Influence of Water on Aging Phenomena of Calendric Stored and Cycled Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell System
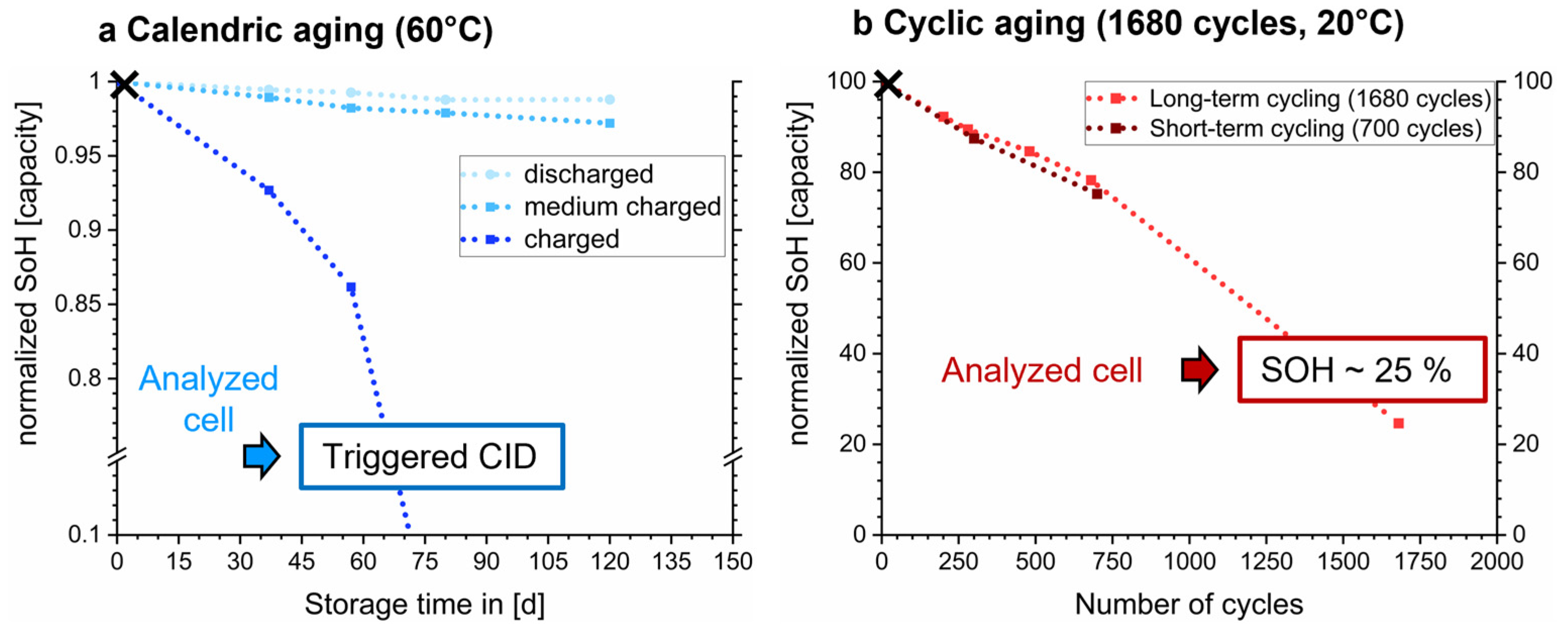
2.2. Aging Scenarios
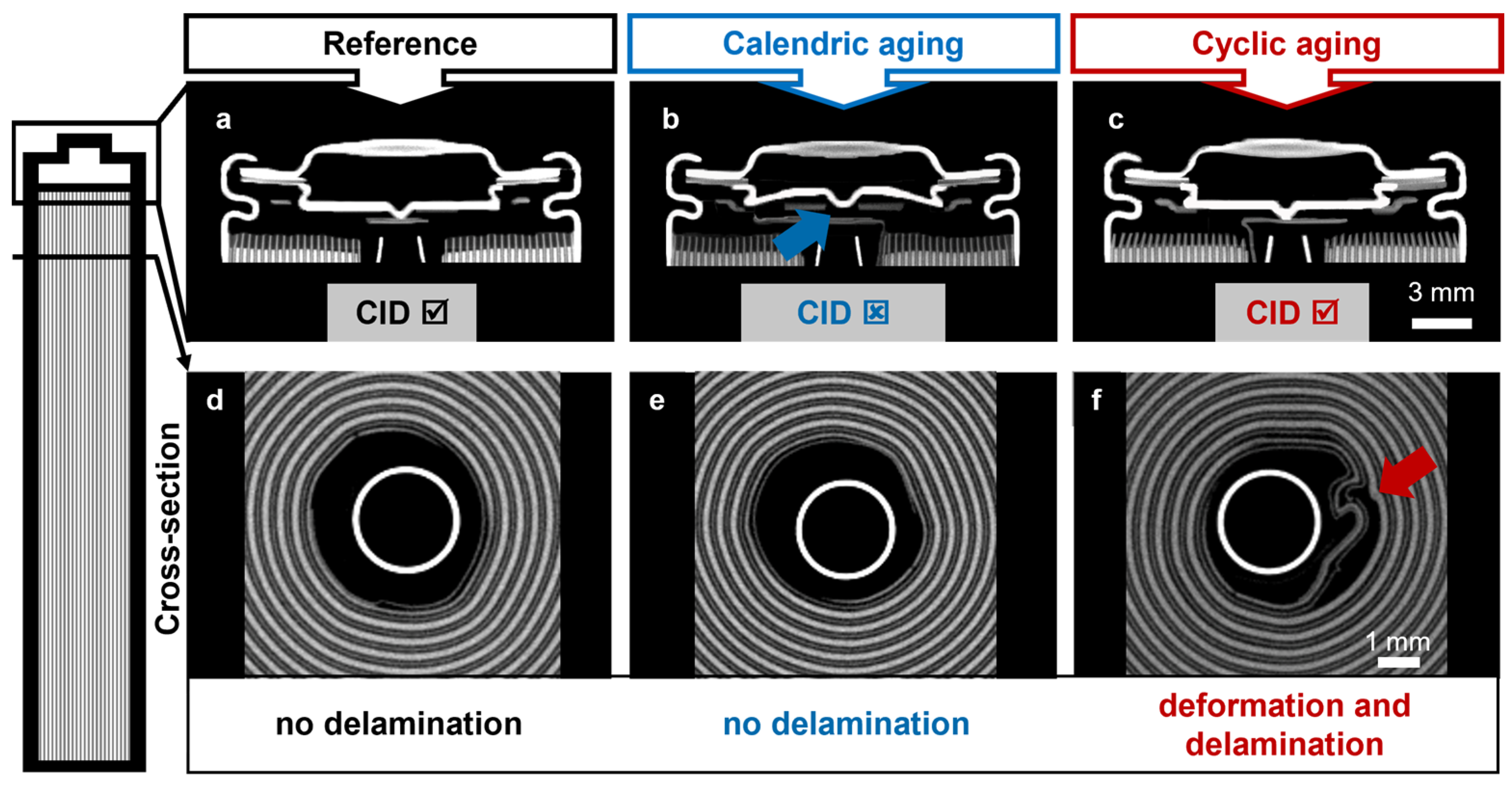
2.3. Computer Tomography (CT)
2.4. Sample Preparation
2.5. Electrolyte Analysis
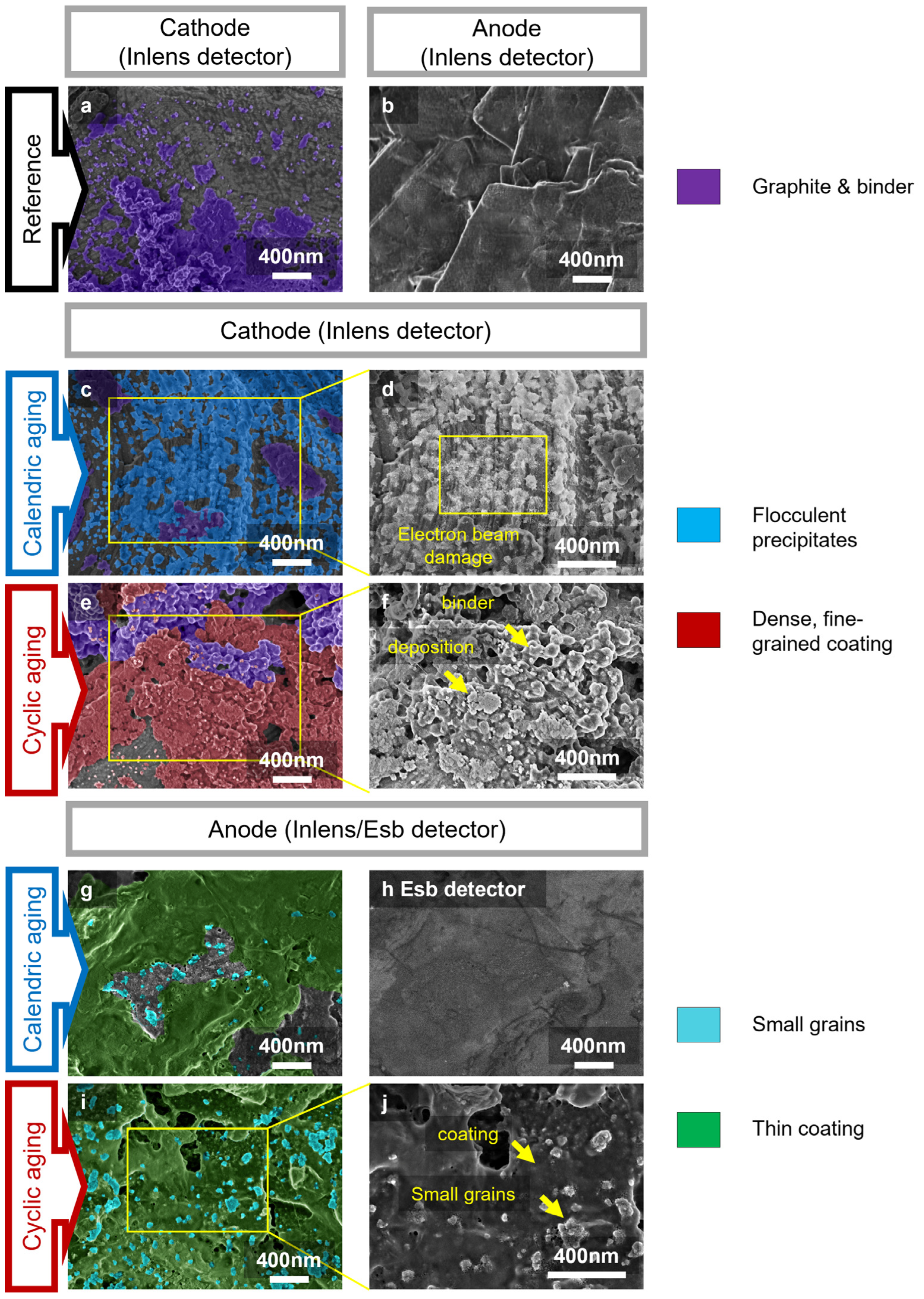
2.6. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
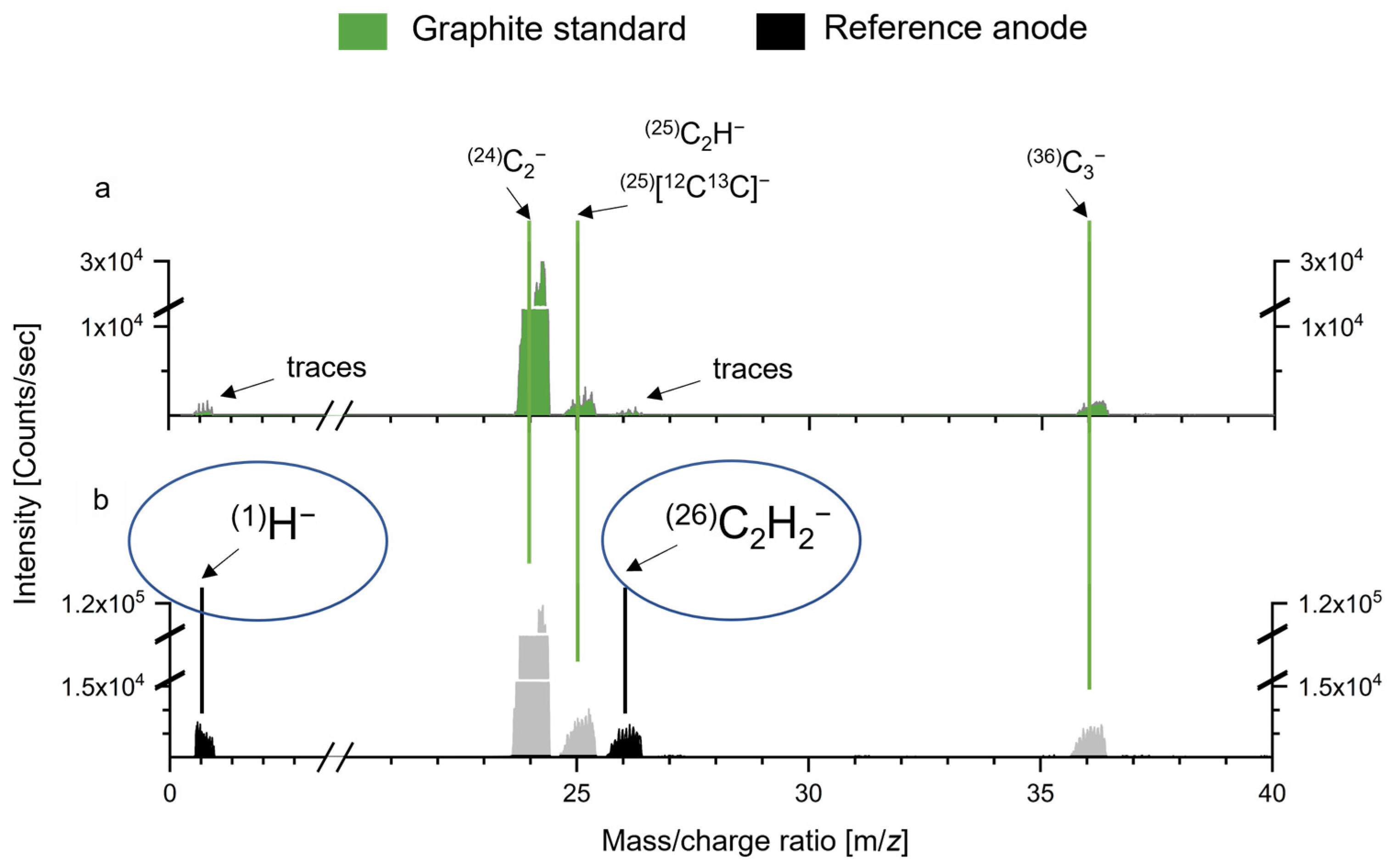
2.7. Secondary Ion Mass Spectrometry (SIMS)
2.8. Karl Fischer Titration
3. Results
3.1. Macroscopic and Microscopic Changes
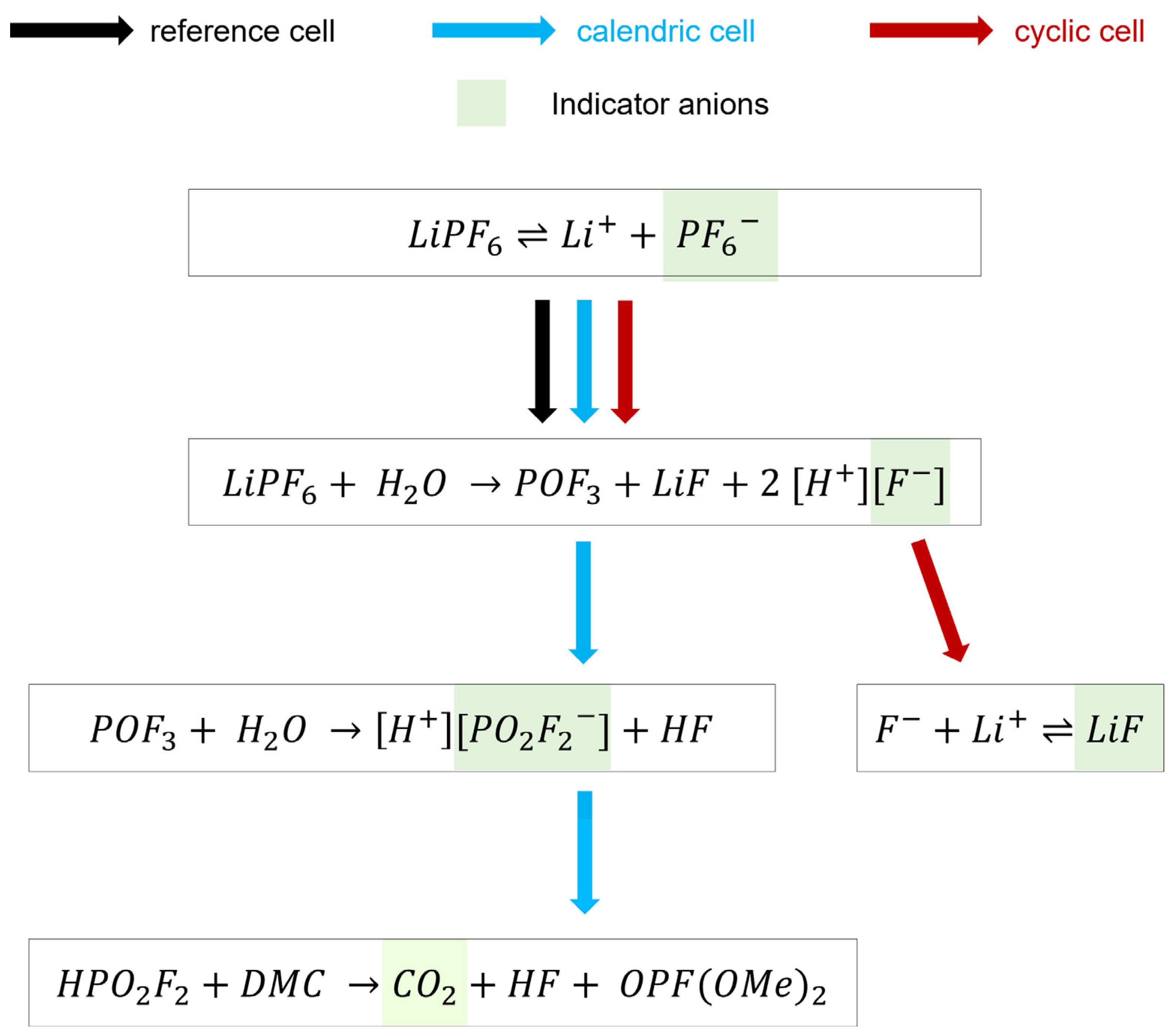
3.2. Presence of Water Causes Electrolyte Degradation
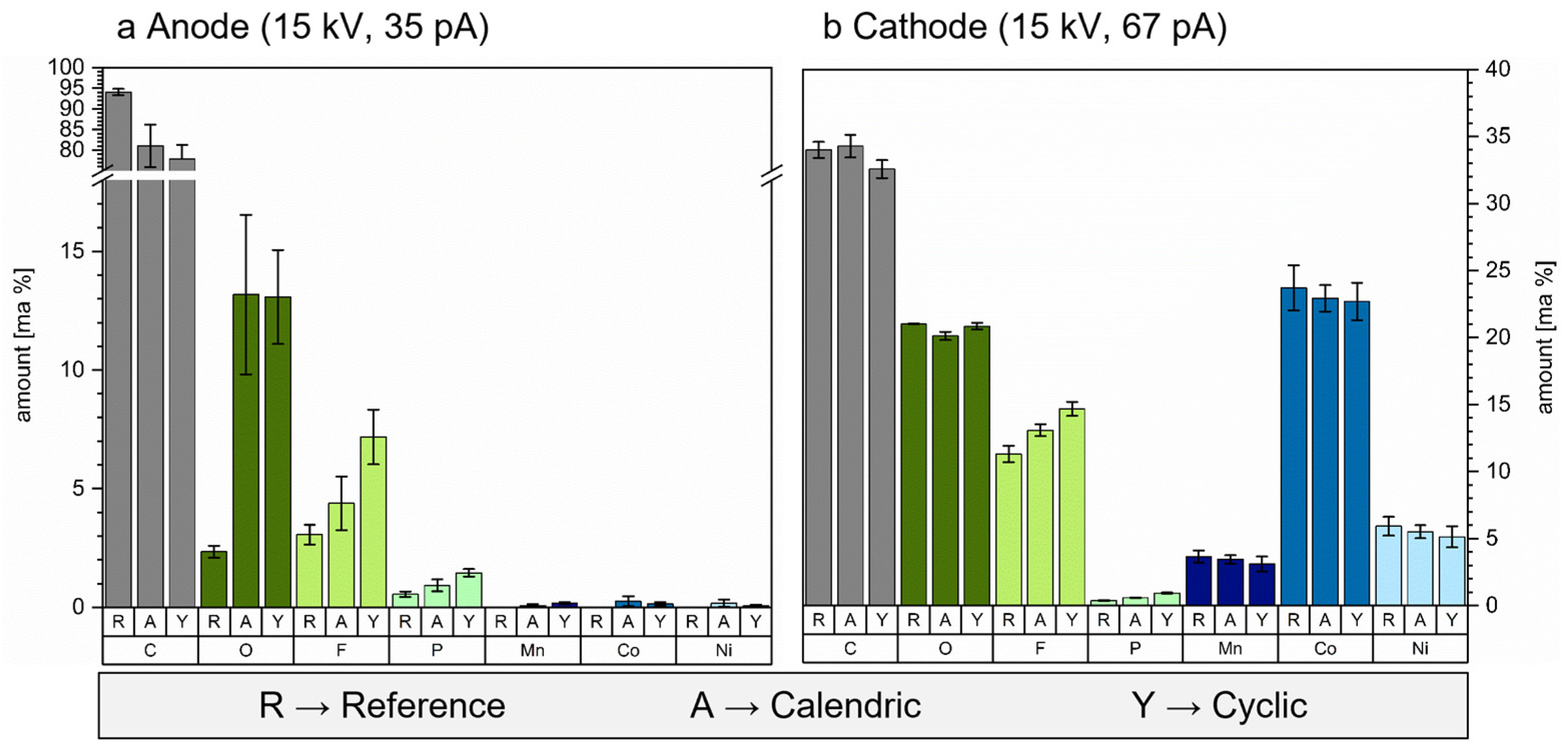
3.3. Water Accelerates Anode and Cathode Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alliata, D.; Kötz, R.; Novak, P.; Siegenthaler, H. Electrochemical SPM investigation of the solid electrolyte interphase film formed on HOPG electrodes. Electrochem. Commun. 2000, 2, 436–440. [Google Scholar] [CrossRef]
- Peled, E.; Tow, D.B.; Merson, A.; Gladkich, A.; Burstein, L.; Golodnitsky, D. Composition, depth profiles and lateral distribution of materials in the SEI built on HOPG-TOF SIMS and XPS studies. J. Power Sources 2001, 97–98, 52–57. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review-SEI: Past, Present and Future. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Levi, E.; Schechter, A. Failure and Stabilization Mechanisms of Graphite Electrodes. J. Phys. Chem. B 1997, 101, 2195–2206. [Google Scholar] [CrossRef]
- Shi, S.; Lu, P.; Liu, Z.; Qi, Y.; Hector, L.G.; Li, H.; Harris, S.J. Direct calculation of Li-ion transport in the solid electrolyte interphase. J. Am. Chem. Soc. 2012, 134, 15476–15487. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Li, F.; Liu, C.; Tan, J.; Cheng, H.-M. New insight into the solid electrolyte interphase with use of a focused ion beam. J. Phys. Chem. B 2005, 109, 22205–22211. [Google Scholar] [CrossRef]
- Edström, K.; Gustafsson, T.; Thomas, J.O. The cathode–electrolyte interface in the Li-ion battery. Electrochim. Acta 2004, 50, 397–403. [Google Scholar] [CrossRef]
- Ceder, G.; Ven, A. Phase diagrams of lithium transition metal oxides: Investigations from first principles. Electrochim. Acta 1999, 45, 131–150. [Google Scholar] [CrossRef]
