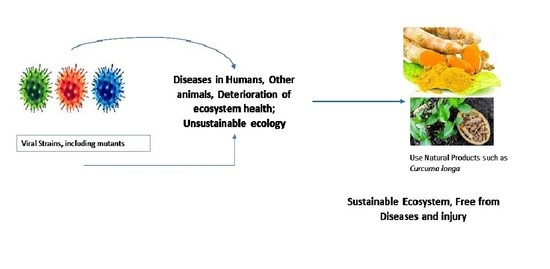
Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa)
Abstract
:1. Introduction
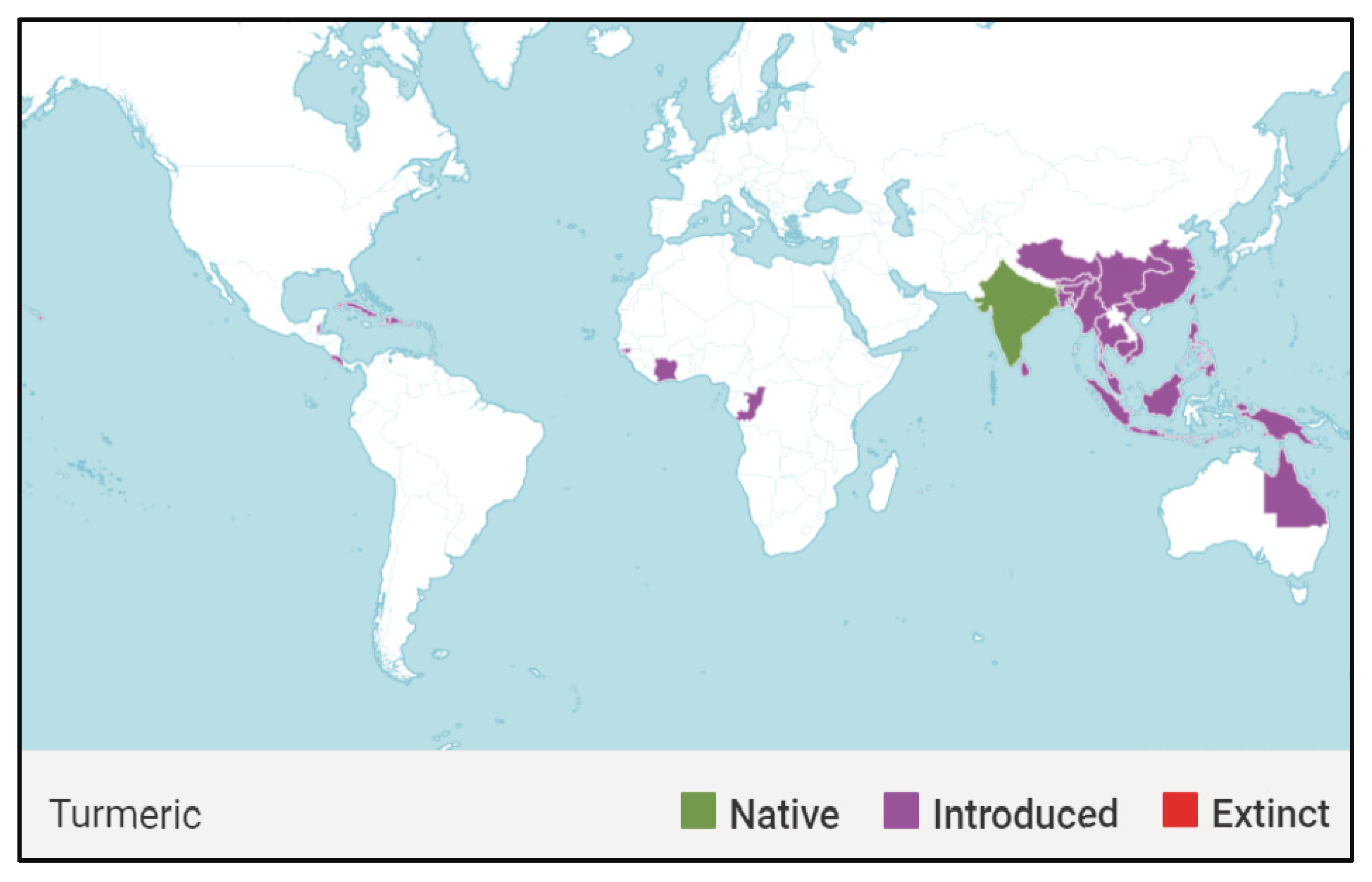
1.1. Global Distribution of Curcuma longa
1.2. Morphology of Curcuma longa
1.3. Botanical Classification of C. longa
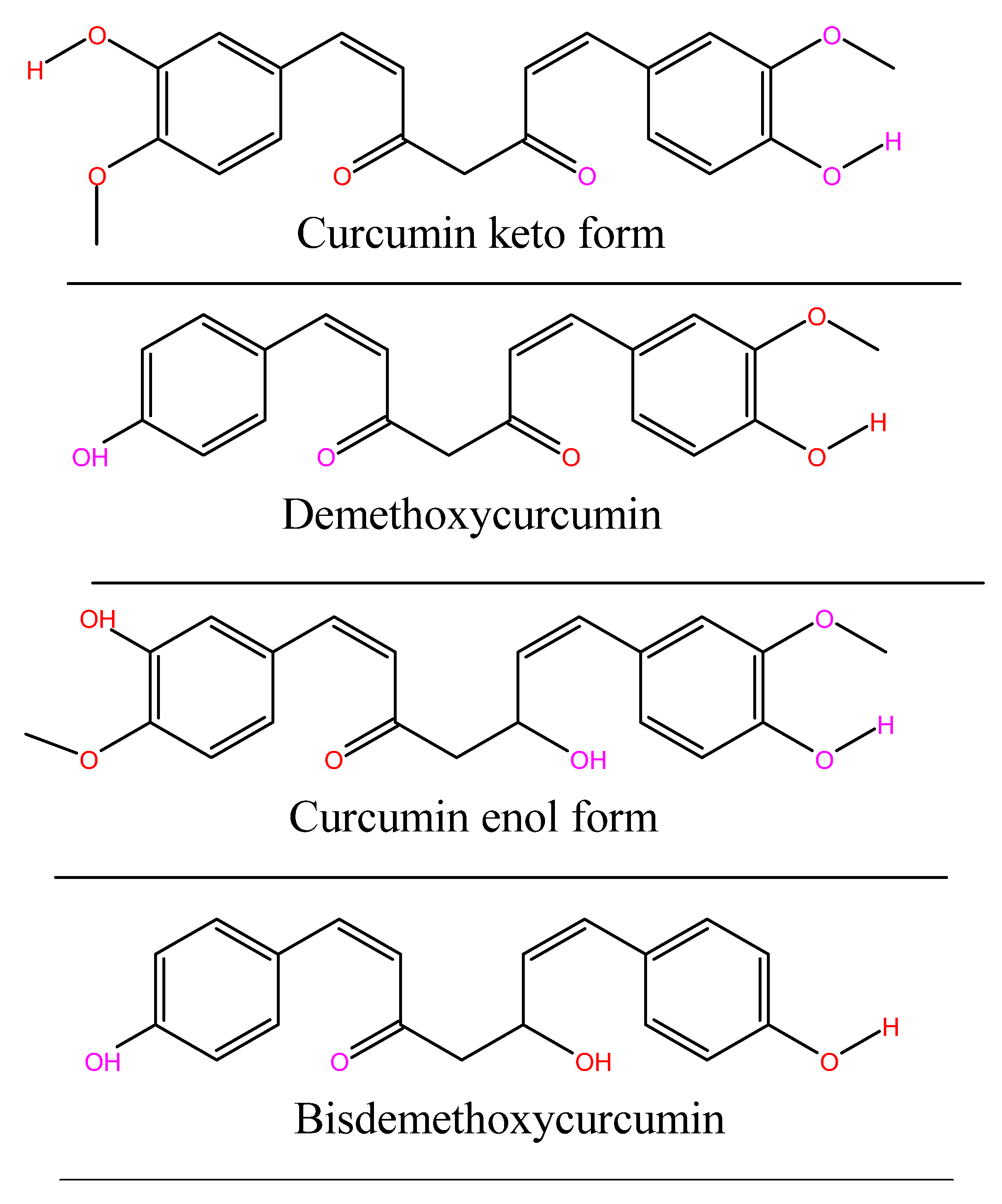
1.4. Chemistry of Curcumin
2. Materials and Methods
3. Pharmacology of Curcumin
4. Mechanism of Curcumin Antiviral Activity
4.1. Inactivate Extracellular Virus Particles
4.2. Prevent Viral Attachment and/or Entry
4.3. Prevent Replication of the Viral Genome
4.4. Curcumin Suppresses Intercellular Signaling Cascades
4.5. Attenuation of PI3K/Akt and NF-κB Signalling Pathways
4.6. Prevent Assembly or Release of New Infectious Virions
4.7. Synergistic Effect
4.8. Inhibition of Cell Binding
4.9. Improvement of Antiviral Activity of Curcumin
4.10. Potential of Curcumin as Antiviral Agent
5. Application of Curcumin
5.1. Traditional Uses and Ethnopharmacology of Curcumin
5.2. Application of Curcumin-Based Nano-Formulations
5.3. Electrostatic Nature and Size of Curcumin-Based Nano-Formulations
5.4. Curcumin Therapy for Mitigation of COVID-19
5.5. User Preference for Curcumin
5.6. Safety Aspects of Curcumin
5.7. Availability of Finished Products
| Purpose of Usage | Remarks | References |
|---|---|---|
| Curcumin is used in the mitigation of inflammatory disorders | This is due to its ability to inhibit different molecules involved in inflammation, such as lipooxygenase, COX-2, interferon-inducible protein, and tumour necrosis factor | [157] |
| Used in the management of diabetes mellitus: | Turmeric rhizome powder is very useful with amla juice and honey in Madhumeha (diabetes mellitus) | [109,157] |
| Used in the mitigation of cardiovascular disorders | This is contributed by the ability of the antioxidants in turmeric to prevent damage to cholesterol, hence its protection against atherosclerosis. | [110] |
| Used in the mitigation of allergic activity | This is due to the ability of curcumin to inhibit nonspecific and specific mast cell-dependent allergic reactions. | [168] |
| Used in the mitigation of dermatophytic activity: | Rhizomes of Haridra fresh juice have the antiparasitic ability in numerous skin affections. | [4] |
| Used in mitigation of drug resistance: | This is due to the ability of curcumin as a potent drug resistance preventer. | [169] |
| Used as additives in other drugs | This is due to the synergism of Curcumin and other drugs. | [110] |
| Used in the management of jaundice (Hepatoprotective) | Due to the synergistic interaction of the rhizome with amla juice and other substances. | [110,170] |
| Used in mitigation of ischemic brain injury | This is attributed to Curcuma oil’s neuroprotective action, which reduces the negative effects of ischemia by reducing nitrosative and oxidative stress. | [171] |
