Y. Porous Organic Frameworks: Advanced Materials in Analytical Chemistry. Adv. Sci. 2018, 5, 1801116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, W.; Astruc, D.; Gu, H. Syntheses and applications of dendronized polymers. Prog. Polym. Sci. 2019, 96, 43–105. [Google Scholar] [CrossRef]
- Lundin, P.M.; Fiser, B.L.; Blackledge, M.S.; Pickett, H.L.; Copeland, A.L. Functionalized Self-Assembled Monolayers: Versatile Strategies to Combat Bacterial Biofilm Formation. Pharmaceutics 2022, 14, 1613. [Google Scholar] [CrossRef]
- Ariga, K.; Lvov, Y.; Decher, G. There is still plenty of room for layer-by-layer assembly for constructing nanoarchitectonics-based materials and devices. PCCP 2022, 24, 4097–4115. [Google Scholar] [CrossRef]
- Singh, R.S. Langmuir and Langmuir–Blodgett films of aromatic amphiphiles. Soft Mater. 2022, 20, 57–98. [Google Scholar] [CrossRef]
- Morallon, E.; Salavagione, H.J.; Barbero, C.A.; Acevedo, D.F.; Lasagni, A.; Mücklich, F. Metodo de Fabricacion de Superficies Metalicas Estructuradas para Usar en Espectroscopia Raman Aumentada por la Superficie y Otras Espectroscopias Relacionadas. Spain P2010000031, 1 December 2010. [Google Scholar]