- Börner, M.; Horsthemke, F.; Kollmer, F.; Haseloff, S.; Friesen, A.; Niehoff, P.; Nowak, S.; Winter, M.; Schappacher, F.M. Degradation effects on the surface of commercial LiNi0.5Co0.2Mn0.3O2 electrodes. J. Power Sources 2016, 335, 45–55. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries. Electrochem. Energ. Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A review of gas evolution in lithium ion batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Liu, Y.-M.; G Nicolau, B.; Esbenshade, J.L.; Gewirth, A.A. Characterization of the Cathode Electrolyte Interface in Lithium Ion Batteries by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 7171–7177. [Google Scholar] [CrossRef]
- Qian, Y.; Niehoff, P.; Börner, M.; Grützke, M.; Mönnighoff, X.; Behrends, P.; Nowak, S.; Winter, M.; Schappacher, F.M. Influence of electrolyte additives on the cathode electrolyte interphase (CEI) formation on LiNi1/3Mn1/3Co1/3O2 in half cells with Li metal counter electrode. J. Power Sources 2016, 329, 31–40. [Google Scholar] [CrossRef]
- Würsig, A.; Buqa, H.; Holzapfel, M.; Krumeich, F.; Novák, P. Film Formation at Positive Electrodes in Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2005, 8, A34–A37. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Q.; Liu, G.; Song, X.; Battaglia, V.S. Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J. Power Sources 2012, 207, 134–140. [Google Scholar] [CrossRef]
- Bessette, S.; Hovington, P.; Demers, H.; Golozar, M.; Bouchard, P.; Gauvin, R.; Zaghib, K. In-Situ Characterization of Lithium Native Passivation Layer in A High Vacuum Scanning Electron Microscope. Microsc. Microanal. 2019, 25, 866–873. [Google Scholar] [CrossRef]
- Otto, S.-K.; Fuchs, T.; Moryson, Y.; Lerch, C.; Mogwitz, B.; Sann, J.; Janek, J.; Henss, A. Storage of Lithium Metal: The Role of the Native Passivation Layer for the Anode Interface Resistance in Solid State Batteries. Appl. Energy Mater. 2021, 4, 12798–12807. [Google Scholar] [CrossRef]
- Lévy, D.; Aléon, J.; Aléon-Toppani, A.; Troadec, D.; Duhamel, R.; Gonzalez-Cano, A.; Bureau, H.; Khodja, H. NanoSIMS Imaging of D/H Ratios on FIB Sections. Anal. Chem. 2019, 91, 13763–13771. [Google Scholar] [CrossRef]
- Lamperti, A.; Bottani, C.E.; Ossi, P.M.; Levi-Setti, R. Focused ion beam-secondary ion mass spectrometry analyses of nanostructured thin films. Surf. Coat. Technol. 2004, 180–181, 323–330. [Google Scholar] [CrossRef]
- Aurbach, D.; Weissmann, I.; Zaban, A.; Dan, P. On the role of water contamination in rechargeable Li batteries. Electrochim. Acta 1999, 45, 1135–1140. [Google Scholar] [CrossRef]