| Used for mitigation of respiratory disorders | The rhizome is used for gargling, and the piece of the rhizome is slightly burnt and given for chewing. | [4] |
| Gastrointestinal disorders: | This is due to the anthelmintic activity of the fresh juice of Haridra. | [172] |
| Used as an additive in poultry diet | Used as a natural growth promoter and disease control. | [106,173] |
| Used for management of Alzheimer’s disease | This is due to the ability of curcumin to reduce oxidative damage and reverse the amyloid pathology. | [107] |
| Used for chemoprotection In tumour cells or tissue | Curcumin is nutraceutical. Chemopreventive ability. | [174] |
| Used in mitigation of cancer | Curcumin possesses anticancer activities via its effect on diverse biological pathways involved in mutagenesis, oncogene expression, cell cycle regulation, apoptosis, tumorigenesis and metastasis. | [175] |
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar]
- Hussain, W.; Haleem, K.S.; Khan, I.; Tauseef, I.; Qayyum, S.; Ahmed, B.; Riaz, M.N. Medicinal plants: A repository of antiviral metabolites. Future Virol. 2017, 12, 299–308. [Google Scholar]
- Krishnaswamy, K. Traditional Indian spices and their health significance. Asia Pac. J. Clin. Nutr. 2008, 17, 265–268. [Google Scholar] [PubMed]
- Pandey, G. Dravyaguna Vijnana, 2nd ed.; Krishnadas Academy Publisher: Varanasi, India, 2002; Volume 1, pp. 737–746. [Google Scholar]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kapoor, S.; Lata Saraf, S. Topical Herbal Therapies an Alternative and Complementary Choice. Res. J. Med. Plant 2011, 5, 650–669. [Google Scholar]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Ody, P. Turmeric: Nature’s Miracle Healer: Fact or Fiction; Souvenir Press: London, UK, 2018. [Google Scholar]
- Mathew, A.; Pushpanath, S. Indian Spices; Dee Bee Info Publications: Kottayam, India, 2005. [Google Scholar]
- Institute, N.C. Clinical development plan: Curcumin. Cell. Biochem. 1996, 26, 72–85. [Google Scholar]
- Sasikumar, B. Genetic resources of Curcuma: Diversity, characterization and utilization. Plant Genet. Resour. 2005, 3, 230–251. [Google Scholar]
- Easmin, M.S.; Sarker, M.Z.; Ferdosh, S.; Shamsudin, S.H.; Yunus, K.B.; Uddin, M.S.; Sarker, M.M.R.; Akanda, M.J.H.; Hossain, M.S.; Khalil, H.A. Bioactive compounds and advanced processing technology: Phaleria macrocarpa (sheff.) Boerl, a review. J. Chem. Technol. Biotechnol. 2015, 90, 981–991. [Google Scholar]
- Van Andel, T.; Carvalheiro, L.G.; Medicine, A. Why urban citizens in developing countries use traditional medicines: The case of Suriname. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar]
- Kew Science. Curcuma longa L. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:796451-1 (accessed on 19 June 2022).