- Lasagni, A.F. Apparatus and Methods for the Interference Patternimg of Planar Samples. U.S. Patent 9.233,435 B2, 12 January 2016. [Google Scholar]

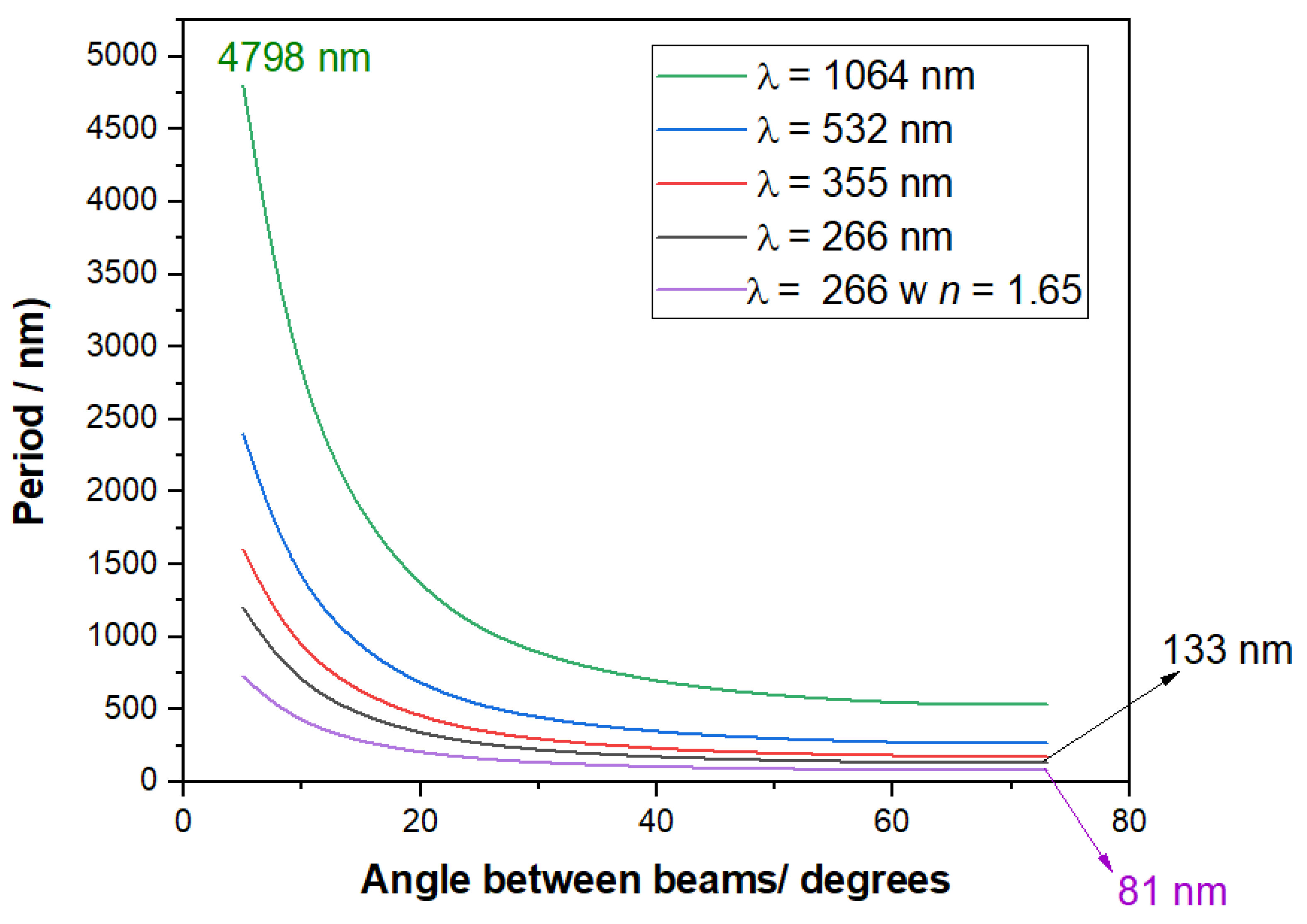
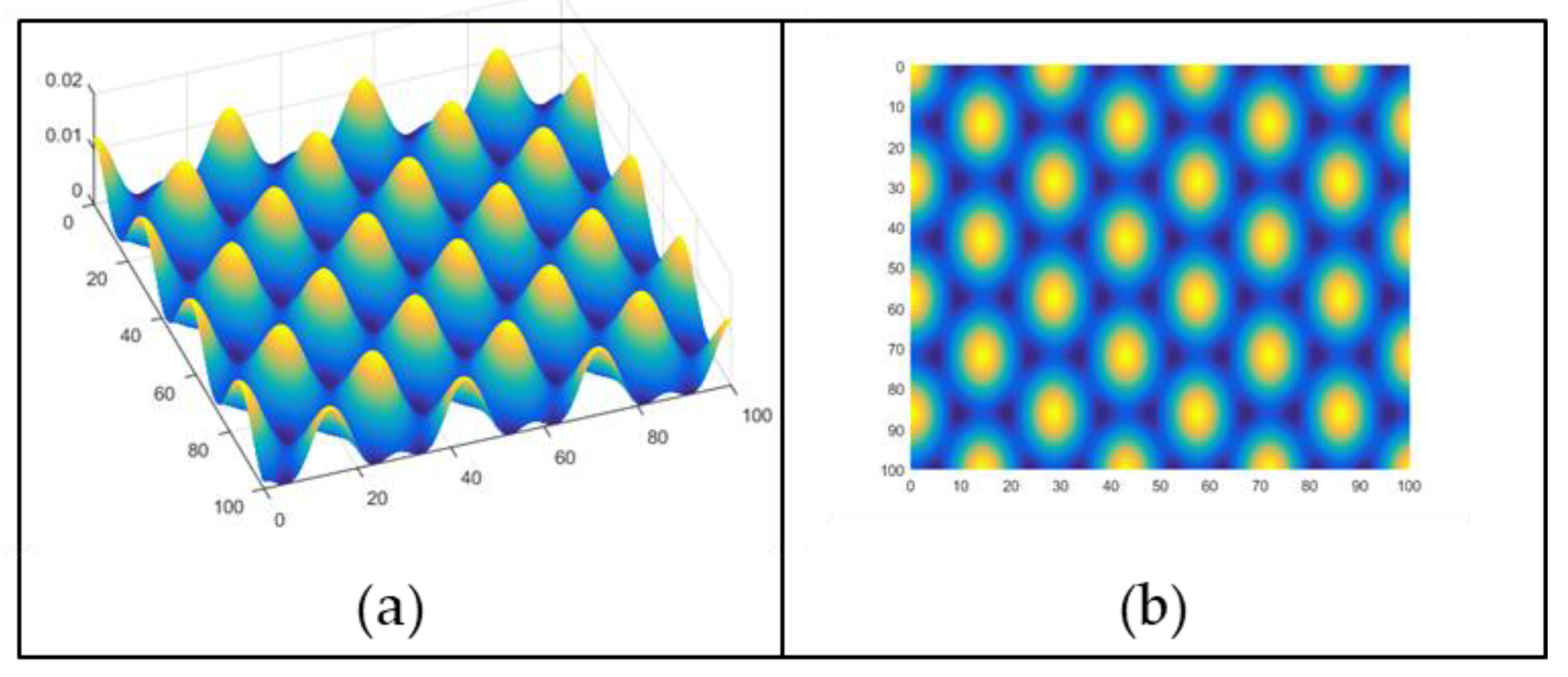
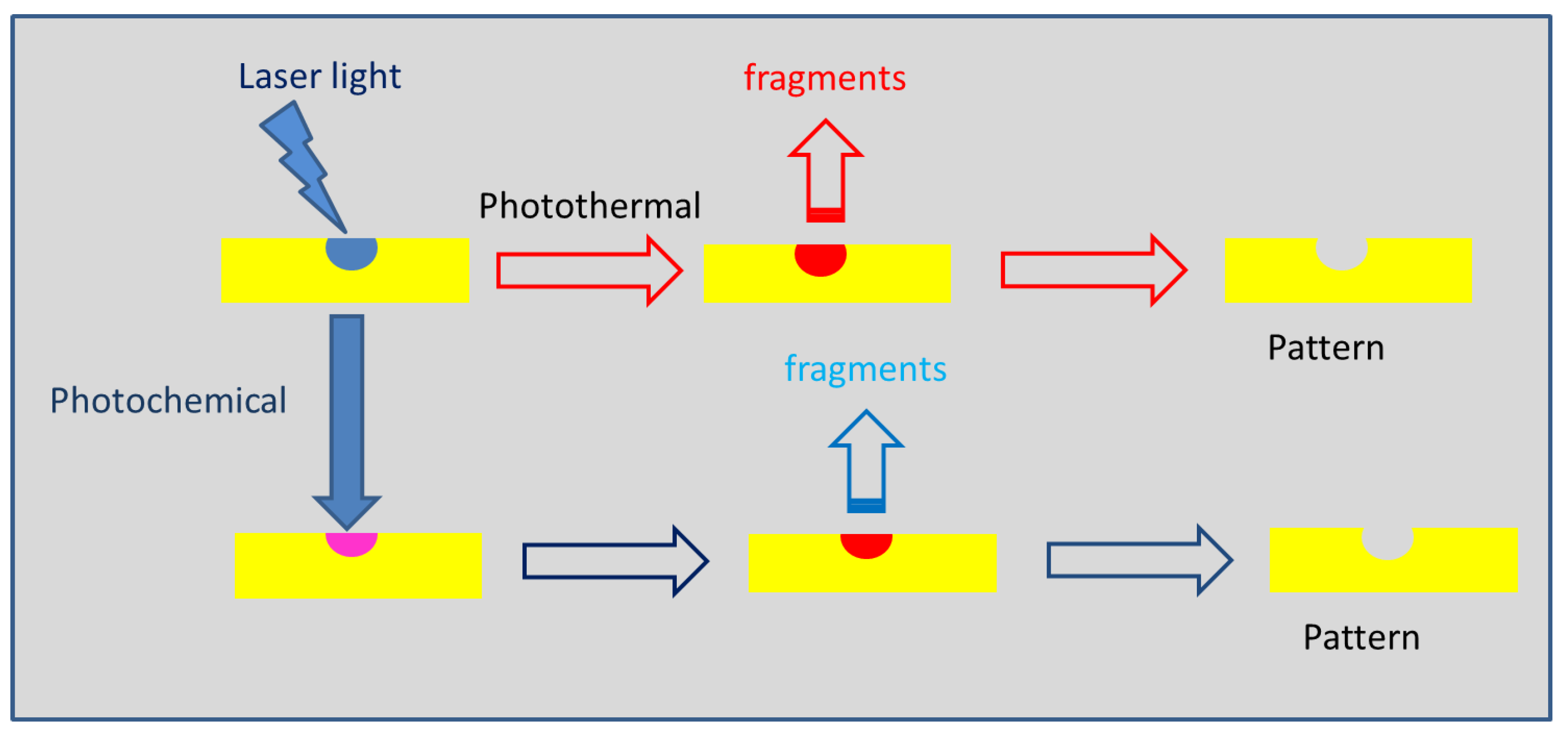
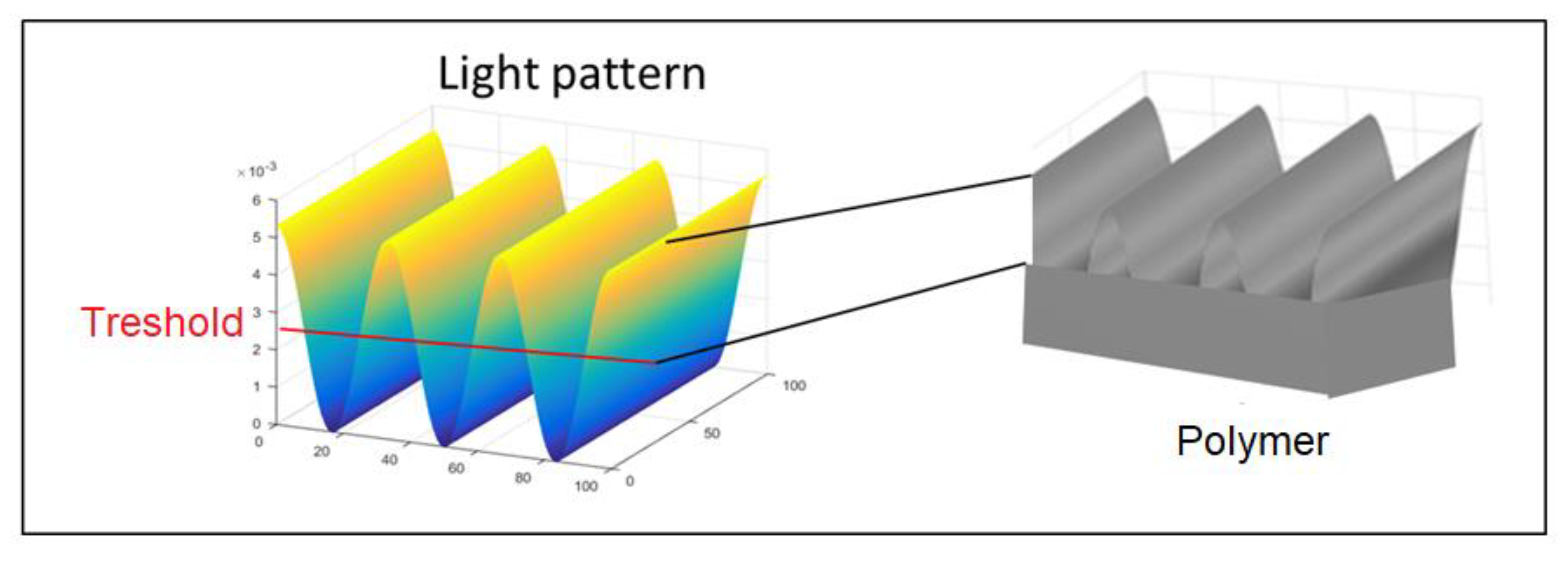
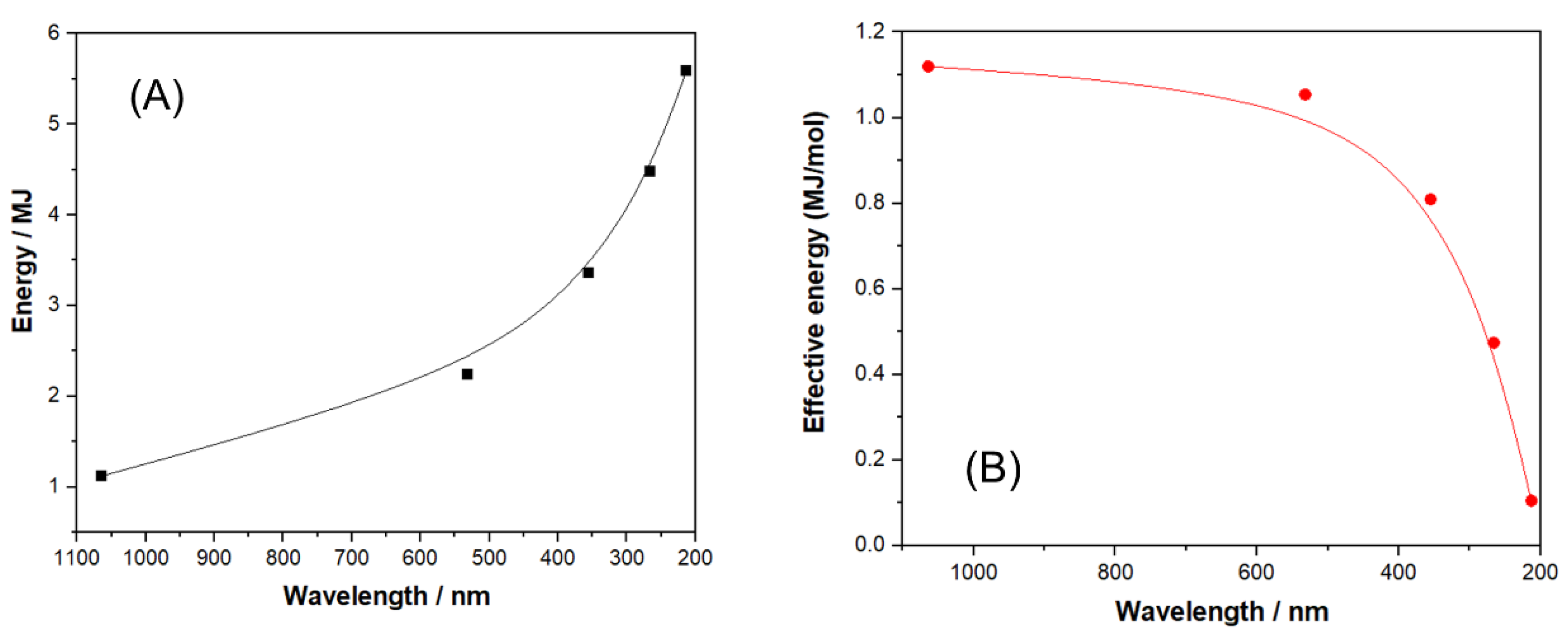

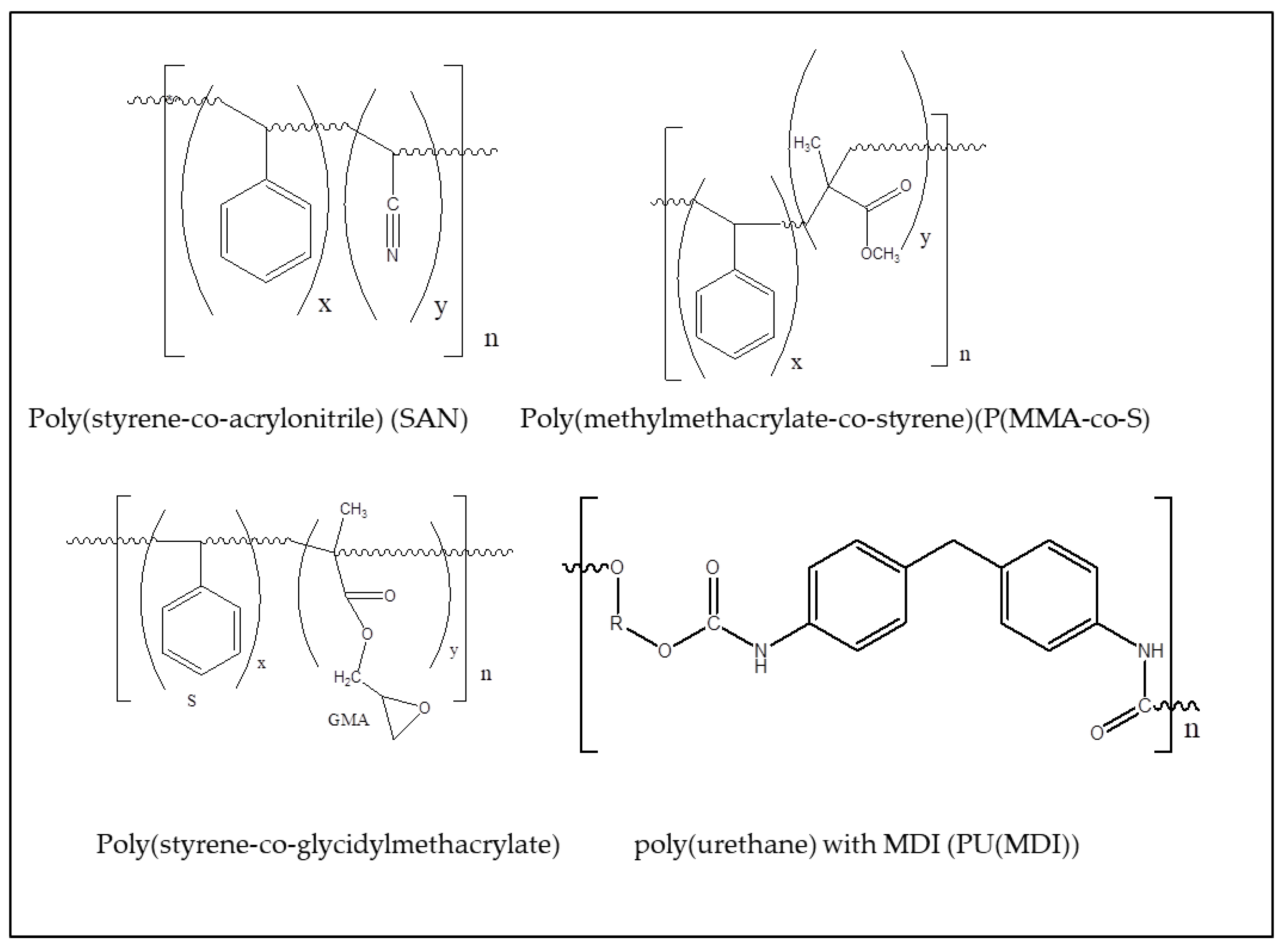
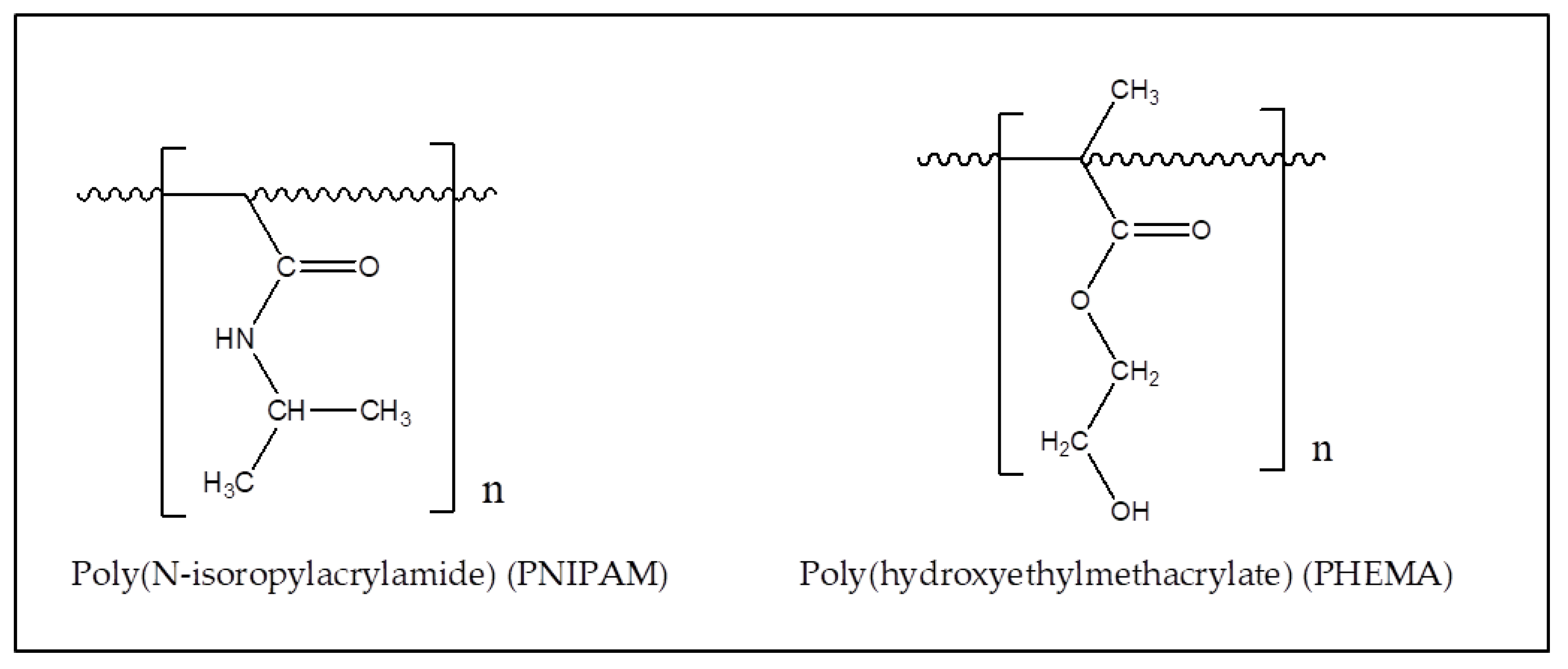
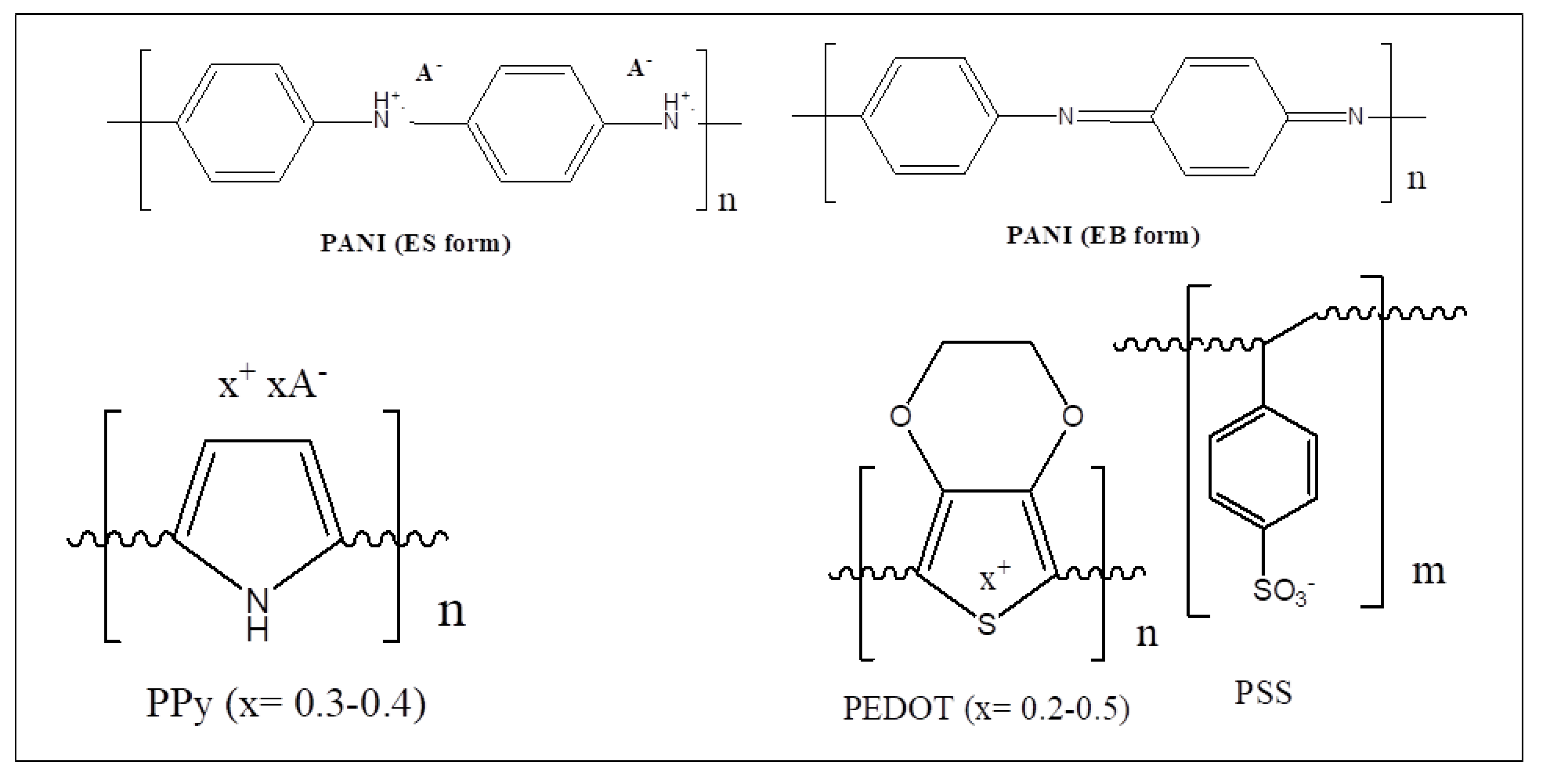
| Polymer | λmax (nm) | α (cm−1) | Source |
|---|---|---|---|
| PMMA | 215 | 6500 | [27] |
| PMMA | 250 | 50 | [27] |
| PMMA | 248 | 65 | [28] |
| PMMA | 308 | <10 | [27] |
| PVAc | 215 | 2000 | [29] |
| PVAc | 250 | 100 | [29] |
| PVAc | 308 | <10 | [27] |
| PI | 230 | 2.5 105 | [27] |
| PI | 248 | 14.7 104 | [27] |
| PI | 248 | 2.8 105 | [27] |
| PI | 308 | 1.2 105 | [27] |
| PS | 248 | 2.4 104 | [29] |
| P(α-MeS) | 248 | 6.5 103 | [27] |