- Dubouis, N.; Serva, A.; Salager, E.; Deschamps, M.; Salanne, M.; Grimaud, A. The Fate of Water at the Electrochemical Interfaces: Electrochemical Behavior of Free Water Versus Coordinating Water. J. Phys. Chem. Lett. 2018, 9, 6683–6688. [Google Scholar] [CrossRef]
- Kitz, P.G.; Novák, P.; Berg, E.J. Influence of Water Contamination on the SEI Formation in Li-Ion Cells: An Operando EQCM-D Study. ACS Appl. Mater. Interfaces 2020, 12, 15934–15942. [Google Scholar] [CrossRef]
- Stich, M. Wasserverunreinigungen in Lithium-Ionen-Batterien. Ph.D. Thesis, Technische Universität Ilmenau, Ilmenau, Germany, 2018. [Google Scholar]
- Grützke, M.; Weber, W.; Winter, M.; Nowak, S. Structure determination of organic aging products in lithium-ion battery electrolytes with gas chromatography chemical ionization mass spectrometry (GC-CI-MS). RSC Adv. 2016, 6, 57253–57260. [Google Scholar] [CrossRef]
- Henschel, J.; Schwarz, J.L.; Glorius, F.; Winter, M.; Nowak, S. Further Insights into Structural Diversity of Phosphorus-Based Decomposition Products in Lithium Ion Battery Electrolytes via Liquid Chromatographic Techniques Hyphenated to Ion Trap-Time-of-Flight Mass Spectrometry. Anal. Chem. 2019, 91, 3980–3988. [Google Scholar] [CrossRef]
- Mönnighoff, X.; Friesen, A.; Konersmann, B.; Horsthemke, F.; Grützke, M.; Winter, M.; Nowak, S. Supercritical carbon dioxide extraction of electrolyte from spent lithium ion batteries and its characterization by gas chromatography with chemical ionization. J. Power Sources 2017, 352, 56–63. [Google Scholar] [CrossRef]
- Schultz, C.; Vedder, S.; Streipert, B.; Winter, M.; Nowak, S. Quantitative investigation of the decomposition of organic lithium ion battery electrolytes with LC-MS/MS. RSC Adv. 2017, 7, 27853–27862. [Google Scholar] [CrossRef]
- Stenzel, Y.P.; Wiemers-Meyer, S.; Edel, J.; Winter, M.; Nowak, S. Analysis of acidic organo(fluoro)phosphates as decomposition product of lithium ion battery electrolytes via derivatization gas chromatography-mass spectrometry. J. Chromatogr. A 2019, 1592, 188–191. [Google Scholar] [CrossRef]
- Wiemers-Meyer, S.; Winter, M.; Nowak, S. Mechanistic insights into lithium ion battery electrolyte degradation—A quantitative NMR study. Phys. Chem. Chem. Phys. 2016, 18, 26595–26601. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, G.V.; Ross, P.N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature dependent ageing mechanisms in Lithium-ion batteries—A Post-Mortem study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]