- Kandiannan, K.; Anandaraj, M.; Prasath, D.; Zachariah, T.J.; Krishnamurthy, K.; Srinivasan, V. Evaluation of short and tall true turmeric (Curcuma longa) varieties for growth, yield and stability. Indian J. Agric. Sci. 2015, 85, 718–720. [Google Scholar]
- Nair, R.R.; Shiva, K.N.; Anchu, S.; Zachariah, T.J. Characterization of open-pollinated seedling progenies of turmeric (Curcuma longa L.) based on chromosome number, plant morphology, rhizome yield and rhizome quality. Cytologia 2010, 75, 443–449. [Google Scholar] [CrossRef]
- Kim, S.; Ko, S.-C.; Kim, Y.-S.; Ha, S.-K.; Park, H.-Y.; Park, Y.; Lee, S.-H. Determination of Curcuma longa L.(Turmeric) leaf extraction conditions using response surface methodology to optimize extraction yield and antioxidant content. J. Food Qual. 2019, 2019, 1–8. [Google Scholar]
- Kumar, A.; Singh, M.; Singh, P.P.; Singh, S.K.; Raj, P.; Pandey, K.D. Antioxidant efficacy and curcumin content of turmeric (Curcuma-longa L.) flower. Int. J. Curr. Pharm. Res. 2016, 8, 112–114. [Google Scholar]
- Fujisawa, S.; Murakami, Y. Eugenol and its role in chronic diseases. Drug Discov. Mother Nat. 2016, 929, 45–66. [Google Scholar]
- Khopde, S.M.; Indira Priyadarsini, K.; Palit, D.K.; Mukherjee, T. Effect of Solvent on the Excited-state Photophysical Properties of Curcumin. Photochem. Photobiol. 2000, 72, 625–631. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar]
- Baker, M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar]
- Bisson, J.; McAlpine, J.B.; Friesen, J.B.; Chen, S.-N.; Graham, J.; Pauli, G.F. Can invalid bioactives undermine natural product-based drug discovery? J. Med. Chem. 2016, 59, 1671–1690. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Hu, G.; Sun, H.; Kong, Q. Bioactivities of EF24, a novel curcumin analog: A review. Front. Oncol. 2018, 8, 614. [Google Scholar]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. BioFactors 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef] [Green Version]
- Santezi, C.; Reina, B.D.; de Annunzio, S.R.; Calixto, G.; Chorilli, M.; Dovigo, L.N. Photodynamic potential of curcumin in bioadhesive formulations: Optical characteristics and antimicrobial effect against biofilms. Photodiagnosis Photodyn. Ther. 2021, 35, 102416. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Brouet, I.; Ohshima, H. Curcumin, an anti-tumor promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys. Res. Commun. 1995, 206, 533–540. [Google Scholar] [CrossRef]
- Sreejayan, N.; Rao, M.; Priyadarsini, K.; Devasagayam, T. Inhibition of radiation-induced lipid peroxidation by curcumin. Int. J. Pharm. 1997, 151, 127–130. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.; Francis, G.; Becker, K. Phorbol esters: Structure, biological activity, and toxicity in animals. Int. J. Toxicol. 2007, 26, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dhawan, S.; Hardegen, N.J.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-κB activation. Biochem. Pharmacol. 1998, 55, 775–783. [Google Scholar] [CrossRef]
- Bharti, A.C.; Donato, N.; Singh, S.; Aggarwal, B.B. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor–κB and IκBα kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood J. Am. Soc. Hematol. 2003, 101, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer. Res. 2004, 24, 2783–2840. [Google Scholar]
- Malkin, E.Z.; Bratman, S.V. Bioactive DNA from extracellular vesicles and particles. Cell Death Dis. 2020, 11, 584. [Google Scholar] [CrossRef]
- Lam, J.H.; Smith, F.L.; Baumgarth, N. B cell activation and response regulation during viral infections. Viral Immunol. 2020, 33, 294–306. [Google Scholar] [CrossRef]
- Gao, Y.; Wijewardhana, C.; Mann, J.F. Virus-like particle, liposome, and polymeric particle-based vaccines against HIV-1. Front. Immunol. 2018, 9, 345. [Google Scholar] [CrossRef]
- Bello-Morales, R.; Ripa, I.; López-Guerrero, J.A. Extracellular vesicles in viral spread and antiviral response. Viruses 2020, 12, 623. [Google Scholar] [CrossRef]
- Magwa, M.L.; Gundidza, M.; Gweru, N.; Humphrey, G. Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Liu, Z.; Ying, Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. J. Front. Cell Dev. Biol. 2020, 8, 479. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Li, S.; Dang, W.; Xin, S.; Long, S.; Zhang, W.; Cao, P.; Lu, J. Extracellular Vesicles Regulated by Viruses and Antiviral Strategies. Front. Cell Dev. Biol. 2021, 9, 2842. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; Chen, D.-Y.; Wen, H.-W.; Ou, J.-L.; Chiou, S.-S.; Chen, J.-M.; Wong, M.-L.; Hsu, W.-L. Inhibition of enveloped viruses infectivity by curcumin. PLoS ONE 2013, 8, e62482. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Przekora, A. Modifications of wound dressings with bioactive agents to achieve improved pro-healing properties. Appl. Sci. 2021, 11, 4114. [Google Scholar] [CrossRef]
- Mathew, D.; Hsu, W.-L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Hu, C.; Fast, D.; Zhang, L.; Yang, B.; Kan, J.; Du, J. Curcumin attenuates poly (I: C)-induced immune and inflammatory responses in mouse macrophages by inhibiting TLR3/TBK1/IFNB cascade. J. Funct. Foods 2022, 89, 104949. [Google Scholar] [CrossRef]