| P(α-MeS) | 308 | 8.0 101 | [27] |
| PET | 250 | 4.9 105 | [30] |
| PET | 308 | 5 104 | [27] |
| PEN | 225 | 3.8 105 | [27] |
| PC (BPA) | 248 | 1.0 104 | [27] |
| PC (BPA) | 308 | 22 | [27] |
| ABS | 248 | 9.8 104 | [27] |
| SAN | 248 | 7.5 104 | [29] |
| PE | 248 and 308 | <10 | [27] |
| PP | 248 and 308 | <10 | [27] |
| PTFE | 248 | 14 | [27] |
| PTFE | 308 | <10 | [27] |
| PHEMA | 222 | 2900 | [31] |
| PET | 308 | 13 | [30] |
| Polymer | Laser | λ (nm) | Max Fluence (mJ/cm2) | Pulse Duration (ns)/nr | Setup | Refs. | Application |
|---|---|---|---|---|---|---|---|
| PET, PI | KrF | 248 | 200 | 25/mul | Michelson interferometer | [17] | Optical gratings |
| PI | KrF | 248 | 58 | -/mul | Talbot interferometer | [52,53] | Semiconductor processing |
| PI | ArF | 193 | 300 | -/mul | Talbot interferometer | [54] | Optical gratings |
| Triazene polymer & | Nd:YAG | 355 | 240 | 1–10 | Michelson interferometer | [55] | Optical gratings |
| PC | KrF | 248 | - | 0.5/single | Diffractive variable delay generator | [56] | Photonic crystals |