- Chen, R.; Liu, F.; Chen, Y.; Ye, Y.; Huang, Y.; Wu, F.; Li, L. An investigation of functionalized electrolyte using succinonitrile additive for high voltage lithium-ion batteries. J. Power Sources 2016, 306, 70–77. [Google Scholar] [CrossRef]
- Wang, X.; Xue, W.-D.; Hu, K.; Li, Y.; Huang, R.-Y. Adiponitrile as Lithium-ion battery electrolyte additive: A positive and peculiar effect on high-voltage system. ACS Appl. Energy Mater. 2018, 1, 5347–5354. [Google Scholar] [CrossRef]
- Wiemers-Meyer, S.; Winter, M.; Novak, S. Battery Cell for In Situ NMR Measurements of Liquid Electrolytes. Phys. Chem. Chem. Phys. 2017, 19, 4962–4966. [Google Scholar] [CrossRef]
- Geisbauer, C.; Wöhrl, K.; Koch, D.; Wilhelm, G.; Schneider, G.; Schweiger, H.-G. Comparative Study on the Calendar Aging Behavior of Six Different Lithium-Ion Cell Chemistries in Terms of Parameter Variation. Energies 2021, 14, 3358. [Google Scholar] [CrossRef]
- Niedermeier, J.; Kopp, A.; Schmidt, J.; Schmidt, P.; Bernthaler, T.; Schneider, G. Metrologische Computertomografie zur seriennahen Anwendung an großformatigen Batteriezellen zur Qualitäts- und Funktionsbewertung. In Proceedings of the DGZfP-Jahrestagung, Leipzig, Germany, 7–9 May 2018. [Google Scholar]
- Kermani, G.; Keshavarzi, M.M.; Sahraei, E. Deformation of lithium-ion batteries under axial loading: Analytical model and Representative Volume Element. Energy Rep. 2021, 7, 2849–2861. [Google Scholar] [CrossRef]
- Waldmann, T.; Gorse, S.; Samtleben, T.; Schneider, G.; Knoblauch, V.; Wohlfahrt-Mehrens, M. A Mechanical Aging Mechanism in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1742–A1747. [Google Scholar] [CrossRef]
- Zhu, J.; Koch, M.M.; Lian, J.; Li, W.; Wierzbicki, T. Mechanical Deformation of Lithium-Ion Pouch Cells under In-Plane Loads—Part I: Experimental Investigation. J. Electrochem. Soc. 2020, 167, 90533. [Google Scholar] [CrossRef]
- Garitagoitia Cid, A.; Rosenkranz, R.; Zschech, E. Optimization of the SEM Working Conditions: EsB Detector at Low Voltage. Adv. Eng. Mater. 2016, 18, 185–193. [Google Scholar] [CrossRef]
- Steigerwald, M.D.G.; Arnold, R.; Bihr, J.; Drexel, V.; Jaksch, H.; Preikszas, D.; Vermeulen, J.P. New Detection System for GEMINI. Microsc Microanal 2004, 10, 1372–1373. [Google Scholar] [CrossRef]