- Pécheur, E.-I. Curcumin against hepatitis C virus infection: Spicing up antiviral therapies with ‘nutraceuticals’? Gut 2014, 63, 1035–1037. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.T.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G.J.H. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Deb, L.; Prasad, S.J.M. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Yang, M.; Lee, G.; Si, J.; Lee, S.-J.; You, H.J.; Ko, G. Curcumin shows antiviral properties against norovirus. Molecules 2016, 21, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.L.; Mizushina, Y.; Wang, S.Y.; Chuang, D.Y.; Nadar, M.; Hsu, W.L. Structure–activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013, 280, 5829–5840. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-H.; Vaidya, B.; Cho, S.-Y.; Park, M.-A.; Kaewintajuk, K.; Kim, S.R.; Oh, M.-J.; Choi, J.-S.; Kwon, J.; Kim, D. Identification of regulators of the early stage of viral hemorrhagic septicemia virus infection during curcumin treatment. Fish Shellfish Immunol. 2015, 45, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Vajragupta, O.; Boonchoong, P.; Morris, G.M.; Olson, A.J. Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg. Med. Chem. Lett. 2005, 15, 3364–3368. [Google Scholar] [CrossRef]
- Sui, Z.; Salto, R.; Li, J.; Craik, C.; de Montellano, P.R.O. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993, 1, 415–422. [Google Scholar] [CrossRef]
- Narayanan, A.; Kehn-Hall, K.; Senina, S.; Lundberg, L.; Van Duyne, R.; Guendel, I.; Das, R.; Baer, A.; Bethel, L.; Turell, M. Curcumin inhibits Rift Valley fever virus replication in human cells. J. Biol. Chem. 2012, 287, 33198–33214. [Google Scholar] [CrossRef]
- Ali, A.; Banerjea, A.C. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci. Rep. 2016, 6, 27539. [Google Scholar] [CrossRef]
- Jafari, M.; Ghadami, E.; Dadkhah, T.; Akhavan-Niaki, H. PI3k/AKT signaling pathway: Erythropoiesis and beyond. J. Cell. Physiol. 2019, 234, 2373–2385. [Google Scholar] [CrossRef]
- Diehl, N.; Schaal, H. Make yourself at home: Viral hijacking of the PI3K/Akt signaling pathway. Viruses 2013, 5, 3192–3212. [Google Scholar] [CrossRef]
- Shi, Q.; Hoffman, B.; Liu, Q. PI3K-Akt signaling pathway upregulates hepatitis C virus RNA translation through the activation of SREBPs. Virology 2016, 490, 99–108. [Google Scholar] [CrossRef]
- Lee, P.J.; Alam, J.; Wiegand, G.W.; Choi, A. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. USA 1996, 93, 10393–10398. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.K.; Ikizler, M.R.; Wallen, C.; Wright, P.F.; Harth, E. Effective delivery of IgG-antibodies into infected cells via dendritic molecular transporter conjugate IgGMT. Mol. Biosyst. 2008, 4, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C.; Sobrino, F. Animal Viruses: Molecular Biology; Caister Academic Press: Norfolk, VA, USA, 2008; Volume 1. [Google Scholar]
- Richart, S.M.; Li, Y.-L.; Mizushina, Y.; Chang, Y.-Y.; Chung, T.-Y.; Chen, G.-H.; Tzen, J.T.-C.; Shia, K.-S.; Hsu, W.-L. Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection. J. Food Drug Anal. 2018, 26, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef]
- Phansalkar, P.S.; Zhang, Z.; Verenich, S.; Gerk, P.M. Pharmacokinetics and bioavailability enhancement of natural products. In Natural Products for Cancer Chemoprevention; Springer: Berlin/Heidelberg, Germany, 2020; pp. 109–141. [Google Scholar]
- Bawa, G.; Khurana, N.; Singh, A. Clinical Uses of Piperine: A Review. World J. Pharm. Res. 2021, 10, 2232–2270. [Google Scholar]
- Lin, C.J.; Chang, L.; Chu, H.W.; Lin, H.J.; Chang, P.C.; Wang, R.Y.; Unnikrishnan, B.; Mao, J.Y.; Chen, S.Y.; Huang, C.C. High amplification of the antiviral activity of curcumin through transformation into carbon quantum dots. Small 2019, 15, 1902641. [Google Scholar] [CrossRef]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int. J. Nanomed. 2020, 15, 2699. [Google Scholar] [CrossRef]
- Kharisma, V.D.; Agatha, A.; Ansori, A.N.M.; Widyananda, M.H.; Rizky, W.C.; Dings, T.G.A.; Marina Derkho8, I.L.; Antonius, Y.; Rosadi, I.; Zainul, R. Herbal combination from Moringa oleifera Lam. and Curcuma longa L. as SARS-CoV-2 antiviral via dual inhibitor pathway: A viroinformatics approach. J. Pharm. Pharmacogn. Res. 2021, 10, 138–146. [Google Scholar] [CrossRef]
- Nabila, N.; Suada, N.K.; Denis, D.; Yohan, B.; Adi, A.C.; Veterini, A.S.; Anindya, A.L.; Sasmono, R.T.; Rachmawati, H. Antiviral action of curcumin encapsulated in nanoemulsion against four serotypes of dengue virus. Pharm. Nanotechnol. 2020, 8, 54–62. [Google Scholar] [CrossRef]
- Singh, R.K.; Rai, D.; Yadav, D.; Bhargava, A.; Balzarini, J.; De Clercq, E. Synthesis, antibacterial and antiviral properties of curcumin bioconjugates bearing dipeptide, fatty acids and folic acid. Eur. J. Med. Chem. 2010, 45, 1078–1086. [Google Scholar] [CrossRef]
- Ting, D.; Dong, N.; Fang, L.; Lu, J.; Bi, J.; Xiao, S.; Han, H. Multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018, 1, 5451–5459. [Google Scholar] [CrossRef]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat. Prod. Commun. 2010, 5, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, C.M.d.; Monteleoni, L.F.; Calmon, M.d.F.; Cândido, N.M.; Provazzi, P.J.S.; Lino, V.d.S.; Rabachini, T.; Sichero, L.; Villa, L.L.; Quintana, S.M. Antiviral activity of curcumin-nanoemulsion associated with photodynamic therapy in vulvar cell lines transducing different variants of HPV-16. Artif. Cells Nanomed. Biotechnol. 2020, 48, 515–524. [Google Scholar] [CrossRef]
- Ma, Y.; Frutos-Beltrán, E.; Kang, D.; Pannecouque, C.; De Clercq, E.; Menéndez-Arias, L.; Liu, X.; Zhan, P. Medicinal chemistry strategies for discovering antivirals effective against drug-resistant viruses. Chem. Soc. Rev. 2021, 50, 4514–4540. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, L.; Li, S.; Sun, Y.; Ding, M.; Wang, Y.; Zhao, Y.; Wu, Y.; Shang, W.; Jiang, X.J.P.; et al. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein Cell 2020, 11, 723–739. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Lee, S.-Y.; Kim, B.-J. Naturally occurring hepatitis B virus reverse transcriptase mutations related to potential antiviral drug resistance and liver disease progression. World J. Gastroenterol. 2018, 24, 1708. [Google Scholar] [CrossRef]