| PET @ | Nd:YAG | 266 | 500 | 10/1–10 | MMI * | [57,58] | Biological cell adhesion/growth |
| PET | Nd:YAG | 266 | 20–400 | 10/1 | MMI | [59] | Solar cell |
| PET | Nd:YAG | 266 | 100–150 | 10/1 | MMI | [60] | Optical gratings |
| PET | Nd:YAG | 266 | 20–400 | 10/1 | Diffractive | [61] | Optical gratings |
| PI, PS | Nd:YAG | 266&355 | 500 | 10/1 | MMI | [62] | Inhibition biofilm |
| PI | fs | 1030 | 0.5 | <0.5/5–10 | Diffractive | [63] | DLIP + LIPSS |
| PI | Nd:YAG | 355 | - | 10/1 | MMI | [64] | Antifouling/ antibacterial |
| PS | Nd:YAG | 266 | 500 | 10/1 | MMI | [65] | functionalization of surfaces |
| PS, PI, PET | Nd:YAG | 266 | 500 | 10/1 | MMI | [66] | Bacterial anti-biofouling |
| PS | ps | 1064 | 0.001 | 10 × 10−3/1 | DOE | [67] | Surface patterning |
| PTT | Nd:YAG | 266 | - | 10/1 | MMI | [68] | Proof of concept |
| PC | Nd:YAG | 266 | >300 | 10/1 | MMI | [69] | SERS |
| PC, C@PC | DPSS | 263 | 0.05 | 3/1 | DOE | [70,71] | Modelling |
| PC | Nd:YAG Nd:YAG DPSS | 355 266 263 | 0.05 180 0.05 | 0.01/1 <10/1 <3/1 | DOE DOE DOE | [26] | Micromachining |