- Vickerman, J.C.; Gilmore, I.S. Molecular Surface Mass Spectrometry by SIMS: The Principal Techniques, 2nd ed.; Wiley: Chichester, UK, 2009; ISBN 978-0-470-01763-0. [Google Scholar]
- De Hoffmann, E. Analytical Methods: Mass spectrometry. ECT 2005, 2, 586–683. [Google Scholar]
- Jung, E.Y.; Park, C.-S.; Lee, J.C.; Park, J.-B.; Suh, K.J.; Hong, T.E.; Lee, D.H.; Chien, S.-I.; Tae, H.-S. Influences of graphite electrode on degradation induced by accelerated charging-discharging cycling in lithium-ion battery. Mol. Cryst. Liq. Cryst. 2018, 663, 90–98. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Friesen, A.; Mönnighoff, X.; Börner, M.; Haetge, J.; Schappacher, F.M.; Winter, M. Influence of temperature on the aging behavior of 18650-type lithium ion cells: A comprehensive approach combining electrochemical characterization and post-mortem analysis. J. Power Sources 2017, 342, 88–97. [Google Scholar] [CrossRef]
- Golla-Schindler, U.; Zeibig, D.; Prickler, L.; Behn, S.; Bernthaler, T.; Schneider, G. Characterization of degeneration phenomena in lithium-ion batteries by combined microscopic techniques. Micron 2018, 113, 10–19. [Google Scholar] [CrossRef]
- Marchesini, S.; Reed, B.P.; Jones, H.; Matjacic, L.; Rosser, T.E.; Zhou, Y.; Brennan, B.; Tiddia, M.; Jervis, R.; Loveridge, M.J.; et al. Surface Analysis of Pristine and Cycled NMC/Graphite Lithium-Ion Battery Electrodes: Addressing the Measurement Challenges. ACS Appl. Mater. Interfaces 2022, 14, 52779–52793. [Google Scholar] [CrossRef]
- Schuster, S.F.; Bach, T.; Fleder, E.; Müller, J.; Brand, M.; Sextl, G.; Jossen, A. Nonlinear aging characteristics of lithium-ion cells under different operational conditions. J. Energy Storage 2015, 1, 44–53. [Google Scholar] [CrossRef]
- Stockhausen, R.; Gehrlein, L.; Müller, M.; Bergfeldt, T.; Hofmann, A.; Müller, F.J.; Maibach, J.; Ehrenberg, H.; Smith, A. Investigating the dominant decomposition mechanisms in lithium-ion battery cells responsible for capacity loss in different stages of electrochemical aging. J. Power Sources 2022, 543, 231842. [Google Scholar] [CrossRef]
- Weisenberger, C.; Meir, B.; Röhler, S.; Harrison, D.K.; Knoblauch, V. A post-mortem study of commercial 18650 lithium-ion cells with LiNi0.5Co0.2Mn0.3O2//Graphite chemistry after prolonged cycling (> 7000 cycles) with low C-rates. Electrochim. Acta 2021, 379, 138145. [Google Scholar] [CrossRef]