- Rhee, S.-Y.; Taylor, J.; Wadhera, G.; Ben-Hur, A.; Brutlag, D.L.; Shafer, R.W. Genotypic predictors of human immunodeficiency virus type 1 drug resistance. Proc. Natl. Acad. Sci. USA 2006, 103, 17355–17360. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Choudhury, M.D.; Bhattacharya, M.K.; Upadhyaya, H. Curcumin as potential therapeutic natural product: A nanobiotechnological perspective. J. Pharm. Pharmacol. 2016, 68, 1481–1500. [Google Scholar] [CrossRef]
- Talodthaisong, C.; Plaeyao, K.; Mongseetong, C.; Boonta, W.; Srichaiyapol, O.; Patramanon, R.; Kayunkid, N.; Kulchat, S.J.N. The Decoration of ZnO Nanoparticles by Gamma Aminobutyric Acid, Curcumin Derivative and Silver Nanoparticles: Synthesis, Characterization and Antibacterial Evaluation. Nanomaterials 2021, 11, 442. [Google Scholar] [CrossRef]
- Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Tomita, M.; Kawakami, H.; Uchihara, J.-n.; Okudaira, T.; Masuda, M.; Takasu, N.; Matsuda, T.; Ohta, T.; Tanaka, Y.; Mori, N. RETRACTED: Curcumin suppresses constitutive activation of AP-1 by downregulation of JunD protein in HTLV-1-infected T-cell lines. Leuk. Res. 2006, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Divya, C.S.; Pillai, M.R. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2006, 45, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Prusty, B.K.; Das, B.C. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int. J. Cancer 2005, 113, 951–960. [Google Scholar] [CrossRef]
- Dutta, K.; Ghosh, D.; Basu, A. Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin–proteasome system. J. Neuroimmune Pharmacol. 2009, 4, 328–337. [Google Scholar] [CrossRef]
- Si, X.; Wang, Y.; Wong, J.; Zhang, J.; McManus, B.M.; Luo, H. Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J. Virol. 2007, 81, 3142–3150. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, K.H.; Kim, H.Y.; Cho, H.K.; Sakamoto, N.; Cheong, J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010, 584, 707–712. [Google Scholar] [CrossRef]
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antivir. Res. 1999, 42, 219–226. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Shien, J.-H.; Tiley, L.; Chiou, S.-S.; Wang, S.-Y.; Chang, T.-J.; Lee, Y.-J.; Chan, K.-W.; Hsu, W.-L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, L.J.; Dezube, B.J.; Crumpacker, C.S.; Pardee, A.B. Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc. Natl. Acad. Sci. USA 1993, 90, 1839–1842. [Google Scholar] [CrossRef]
- Mazumder, A.; Raghavan, K.; Weinstein, J.; Kohn, K.W.; Pommier, Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995, 49, 1165–1170. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; An, Z.; Chen, H.; Wang, Z.; Liu, L. Mechanism of curcumin resistance to human cytomegalovirus in HELF cells. BMC Complement. Altern. Med. 2014, 14, 284. [Google Scholar] [CrossRef]
- Ferreira, V.H.; Nazli, A.; Dizzell, S.E.; Mueller, K.; Kaushic, C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE 2015, 10, e0124903. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ding, X.; Zhang, D.; Ch, Y.; Wang, J.; Ndegwa, E.; Zhu, G. Curcumin inhibits bovine herpesvirus type 1 entry into MDBK cells. Acta Virol. 2015, 59, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Kojima, T.; Masaki, T.; Okabayashi, T.; Yokota, S.; Hirakawa, S.; Nomura, K.; Takasawa, A.; Murata, M.; Tanaka, S. Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS ONE 2013, 8, e70225. [Google Scholar] [CrossRef]
- Manoharan, Y.; Haridas, V.; Vasanthakumar, K.C.; Muthu, S.; Thavoorullah, F.F.; Shetty, P. Curcumin: A Wonder Drug as a Preventive Measure for COVID-19 Management. Indian J. Clin. Biochem. 2020, 35, 373–375. [Google Scholar] [CrossRef]
- D’Souza, S.P.; Chavannavar, S.V.; Kanchanashri, B.; Niveditha, S.B. Pharmaceutical perspectives of spices and condiments as alternative antimicrobial remedy. J. Evid.-Based Integr. Med. 2017, 22, 1002–1010. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Javdani, M.; Nikousefat, Z.; Selvaggi, M.; Tufarelli, V.; Laudadio, V. The use of turmeric (Curcuma longa) in poultry feed. World’s Poult. Sci. J. 2012, 68, 97–103. [Google Scholar] [CrossRef]
- Rao, R.V.; Descamps, O.; John, V.; Bredesen, D.E. Ayurvedic medicinal plants for Alzheimer’s disease: A review. Alzheimer’s Res. Ther. 2012, 4, 22. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F.; et al. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid.-Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed]
- Malgaonkar, M.; Shirolkar, A.; Murthy, S.N.; Pawar, S. Ayurvedic Plants with Antidiabetic Potential. In Medicinal Plants-Recent Advances in Research and Development; Springer: Berlin/Heidelberg, Germany, 2016; pp. 439–468. [Google Scholar]
- Krup, V.; Prakash, L.H.; Harini, A. Pharmacological activities of turmeric (Curcuma longa Linn): A review. J. Homeopath. Ayurvedic Med. 2013, 2, 1000133. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Rajkumari, S.; Sanatombi, K. Nutritional value, phytochemical composition, and biological activities of edible Curcuma species: A review. Int. J. Food Prop. 2017, 20, S2668–S2687. [Google Scholar] [CrossRef]
- Gilani, S.J.; Imam, S.S.; Jafar, M.; Alshehri, S.; Taleuzzaman, M.; Jahangir, M.A. Curcumin Nanomedicine and Their Application in the Management of Disease. In Biomarkers as Targeted Herbal Drug Discovery; Apple Academic Press: Palm Bay, FL, USA, 2021; pp. 43–63. [Google Scholar]
- Khezri, K.; Saeedi, M.; Mohammadamini, H.; Zakaryaei, A.S. A comprehensive review of the therapeutic potential of curcumin nanoformulations. Phytother. Res. 2021, 35, 5527–5563. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, Y.; Yang, X.; Ling, G.; Zhang, P. Application of curcumin nanoformulations in Alzheimer’s disease: Prevention, diagnosis and treatment. Nutr. Neurosci. 2022, 1–16. [Google Scholar] [CrossRef]