| PC | DPSS Yb:YAG Nd:VAN | 263 343 355 | 0.05 2–4 2–4 | 3/1 0.007/1000 0.010/1000 | DOE (DLIP) 1 beam (LIPSS) 1 beam (LIPSS) | [55] | Hierarchical micro-/nanostructures |
| PC | KrF | 248 | - | <0.0001/1 | DVDG | [72] | Photonic crystals |
| PMMA, PS, P(MMA-co-S), PI, PC | Nd:YAG | 266 355 | 300 | 10/1 | MMI | [72] | Effect of polymer and fluence |
| Doped PS | Nd:YAG | 266 355 | 300 | 10/1 | MMI | [73] | Cell growth |
| PI, PEEK | Nd:YAG | 355 | 1.2 | 38/1 | MMI | [74] | Embossing of PDMS for guiding neurons |
| PMMA with Ag Nc | Nd:YAG | 355 | 800 | 6/1 | MMI | [75] | Fluorescent patterning |
| PEEK-CF composite | DPSS | 1053 263 | 1.4, 2.9 2 | 15/1 4/1 | DOE | [76] | Superhydrophobic surfaces |
| Period (μm) | Contact Angle/o | Roughness (nm) | Cells per mL × 10−7 |
|---|---|---|---|
| 0 (flat) | 65 | 1.4 | 49.5 |
| 1 | 101 | 33.4 | 4.3 |
| 2 | 92 | 28.9 | 2.76 |
| 10 | 72 | 19.4 | 5.88 |
| Copolymer | Laser | λ (nm) | Fluence (mJ/cm2) | Pulse Duration (ns)/nr | Setup | Refs. | Application |