- Lu, P.; Li, C.; Schneider, E.W.; Harris, S.J. Chemistry, Impedance, and Morphology Evolution in Solid Electrolyte Interphase Films during Formation in Lithium Ion Batteries. J. Phys. Chem. C 2014, 118, 896–903. [Google Scholar] [CrossRef]
- Björklund, E.; Xu, C.; Dose, W.M.; Sole, C.G.; Thakur, P.K.; Lee, T.-L.; de Volder, M.F.L.; Grey, C.P.; Weatherup, R.S. Cycle-Induced Interfacial Degradation and Transition-Metal Cross-Over in LiNi0.8Mn0.1Co0.1O2-Graphite Cells. Chem. Mater. 2022, 34, 2034–2048. [Google Scholar] [CrossRef]
- Gorse, S.; Kugler, B.; Schneider, G.; Knoblauch, V.; Samtleben, T.; Waldmann, T.; Wohlfahrt-Mehrens, M. An Explanation of the Ageing Mechanism of Li-Ion Batteries by Metallographic and Material Analysis. Pract. Metallogr. 2014, 51, 829. [Google Scholar] [CrossRef]
- Börger, E.M.; Jochler, E.; Kaufmann, J.; Ramme, R.; Grimm, A.; Nowak, S.; Schappacher, F.M.; Rodehorst, U.; Voigt, A.-C.; Passerini, S.; et al. Aging of ceramic coated graphitic negative and NCA positive electrodes in commercial lithium-ion battery cells—An ex-situ study of different states of health for identification and quantification of aging influencing parameters. J. Energy Storage 2017, 13, 304–312. [Google Scholar] [CrossRef]
- Jayawardana, C.; Rodrigo, N.D.; Rynearson, L.; Lucht, B.L. Difluorophosphoric Acid Generation and Crossover Reactions in LiNixCoyMnzO2 Cathodes Operating at High Voltage. J. Electrochem. Soc. 2022, 169, 60509. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, D.-H.; Shin, J.H.; Jang, J.E.; Ahn, K.H.; Lee, C.; Lee, H. Ionic Conduction and Solution Structure in LiPF 6 and LiBF 4 Propylene Carbonate Electrolytes. J. Phys. Chem. C 2018, 122, 19438–19446. [Google Scholar] [CrossRef]
- Jin, Y.; Kneusels, N.-J.H.; Marbella, L.E.; Castillo-Martínez, E.; Magusin, P.C.M.M.; Weatherup, R.S.; Jónsson, E.; Liu, T.; Paul, S.; Grey, C.P. Understanding Fluoroethylene Carbonate and Vinylene Carbonate Based Electrolytes for Si Anodes in Lithium Ion Batteries with NMR Spectroscopy. J. Am. Chem. Soc. 2018, 140, 9854–9867. [Google Scholar] [CrossRef]