- Quispe, C.; Herrera-Bravo, J.; Javed, Z.; Khan, K.; Raza, S.; Gulsunoglu-Konuskan, Z.; Daştan, S.D.; Sytar, O.; Martorell, M.; Sharifi-Rad, J.; et al. Therapeutic applications of curcumin in diabetes: A review and perspective. BioMed Res. Int. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M.; et al. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef]
- Haghnegahdar, N.; Abbasi Tarighat, M.; Dastan, D. Curcumin-functionalized nanocomposite AgNPs/SDS/MWCNTs for electrocatalytic simultaneous determination of dopamine, uric acid, and guanine in co-existence of ascorbic acid by glassy carbon electrode. J. Mater. Sci. Mater. Electron. 2021, 32, 5602–5613. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, L.; Ye, S.; Kang, Y.; Liu, J.; Zeng, S.; Yu, L. Research and development of drug delivery systems based on drug transporter and nano-formulation. Asian J. Pharm. Sci. 2020, 15, 220–236. [Google Scholar] [CrossRef]
- Walker, D.A.; Kowalczyk, B.; de la Cruz, M.O.; Grzybowski, B.A. Electrostatics at the nanoscale. Nanoscale 2011, 3, 1316–1344. [Google Scholar] [CrossRef] [PubMed]
- Halevas, E.; Mavroidi, B.; Swanson, C.H.; Smith, G.C.; Moschona, A.; Hadjispyrou, S.; Salifoglou, A.; Pantazaki, A.A.; Pelecanou, M.; Litsardakis, G. Magnetic cationic liposomal nanocarriers for the efficient drug delivery of a curcumin-based vanadium complex with anticancer potential. J. Inorg. Biochem. 2019, 199, 110778. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, Y.; Rana, S.; Miranda, O.R.; Dubin, P.L.; Rotello, V.M.; Sun, L.; Guo, X. Electrostatic selectivity in protein-nanoparticle interactions. Biomacromolecules 2011, 12, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; Van der Meeren, P. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Greer, J.M.; Fenton, O.S.; Kreuels, K.; Anderson, D.G.; Langer, R. Exploiting Electrostatic Interactions in Polymer-Nanoparticle Hydrogels. ACS Macro Lett. 2015, 4, 848–852. [Google Scholar] [CrossRef]
- Sims, K.R., Jr.; He, B.; Koo, H.; Benoit, D.S.W. Electrostatic Interactions Enable Nanoparticle Delivery of the Flavonoid Myricetin. ACS Omega 2020, 5, 12649–12659. [Google Scholar] [CrossRef]
- Chen, S.; McClements, D.J.; Jian, L.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Core-Shell Biopolymer Nanoparticles for Co-Delivery of Curcumin and Piperine: Sequential Electrostatic Deposition of Hyaluronic Acid and Chitosan Shells on the Zein Core. ACS Appl. Mater. Interfaces 2019, 11, 38103–38115. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sklapani, A. Xanthan-based polysaccharide/protein nanoparticles: Preparation, characterization, encapsulation and stabilization of curcumin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100075. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhao, C.; Zhang, H.; Chi, Y.; Wang, L.; Zhang, H.; Bai, S.; Zhang, X. Entrapment of curcumin in soy protein isolate using the pH-driven method: Nanoencapsulation and formation mechanism. LWT 2022, 153, 112480. [Google Scholar] [CrossRef]
- Vatanparast, H.; Shahabi, F.; Bahramian, A.; Javadi, A.; Miller, R. The Role of Electrostatic Repulsion on Increasing Surface Activity of Anionic Surfactants in the Presence of Hydrophilic Silica Nanoparticles. Sci. Rep. 2018, 8, 7251. [Google Scholar] [CrossRef]
- Bian, T.; Gardin, A.; Gemen, J.; Houben, L.; Perego, C.; Lee, B.; Elad, N.; Chu, Z.; Pavan, G.M.; Klajn, R. Electrostatic co-assembly of nanoparticles with oppositely charged small molecules into static and dynamic superstructures. Nat. Chem. 2021, 13, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Karatas, D.; Tekin, A.; Bahadori, F.; Celik, M.S. Interaction of curcumin in a drug delivery system including a composite with poly(lactic-co-glycolic acid) and montmorillonite: A density functional theory and molecular dynamics study. J. Mater. Chem. B 2017, 5, 8070–8082. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Celik, C.; Gencay, A.; Ocsoy, I. Can food and food supplements be deployed in the fight against the COVID-19 pandemic? Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129801. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. The effect of curcumin on the risk of mortality in patients with COVID-19: A systematic review and meta-analysis of randomized trials. Phytother. Res. 2022. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Xie, Y.; Tang, L.S.; Pu, D.; Zhu, Y.J.; Liu, J.Y.; Ma, X.L. Therapeutic targets and interventional strategies in COVID-19: Mechanisms and clinical studies. Signal Transduct. Target. Ther. 2021, 6, 317. [Google Scholar] [CrossRef]
- Krumm, Z.A.; Lloyd, G.M.; Francis, C.P.; Nasif, L.H.; Mitchell, D.A.; Golde, T.E.; Giasson, B.I.; Xia, Y. Precision therapeutic targets for COVID-19. Virol. J. 2021, 18, 66. [Google Scholar] [CrossRef]
- Matthews, D.A.; Dragovich, P.S.; Webber, S.E.; Fuhrman, S.A.; Patick, A.K.; Zalman, L.S.; Hendrickson, T.F.; Love, R.A.; Prins, T.J.; Marakovits, J.T.; et al. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. USA 1999, 96, 11000–11007. [Google Scholar] [CrossRef]
- Bruningk, S.C.; Klatt, J.; Stange, M.; Mari, A.; Brunner, M.; Roloff, T.-C.; Seth-Smith, H.; Schweitzer, M.; Leuzinger, K.; Sogaard, K.K. Determinants of SARS-CoV-2 transmission to guide vaccination strategy in an urban area. Virus Evol. 2022, 8, veac002. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Bhattacharjee, P. Promising role of curcumin against viral diseases emphasizing COVID-19 management: A review on the mechanistic insights with reference to host-pathogen interaction and immunomodulation. J. Funct. Foods 2021, 82, 104503. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.; Freire, D.T.; Pereira, D.T.; Amaral-Machado, L.; Alencar, N.; de Barros, A.L.B.; Egito, E.S.T. Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials? Biomed. Pharmacother. 2021, 139, 111578. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef]