|---|---|---|---|---|---|---|---|
| PU | Nd:YAG | 266 | 100–600 | 10/1 | MMI | [117] | Wettability control |
| SAN (p(S-co-AN) | Nd:YAG | 266 | 500 | 10/1 | MMI | [118] | Chemically patterned surfaces |
| p(MMA-co-S) | Nd:YAG | 266 | 500 | 10/1 | MMI | [73] | Structuring PMMA |
| P(S-co-EGMA) | Nd:YAG | 266 | 500 | 10/1 | MMI | [64] | Chemical pattern reactivity |
| Polymer | Laser | λ (nm) | Fluence (mJ/cm2) | Pulse Duration (ns)/Number | Setup | Refs. | Application |
|---|---|---|---|---|---|---|---|
| d-PNIPAM | Nd:YAG | 355 | 800 | 10/1 | MMI | [134] | Cell growth |
| Safrofilcon | -- | 263 | 470 | 4/4–7 | DOE | [135] | Ophthalmic lenses |
| PANI@ PNIPAM | Nd:YAG | 266 | 400–800 | 10/1 | MMI | [136] | Remote triggering |
| PHEMA PHEMA-UV | Nd:YAG | 266 | 10/1 | MMI | [137] | Ophthalmic diffraction gratings |
| Polymer | Laser | λ (nm) | Fluence (mJ/cm2) | Pulse Duration (ns)/Number | Setup | Refs. | Application |
|---|---|---|---|---|---|---|---|
| PANI | Nd:YAG | 355 | 174–325 | 10/1 | MMI | [147] | Conductive nanowires |