- Michan, A.L.; Parimalam, B.S.; Leskes, M.; Kerber, R.N.; Yoon, T.; Grey, C.G.; Lucht, B.L. Fluoroethylene Carbonate and Vinylene Carbonate Reduction: Understanding Lithium-ion Battery Electrolyte Additives and Solid Electrolyte Interphase Formation. Chem. Mater. 2016, 28, 8149–8159. [Google Scholar] [CrossRef]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energ. Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Sloop, S.E.; Kerr, J.B.; Kinoshita, K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge. J. Power Sources 2003, 119–121, 330–337. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Tesfamhret, Y. Transition Metal Dissolution from Li-Ion Battery Cathodes. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2022. [Google Scholar]
- Hunter, J. Preparation of a New Crystal Form of Manganese Dioxide: Y-MnO2. J. Solid State Chem. 1981, 39, 142–147. [Google Scholar] [CrossRef]
- Besli, M.M.; Xia, S.; Kuppan, S.; Huang, Y.; Metzger, M.; Shukla, A.K.; Schneider, G.; Hellstrom, S.; Christensen, J.; Doeff, M.M.; et al. Mesoscale Chemomechanical Interplay of the LiNi0.8Co0.15Al0.05O2 Cathode in Solid-State Polymer Batteries. Chem. Mater. 2019, 31, 491–501. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Z.; Baggetto, L.; Veith, G.M.; More, K.L.; Unocic, R.R. Unraveling manganese dissolution/deposition mechanisms on the negative electrode in lithium ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 10398–10402. [Google Scholar] [CrossRef]
- Meunier, V.; de Souza, M.L.; Morcrette, M.; Grimaud, A. Electrochemical Protocols to Assess the Effects of Dissolved Transition Metal in Graphite/LiNiO2 Cells Performance. J. Electrochem. Soc. 2022, 169, 70506. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the Degradation Mechanisms of LiNi0.5Co0.2Mn0.3O2 Cathode Material in Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A review of Ni-based layered oxides for reachargeable Li-ion batteries. J. Mater. Chem. A 2017, 5, 874–901. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Soto, F.A.; Nie, A.; Yuan, Y.; Asayesh-Ardakani, H.; Foroozan, T.; Yurkiv, V.; Song, B.; Mashayek, F.; Klie, R.F.; et al. Facet-Dependent Thermal Instability in LiCoO2. Nano Lett. 2017, 17, 2165–2171. [Google Scholar] [CrossRef]
- Jung, D.-H.; Umirov, N.; Kim, T.; Bakenov, Z.; Kim, J.-S.; Kim, S.-S. Thermal and Structural Stabilities of Lix CoO2 Cathode for Li Secondary Battery Studied by a Temperature Programmed Reduction. Eurasian Chem.-Technol. J. 2019, 21, 3–12. [Google Scholar] [CrossRef]
- Kikkawa, J.; Shohei Terada, S.; Gunji, A.; Nagai, T.; Kurashima, K.; Kimoto, K. Chemical States of Overcharged LiCoO2 Particle Surfaces and Interiors Observed Using Electron Energy-Loss Spectroscopy. J. Phys. Chem. 2015, 119, 15823–15830. [Google Scholar] [CrossRef]