- Widjaja, S.S.; Rusdiana, R.; Amelia, R. Curcumin: Boosting the immunity of COVID-19-vaccinated populations. J. Adv. Pharm. Technol. Res. 2020, 13, 187. [Google Scholar] [CrossRef]
- Suresh, P.S. Curcumin and Coagulopathy in the COVID-19 Era. Indian J. Clin. Biochem. 2020, 35, 504–505. [Google Scholar] [CrossRef]
- Hassaniazad, M.; Eftekhar, E.; Inchehsablagh, B.R.; Kamali, H.; Tousi, A.; Jaafari, M.R.; Rafat, M.; Fathalipour, M.; Nikoofal-Sahlabadi, S.; Gouklani, H.; et al. A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother. Res. 2021, 35, 6417–6427. [Google Scholar] [CrossRef]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral Curcumin with Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front. Pharmacol. 2021, 12, 669362. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Azimi, M.; Haddadi-Asl, V.; Ahmadi, H.; Gholamzad, M.; Ghorbanpour, S.; Bahador, A. Robust antimicrobial photodynamic therapy with curcumin-poly (lactic-co-glycolic acid) nanoparticles against COVID-19: A preliminary in vitro study in Vero cell line as a model. Photodiagnosis Photodyn. Ther. 2021, 34, 102286. [Google Scholar] [CrossRef]
- Rattis, B.A.C.; Ramos, S.G.; Celes, M.R.N. Curcumin as a Potential Treatment for COVID-19. Front. Pharmacol. 2021, 12, 675287. [Google Scholar] [CrossRef]
- Bormann, M.; Alt, M.; Schipper, L.; van de Sand, L.; Le-Trilling, V.T.K.; Rink, L.; Heinen, N.; Madel, R.J.; Otte, M.; Wuensch, K.; et al. Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro. Viruses 2021, 13, 1914. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Sahebkar, A.; Soleimani, D.; Mahdavi, A.; Rafiee, S.; Majeed, M.; Khorvash, F.; Iraj, B.; Elyasi, M.; Rouhani, M.H. The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: A randomized double-blind, placebo-controlled trial. Trials 2022, 23, 472. [Google Scholar] [CrossRef] [PubMed]
- Askari, G.; Alikiaii, B.; Soleimani, D.; Sahebkar, A.; Mirjalili, M.; Feizi, A.; Iraj, B.; Bagherniya, M.J.T. Effect of curcumin-pipeine supplementation on clinical status, mortality rate, oxidative stress, and inflammatory markers in critically ill ICU patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials 2021, 22, 434. [Google Scholar] [CrossRef]
- Schraufstätter, E.; Bernt, H. Antibacterial action of curcumin and related compounds. Nature 1949, 164, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–546. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Alafiatayo, A.A.; Lai, K.-S.; Syahida, A.; Mahmood, M.; Shaharuddin, N.A.; Medicine, A. Phytochemical evaluation, embryotoxicity, and teratogenic effects of Curcuma longa extract on zebrafish (Danio rerio). Evid.-Based Complement. Altern. Med. 2019, 2019, 3807207. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Cheng, A.-L. Clinical studies with curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2007; pp. 471–480. [Google Scholar]
- Kuptniratsaikul, V.; Thanakhumtorn, S.; Chinswangwatanakul, P.; Wattanamongkonsil, L.; Thamlikitkul, V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J. Altern. Complement. Med. 2009, 15, 891–897. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Kumar, N.V.A.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Izumi, Y.; Asakura, K.; Hayashi, Y.; Nomori, H. Curcumin induces autophagy in ACC-MESO-1 Cells. Phytother. Res. 2012, 26, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.N.; Bhatt, M.; Purohit, K.; Acharya, J.; Kumar, R.; Garg, R.J. Clinical and radiological evaluation of turmeric powder as a pulpotomy medicament in primary teeth: An in vivo study. Int. J. Clin. Pediatr. Dent. 2017, 10, 37. [Google Scholar]
- Ullah, F.; Liang, A.; Rangel, A.; Gyengesi, E.; Niedermayer, G.; Münch, G. High bioavailability curcumin: An anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol. 2017, 91, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- AloK, A.; Singh, I.D.; Singh, S.; Kishore, M.; Jha, P.C. Curcumin–pharmacological actions and its role in oral submucous fibrosis: A review. J. Clin. Diagn. Res. 2015, 9, ZE01. [Google Scholar] [CrossRef]
- Booker, A.; Chatzinasiou, L.; Heinrich, M.; MacLennan, E.; Mackonochie, M. Turmeric (Curcuma longa L.) products: What quality differences exist? J. Herb. Med. 2019, 17, 100281. [Google Scholar]
- Choi, Y.-H.; Yan, G.-H.; Chai, O.H.; Song, C.H. Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation. Anat. Cell Biol. 2010, 43, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Tian, W.; Shen, H. Curcumin prevents induced drug resistance: A novel function? Chin. J. Cancer Res. 2011, 23, 218–223. [Google Scholar] [CrossRef]
- Bhatt, N.A.; Singh, A.; Sharma, R. Pharmacological Activities of Curcuma Longa: A Review. Eur. J. Mol. Clin. Med. 2021, 8, 2021. [Google Scholar]
- Dohare, P.; Garg, P.; Jagannathan, N.; Ray, M. Neuroprotective efficacy and therapeutic window of curcuma oil: In rat embolic stroke model. BMC Complementary Altern. Med. 2008, 8, 55. [Google Scholar] [CrossRef]
- Dhiman, A.K. Common Drug Plants and Ayurvedic Remedies; Reference Press: New Delhi, India, 2004. [Google Scholar]
- Oluwafemi, R.; Olawale, I.; Alagbe, J.; Sciences, V. Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition—A review. Res. Agric. Vet. Sci. 2020, 4, 5–11. [Google Scholar]
- Park, J.; Conteas, C.N. Anti-carcinogenic properties of curcumin on colorectal cancer. World J. Gastrointest. Oncol. 2010, 2, 169. [Google Scholar] [CrossRef] [PubMed]
- Parasramka, M.A.; Gupta, S.V. Synergistic effect of garcinol and curcumin on antiproliferative and apoptotic activity in pancreatic cancer cells. J. Oncol. 2012, 2012, 709739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Taxa | Name |
|---|---|
| Domain | Eukaryota |
| Kingdom | Plantae |
| Phylum | Spermatophyta |
| Subphylum | Angiospermae |