| PANI | Nd:YAG | 355 | 174–325 | 10/1 | MMI | [148] | Conductive arrays |
| PEDOT:PSS | Nd:YAG | 355 | 54–296 | 10/1 | MMI | [149] | Biomedicine |
| PPy | Nd:YAG | 355 | 1200 | 10/1 | MMI | [150] | Sensors |
| Polymer | Pulse Duration | λ (nm) | Doping | Ref. |
|---|---|---|---|---|
| PTFE | 308 266 | Perfluoro-s-triazines | [184] | |
| PVC | 4–6 ns | 266 | None | [185] |
| Polyamide (Nylon 6,6) | 17 ns 400 μs | 308 1060 | None | [186] |
| P(α-MeS) | 15 ns | 193 | None | [187] |
| ABS | 15 ns 100 fs | 248 800 | None None | [188] |
| ABS/PVC | 60 ps | 266 | None | [189] |
| Polyetherimide | 60 ns | 355 | None | [190] |
| PP | 130 fs | 800 | None | [191] |
| PE | 64 ns | 1064 | Black pigment | [192] |
| Polythiophene | CW | 325 | None | [193] |
| PCL | 35 ns 220 fs | 193 800 | None | [194] |
| PCL/gelatin nanofiber | 150 fs | 775 | None | [195] |
| Nitrocellulose | 5 ns | 337 | Stilbene 420, Coumarin 120, Rhodamine 6G | [33] |