- Fuller, E.J.; Ashby, D.S.; Polop, C.; Salagre, E.; Bhargava, B.; Song, Y.; Vasco, E.; Sugar, J.D.; Albertus, P.; Menteş, T.O.; et al. Imaging Phase Segregation in Nanoscale LixCoO2 Single Particles. ACS Nano 2022, 16, 16363–16371. [Google Scholar] [CrossRef]


| Reference | |
|---|---|
| ethylene carbonate (EC) [ma %] | 25.2 |
| ethyl methyl carbonate (EMC) [ma %] | 17.2 |
| dimethyl carbonate (DMC) [ma %] | 52.6 |
| diethylcarbonate (DEC) [ma %] | 0.35 |
| fluoroethylene carbonate (FEC) [ma %] | 2.7 |
| succinonitrile [ma %] | 0.4 |
| adiponitrile | Peak overlap with EMDOHC |
| Anode | Cathode | |
|---|---|---|
| Acceleration voltage [kV] | 15 | 15 |
| Current [pA] | 35 | 67 |
| Working distance [mm] | 5 | 5 |
| Magnification | 5000 | 5000 |
| Reference | Calendric Aged | Cyclic Aged | |
|---|---|---|---|
| Fluoride (F−) | 0.15 mol/L | <LOD 1 | <LOD 1 |
| Hexafluorophosphate (PF6−) | 0.79 mol/L | 1.17 mol/L | 1.34 mol/L |
| Difluorophosphate (PO2F2−) | <LOD 1 | 33 mmol/L | <LOD 1 |
| Fluoroethylene carbonate (FEC) | 2.7 ma % | 2.4 ma % | <LOD 1 |
| Succinonitrile | 0.4 ma % | <LOD 1 | <LOD 1 |
| Adiponitrile | detected | <LOD 1 | <LOD 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilhelm, G.; Golla-Schindler, U.; Wöhrl, K.; Geisbauer, C.; Cooke, G.; Bernthaler, T.; Schweiger, H.-G.; Schneider, G. Influence of Water on Aging Phenomena of Calendric Stored and Cycled Li-Ion Batteries. Nanoenergy Adv. 2024, 4, 174-195. https://doi.org/10.3390/nanoenergyadv4020011
Wilhelm G, Golla-Schindler U, Wöhrl K, Geisbauer C, Cooke G, Bernthaler T, Schweiger H-G, Schneider G. Influence of Water on Aging Phenomena of Calendric Stored and Cycled Li-Ion Batteries. Nanoenergy Advances. 2024; 4(2):174-195. https://doi.org/10.3390/nanoenergyadv4020011
Chicago/Turabian StyleWilhelm, Gudrun, Ute Golla-Schindler, Katharina Wöhrl, Christian Geisbauer, Graham Cooke, Timo Bernthaler, Hans-Georg Schweiger, and Gerhard Schneider. 2024. "Influence of Water on Aging Phenomena of Calendric Stored and Cycled Li-Ion Batteries" Nanoenergy Advances 4, no. 2: 174-195. https://doi.org/10.3390/nanoenergyadv4020011
APA StyleWilhelm, G., Golla-Schindler, U., Wöhrl, K., Geisbauer, C., Cooke, G., Bernthaler, T., Schweiger, H.-G., & Schneider, G. (2024). Influence of Water on Aging Phenomena of Calendric Stored and Cycled Li-Ion Batteries. Nanoenergy Advances, 4(2), 174-195. https://doi.org/10.3390/nanoenergyadv4020011