| Class | Monocotyledonae |
| Order | Zingiberales |
| Family | Zingiberaceae |
| Genus | Curcuma |
| Species | Curcuma longa |
| Curcumin Based Drug | Output | Remarks | References |
|---|---|---|---|
| Nanocurcumin was generated by a self-nanoemulsion technique that resulted in a nanoemulsion with uniform distribution of average droplet size of 40.85 ± 0.919 nm. | Nanocurcum prepared exhibited higher cell cytotoxicity than curcumin solution and may be explained by better cell uptake. Nanocurcumin treatment suppressed dengue virus (DENV) growth, although no significant difference was observed compared to the curcumin solution counterpart. Greater virus reduction was observed for DENV-1 and DENV-2. | Synthesized nano curcumin improved curcumin physicochemical properties with potential as an antiviral against DENV | [76] |
| Curcumin Modified Silver Nanoparticles | Curcumin-modified silver nanoparticles (cAgNPs) exhibit a highly effective inhibitory impact against respiratory syncytial virus (RSV) infection, resulting in a two-order-of-magnitude reduction in viral titres at the 15 AgNP doses tested, with no damage to the host cells. | Uniform and stable curcumin silver nanoparticles (AgNPs) with antiviral properties | [70] |
| Synthesized curcumin bioconjugates bearing dipeptide, fatty acids and folic acid | The synthesized curcumin bioconjugates have antiviral activities against HSV, VSV, FIPV, PIV-3, RSV and FHV, and di-O-tryptophanyl molecules phenylalanine curcumin (2), di-O-decanoyl curcumin (3), had good results with EC50 0.011 mM and 0.029 mM against VSV and FIPV/FHV, respectively. | The increased antiviral activities against a wide range of viruses | [77] |
| Synthesized uniform and stable cationic carbon dots (CCM-CDs) | Porcine epidemic diarrhoea virus (PEDV) is used as a coronavirus model. The cationic CCM-CDs treatment could inhibit the proliferation of PEDV compared with the common CDs (EDA-CDs). The CCM-CDs treatment changes the surface protein structure in viruses, thereby inhibiting viral entry, suppressing the synthesis of negative-strand RNA, budding of the virus, and accumulation of reactive oxygen species (ROS) by PEDV. F CCM-CDs treatment was also found to suppress viral replication by stimulating the production of interferon-stimulating genes (ISGs) and pro-inflammatory cytokines. | Development of CCM-CDs as a potential antiviral agent for the treatment of coronavirus infections, including PEDV | [78] |
| Curcumin, gallium-curcumin, Cu-curcumin | curcumin and its new derivatives have remarkable antiviral effects on HSV-1 in cell culture. | Reduction of HSV-1 replication | [79,80] |
| Curcumin, curcumin boron complexes | Inhibition of HIV-1 and HIV-2 proteases | [60] | |
| Empty nanoemulsion (NE-V) and the nanoemulsion of curcumin (NE-CUR) | The cell viability assay showed that the empty nanoemulsion (NE-V) and the curcumin nanoemulsion (NE-CUR) had little effect on cell viability compared to control cells. Additionally, we observed that cells irradiated in NE-CUR presence presented 90% of cell death. The apoptosis assay further revealed a significant increase in caspases 3 and 7 in A431 cells expressing both HPV-16 E6 variants after treatment with NE-CUR, indicating a potential antiviral effect. | HPV | [81] |
| Substance | Antiviral Activity | Virus | References |
|---|---|---|---|
| Curcumin | Decreased regulation of Jun D protein in HTLV-1-infected T-cell lines | HTLV-1 | [89] |
| Curcumin | Inhibition expression of viral oncoproteins of E6 and E7 and Decreased regulation effect on the transcription of HPV-18 | HPV | [90,91] |
| Curcumin | Reduced production of infective viral particles | JEV | [92] |
| Curcumin | HCV replication decreased by the Akt-SREBP-1 pathway suppression | HVC | [7] |
| Curcumin | Replication inhibition through UPS dysregulation | Coxsackievirus | [93] |
| aqueous Curcumin extract | Increasing the p53 level leads to suppression of HBV replication | HBV | [94] |
| Curcumin | Significant protection against HSV-2 in a mouse model | HSV-2 | [95] |
| Curcumin | Haemagglutination inhibition | Influenza | [96] |
| Curcumin | Inhibition of HIV-1 LTR-directed gene expression, Inhibition of HIV-1 Integrase and Curcumin, Inhibition of Tat protein acetylation | HIV | [97,98,99] |
| Curcumin | Immediate early antigen (IEA) and UL83A expression | Human cytomegalovirus (HCMV) | [100] |
| Curcumin | BZLF-1 inhibitor | Epstein-Barr virus (EBV) | [101] |
| Curcumin | Inhibition of viral entry | Bovine herpesvirus 1 (BHV 1) | [102] |
| Curcumin | Interfered with viral life cycle stages | Human norovirus (HuNoV) | [56] |
| Curcumin | Virucidal effect (virus inactivation) | Respiratory syncytial virus (RSV) | [56,103] |
| Curcumin | Viral entry and viral replication | Fish viral haemorrhagic septicaemia virus (VHSV) | [58] |
| Curcumin | Virucidal effect (attack envelope), inhibition of viral entry, and viral replication | Influenza A virus (IAV) | [48,97] |
| Curcumin | [54,104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, B.B.L.; Ripanda, A.S.; Mwanga, H.M. Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa). Compounds 2022, 2, 200-221. https://doi.org/10.3390/compounds2030017
Srivastava BBL, Ripanda AS, Mwanga HM. Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa). Compounds. 2022; 2(3):200-221. https://doi.org/10.3390/compounds2030017
Chicago/Turabian StyleSrivastava, Bajarang Bal Lal, Asha Shabani Ripanda, and Hossein Miraji Mwanga. 2022. "Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa)" Compounds 2, no. 3: 200-221. https://doi.org/10.3390/compounds2030017
APA StyleSrivastava, B. B. L., Ripanda, A. S., & Mwanga, H. M. (2022). Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa). Compounds, 2(3), 200-221. https://doi.org/10.3390/compounds2030017