| Polyurethane (PU) + Poly(lactic-co-glycolic acid) (PLGA) + Polylactide-polyethylene glycol-polylactide (PPP) | 200 fs | 75 | None | [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, C.A.; Acevedo, D.F. Manufacturing Functional Polymer Surfaces by Direct Laser Interference Patterning (DLIP): A Polymer Science View. Nanomanufacturing 2022, 2, 229-264. https://doi.org/10.3390/nanomanufacturing2040015
Barbero CA, Acevedo DF. Manufacturing Functional Polymer Surfaces by Direct Laser Interference Patterning (DLIP): A Polymer Science View. Nanomanufacturing. 2022; 2(4):229-264. https://doi.org/10.3390/nanomanufacturing2040015
Chicago/Turabian StyleBarbero, Cesar Alfredo, and Diego Fernando Acevedo. 2022. "Manufacturing Functional Polymer Surfaces by Direct Laser Interference Patterning (DLIP): A Polymer Science View" Nanomanufacturing 2, no. 4: 229-264. https://doi.org/10.3390/nanomanufacturing2040015
APA StyleBarbero, C. A., & Acevedo, D. F. (2022). Manufacturing Functional Polymer Surfaces by Direct Laser Interference Patterning (DLIP): A Polymer Science View. Nanomanufacturing, 2(4), 229-264. https://doi.org/10.3390/nanomanufacturing2040015







